Abstract
PURPOSE
To report on the biometric findings of adults and children with Marfan syndrome (MFS) recruited from 2 annual National Marfan Foundation conferences (2012 and 2015).
DESIGN
Cross-sectional study.
METHODS
Subjects diagnosed with MFS by Ghent 2 nosology were included for analysis. Subjects were divided into “adults” (≥16 years of age) and “children” (5–15 years of age). Biometric data included values for refractive error, axial length (AL), corneal curvature, anterior chamber depth, lens thickness, and central corneal thickness.
RESULTS
Of the 117 subjects evaluated, 74 (35 adults, 32 children, and 7 children <5 years of age) had a definite diagnosis of MFS and were included in the study. The AL was longer (25.25 ± 0.32 mm vs 24.24 ± 0.33 mm, P [ .03) and the lens was thicker (3.94 ± 0.09 mm vs 3.62 ± 0.10 mm, P [ .03) in adults. Both groups had flat corneas (average keratometry [Kmed] of 41.59 ± 0.35 diopters [D] in adults vs 40.89 ± 0.36 D in children, P [ .17). A negative correlation was found between AL and Kmed (L0.33, P < .001). The corneas of patients with MFS with ectopia lentis (EL) were significantly flatter and with higher degree of corneal astigmatism compared to patients without EL (Kmed of 40.68 ± 0.31 D vs 41.75 ± 0.28 D, P < .01 and corneal astigmatism of 1.68 ± 0.16 D vs 1.13 ± 0.14 D, P =.01).
CONCLUSIONS
Children with established MFS have flat corneas at least to the same degree as adults. Corneas of patients with MFS with EL are flatter and have a higher degree of corneal astigmatism. We strongly suggest that corneal parameters should be measured if MFS is suspected, especially in children that may not yet have developed EL.
MARFAN SYNDROME (MFS) IS AN AUTOSOMAL dominant connective tissue disorder typically involving the cardiovascular, skeletal, and ocular systems.1 According to the fourth set of criteria for the diagnosis of MFS from 1996, also known as the Ghent 1 criteria,2 ectopia lentis (EL) was the only ocular major criterion present in about 60% of patients.3 Three minor criteria included abnormally flat cornea, increased axial length (AL) and hypoplastic iris. In the revised Ghent criteria for the diagnosis of MFS from 2010, the Ghent 2,4 EL, together with aortic root aneurysm or dissection, are the cardinal features. The only other ocular feature in the revised nosology is myopia >3 diopters (D). Other known ocular features of patients with MFS that are not part of the criteria are early cataract, glaucoma, and retinal detachment.1,2
Descriptive biometric and refractive data on patients with MFS have been reported.3,5–12 However, most studies were performed on a defined European population, mainly from Scandinavia,5–8,11 and those studies used the Ghent 1 or older criteria for the diagnosis of MFS. To the best of our knowledge, other than the seminal paper by Maumenee in 1981,3 the only descriptive biometric study on an American population—also using older criteria for the diagnosis of MFS—was a retrospective study that focused specifically on keratometry and central corneal thickness (CCT).9 In this study, we report the biometric findings of a well-described adult and pediatric population with verified MFS diagnosis based on the Ghent 2 criteria and recruited from 2 annual National Marfan Foundation conferences (2012 and 2015).
METHODS
IN THIS CROSS-SECTIONAL STUDY, PATIENTS WERE EXAMined during the 28th (August 2012) and 31st (August 2015) National Marfan Foundation annual conferences in Chicago, Illinois. As part of the annual conference, the Marfan Eye Consortium of Chicago—a collaborative effort between Ann & Robert H. Lurie Children’s Hospital of Chicago, Northwestern Memorial Hospital, and the University of Illinois—was created to study the ocular manifestations of patients with MFS. Institutional review board approvals were obtained from the 3 institutions. All procedures and data collection were conducted in a manner compliant with the Health Insurance Portability and Accountability Act. Written informed consent was obtained from each participant. A parent or guardian provided the consent for patients <18 years of age. Exclusion criteria were unverified MFS by the Ghent 2 criteria, the presence of other connective tissue disease, or the lack of informed consent. Patients were divided into 2 groups: “adults” (≥16 years of age) and “children” (5–15 years of age). Several younger children were also evaluated, and the results will be reported separately. Both eyes of each participant were included.
Subjects were examined by ophthalmic technicians who were specially trained in the use of all the devices and by Board-certified ophthalmologists. Values for AL, anterior chamber depth, and lens thickness (LT) were obtained with an IOL Master (Carl Zeiss Jena GmbH, Jena, Germany) or contact ultrasonic A-scan biometry (Ellex Medical Pty, Adelaide, Australia). Minimum and maximum values of corneal curvature were measured using either a Marco Keratometer I (Jacksonville, FL) or an IOL Master. The Kmed value was calculated in D as the average values of Kmin and Kmax, and the corneal astigmatism (Cast) was calculated as Kmax–Kmin. CCT was measured with the 300P PacScan Pachymeter (Sonomed Escalon, Wayne, PA). All patients were dilated using tropicamide 1%, phenylephrine 2.5%, and, in children <12 years of age, cyclopentolate 1%. For the calculation of the spherical equivalent (SE), we used only phakic eyes. We also excluded phakic eyes with severe EL requiring aphakic correction. For anterior chamber depth and LT analysis, only phakic patients without EL were included. Finally, because most patients who underwent surgery for the repair of retinal detachment did not necessarily know if the procedure included a scleral buckle, all eyes that had a history of retinal detachment were excluded from the AL calculations.
Descriptive statistics for categorical data are presented in frequencies and percentages. Analyses include both eyes per patient. Analyses used linear mixed models to determine statistical significance for differences in age groups (adults and children) and lens status (with and without EL) for the relevant biometric values. The mixed model included a random patient effect to account for correlation between 2 eyes of an individual in the study. The Pearson correlation coefficient was used to determine the correlation between AL and Kmed.
RESULTS
ONE HUNDRED SEVENTEEN PATIENTS WERE SEEN AND evaluated at the annual conferences of the Marfan Eye Consortium in 2012 and 2015. Of those, 74 patients (148 eyes) had a definite diagnosis of MFS according to the Ghent 2 criteria and were included in the study. The adults group included 35 patients (70 eyes) and the children group included 32 patients (64 eyes). The demographic data of both groups is shown in Table 1. An additional 7 patients (14 eyes) were <5 years of age (range 2–4 years) and are reported separately below.
TABLE 1.
Demographic Data of Patients With Marfan Syndrome
| Demographic | Group 1 (Adults) | Group 2 (Children) |
|---|---|---|
| No. of patients (no. of eyes) | 35 (70) | 32 (64) |
| Age, y (range) | 33 (16–70) | 11 (5–15) |
| Females, n (%) | 11 (31) | 16 (50) |
Among all eyes of patients ≥5 years of age, 59 of 134 (44%; 29/70 [41%] of adult eyes and 33/64 [52%] of children’s eyes) had EL and 71 of 134 (53%) did not. In 4 eyes (3%) the information was missing. Out of all eyes of patients ≥5 years of age, 113 of 134 (84%) were phakic, 9 of 134 (7%) were aphakic, and 11 of 134 (8%) were pseudophakic. The lens status was unknown in 1 eye. Ten of 134 (7%) eyes (1 child eye, 9 adult eyes) underwent surgery for retinal detachment.
The biometric data of both groups are shown in Table 2. There was no statistically significant difference between the groups in the following parameters: SE, Kmed, Cast, CCT, and anterior chamber depth. AL was significantly longer, and LT was significantly thicker in the adults group. Further analysis was performed for Kmed, Cast, and CCT. Forty-one percent of adult eyes had Kmed <41.5 D compared to 53% of children’s eyes (P .17, χ2; Figure 1, top). Eighteen percent of adult eyes had a CCT <500 μm compared to 11% of children’s eyes (P .26, χ2; Figure 1, middle). Finally, 26% of adult eyes had Cast of >1.5 D compared to 32% of children’s eyes (P .43, χ2; Figure 1, bottom). There was a negative association between the AL and the Kmed (correlation −0.33, P < .001 Pearson correlation coefficient; Figure 2).
TABLE 2.
Biometric Data of Adults and Children With Marfan Syndrome
| Adults | Children | P Value | |
|---|---|---|---|
| Spherical equivalent (D), ±SEa | −5.57 ± 1.36 | −7.85 ± 1.30 | .23 |
| Axial length (mm), ±SEb | 25.25 ± 0.32 | 24.24 ± 0.33 | .03 |
| Kmed (D), ±SE | 41.59 ± 0.35 | 40.89 ± 0.36 | .17 |
| Cast (D), ±SEc | 1.26 ± 0.17 | 1.44 ± 0.17 | .46 |
| CCT (μm), ±SE | 543.00 ± 7.31 | 541.56 ± 8.04 | .90 |
| ACD (mm), ±SEd | 3.47 ± 0.09 | 3.54 ± 0.09 | .62 |
| Lens thickness (mm), ±SEe | 3.94 ± 0.09 | 3.62 ± 0.10 | .03 |
ACD = anterior chamber depth; Cast = corneal astigmatism; CCT = central corneal thickness; D = diopters; K = keratometry; LT = lens thickness.
Pseudophakic and aphakic eyes were excluded. Phakic eyes with aphakic correction (ie, the patient looking through “aphakia” in case of ectopia lentis) were also excluded.
Eyes status postsurgery for retinal detachment were excluded.
Calculated as Kmax–Kmin.
Only phakic eyes were included.
Only phakic eyes without ectopia lentis were included.
FIGURE 1.
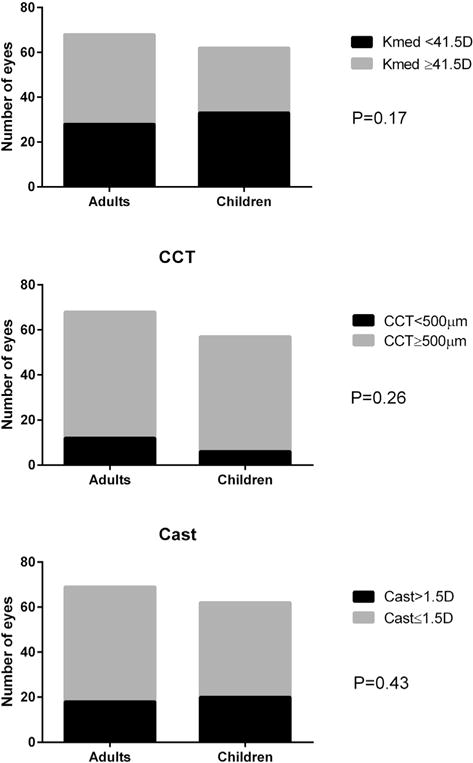
Categorical analyses of corneal parameters between adults and children with Marfan syndrome. Percentage of patients with flat corneas (Kmed, top) central corneal thickness (CCT, middle) and corneal astigmatism (Cast, bottom) was not statistically significant.
FIGURE 2.
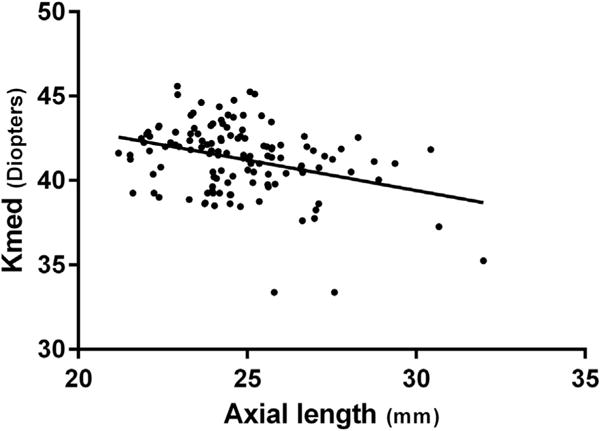
A negative correlation between the axial length and the corneal curvature (Kmed) in patients with Marfan syndrome (r = −0.33, P < .001).
Seven patients were <5 years of age, and the biometric results of these 7 patients are shown in Table 3.
TABLE 3.
Biometric Data of Children <5 Years of Age With Marfan Syndrome
| Patient No. | Eye | Age (y) | Gender | SE (D) | AL (mm) | Cast (D) | Kmed (D) | CCT (μ) | ACD (mm) | LT (mm) | EL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | OD | 2 | Male | −15.00 | NA | NA | NA | NA | NA | NA | Yes |
| OS | −19.75 | NA | NA | NA | NA | NA | NA | Yes | |||
| 2 | OD | 2 | Female | NA | 19.89 | 1.75 | 45.25 | 463 | 3.18 | 3.97 | No |
| OS | NA | 19.97 | 4.00 | 44.25 | 460 | 3.23 | 4.08 | No | |||
| 3 | OD | 3 | Female | NA | 23.09 | 2.75 | 40.88 | 533 | 3.81 | 3.79 | No |
| OS | NA | 23.31 | 1.75 | 39.88 | 541 | 3.61 | 3.95 | No | |||
| 4 | OD | 3 | Male | −9.75 | NA | 1.50 | 38.50 | NA | NA | NA | Yes |
| OS | −10.00 | NA | 2.00 | 39.00 | NA | NA | NA | Yes | |||
| 5 | OD | 4 | Female | −26.00 | 31.29 | 0.50 | 39.00 | 480 | NA | NA | No |
| OS | −26.00 | 28.60 | 0.50 | 38.75 | 491 | NA | NA | No | |||
| 6 | OD | 4 | Female | NA | NA | 0.75 | 42.13 | NA | NA | NA | No |
| OS | NA | NA | 0.50 | 41.75 | NA | NA | NA | No | |||
| 7 | OD | 4 | Female | −3.75 | 23.23 | 0.75 | 39.88 | NA | NA | NA | Yes |
| OS | −8.00 | 25.10 | 0.50 | 40.25 | NA | NA | NA | Yes |
ACD = anterior chamber depth; AL = axial length; Cast = corneal astigmatism; CCT = central corneal thickness; D = diopters; EL = ectopia lentis; Kmed = keratometry; LT = lens thickness; OD = oculus dexter; OS = oculus sinister; SE = spherical equivalent
The following parameters were compared between the subjects with and without EL: age, sex, AL, Kmed, Cast, and CCT (Table 4). Kmed was significantly lower, and Cast was significantly higher in the EL group.
TABLE 4.
Biometric Data of Patients With Marfan Syndrome With or Without Ectopia Lentis
| Ectopia Lentis | ||||
|---|---|---|---|---|
|
|
|
|||
| Without | With | P Value | ||
| Age (y), ±SE | 20.45 ± 2.75 | 24.53 ± 2.45 | .15 | |
| Females (%) | 58% | 76% | .57 | |
| Axial length (mm), ±SE | 24.70 ± 0.32 | 24.81 ± 0.34 | .80 | |
| Kmed (D), ±SE | 41.75 ± 0.28 | 40.68 ± 0.31 | <.01 | |
| Cast (D), ±SEa | 1.13 ± 0.14 | 1.68 ± 0.16 | .01 | |
| CCT (μm), ±SE | 535.30 ± 6.58 | 548.41 ± 7.32 | .15 | |
Cast = corneal astigmatism; CCT = central corneal thickness; D = diopters; K = keratometry; SE = standard error.
Calculated as Kmax–Kmin.
DISCUSSION
IN THIS STUDY, WE REPORT ON THE BIOMETRIC OCULAR characteristics of 158 eyes of 74 patients with MFS. This is the largest biometric study of MFS patients in the United States and the largest biometric study according to the newer Ghent 2 criteria for the diagnosis of MFS. This is also the first study to compare children with MFS to adults with MFS. We found that with the exception of the expected longer AL and thicker crystalline lenses in adults with MFS compared to children with MFS, other biometric characteristics were not statistically different between the 2 groups using 2 different sets of statistical analyses. We also found that eyes with EL had flatter corneas and higher astigmatism compared to eyes without EL. Finally, eyes with longer AL were significantly correlated with flatter corneal measurements.
In the current Ghent criteria, only myopia >3 D, which depends on the mutual effect of several factors, is considered as a systemic criterion for MFS. The refractive state of the eye depends on the interaction of a number of parameters—a longer AL will result in a more myopia, flatter corneas will result in more hyperopia, and advanced EL will result in higher myopia and astigmatism. Previous studies on patients with MFS showed an average AL of 24.0–25.0 mm.3,5,7,11,12 This AL is relatively longer compared to population studies on healthy patients, which suggest a normal AL of approximately 23.5 mm.13,14 Our study confirms these previous reports, with an average AL of 25.25 mm in adults and 24.24 mm in children. According to the older Ghent criteria, an axial length of >23.5 mm was considered a minor criterion. Moreover, because myopia is a relatively common finding in the general population, it was attributed only 1 point in the systemic score for the diagnosis of MFS. In our study, most eyes met this criterion (84% of adult eyes and 64% of children’s eyes). Although it is no longer considered a criterion for MFS, it should be suspected in patients with longer AL and a flat cornea.7 According to previous reports, myopia >3 D is a nonspecific criterion for MFS, with a prevalence of about 30–40% in patients with MFS.3,5,7 However, the refractive error of all MFS subjects <5 years of age (Table 3), when it was known, showed high to very severe myopia (range −3.75 to −26 D). This might strengthen the conclusion that MFS should be considered among other etiologies in young children with high myopia.
We found that our cohort of patients with MFS had decreased corneal curvature. This finding has been previously described.3,5,8,10,15 We also found that children with MFS may have even flatter corneas, with an average Kmed of 40.89 D vs 41.49 D in adults (compared to values around 43.5 D in the normal population13), with more children having a Kmed ≤41.50 D. It is also notable that the cornea was even flatter in our group of very young children, with 50% of eyes with a Kmed of <40 D (Table 3), although statistical analysis could not be conducted because of the small sample size. According to the updated diagnostic criteria for MFS—the Ghent 2 criteria—myopia exceeding 3 D is included as a diagnostic sign if EL is not present. Therefore, it has been claimed that there is a risk of not diagnosing all cases of MFS when EL is not present because these flat corneas may counteract the myopia with increased AL in MFS, causing a less than expected myopic error.5 In this study, we corroborated this finding by showing the negative correlation between AL and Kmed. In mild forms of MFS, a patient with a longer AL might not be as myopic as expected because of a flatter than normal cornea. When combined with children that may not have severe EL, the risk of not diagnosing MFS in children is even more pronounced. Therefore, given the risk of systemic complications of MFS, it might be important to measure the AL and corneal curvature in patients with suspected MFS without EL or high myopia.
With regard to CCT, results in the literature are conflicting. Some studies have shown that patients with MFS have thinner corneas compared to the normal population8,10; other studies have shown no significant difference.3,7–9,11,12 Another reported finding is that patients with MFS and EL have thinner corneas than patients with MFS but without EL.10,12 In our study, we found that CCT was not significantly different between adults and children with MFS or between MFS with or without EL. It seems that CCT is of less diagnostic importance than other biometric parameters, with significant overlap in the values between patients with and without MFS.9
It is expected that eyes with EL will have severe lenticular astigmatism because of a displacement of the center of the lens from its natural optical axis and lens tilting. However, we show that eyes also show increased corneal astigmatism.8 The mechanism of this finding is still unclear. It might be that the same mechanism that causes zonular instability and EL also causes defects in the corneal tissue and therefore increased corneal astigmatism.8,9
EL is a major diagnostic criterion for MFS. The prevalence of patients with current phakic EL or a history of surgical correction for EL was 59 of 148 (39.9%) of eyes. Other studies have found the frequency of EL to range from 30.2– 87%.3,5,7–12,16–18 The variability could be attributed to different study designs and selection criteria. For example, some studies used “eyes” and some used “patients.” This alone can affect the results, because a minority of patients can have unilateral EL.2,16,19,20 Other variables include the use of an eye examination vs a questionnaire and the different age ranges assessed in different studies. In this study, we found that the frequency of EL was higher in children (52%) than in adults (41%). However, we believe that this is related to a selection bias—it might be that more children with EL (which may represent a more advanced disease21,22) attended these national conferences, therefore accounting for the higher frequency of EL among children. In addition, the true prevalence of EL is probably greater in both groups. Although special attention was given to identify subtle findings, such as iridodonesis and phacodonesis, it is possible to miss minimal subluxation when the pupil cannot maximally dilate, as is frequently the case in patients with MFS.
Previous studies have shown that in patients with MFS and EL there is a difference in some biometric parameters compared to patients with MFS but without EL. For example, AL has been reported to be longer in MFS with EL compared to MFS without EL.3,7,12 Our cohort, as well others,5 did not show that difference. Another example is CCT; some studies have reported that eyes with EL have thinner corneas.10,12 In our cohort, as well as in other studies,7–9,11 this finding was not corroborated. On the other hand, we did find that patients with MFS and EL have flatter corneas and higher corneal astigmatism compared to patients with MFS but without EL, as has been reported.8,9
The strength of our study is that we present ocular biometric characteristics in the largest cohort according to the Ghent 2 criteria and provide a separate analysis of children and adults. In addition, previous studies have focused mainly on MFS from Scandinavia, while our study was performed on a more diverse American population, although we do not have these demographic data available for analysis. A limitation of our study is the lack of a control group matched for age and SE. However, this is not necessary when comparing eyes with EL to eyes without EL. Another limitation of the study is that multiple examiners took the measurements and different equipment was used between patients. An additional limitation is that current spectacle refraction was used as a surrogate for manifest refraction. Finally, all ocular measurements could not be obtained from every subject because many were young children at the time of the examination.
MFS is a condition associated with a shortened life expectancy, mostly because of aortic dissection that often occurs before 40 years of age.23 Today, this can increasingly be prevented and diagnosed early because of regular cardiac ultrasound examinations in patients with MFS. Because the diagnosis of MFS is heavily defined by the ophthalmic features, it is imperative to perform all eye studies within reason in order to make the diagnosis of MFS. This is especially true with children because EL—the classic and diagnostic finding in MFS—may not have developed yet. In addition, the minor diagnostic criteria of myopia >3 D also may not be present because of the compensatory effects of a flattened cornea that balance the increased AL in terms of refractive error. We found that children with established MFS have flat corneas to the same degree as adults, if not flatter. Therefore, although AL and corneal curvature are not included in the updated diagnostic criteria for MFS (it was stated that they had unclear specificity and were not routinely measured by ophthalmologists4), we strongly suggest that both parameters, which are obtainable in clinical settings even in young children, should be measured if MFS is suspected.
Supplementary Material
Acknowledgments
FUNDING/SUPPORT: PUBLICATION OF THIS ARTICLE WAS SUPPORTED BY A GRANT FROM RESEARCH TO PREVENT BLINDNESS, Inc, New York, New York (to the University of Illinois at Chicago); a grant from the Children’s Surgical Foundation, Chicago, Illinois (to Ann & Robert H. Lurie Children’s Hospital of Chicago); and National Eye Institute grants K12 EY021475 and K08EY024645 (to I.S.K.). Financial Disclosures: The following authors have no financial disclosures: Michael Kinori, Sarah Wehrli, Iris S. Kassem, Nathalie F. Azar, Irene H. Maumenee, and Marilyn B. Mets. All authors attest that they meet the current ICMJE criteria for authorship.
Footnotes
Supplemental Material available at AJO.com.
References
- 1.Judge DP, Dietz HC. Marfan’s syndrome. Lancet. 2005;366(9501):1965–1976. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62(4):417–426. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Maumenee IH. The eye in the Marfan syndrome. Trans Am Ophthalmol Soc. 1981;79:684–733. [PMC free article] [PubMed] [Google Scholar]
- 4.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(7):476–485. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 5.Konradsen TR, Zetterstrom C. A descriptive study of ocular characteristics in Marfan syndrome. Acta Ophthalmol. 2013;91(8):751–755. doi: 10.1111/aos.12068. [DOI] [PubMed] [Google Scholar]
- 6.Konradsen TR, Koivula A, Kugelberg M, Zetterstrom C. Accommodation measured with optical coherence tomography in patients with Marfan’s syndrome. Ophthalmology. 2009;116(7):1343–1348. doi: 10.1016/j.ophtha.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Drolsum L, Rand-Hendriksen S, Paus B, Geiran OR, Semb SO. Ocular findings in 87 adults with Ghent-1 verified Marfan syndrome. Acta Ophthalmol. 2015;93(1):46–53. doi: 10.1111/aos.12448. [DOI] [PubMed] [Google Scholar]
- 8.Konradsen TR, Koivula A, Kugelberg M, Zetterstrom C. Corneal curvature, pachymetry, and endothelial cell density in Marfan syndrome. Acta Ophthalmol. 2012;90(4):375–379. doi: 10.1111/j.1755-3768.2010.01996.x. [DOI] [PubMed] [Google Scholar]
- 9.Heur M, Costin B, Crowe S, et al. The value of keratometry and central corneal thickness measurements in the clinical diagnosis of Marfan syndrome. Am J Ophthalmol. 2008;145(6):997–1001. doi: 10.1016/j.ajo.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Sultan G, Baudouin C, Auzerie O, et al. Cornea in Marfan disease: Orbscan and in vivo confocal microscopy analysis. Invest Ophthalmol Vis Sci. 2002;43(6):1757–1764. [PubMed] [Google Scholar]
- 11.Setala K, Ruusuvaara P, Karjalainen K. Corneal endothelium in Marfan syndrome. A clinical and specular microscopic study. Acta Ophthalmol (Copenh) 1988;66(3):334–340. doi: 10.1111/j.1755-3768.1988.tb04606.x. [DOI] [PubMed] [Google Scholar]
- 12.Kara N, Bozkurt E, Baz O, et al. Corneal biomechanical properties and intraocular pressure measurement in Marfan patients. J Cataract Refract Surg. 2012;38(2):309–314. doi: 10.1016/j.jcrs.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 13.Olsen T, Arnarsson A, Sasaki H, Sasaki K, Jonasson F. On the ocular refractive components: The Reykjavik Eye Study. Acta Ophthalmol Scand. 2007;85(4):361–366. doi: 10.1111/j.1600-0420.2006.00847.x. [DOI] [PubMed] [Google Scholar]
- 14.Shufelt C, Fraser-Bell S, Ying-Lai M, Torres M, Varma R, Los Angeles Latino Eye Study Group Refractive error, ocular biometry, and lens opalescence in an adult population: The Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2005;46(12):4450–4460. doi: 10.1167/iovs.05-0435. [DOI] [PubMed] [Google Scholar]
- 15.Fotedar R, Wang JJ, Burlutsky G, et al. Distribution of axial length and ocular biometry measured using partial coherence laser interferometry (IOL Master) in an older white population. Ophthalmology. 2010;117(3):417–423. doi: 10.1016/j.ophtha.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 16.Chandra A, Ekwalla V, Child A, Charteris D. Prevalence of ectopia lentis and retinal detachment in Marfan syndrome. Acta Ophthalmol. 2014;92(1):e82–e83. doi: 10.1111/aos.12175. [DOI] [PubMed] [Google Scholar]
- 17.Faivre L, Collod-Beroud G, Child A, et al. Contribution of molecular analyses in diagnosing Marfan syndrome and type I fibrillinopathies: An international study of 1009 probands. J Med Genet. 2008;45(6):384–390. doi: 10.1136/jmg.2007.056382. [DOI] [PubMed] [Google Scholar]
- 18.Arbustini E, Grasso M, Ansaldi S, et al. Identification of sixty-two novel and twelve known FBN1 mutations in eighty-one unrelated probands with Marfan syndrome and other fibrillinopathies. Hum Mutat. 2005;26(5):494. doi: 10.1002/humu.9377. [DOI] [PubMed] [Google Scholar]
- 19.Nemet AY, Assia EI, Apple DJ, Barequet IS. Current concepts of ocular manifestations in Marfan syndrome. Surv Ophthalmol. 2006;51(6):561–575. doi: 10.1016/j.survophthal.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Cross HE, Jensen AD. Ocular manifestations in the Marfan syndrome and homocystinuria. Am J Ophthalmol. 1973;75(3):405–420. doi: 10.1016/0002-9394(73)91149-5. [DOI] [PubMed] [Google Scholar]
- 21.Traboulsi EI, Whittum-Hudson JA, Mir SH, Maumenee IH. Microfibril abnormalities of the lens capsule in patients with Marfan syndrome and ectopia lentis. Ophthalmic Genet. 2000;21(1):9–15. [PubMed] [Google Scholar]
- 22.Wheatley HM, Traboulsi EI, Flowers BE, et al. Immunohisto-chemical localization of fibrillin in human ocular tissues. Relevance to the Marfan syndrome. Arch Ophthalmol. 1995;113(1):103–109. doi: 10.1001/archopht.1995.01100010105028. [DOI] [PubMed] [Google Scholar]
- 23.Attias D, Stheneur C, Roy C, et al. Comparison of clinical presentations and outcomes between patients with TGFBR2 and FBN1 mutations in Marfan syndrome and related disorders. Circulation. 2009;120(25):2541–2549. doi: 10.1161/CIRCULATIONAHA.109.887042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


