Abstract
Plants that undergo C4 photosynthesis, such as maize, are enriched in the stable isotope of carbon (13C) compared with other dietary plants and foods. Consumption of maize that has been biofortified to contain elevated levels of provitamin A carotenoids (orange maize) increased the abundance of 13C in serum retinol of Mongolian gerbils. We evaluated this method in humans to determine if it has potential for further use in intervention effectiveness studies. A random subset of samples from a two-month randomized controlled feeding trial of rural three- to five-year old Zambian children were used to determine the impact of orange maize intake on serum carotenoid concentrations (n = 88) and 13C-natural abundance in serum retinol (n = 77). Concentrations of β-cryptoxanthin (a xanthophyll provitamin A carotenoid) and the dihydroxy xanthophylls lutein and zeaxanthin, which do not have vitamin A activity, were elevated in children consuming orange maize compared with those consuming a white maize control (P < 0.001), while β-carotene was not different (P > 0.3). Furthermore, 13C natural abundance was higher after two months’ intervention in the orange maize group compared with the white maize group (P = 0.049). Predictions made from equations developed in the aforementioned gerbil study estimated that maize provided 11% (2–21%, 95% confidence interval) of the recent dietary vitamin A to these children. These results demonstrate that orange maize is efficacious at providing retinol to the vitamin A pool in children through provitamin A carotenoids, as monitored by the change in 13C enrichment, which was not reflected in serum β-carotene concentrations. Further effectiveness studies in countries who have adopted orange maize should consider determining differences in retinol 13C-enrichment among target groups in addition to profiling serum xanthophyll carotenoids with specific emphasis on zeaxanthin.
Impact statement
Maize biofortified with provitamin A carotenoids (orange) has been released in some African markets. Responsive and sensitive methods to evaluate dissemination effectiveness are needed. This study investigated methods to evaluate effectiveness of orange maize consumption using serum from Zambian children fed orange maize for two months. Many varieties of orange maize contain higher amounts of the xanthophyll carotenoids in addition to β-carotene compared with typical varieties. This study uniquely showed higher concentrations of the maize xanthophylls lutein, zeaxanthin, and β-cryptoxanthin in children who consumed orange maize compared with white. Furthermore, maize is a C4 plant and is therefore naturally enriched with 13C. Higher 13C was detected in the serum retinol of the orange maize consumers with no change in serum β-carotene concentration suggesting preferential bioconversion to retinol. The combined analyses of serum zeaxanthin specifically and 13C-natural abundance of retinol could prove useful in effectiveness studies between orange maize adopters and non-adopters.
Keywords: Biomarkers, lutein, natural abundance, retinol isotope dilution, vitamin A, zeaxanthin
Introduction
In 2011, earlier guidelines were confirmed by the World Health Organization to provide children 6–59 months of age in developing countries with vitamin A (VA) as periodic high-dose supplements to reduce the effects of VA deficiency, including growth faltering, immune system defects, and overall childhood mortality.1 Many of the participating countries also implemented fortification programs, in which preformed VA is added to staples, such as flour, sugar, or oil, in order to increase VA consumption among most members of the population.2 Since then, simulations have determined that VA intake among some groups can exceed the tolerable upper limit of intake in countries with fortification of multiple foods.3 Although minimally invasive biomarkers for high levels of total body VA are still being established,4 a study in Zambian children (n = 133) showed that 59% had baseline VA liver reserves above that considered to be hypervitaminotic (>1 µmol/g liver) determined by retinol isotope dilution (RID).5 This finding was attributed to a combination of a successful supplementation program, mandatory table sugar fortification, and a diet naturally high in provitamin A carotenoids.6,7
Over half of caloric intake in African countries can come from staple foods.8 Biofortification of staple crops (e.g. maize, rice, sorghum) with β-carotene and β-cryptoxanthin has been proposed as a method to address VA deficiency while mitigating the risk of VA overconsumption through fortified foods.3 This approach has several key advantages over supplementation and fortification programs with preformed VA. First, these VA precursors have additional steps of regulation through modulation of absorption and bioconversion to retinal, which is responsive to body VA stores through retinoic acid signaling.9 In Mongolian gerbils, cleavage of provitamin A carotenoids to VA occurs at a slower rate when liver stores are >0.4 µmol retinol/g, giving evidence that regulation occurs on an organism-wide scale.10
Efficacy trials of biofortified crops have used serum retinol concentrations and/or the modified relative dose response (MRDR)4 test in South Africa,11 Zambia,12,13 and Kenya.14 These methods estimate VA deficiency prevalence but do not determine total body stores.4 The RID method, originally described by Bausch and Rietz15 and updated for use with 13C,16 quantitatively determines and compares total body VA stores before and after interventions. The 13C-RID test used in Zambia5 involved administration of an extrinsic, synthetic 13C-retinyl acetate dose and determination of the ratio of unlabeled to labeled retinol using mass spectrometry, followed by appropriate calculations.17 Increases in 13C enrichment of serum retinol in response to doses of 13C-retinyl acetate form the basis of the RID method. On the other hand, relatively little work has been done on changes in the natural abundance of 13C in serum retinol in response to dietary changes.18 Plants that undergo C4 photosynthesis, like maize and sorghum, are enriched in 13C.19 In maize, 13C makes up 1.09% of total carbon, compared with 1.07% in carrots.18 A 60-day study in male Mongolian gerbils, showed an increase in 13C enrichment in serum retinol in those consuming provitamin A carotenoids from orange maize compared with those consuming carotenoids from carrots.18
A three-month vegetable (predominantly C3 plants) intervention study in overweight adults demonstrated a shift to lower 13C in serum retinol.20 Therefore, higher 13C enrichment of serum retinol compared with an appropriate control may serve as a useful biomarker for effectiveness studies of provitamin A carotenoid-enriched maize. In this study, we hypothesized that orange maize consumption will show higher 13C-enrichment in serum over that of children consuming white maize devoid of provitamin A carotenoids after a two-month intervention in Zambia. Given that orange maize has been released in some African markets21 and some VA biomarkers are not sensitive (e.g. serum retinol, retinol-binding protein), and others require VA dosing and time constraints (e.g. dose response, RID tests),4 responsive, sensitive, and minimally invasive methods are needed to measure effectiveness. Biofortification is a desirable, sustainable intervention because providing VA as provitamin A carotenoids is safer than preformed VA fortification;10 therefore, measuring biofortification impact is important.
Subjects and methods
Subjects and study design
The feeding protocol used in this trial was previously published,22 and is summarized below. This study examined the two month interval of that trial during which provitamin A carotenoid biofortified (orange) or traditional white maize was fed. The orange maize (CI7XDexp3) was approximately half of the target level of 15 µg β-carotene equivalents/g.23 All procedures involving human subjects were approved by the University of Wisconsin-Madison (UW) Health Sciences Human Subjects Institutional Review Board and the Zambian Tropical Diseases Research Center Ethics Review Committee. Children (4.5 ± 0.9 y; n = 195) were recruited for the randomized, controlled maize feeding trial. Baseline anthropometric and biochemical characteristics are listed in Table 1. Written informed consent was obtained from the participants’ caregivers. Exclusion criteria included severe malnutrition and anemia.12
Table 1.
Baseline anthropometric measurements by intervention and analysis group.22
| Group |
Orange maize |
White maize |
||
|---|---|---|---|---|
| Measurement | Main study (n = 91a) | Substudy (n = 47a) | Main study (n = 87) | Substudy (n = 47) |
| Weight (kg) | 14.8 ± 1.9b | 14.7 ± 1.9c | 14.8 ± 1.9 | 14.9 ± 2.1c |
| Height (cm) | 98.3 ± 7.0 | 98.1 ± 6.7 | 98.6 ± 7.0 | 98.4 ± 7.4 |
| Age (mo) | 52.9 ± 10.2 | 52.7 ± 10.7 | 54.5 ± 10.3 | 53.4 ± 10.0 |
| C-reactive protein (mg/L) | 3.22 ± 3.79 | 3.71 ± 4.05 | 2.52 ± 3.79 | 2.73 ± 3.69 |
| α1-acid glycoprotein (g/L) | 1.44 ± 0.48 | 1.50 ± 0.52 | 1.40 ± 0.48 | 1.30 ± 0.51 |
| Inflammationd (%) | 70.3 | 70.8 | 59.8 | 48.9 |
| Low liver VA storese (%) | 8.7 | 6.3 | 13.7 | 14.9 |
One analyzed subject in the orange maize main study and substudy is not included in this analysis due to missing anthropometric data.
Values are means ± SD.
All values in each substudy were determined to be not significantly different from the full group of subjects, P > 0.3 by two-tailed Student’s t-test or by two-proportion Z-test as was appropriate.
Defined as CRP > 5 mg/L and/or AGP > 1.2 g/L, indicative of acute or chronic inflammation.24
Defined as modified relative dose response value >0.060.25
For the study reported herein, participants were fed breakfast, lunch, and snack 6 days/week for 46 days (excludes Sundays). The only difference between the two groups was the type of maize used in the standardized menu. A single genotype of biofortified maize was fed, which did lose some provitamin A activity during storage.22 Dietary intakes of orange and white maize were similar; data on the adaptation of the children to consuming the orange maize22 and the impact of malaria on dietary intake have been published.26
Blood samples were collected after two months to determine mid-term intervention serum carotenoids and 13C enrichment of serum retinol. This blood sample was taken mid-intervention because a one-week washout period was included at the end of the main study and would have interfered with peak carotenoid and 13C measurements at endline. Samples were centrifuged at 2800 × g for 15 min. Serum aliquots were stored at −80℃ in Zambia until shipped to UW under nitrogen. Acute phase proteins, i.e. C-reactive protein (CRP) and α1-acid glycoprotein (AGP), were analyzed at baseline (Table 1) and the end of the three month main trial.13 If a child had either CRP or AGP elevated at both timepoints, they were considered to have chronic inflammation.
Carotenoid analysis
Maize carotenoid concentrations were determined as previously described.27,28 For serum carotenoid analysis, 500 µL serum was mixed with 500 µL ethanol to denature proteins and β-apo-8′-carotenal was used as an internal standard. Three 1 mL hexane extractions were performed, pooled, and dried under nitrogen. Samples were resuspended in 100 µL methanol, and 50 µL was injected onto a modified HPLC system from Yeum et al.29 Differences included a Waters 1525 binary pump and 996 photodiode array detector (Waters, Milford, MA, USA) equipped with a YMC carotenoid column (2.0 × 150 mm, Wilmington, NC, USA). Solvent A was methanol:methyl-tert-butyl ether:water (83:15:2, v/v/v, 1.5% ammonium acetate in the water) and solvent B was methanol:methyl-tert-butyl ether:water (8:90:2, v/v/v, 1% ammonium acetate in the water). The gradient used a 0.5 mL/min flow rate: (1) 100% A for 5 min; (2) a 5-min linear gradient to 70% A; (3) a 15-min linear gradient to 45% A; (4) a 3-min hold at 45% A; (5) a 2-min linear gradient to 100% A.
Serum retinol 13C enrichment
Serum retinol was analyzed for 13C enrichment by gas chromatography/combustion/isotope ratio-mass spectrometry (GC-C-IRMS) as previously described.5
Statistical analysis
Statistical analyses were performed with Excel (Microsoft, Redmond, WA, USA). Differences between intervention and control serum carotenoids and serum retinol 13C enrichment were determined by one-tailed Student’s t-test due to the predetermined nature of the intervention as an enrichment over control. The effect of infection status, qualitative VA liver stores, or carotenoid concentrations on serum retinol 13C enrichment was analyzed post hoc by multi-way ANOVA with SAS software (version 9.4; SAS Institute, Cary, NC, USA). The two-tailed Student’s t-test and two-proportion Z-test were used to compare anthropomorphic data between the larger study’s intervention groups and the subjects analyzed in this substudy. Values are expressed as means ± SD, significance was set at α = 0.05. For future effectiveness studies, power calculations were performed to determine the number of subjects needed per evaluation group to show significance using a two-tailed test given the difference observed using the formula:
| (1) |
where α is the desired probability of a false positive (i.e. 0.05), β is the desired probability of a false negative (generally 0.2), f(α,β) is a factor based on α and (1–β), here 10.5, σ is the standard deviation of the measurements of all of the subjects and μw and μo are the means of the serum retinol 13C atom percent values for the white and orange maize-consuming groups, respectively.
Results
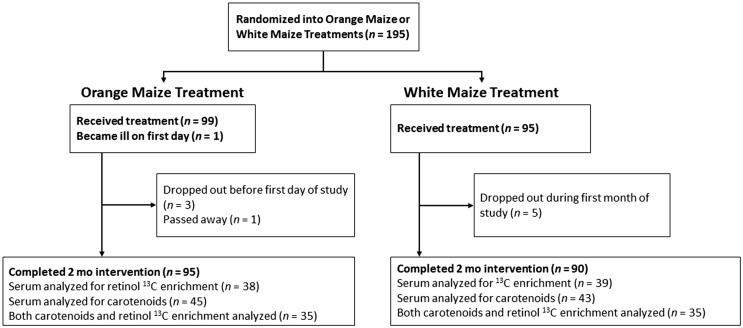
Ninety-five percent of children enrolled completed the feeding study up to two months (185 subjects, Figure 1). Reasons for discontinuation of the study occurred due to non-compliance with requirements and persistent, severe illnesses. Anthropometric and biochemical characteristics did not differ from those of the total subjects enrolled (P > 0.3). Due to sample volume restrictions in these young children, approximately half (47 in each group) of the subjects’ samples were available for either serum carotenoids, 13C-retinol, or both analyses. The assay chosen for serum carotenoids by HPLC required 0.5 mL and the 13C-enrichment in serum retinol by GC-C-IRMS required 1 to 1.5 mL for measurements at natural abundance.
Figure 1.
CONSORT flow diagram of subjects who initiated and completed the two-month intervention and were analyzed for carotenoids and/or 13C-enrichment in serum retinol
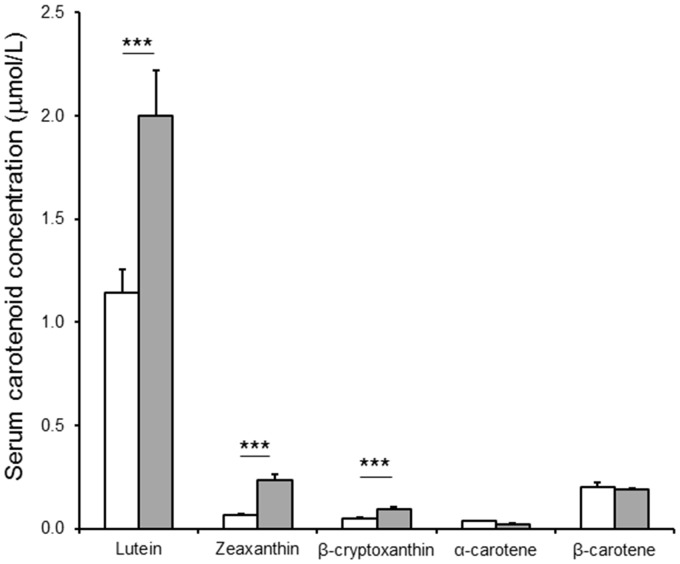
Maize carotenoid concentrations are reported in Table 2. After 46 days of consuming either orange or white maize, serum lutein, zeaxanthin, and β-cryptoxanthin were significantly elevated in the orange maize group over white maize controls (P < 0.001), while α- and β-carotene did not differ (P > 0.2) (Figure 2).
Table 2.
Carotenoid composition of orange maize used in the trial and typical Zambian white maize
| Maize | Typical Zambian white maize30a | Provitamin A carotenoid- biofortified orange maize22 |
|---|---|---|
| Zeaxanthin (µg/g) | 0.02 ± 0.01 b | 2.04 ± 0.41 |
| Lutein (µg/g) | 0.02 ± 0.03 | 5.96 ± 1.46 |
| β-carotene (µg/g) | NDc | 4.80 ± 1.30 |
| β-cryptoxanthin (µg/g) | ND | 0.32 ± 0.05 |
| α-carotene (µg/g) | ND | 0.14 ± 0.04 |
| Theoretical vitamin A (µg/g) | Negligibled | 5.36 ± 1.44 |
Typical Zambian white maize described here was obtained from the same region, two years after the study presented in this paper and is included for reference only.
Values are means ± SD.
ND; Not detectable. The limit of detection was 0.005 µg/g.
Considering non-detectable levels of provitamin A carotenoids, little vitamin A activity is expected.
Figure 2.
Serum carotenoid concentrations of subjects following 46 days of consumption of white (n = 45, white bars) or provitamin A carotenoid-biofortified orange (n = 43, grey bars) maize. Values shown as mean ± SEM. ***P < 0.001; one-tailed Student’s t-test
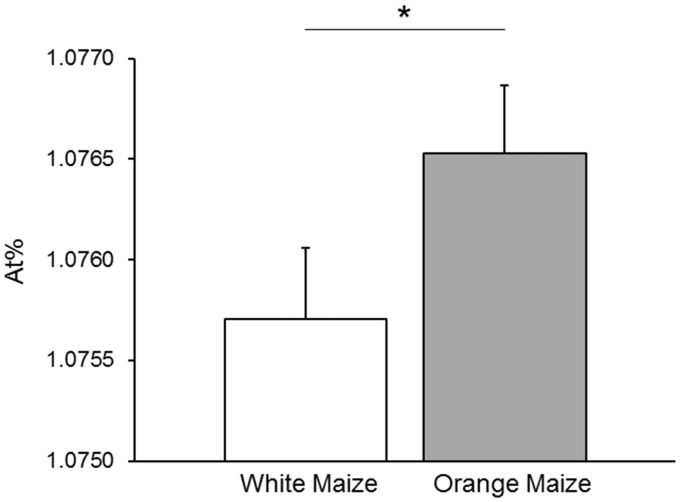
In this controlled feeding study, the orange maize group showed a significantly higher 13C enrichment than the white maize group (Figure 3; P = 0.049). Serum retinol 13C enrichment was not affected by chronic infection, defined as AGP >1.2 g/L or CRP >5 mg/L at both baseline and endpoint, (P > 0.3) or low baseline liver stores of VA (MRDR value > 0.060, P > 0.3) as determined in the main study.12 Total serum carotenoid concentration did not correlate with serum retinol 13C-enrichment with or without grouping by intervention (P > 0.3).
Figure 3.
13C natural abundance in serum retinol (mean ± SEM) expressed as At% in subjects following 46 days of control white (n = 38) or provitamin A carotenoid-biofortified orange maize (n = 39) consumption. *P < 0.05; one-tailed Student’s t-test. At%, atom percent of 13C in total carbon determined by GC-C-IRMS
Previously, a study in Mongolian gerbils that received VA from C3 orange carrots, C4 orange maize, a combination, or no VA demonstrated that serum retinol 13C enrichment could be used as a biomarker for orange maize consumption.18 Prediction equations were generated from these gerbils to determine the proportion of dietary VA coming from orange maize. Using this concept, the serum retinol 13C enrichment values for the white maize-consuming children were plotted as 0% dietary VA and the original gerbil data point after consuming only orange maize was assigned 100%, resulting in the following equation
| (2) |
where At% is the atom percent of 13C in total carbon determined by GC-C-IRMS.
This equation was applied to the data from the orange maize-consuming children to predict that 11% (2–21%, 95% confidence interval) of dietary VA came from the orange maize.
Discussion
Previous work has shown that long-term consumption of carotenoids will result in increased concentrations in the serum,13,31 making them a potential biomarker of provitamin A carotenoid-biofortified crop effectiveness. In this study, the xanthophyll concentrations were higher in the orange maize-fed group versus the white group, but β-carotene was not higher after feeding mid-provitamin A target level maize for 46 days. In an intervention with orange maize that had much higher β-carotene concentrations, β-carotene concentrations increased in Zambian children without a change in serum retinol compared with white maize control.13 Furthermore, β-carotene increased in Kenyan children fed biofortified yellow cassava with a modest increase in serum retinol.14 Notably, however, β-carotene did not accumulate in serum in the current study despite being the main provitamin A carotenoid present in the orange maize,22 likely reflecting its bioconversion and contribution to the retinol pool, demonstrated by the positive 13C enrichment change in serum retinol. Considering that the level of carotenoid biofortification in the maize was relatively low compared with that used in older Zambian children,5,13 more complete bioconversion of the β-carotene would be expected during controlled carotenoid-restricted feeding. The serum β-carotene concentrations in these younger children (0.19 ± 0.31 µmol/L) were identical to that found in older Zambian children from a different area (0.18 µmol/L),13 and lower than that measured in older children (0.57–0.74 µmol/L) from the same villages.6 Thus, serum carotenoid concentrations alone are not sufficient to determine the contribution of an intervention to retinol stores.
The changes observed in lutein, zeaxanthin, and β-cryptoxanthin reflected the increased concentration of these carotenoids in the orange maize over white maize. β-Cryptoxanthin and zeaxanthin specifically were found to be quite low in the serum of older Zambian children from the same community,6 indicating that these carotenoids are not a normal part of the traditional diet. The increased presence of these compounds in the serum after the orange maize intervention, therefore, makes them candidates for biomarkers of intervention effectiveness. While serum β-cryptoxanthin would likely have similar variation as β-carotene due to its regulated bioconversion to retinal,9 increases in the xanthophyll zeaxanthin in the diet would be a direct qualitative method to measure consumption of orange maize varieties that have been released in Zambia.21 Another important component of this approach is monitoring other potential sources of zeaxanthin in the diet, such as eggs from hens fed biofortified maize,32 some green leafy vegetables, and various fruits.33
In combination with serum zeaxanthin, enrichment of serum retinol 13C natural abundance shows definite promise as a biomarker for effectiveness of orange maize dissemination and adoption, and will require further research to strengthen the association between consumption of orange maize over time and accumulation of 13C-enriched retinol. Our positive results reflect the opposite trend observed in another study where consumption of provitamin A carotenoids from C3 plants decreased the ratio of 13C in serum retinol in a three-month vegetable intervention to promote weight loss in humans.20 Furthermore, our study corroborates a gerbil study in which provitamin A carotenoids from C4 orange maize increased 13C in serum retinol.18 The serum retinol 13C enrichment observed in the gerbils was much more dramatic than that measured in our study,18 likely for two reasons.
First, the gerbils were initially fed a VA-deficient feed and thus any additional intake above the feed would make up a larger percentage of the total VA stores, influencing the mean enrichment. After completing a second study in the same area in Zambia, we ascertained that it is possible for older Zambian children to have high VA levels corresponding to approximately 700 µmol total body retinol stores.5 In comparison, the intake in this study was the equivalent of ∼1 µmol retinol through orange maize provitamin A carotenoids each day.22 Thus, this low intake, even over two month, would make up about 10% of high VA stores. Indeed, application of equation (2) confirmed this estimate with a value of 11% of dietary VA coming from the maize. The second Zambian trial was performed two years later in older children and VA stores accumulate over time on the background of adequate status and widespread fortification.10 In these younger children, low liver stores were determined by the MRDR test in 19% of the children at the end of this study.12 Thus, the positive shift in 13C retinol and no increases in serum β-carotene support bioconversion to retinol in these children.
Second, biofortified maize was 50% of the gerbils’ feed without other confounding sources of VA present in the maize-only group, unlike these children who were fed dried whole fish and pumpkin leaves (both low 13C VA sources) in the controlled rotating menu two days/week.22 This dilution of 13C enriched provitamin A carotenoid intake by C3-plants and preformed VA likely decreased the 13C proportion of total carbon in retinol, diminishing the difference between the intervention and control groups. The study menu was designed to reflect routine food intake patterns of this community.34
Decreasing VA stores or completely eliminating other VA intake among children in an intervention study is difficult and somewhat unethical, even in a highly controlled study. Increased power must therefore come from somewhere other than a change in the difference between the two groups. Considering the calculation for sample size in equation (1), the most viable source of statistical power would be to increase n, given α = 0.05 and assuming the standard deviation σ is unlikely to change due to heterogeneity of the population. Practically, we recommend increasing sample size to accommodate the smaller differences in human populations without controlled diets. In this case, we calculated that increasing n above 110 subjects per group will increase the probability of detecting a difference of the size seen in this study from about 40% to 80% and the ability to use two-tailed statistical methods for effectiveness studies where the interventions are not controlled. Development of a more precise method of measuring 13C abundance could also reduce error due to instrument variation. Statistical power could also be increased by collecting baseline serum samples and assaying 13C enrichment before intervention studies or biofortified crop release. In this case, comparing changes in enrichment over time instead of comparison of enrichment post-study would result in decreased variance and provide a greater ability to detect intervention changes.
Another factor to consider is the time requirement for typical effectiveness studies, which is often >1 year after introduction of an intervention into a population. This study used two-month data from an efficacy study to evaluate potential biomarkers. A longer effectiveness study that simultaneously measures habitual dietary intake of orange maize and targets communities with high adoption rates allows more time for the body pool to accurately reflect the diet.
Conclusion
Analysis of 13C enrichment in serum retinol will provide valuable information about the effectiveness of provitamin A carotenoid-biofortified maize interventions in treating VA deficiency worldwide. Serum provitamin A carotenoids are more qualitative and will not necessarily detect an effect on VA deficiency if preferentially bioconverted to retinol with no accumulation. We have demonstrated a significant change in the 13C-retinol biomarker in response to consumption of orange maize for two months; however, future studies should increase sample size and study length to have more power to detect differences in effectiveness trials after crop dissemination. Further refinements in study design could also increase power of this technique to demonstrate the contribution of the biofortified foods to retinol stores; however, these changes must be balanced against cost and time constraints.
Acknowledgements
The authors thank Peter Crump, UW-Madison College of Agricultural and Life Sciences Statistical Consulting Service, for statistical analysis consultation; Emily Nuss for analyzing the carotenoids; and Kevin Pixley and Natalia Palacios-Rojas for overseeing the orange maize production used in this study. This work was supported by Global Health funds at UW-Madison during manuscript preparation and HarvestPlus contract number 8217 for the fieldwork. HarvestPlus (www.harvestplus.org) is a global alliance of agriculture and nutrition research institutions working to increase the micronutrient density of staple food crops through biofortification. The views expressed do not necessarily reflect those of HarvestPlus.
Authors’ contributions
JS wrote the manuscript. BG and CD performed 13C-retinol GC-C-IRMS analysis on the serum samples. JS and BG analyzed the data. SAT designed the study and revised the manuscript. All authors reviewed and approved this paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.WHO. Guideline: vitamin A supplementation in infants and children 6–59 months of age, Geneva, Switzerland: World Health Organization, 2011. [PubMed] [Google Scholar]
- 2.Wirth JP, Petry N, Tanumihardjo SA, Rogers LM, McLean E, Greig A, Garrett GS, Klemm RDW, Rohner F. Vitamin A supplementation programs and country-level evidence of vitamin A deficiency. Nutrients 2017; 9: 190–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanumihardjo SA, Mokhtar N, Haskell MJ, Brown KH. Assessing the safety of vitamin A delivered through large-scale intervention programs. Food Nutr Bull 2016; 37: S63–S74. [DOI] [PubMed] [Google Scholar]
- 4.Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of nutrition for development (BOND)-vitamin A review. J Nutr 2016; 146: 1816S–48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gannon BM, Kaliwile C, Arscott SA, Schmaelzle S, Chileshe J, Kalungwana N, Mosonda M, Pixley K, Masi C, Tanumihardjo SA. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: a community-based, randomized placebo-controlled trial. Am J Clin Nutr 2014; 100: 1541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mondloch S, Gannon BM, Davis CR, Chileshe J, Kaliwile C, Masi C, Rios-Avila L, Gregory JF, III, Tanumihardjo SA. High provitamin A carotenoid serum concentrations, elevated retinyl esters, and saturated retinol-binding protein in Zambian preschool children are consistent with the presence of high liver vitamin A stores. Am J Clin Nutr 2015; 102: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanumihardjo SA. Vitamin A fortification efforts require accurate monitoring of population vitamin A status to prevent excessive intakes. Procedia Chem 2015; 14: 398–407. [Google Scholar]
- 8.Benson TD. Africa's food and nutrition security situation: where are we and how did we get here?, Washington, DC: International Food Policy Research Institute, 2004. [Google Scholar]
- 9.Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, von Lintig J. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem 2013; 288: 9017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanumihardjo SA. Food-based approaches for ensuring adequate vitamin A nutrition. Compr Rev Food Sci Food Saf 2008; 7: 373–81. [Google Scholar]
- 11.van Jaarsveld PJ, Faber M, Tanumihardjo SA, Nestel P, Lombard CJ, Benadé AJS. β-carotene-rich orange-fleshed sweet potato improves the vitamin A status of primary school children assessed with the modified-relative-dose-response test. Am J Clin Nutr 2005; 81: 1080–7. [DOI] [PubMed] [Google Scholar]
- 12.Bresnahan KA, Chileshe J, Arscott S, Nuss E, Surles R, Masi C, Kafwembe E, Tanumihardjo SA. The acute phase response affected traditional measures of micronutrient status in rural Zambian children during a randomized, controlled feeding trial. J Nutr 2014; 144: 972–8. [DOI] [PubMed] [Google Scholar]
- 13.Palmer AC, Siamusantu W, Chileshe J, Schulze KJ, Barffour M, Craft NE, Molobeka N, Kalungwana N, Arguello MA, Mitra M, Caswell B, Klemm RD, West KP., Jr. Provitamin A-biofortified maize increases serum β-carotene, but not retinol, in marginally nourished children: a cluster-randomized trial in rural Zambia. Am J Clin Nutr 2016; 104: 181–90. [DOI] [PubMed] [Google Scholar]
- 14.Talsma EF, Brouwer ID, Verhoef H, Mbera GN, Mwangi AM, Demir AY, Maziya-Dixon B, Boy E, Zimmermann MB, Melse-Boonstra A. Biofortified yellow cassava and vitamin A status of Kenyan children: a randomized controlled trial. Am J Clin Nutr 2016; 103: 258–67. [DOI] [PubMed] [Google Scholar]
- 15.Bausch J, Rietz P. Method for the assessment of vitamin A liver stores. Acta Vitaminol Enzymol 1977; 31: 99–112. [PubMed] [Google Scholar]
- 16.Tanumihardjo SA. Vitamin A status assessment in rats with 13C4-retinyl acetate and gas chromatography/combustion/isotope ratio mass spectrometry. J Nutr 2000; 130: 2844–9. [DOI] [PubMed] [Google Scholar]
- 17.Gannon BM, Tanumihardjo SA. Comparisons among equations used for retinol isotope dilution in the assessment of total body stores and total liver reserves. J Nutr 2015; 145: 847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gannon BM, Pungarcher I, Mourao L, Davis CR, Simon P, Pixley KV, Tanumihardjo SA. 13C natural abundance of serum retinol is a novel biomarker for evaluating provitamin A carotenoid-biofortified maize consumption in male Mongolian gerbils. J Nutr 2016; 146: 1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Leary MH. Carbon isotopes in photosynthesis. Bioscience 1988; 38: 328–36. [Google Scholar]
- 20.Howe JA, Valentine AR, Hull AK, Tanumihardjo SA. 13C natural abundance in serum retinol acts as a biomarker for increases in dietary provitamin A. Exp Biol Med 2009; 234: 140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanumihardjo SA, Ball AM, Kaliwile C, Pixley KV. The research and implementation continuum of biofortified sweet potato and maize in Africa. Ann N Y Acad Sci 2017; 1390: 88–103. [DOI] [PubMed] [Google Scholar]
- 22.Nuss ET, Arscott SA, Bresnahan K, Pixley KV, Rocheford T, Hotz C, Siamusantu W, Chileshe J, Tanumihardjo SA. Comparative intake of white- versus orange-colored maize by Zambian children in the context of promotion of biofortified maize. Food Nutr Bull 2012; 33: 63–71. [DOI] [PubMed] [Google Scholar]
- 23.Bouis HE, Hotz C, McClafferty B, Meenakshi JV, Pfeiffer WH. Biofortification: a new tool to reduce micronutrient malnutrition. Food Nutr Bull 2011; 32: S31–40. [DOI] [PubMed] [Google Scholar]
- 24.Bresnahan KA, Tanumihardjo SA. Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Adv Nutr 2014; 5: 702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valentine AR, Tanumihardjo SA. Adjustments to the modified relative dose response (MRDR) test for assessment of vitamin A status minimize the blood volume used in piglets. J Nutr 2004; 134: 1186–92. [DOI] [PubMed] [Google Scholar]
- 26.Bresnahan KA, Chileshe J, Tanumihardjo SA. Quantification of food and nutrient intakes in Zambian children with and without malaria under controlled feeding conditions. Exp Biol Med 2014; 239: 45–51. [DOI] [PubMed] [Google Scholar]
- 27.Howe JA, Tanumihardjo SA. Evaluation of analytical methods for carotenoid extraction from biofortified maize (Zea mays sp.). J Agric Food Chem 2006; 54: 7992–7. [DOI] [PubMed] [Google Scholar]
- 28.Davis CR, Howe JA, Rocheford TR, Tanumihardjo SA. The xanthophyll composition of biofortified maize (Zea mays sp.) does not influence the bioefficacy of provitamin A carotenoids in Mongolian gerbils (Meriones unguiculatus). J Agric Food Chem 2008; 56: 6745–50. [DOI] [PubMed] [Google Scholar]
- 29.Yeum K-J, Ahn S-H, Rupp de Paiva SA, Lee-Kim YC, Krinsky NI, Russell RM. Correlation between carotenoid concentrations in serum and normal breast adipose tissue of women with benign breast tumor or breast cancer. J Nutr 1998; 128: 1920–6. [DOI] [PubMed] [Google Scholar]
- 30.Gannon BM, Pixley KV, Tanumihardjo SA. Maize milling method affects growth and zinc status but not provitamin A carotenoid bioefficacy in male Mongolian gerbils. J Nutr 2017; 147: 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Institute of Medicine Food and Nutrition Board. Vitamin A. In: Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academies Press, 2001, pp.82–161. [PubMed]
- 32.Heying EK, Tanumihardjo JP, Vasic V, Cook M, Palacios-Rojas N, Tanumihardjo SA. Biofortified orange maize enhances β-cryptoxanthin concentrations in egg yolks of laying hens better than tangerine peel fortificant. J Agric Food Chem 2014; 62: 11892–900. [DOI] [PubMed] [Google Scholar]
- 33.Sommerburg O, Keunen JEE, Bird AC, van Kuijk FJGM. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br J Ophthalmol 1998; 82: 907–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotz C, Chileshe J, Siamusantu W, Palaniappan U, Kafwembe E. Vitamin A intake and infection are associated with plasma retinol among pre-school children in rural Zambia. Pub Health Nutr 2012; 15: 1688–96. [DOI] [PubMed] [Google Scholar]