Abstract
Objective
This review focuses on the relationship between obesity and aging and how these interact together to affect cognitive function. The topics covered are guided by the Scaffolding Theory of Aging and Cognition (STAC; Park & Reuter-Lorenz, 2009—a conceptual model designed to relate brain structure and function to one’s level of cognitive ability.
Methods
The initial literature search was focused on normal aging and was guided by the key words, “aging, cognition, and obesity” in “PUBMED”. In a second search we added key words related to neuropathology including words “Alzheimer’s Disease”, “Vascular dementia” (VaD) and “Mild Cognitive Impairment” (MCI).
Results
The data suggest that being overweight or obese in midlife may be more detrimental to subsequent age-related cognitive decline than being overweight or obese at later stages of the lifespan. These effects are likely mediated by the accelerated effects obesity has on the integrity of neural structures, including both gray and white matter. Further epidemiological studies have provided evidence that obesity in mid-life is linked to an increased risk for AD and VaD, most likely via an increased accumulation of AD pathology.
Conclusion
While it is clear that obesity negatively affects cognition, more work is needed to better understand how aging plays a role and how brain structure and brain function might mediate the relationship of obesity and age on cognition. Guided by the STAC and the STAC-R models, we provide a roadmap for future investigations of the role of obesity on cognition across the lifespan.
Keywords: Obesity, aging, adult lifespan, cognitive and brain function, Alzheimer’s Disease
Introduction
Obesity is a growing global health issue with an increasing prevalence in both children (1) and adult populations. There are more than 1.4 billion overweight adults and an additional 500 million adults classified as obese world-wide (2). The World Health Organization distinguishes between overweight and obese individuals. Individuals with a BMI index of ≥ 30 kg/m2 are defined as obese, whereas individuals with a BMI index of 25 to 29 kg/m2 are considered overweight. In addition to BMI, these weight classifications can also be measured by waist circumference. Obesity is important to study because it is associated with a risk to develop a number of medical conditions. For instance, obesity is a major risk factor for type 2 diabetes which is linked to metabolic syndrome, an even more complex disorder that includes a constellation of symptoms such as hypertension and dyslipidemia (3). Additionally, prospective cohort studies have reliably linked overweight status and obesity to coronary artery disease, congestive heart failure, and gallbladder disease using waist circumference (WS) to determine overweight and obese status (4).
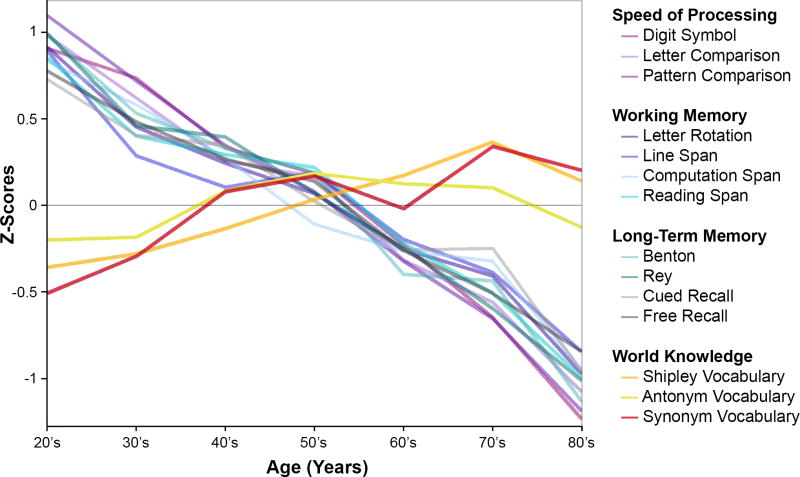
Besides increasing the risk for a number of concomitant medical conditions, obesity and type 2 diabetes are also associated with cognitive decline (5–9), and even with a diagnosis of dementia in later life (10). These findings are of considerable concern, as it is well-documented that even the healthiest adults show age-related declines in basic cognitive operations, including speed of processing, working memory, reasoning, and episodic memory, as shown in Figure 1. If obesity status incurs additional decline in cognition, the effects of obesity should have the greatest impact in old age, when cognitive resources have dwindled. Figure 1 also displays a reliable age-related increase in world knowledge, suggesting that increased experience and knowledge accrue with age and might confer some support for preserving the ability to function in everyday life (11–15).
Figure 1.
Cross-sectional aging data adapted from Park et al. (2002) showing behavioral performance on measures of processing speed (i.e., Digit Symbol, Letter Comparison, Pattern Comparison), working memory (i.e., Letter Rotation, Line span, Computation Span, Reading Span), Long-Term Memory (i.e., Benton, Rey, Cued Recall, Free Recall), and world knowledge (i.e., Shipley Vocabulary, Antonym Vocabulary, Synonym Vocabulary). Almost all measures of cognitive function showed declines with age, except world knowledge, which showed slight improvements.
Although obesity and aging are both associated with cognitive decline, very little is understood about how they interact together to affect cognitive function across the lifespan. Of particular concern is whether there is a synergistic effect of obesity and older age that could accelerate normal aging processes or even increase the risk for pathological aging processes such as dementia. In the current review, we consider the limited literature addressing the joint effects of obesity and age on cognitive function, as well as the effect of obesity and age on underlying neural structures and functional brain activity. This review is guided by the Scaffolding Theory of Aging and Cognition (STAC)—a conceptual model that relates structural and functional age-related changes to the ability of the brain to respond to these biological aging processes through compensatory scaffolding (e.g., additional bilateral recruitment, increased recruitment of frontal-parietal areas, neurogenesis) (16, 17).
We then consider whether obesity increases the risk for neurodegenerative disease such as Alzheimer’s Disease (AD) and vascular dementia (VaD) in late adulthood and examine different approaches that have been taken in an effort to understand the impact of obesity and related comorbidities on disease progression to AD. It is critical to understand who may be most at risk for AD as that understanding will ultimately shape and improve the design and effectiveness of intervention strategies. We close this review by providing a roadmap for future investigations of the role of obesity on cognition across the adult lifespan.
1. The Scaffolding Theory of Aging and Cognition (STAC)
The last decade has been one of remarkable discovery of how the structure and function of the brain changes in response to aging, and how these changes affect humans’ ability to process information and maintain cognitive function as they age. Early neuroimaging tools initially permitted scientists to see the structure of the human brain and relate it to cognitive function. Structural studies demonstrated that many structures of the brain decreased in volume with age, particularly the frontal cortex, and that these volumetric changes affected cognition (18).
Eventually, in the 1990’s, the ability to see functional activity in the brain became available. Pioneering studies that examined neural circuitry with age demonstrated that older adults showed greater neural activity in frontal cortex than young, and in particular, that older adults tended to activate prefrontal regions bilaterally for verbal encoding and working memory tasks, compared to young adults who showed activation primarily in left prefrontal regions (19–21). At the same time that functional work on the aging brain was developing, there was also a focus on white matter structure and integrity in the aging brain. This research demonstrated that small white matter lesions or hyperintensities that occurred with age negatively affected cognitive function (22). More recently, diffusion tensor imaging techniques have become available that allow for even more microscopic measurement of white matter integrity (23). In addition, molecular imaging was making strides to allow scientists to measure amyloid protein deposition (the plaques associated with Alzheimer’s disease) (24) on the brain.
The initial work in the field of cognitive neuroscience of aging was focused on separately investigating different aspects of neural structure and function that contributed to age-related differences in cognitive performance in older adults. As the field matured, in order to develop a better understanding of both neural degradation and plasticity with age, it became increasingly important to begin to integrate and understand how measures from these imaging modalities interact together to produce cognitive behaviors.
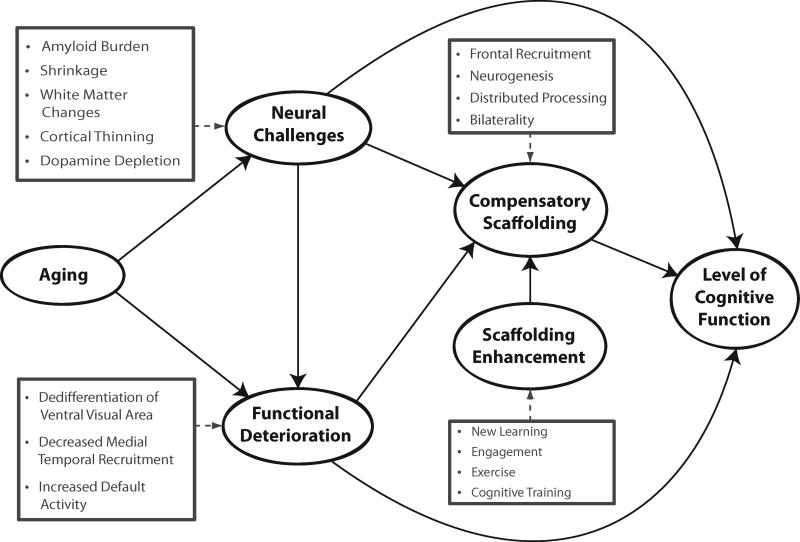
STAC was developed by Park and Reuter-Lorenz (2009) and was aimed to integrate measures of both brain structure and function to understand how these factors might operate in concert to control the expression of cognitive aging in older adults. STAC posits that even in healthy adults, there is some degradation of both neural structure and function with age (see Figure 2). Structural declines include volumetric reductions of brain tissue, particularly in frontal regions (18), decreases in dopamine receptors(25, 26), and increases in amyloid deposition(27). Declines in neural function are evidenced by dedifferentiation (a decreased specialization of neural tissue to visual categories such as faces, and houses (28)), decreased control of brain networks, particularly the default network(29), and decreased hippocampal activity (30). The scaffolding model suggests that individuals who have accrued multiple neural insults will perform most poorly. Thus one would predict that with frail older adults are likely to have accumulated multiple neural insults thus would show steeper cognitive decline, than those with a lower neural burden.
Figure 2.
A conceptual model of the scaffolding theory of aging and cognition (STAC).
A key element of the model is that the aging brain has the capacity to adapt to brain degradation by developing neural scaffolds (i.e., supportive neural structures and neural activity) that minimize or compensate for some aspects of neural degradation and in turn minimizes the impact of this degradation on cognition. Of particular importance in this model is the compensatory role of enhanced frontal activation, particularly bilateral activity in older adults when younger counterparts show more focal unilateral activations (19, 31). Finally, the model suggests that it may be possible through behavioral interventions such as exercise, cognitive engagement, new learning, or cognitive training, to enhance the development of neural scaffolds and support cognitive function.
2. The Impact of Obesity on Cognition across the Adult Lifespan
The major outcome variable of the STAC model is an individual’s level of cognitive function, which is determined by a host of influences including structural and functional markers of brain aging as well as the ability to adapt to these changes utilizing compensatory scaffolding. Individual differences such as obesity appears to be one of many factors that influences one’s level of cognitive function, thus it is important to examine if evidence exists that shows direct effects of obesity on cognitive function and whether these relationships are differentially expressed across the adult lifespan.
There are a handful of cross-sectional lifespan studies (e.g.. studies that included individuals ranging in age from about 20 to 90 years old) that examine the effect of obesity on cognition and these report overall negative effects of being overweight, or obese, on both global cognitive functions and specific domains of cognition, including attention, executive function, speed of processing and verbal memory (e.g., (32, 33)). In a community-based sample of older adults (Mage = 65.7), obese adults showed lower global cognitive performance after controlling for other risk factors such as hypertension, total cholesterol and cigarette smoking (6). These associations were more pronounced in men than in women. In another study, overweight status was negatively associated with executive function, but not with attention (32), even after controlling for demographic variables such as education, sex, depression and anxiety (N = 408; Mage = 48.67; Age Range: 20–82). In a large population-based study (N = 1959; Medianage = 74), the investigators reported that obesity negatively affected attention, verbal fluency, verbal memory, pre-morbid intelligence, and logical memory even when controlling for comorbidities such as type 2 diabetes, hypertension, dementia, as well as smoking, drinking behavior, and medication intake, and nevertheless (33). Although the effect sizes are moderate, lifespan and population-based studies suggest a general negative effect of obesity on cognitive function.
In the aforementioned studies, the most negative impact occurred in mid-life compared to late life (age > 65). More evidence of this trend comes from a study in which long-term obesity that existed from early middle-adulthood (Mage =41) to late midlife (Mage= 61) was linked to lower scores on the Mini-Mental State Examination Test (MMSE) and to poorer performance on memory and executive functioning tasks (34). Interestingly, adjusting for cardiovascular risk factors (e.g., health behaviors, blood pressure, and cholesterol) in early mid-life attenuated, but did not entirely eliminate, the effect of obesity on lower cognitive performance (34). Thus, the strongest negative effect on cognition in this sample was related to obesity in early midlife.
Some data even suggest that being obese, or overweight, in late life has positive effects on cognition. For example, for individuals at later ages of the lifespan (age 65–94), there is some evidence that being overweight was positively related to reasoning, visuo-spatial abilities and speed of processing. Moreover, individuals classified as obese in late life (Medianage = 73.8) showed superior visuo-spatial abilities and speed of processing performance compared to normal-weight individuals, even after controlling for sex, blood pressure and Type 2 diabetes (35). Other results demonstrated that being overweight and obese in late life (Medianage = 75.4) was associated with less impairment in instrumental activities of daily living and a slower reduction of MMSE scores over 5 years, findings congruent with evidence that there may be some beneficial effects of being overweight or obese in old age (36).
Non-linear and age-dependent effects of obesity on cognition are further confirmed by short-term longitudinal data that studied four cohorts of adults beginning at age 32, 42, 52, and 62, with a second wave of data collected five years later. Results showed that increased BMI at baseline was indicative of cognitive decline in domains of verbal memory and speed of processing, but also provided evidence that change in BMI over the five-year period was not predictive of change in cognitive function over time (5). Midlife obesity (i.e., indivduals aged 40–69) was negatively correlated with visuo-spatial performance and executive function but not memory over a time span of 8 to12 years (8).
The paradoxical finding that greater BMI has a more deleterious effect on cognitive outcomes in late life, when measured in middle age as compared to measurements taking in old age that can be explained via several hypotheses: (1) Advanced age (> 65) has been shown to be particularly vulnerable to loss of skeletal mass which in turn has been associated with lower cognitive functioning (37). Further, loss of skeletal mass has been shown to be negatively associated with BMI (38). These data suggests that specifically in older adults the maintenance of skeletal mass via higher BMI may be protective of cognitive function. (2) Another possibility is that some middle-aged obese adults experience mortality, leaving a healthier sample of obese adults in old age as assessed in cross-sectional studies. (3) Finally, some researchers have argued that BMI measurement neglects the original distribution of lean and fat tissue masses in evaluating adiposity and suggested that central adiposity (i.e., hip-waist ratio or waist circumference) is a better suited measure to assess obesity (39). Interestingly, central adiposity is closely related to cardio-metabolic risk (40) and may be more consistently associated with the negative effects of cognitive function in older age (41). The true nature of this relationship can only be ascertained with careful longitudinal study designs.
We note there are some methodological issues that limit the interpretation of the effects of obesity on cognition. Some studies control for comorbidites of obesity (e.g., blood pressures, vascular disease, Type 2 diabetes, metabolic syndrome) while others prioritize the importance of psycho-social and demograhic variables (e.g., stress, anxiety, depression, perceived health, age, sex, education).
A recent study attempted to disentangle the effect of differential metabolic phenotypes (high triglycerides, high blood pressure, and elevated cholesterol levels) on cognition in participants (Age range: 39–63 years) classified as normal weight, overweight, or obese (42). Similar to other findings, they reported that obesity and abnormal metabolic phenotypes at baseline were negatively associated with cognition. Moreover, mid-life obesity and metabolic abnormalities were associated with cognitive decline over a subsequent period of ten years. These important findings suggest that the effects of BMI and metabolic abnormalities on cognition persist over time and the investigators conclude that it may amplify cognitive decline. Similar synergistic effects of obesity on cognitive function and related metabolic factors were observed for hypertension and obesity on visuo-spatial task and verbal memory tasks (43).
Overall the current data suggest that being overweight or obese at midlife may be more detrimental to subsequent cognitive decline than the same status at later ages of the lifespan. Also, differential cognitive effects associated with obesity are more apparent for executive functioning tasks and neuropsychological tests of speed of processing , which are sensitive to vascular compromise (44) compared to episodic memory tasks (45), which tend to be more sensitive to Alzheimer’s disease. This task-disease correspondence is consistent with the notion that obesity and its accompanying conditions are related specifically to vascular cognitive impairments (8). Lastly, directly assessing the effect of overweight status and obesity on age-related differences in cognitive function warrants the inclusion of covariates (i.e., hypertension, type 2 diabetes, cerebral vascular disease, metabolic abnormalities) that may modify or mediate the relationship of obesity on age-related cognitive differences, either idependently (8) or additively (42).
2a) Aging, Obesity, and Brain Structure
The STAC model proposes that structural brain aging directly affects age-related cognitive functions through neural challenges including volumetric reductions, changes to the macro- and microstructure of the white matter, elevated amyloid burden, cortical thinning and dopamine depletion (17). Obesity and its associated cardio-vascular risk factors may directly increase the amount of neural challenges a person has to sustain. Therefore the relationship of overweight and obesity affect the burden of neural challenges and in turn affect cognitive function.
Initial work on obesity and brain structure examined the association between BMI and global brain volume in cross-sectional study designs (N = 114, Age Range = 40–66 years, Mage = 54.2), and found that higher levels of BMI were associated with lower global brain volume, even after controlling for age, hypertension and cholesterol levels (46). A longitudinal study over six years in older adults (Mage = 59.8) examined predictors of brain atrophy (e.g., age, cholesterol level, hypertension, BMI, fasting glucose) and reported that BMI was the strongest predictor of gray matter volume decline (47). Regional effects of BMI on gray matter differences have been reported recently in a group of older adults (N=94; Mage =75.3) that included regions in the frontal lobes (e.g., the anterior cingulate) and in subcortical regions (e.g., the hippocampus and thalamus) (48). Other regional effects of obesity on brain volume were observed in individuals who maintained obesity status over a five-year period. Specifically, dorsolateral prefrontal cortex (DLPFC) was preferentially vulnerable to atrophy in obese individuals (Mage =75) when compared to individuals who maintained a normal weight (49). Although, only a few studies have examined the relationship of obesity on gray matter differences thus far, it is noteworthy that medial temporal (i.e., hippocampus) and frontal regions (particularly, anterior cingulate, DLPFC) appear to be particularly vulnerable to obesity.
Additionally obesity effects on frontal white matter have been reported in a study of healthy middle-aged adults (N = 50; Mage = 41.7 +/− 8.5 years) using spectroscopy with N-acetyalsparate, a microstructural marker of neural viability (50). High levels of BMI were associated with low neural viability , particularly in frontal and parietal cortex (50). Additional negative effects of BMI were observed in older adults (N =138; Mage = 71.3) for white matter fiber integrity in the genu and the cingulate, as measured by fractional anisotropy, even after controlling for other potential vascular and inflammatory conditions (51). In addition to microstructural changes of the white matter, macrostructural differences, as indexed by white matter hyperintensities, have been linked to increased BMI in the elderly (N=122; Mage = 69.7; Range: 60–83) (52).
We also note that the data reviewed here on the association of obesity to brain structure provide initial evidence that areas most vulnerable to the aging process (i.e., frontal and anterior brain regions) seem to be particularly sensitive to obesity as well, reflecting Ribot’s law: “last in – first out” first introduced by Théodule Ribot, a French philosopher. The theory postulates that brain regions that phylogentically and ontogenetically emerge first are most resistant to the aging process whereas regions that emerge late in the adult development, are more vulnerable to aging.
Thus far, we have provided clear evidence that obesity negatively affects neural structures in both the middle-aged and the elderly. Quite interestingly, a very recent study extended these negative obesity effects to young adults (Mage = 27) when comparing volume differences between obese and normal weighted women (53). Furthermore, a meta-analysis of 67 studies examining the relationship of obesity and neurocognitive function in children indicated that even obese school-aged children exhibit diminished executive functions compared to normal weight children (54). These results indicate obesity exerts a negative effect on brain structure across the lifespan, beginning in childhood, and is associated with both gray and white matter changes.
The mechanisms by which obesity results in differences in brain structure are not well understood. However some hypotheses that have been proposed as follows: 1) Hyperglycemia (higher blood glucose levels) is associated with a high fat diet and has been shown to damage to the vascular system and may indirectly affect brain shrinkage through decreases in neurons or impaired neurogenesis (55). 2) Recent evidence suggests that the increased production of triglycerides and fatty free acids associated with obesity may cause a chronic inflammatory response in the central nervous system which also affects the brain (56). 3) Finally, a reduction of dopamine-related pathway activities has been proposed to underlie patterns of increased activation in reward-related areas during food processing in obese individuals, as well as a lack of inhibitory responses to food in the DLPFC. The consequences of this would be increased consumption of food resulting in obesity (57).
In summary, global and regional age-related gray matter changes as well as microstructural and macrostructural differences in the white matter of the brain are associated with obesity and increased BMI.
The data reviewed thus far suggest that structural effects mediate the relationship between obesity and cognition. However, it is also possible that age interacts with obesity such that a life history of obesity combined with age results in accelerated brain shrinkage and accelerated age-related cognitive decline. Although little work has been done examining this question, initial research suggests that obese older adults show accelerated gray matter shrinkage in DLPFC and decreased performance on the trail-making tasks relative to normal weight controls (50). However, others failed to find a moderating effect of structural gray matter differences on the relationship between obesity and cognitive function, including episodic memory, working memory or processing speed (46). At this point, there is simply insufficient data to draw conclusions about the interactive effects of obesity and age on brain structure and cognition. Important work remains to be done in this domain.
With the advent of functional magnetic resonance imaging (fMRI) great progress has been made in advancing our understanding about the neural function of the aging brain (17, 58). In the next section we will briefly review the general pattern of functional brain aging and will further examine the idea that obesity may alter functional activation patterns across the lifespan.
2b) Aging, Obesity, and Brain Function
Aging is accompanied by differences in brain function across a range of different tasks (59). Neuroimaging studies examining patterns of activation during perception of different object categories (e.g., faces, chairs, houses, etc.) found that young adults showed distinct neural responses to each category in specific areas of the brain. For example, young adults selectively activated the fusiform gyrus to faces, the lateral occipital cortex to objects, and the parahippocampal region to houses (60–63). However, with advanced age (e.g., Age Range = 64–79), these activation patterns become less selective to each category across multiple regions (17, 28, 64, 65). This dedifferentiation of brain activity indicates that, even at the level of perception, age exerts an effect on the brain’s ability to respond to objects early in the processing stream (66). In addition to perceptual processes, memory has been the focus of many research studies designed to understand the neural basis of age-related differences in memory performance, with a focus on the hippocampus and other medial temporal regions (31, 67–74). Studies have revealed that with normal aging, medial temporal recruitment is diminished and may be compensated for by additional recruitment of frontal cortex areas (30).
One of the most intriguing results from functional neuroimaging studies in younger and older adults is evidence of increased frontal activation in older adults compared to young. Young adults typically show primarily left frontal activity to verbal encoding tasks, but older adults show a bilateral activation pattern in homologous frontal regions for the same task (19, 75–78). Researchers have speculated this increased pattern of activity is evidence of a compensatory mechanism for the compromised aging brain structure and that the additional neural activity facilitates task performance (17, 76, 78), although there is still some debate on this issue(79).
In addition to understanding age-related differences in neural function during various cognitive processes, researchers have also mapped the fundamental properties of brain activity in young and old during quiet states (or resting states) when no cognitive task was presented (80–83). Initial studies of young adults showed that activation patterns during resting state resulted in a network of activation that spanned the precuneus, anterior and posterior cingulate, medial prefrontal cortex, lateral temporal, parietal cortex and the hippocampus (84–86). Because of the coherent pattern of activation observed in these regions during a resting state, researchers termed this set of regions the “default mode network” (84). Studies have consistently shown that with advanced age, coordinated activity between the areas of the default mode network decreases, suggesting a disruption of the endogenously evoked brain signal with aging (80, 82, 83). Furthermore, studies have shown that under cognitive challenge, older adults have more difficulty switching out of the default state, resulting in default mode activity that is more active and less suppressed during a task in older adults compared to younger adults (81, 87).
In summary, aging is associated with fundamental changes in brain function including dedifferentiation of the ventral visual cortex to categories, decreased activity in the medial temporal lobe during episodic memory tasks, and increased recruitment of frontal and parietal regions, which may compensate for deficiencies in brain structure and function. Moreover, there is a reliable pattern of age-related difficulties in suppressing the activity of the default mode network during cognitive task performance and less coherence among the regions of the network during resting state.
What is known about the relationship of obesity to these patterns of age-related differences in neural activity? The answer is “very little,” as most neuroimaging studies of obese adults have focused on younger individuals. The majority of neuroimaging studies with obese subjects have focused on comparing their neural activation pattern to food images to that of normal weight individuals (53, 88). A recent meta-analysis on neuroimaging findings during food-related-processing in obese individuals, found robust increased activity predominantly in the right hemisphere relative to individuals of normal weight, including the anterior cingulate, parahippocampal gyrus, inferior frontal gyrus, and precentral gyrus (88), combined with a pattern of reduced activation for the left DLPFC and insular cortex. These different neural activity patterns in obese subjects to food images have been interpreted to be related to a reduction of cognitive control in anticipation of food, and a heightened motivational response to food images associated with an increased activity in reward related areas (88). It is well-recognized that that the cognitive control network is less efficient with age, and it is certainly possible that poor cognitive control associated with obesity to food could generalized to other domains (88). Supporting this speculation, there is evidence that obese children show reduced executive function (cognitive control) compared to normal weight children (54).
Further research should examine the vulnerability of different brain networks to the combined effects of obesity and age and determine if there are additive effects of these variables or whether they operate in a synergistic manner, particularly for executive function. Given evidence that obesity is associated with degraded gray and white matter, as discussed previously, it seems quite important to understand whether obese adults show added functional deterioration, or show it earlier in the lifespan than normal weight adults.
Bilateral activation and additional recruitment of cortex areas during cognitive tasks have been identified as a characteristic feature of advanced aging. As suggested by the STAC model, this bilateral activation may be indicative of reorganization or a compensatory response of the brain to biological aging process, and it is important to understand whether obesity may directly or indirectly alter reorganization processes in the brain. Recently, researchers investigated hippocampal and cerebellar gray matter density and neuron-specific enolase (NSE) serum level, a blood biomarker of neural injury, in obese and normal weighted young adults (89). The authors reported increased serum NSE levels and reduced gray matter density in both hippocampus and cerebellum in young obese individuals. They suggested that declines in hippocampal plasticity due to both impaired structural integrity and increased neurodegenerative markers may be an underlying mechanism to explain the accelerated cognitive decline in obese individuals (89). Thus, it is possible that neural plasticity and the brain’s ability to reorganize in response to structural deterioration or cognitive challenge may be impaired with obesity. It is possible that across the lifespan, efficient adaptive neural mechanisms may fail due to fundamental, obesity-related changes in the neural system. An argument against this hypothesis, are the data presented earlier that suggested that obesity in middle age (Age Range = 30–59) was particularly detrimental to cognition in old age, but could be facilitative of cognition in older age (Age Range = 65–80). The behavioral data suggested that short-term obesity, particularly later in the lifespan, was less detrimental to cognition compared to mid-life or life-long obesity. In sum, neuroimaging studies of obesity have found a dynamic shift of increases and decreases of brain networks associated with reward processing and cognitive control, but more longitudinal and lifespan research is essential to understand the broad influence of obesity on structural and functional brain aging and how the brain may respond to the interactive effect of biological aging and obesity to maintain cognitive functioning.
Concluding this section on functional brain activity, we should note that one of the most urgent issues driving the need to understand the normal aging process is the increasing prevalence of AD in the older population. Age as well as obesity, are both risk factors for AD (90–92), therefore an understanding about how these two factors may enhance the likelihood for disease is important. In this next section, we will review the current state of the relationship of obesity on the differential phases of AD.
3. Impact of Obesity on different phases of AD
More than 35 million individuals in the world are affected by dementia due to Alzheimer’s disease (93). The most predictive factors of the disease are advanced age, carrying the apolipoprotein ε4 (APOE) allele, and family history of AD (94). Whereas AD is the most common form of dementia (i.e., 50–70% of the dementia cases), vascular cognitive impairment associated with VaD and cerebral vascular disease (CVD) is the second most common form of dementia (95). Obesity during mid-life has been shown to be associated with an increased risk for dementia due to AD as well as an increased number of vascular risk factors and diagnosis of vascular dementia (92, 96–100).
A recent meta-analysis on the relationship of obesity to risk for AD suggested a complex relationship between body weight and AD (101). The authors reported that underweight BMI, overweight BMI and obesity are all associated with an increased risk for dementia compared to normal weight individuals. The nonlinear relationship of body weight to dementia risk has also been reported previously (102, 103). There is also some evidence for a significant effect of obesity during early adulthood and mid-life on the likelihood of future diagnosis of vascular dementia (104), although others have not found a significant association in individuals with obesity (101). Given that obesity is associated with an increase in vascular risk factors, including diabetes Type 2, hypertension, and metabolic syndrome, it is not surprising that a relationship between obesity and vascular dementia would exist. Despite the collinearity among diabetes, hypertension, and metabolic syndrome, there is clear evidence for an independent effect of obesity on dementia. A meta-analysis that included only studies that controlled for comorbidities such as hypertension, dyslipidemia, CVD and genetic predispositions (APOE ε4), found that obesity played an independent role in the etiology of AD with some studies showing, as well, an enhanced risk for vascular dementia (102).
Further investigations that assessed whether there was a relationship of very low and high body weight to AD reported a 5-fold (CI 0.9-33.7, p=.001) and a 9-fold (CI 2.4-37.3, p=.001) increased risk, respectively, for AD with odds-ratios of 7.9 (CI 1.0-66.3, p=.056) (underweight) and 12. 6 (CI 2.8-56.5, p=.001) (obese) after controlling for metabolic risk factors (105). In addition to an increased prevalence of obesity in AD, a recent longitudinal study explored the relationship of obesity and metabolic syndromes to prodromal stages of AD (i.e., amnestic mild cognitive impairment; aMCI) (106). The researchers reported that metabolic syndrome increased the likelihood for a diagnosis of aMCI, and among the factors tested, central obesity was the major factor driving this relationship (106). Taken together these findings emphasize an independent role for both obese and underweight status on the prevalence of AD. These findings suggest that a much better understanding is needed of whether the joint effects of obesity and age may result in an accelerated risk of dementia diagnosis as well as cognitive decline accompanied by patterns of brain atrophy.
One dominant pathophysiological view of AD suggests that it results from an imbalance between the production and clearance of amyloid-beta peptides in the initial phase of the disease, which is then followed by multiple neuroinflammatory changes that lead to synaptic loss, tau phosphorylation, and ultimately neural degeneration and cell death (107). A recent autopsy study investigated the neuropathological changes in obese patients (N=12) and non-obese controls (N=10) and reported increased AD pathology in obese patients that included hippocampal amyloid-beta peptides, amyloid precursor proteins, and tau pathology (108). One hypothesis regarding obesity and AD is that the increased risk of AD is a result of chronic inflammation, which leads to increased pro-inflammatory cytokines that contribute to systemic insulin resistance, and also penetrates the cerebral vasculature, impairing synaptic function (95, 109, 110). In support of this hypothesis, an animal model involving obese mice indicated that pro-inflammatory changes associated with a high-fat diet led to an increased level of amyloid precursor protein (111). A review of animal models of Type 1 and Type 2 diabetes suggested a relationship of insulin dysfunction and tau hyperphosphorylation, with increased tau pathology in the diabetic animals (112).
With respect to human studies, correlational evidence for a relationship of aggregates of amyloid precursor protein (i.e., Abeta 40) to body fat in a group of healthy adults was recently reported (113). Our own laboratory reported increased levels of beta-amyloid deposition in hypertensive individuals compared to normotensive participants in a large sample of otherwise healthy older adults (Age Range = 47-89) (114). These relationships were modified by APOE ε4 genotype, where in a dose-response fashion, both risk factors (hypertension and APOE ε4 genotype) were associated with elevated levels of beta-amyloid burden measured in vivo (114). In sum, evidence is rapidly accumulating that relates obesity and its comorbidities (i.e., diabetes, hypertension) to an increased risk for AD pathology (112, 114).
To summarize, epidemiological studies have provided evidence that obesity in mid-life is linked to an increased risk for AD and VaD. An underweight BMI in mid-life also appears to be associated with AD in late life, underscoring the need for further research relating body weight to pathological aging processes. The role of obesity in developing AD pathology has only recently been studied, and animal models and post mortem studies both suggest that obesity enhances the expression of the pathophysiological changes associated with disease progression. Interestingly, comorbidities of obesity (i.e., hypertension or diabetes) have been shown to be associated with increased level of pathology in preclinical stages of the disease. Thus, the current evidence suggests that obesity may enhance the likelihood of AD across the spectrum of the disease from preclinical to advance AD stages. More research is needed to fully elucidate the contributing factors of obesity (e.g., enhanced beta-amyloid pathology, tau pathology, cortical changes) to disease progression.
5. Summary and Future Directions
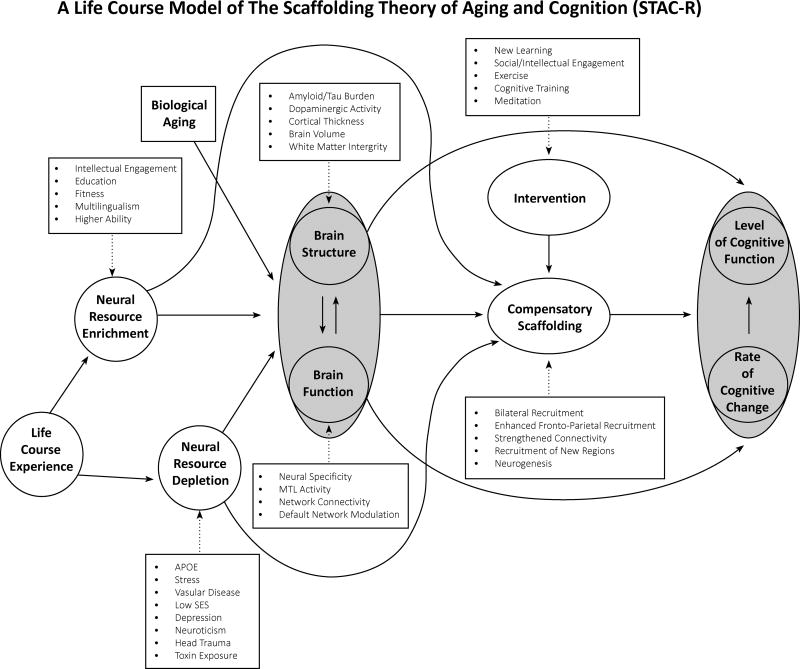
Obesity is a multi-factorial health condition that has widespread consequences on major organs including the heart, liver, and the brain. Less well-recognized, but highlighted in the present paper, is that obesity negatively affects cognitive function from childhood to late-life adulthood. The main conclusions about obesity across the lifespan and cognition are as follows: Whereas midlife overweight status and obesity may be more detrimental to cognitive decline, overweight status and obesity in late life may be neutral with respect to cognitive decline, or possibly even protective. From the perspective of the STAC model it can be concluded, that obesity exacerbates the relationship of age on cognition, however this association is not uniformly negative across all ages, as reviewed here. Importantly, since STAC was published in 2009, Reuter-Lorenz and Park developed an extension of their model termed ‘STAC-R’ (16). The revised version of the model (see Figure 3) adds to the original model the following: 1) Life-course experience are integrated into the model. Life-course variables can be important modifications that are either depleting or enriching of neural resources. Neural enrichments results from intellectual engagement, higher education, cognitive ability, as well as physical fitness and multilingualism. Life-course factors that deplete neural resources include environmental factors such as APOE or Stress respectively. Further, lower socioeconomic status, depression, and neuroticism may also deplete neural resources. 2) Life-course factors are now understood within the context of the rate of cognitive change over time, rather than an individual’s level of cognitive function. The present review suggest that obesity, particularly in middle-aged can be understood as a factor that contributes to neural resource depletion, with negative effects on brain structure and brain function and vitality on cognition. Other factors such as ‘lower SES’ or lower education is associated with obesity and may magnify the depletion effect on cof obesity on cognition (115, 116). Others have reported that education level may exert the effect of SES on increased prevalence of BMI (117). Psychological variables that could lead to neural enrichment have not explicitly modeled in the studies reported here and future studies may isolate resilience factors that may moderate the relationship of obesity on cognition in advanced age.
Figure 3.
A revision of the scaffolding theory of aging and cognition (STAC-R)
Lifestyle factors such as physical activity should be considered as an intervening factor that alters the relationship of obesity on cognition. A recent comprehensive model on the interaction of physical activity and eating behavior on neurocognitive function, suggests that physical activity may improve executive function through increased efficient processing of information in the prefrontal cortex, which in turn may improve inhibitory control over eating behavior (118). This model suggests that interventions that modify some cognitive deficits associated with obesity may also result in better control over obesity and other negative obesity-related consequences.
It is critical to begin investigating the relationships among aging, obesity, cognition, and the brain to establish a basic understanding of these complex associations. The revised version of the STAC model provide a guide for how obesity may serve as a factor of neural depletion factor and how it affects the differential trajectories on an individual’s rate of cognitive change.
Our review also reported that obesity has been shown to be associated with an increased risk for AD dementia and vascular dementia. Future research should focus on studies designed to disentangle the factors that may influence both protective and risk factors associated with obesity on cognitive decline in older age. In general, this review suggests that obesity and age are additive factors for cognitive decline in midlife, with little evidence for an interactive effect, although larger and more focused studies could amend this view. Structural changes to both gray and white matter of the brain have been reported with obesity and increased BMI. These structural changes begin to appear in young adulthood, suggesting that obesity may have a sustained negative effect on brain structure across the lifespan. The data are not clear as to whether obesity and age synergistically or additively exacerbate structural brain aging. With respect to brain function, very little research has been done targeting how obesity affects markers of functional brain aging. We have speculated that obesity may amplify age-related differences in brain function due to evidence suggesting that, even in younger adults, obesity is associated with different patterns of neural activation in cognitive control, reward, and attentional networks. Finally the study of obesity within the framework of STAC and STAC-R may be a helpful guide to gain further insights in the complex nature between obesity, aging, and cognition.
Acknowledgments
This study was supported in part by NIH grants 5R37AG-006265-27, 3R37AG 006265-25S awarded to D C. P. The authors are grateful to Patrick Evans, Jenny Rieck, and Ian McDonough for helpful comments on a previous version of this manuscript.
Abbreviations
- AD
Alzheimer’s disease
- APOE
apolipoprotein
- DLPFC
dorsolateral prefrontal cortex
Footnotes
The authors declare no conflict of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity and Trends in Body Mass Index Among US Children and Adolescents, 1999–2010. Jama-J Am Med Assoc. 2012 Feb 1;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011 Feb 12;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakhan SE, Kirchgessner A. The emerging role of dietary fructose in obesity and cognitive decline. Nutr J. 2013 Aug 8;12 doi: 10.1186/1475-2891-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cournot M, Marquie JC, Ansiau D, Martinaud C, Fonds H, Ferrieres J, Ruidavets JB. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006 Oct 10;67(7):1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- 6.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiology of Aging. 2005 Dec;26:S11–S16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Kilander L, Nyman H, Boberg M, Lithell H. Cognitive function, vascular risk factors and education. A cross-sectional study based on a cohort of 70-year-old men. J Intern Med. 1997 Oct;242(4):313–321. doi: 10.1046/j.1365-2796.1997.00196.x. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: Importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Current Alzheimer Research. 2007 Apr;4(2):111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 9.Sturman MT, de Leon CFM, Bienias JL, Morris MC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology. 2008 Jan 29;70(5):360–367. doi: 10.1212/01.wnl.0000285081.04409.bb. [DOI] [PubMed] [Google Scholar]
- 10.Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, White LR, Launer LJ. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005 Jan;62(1):55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002 Jun;17(2):299–320. [PubMed] [Google Scholar]
- 12.Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: Cross-sectional results from the Berlin Aging Study. Psychology and Aging. 1997 Sep;12(3):410–432. doi: 10.1037//0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- 13.Lindenberger U, Baltes PB. Sensory Functioning and Intelligence in Old-Age - a Strong Connection. Psychology and Aging. 1994 Sep;9(3):339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 14.Baltes PB, Staudinger UM, Lindenberger U. Lifespan psychology: Theory and application to intellectual functioning. Annu Rev Psychol. 1999;50:471–507. doi: 10.1146/annurev.psych.50.1.471. [DOI] [PubMed] [Google Scholar]
- 15.Baltes PB, Smith J. New frontiers in the future of aging: From successful aging of the young old to the dilemmas of the fourth age. Gerontology. 2003 Mar-Apr;49(2):123–135. doi: 10.1159/000067946. [DOI] [PubMed] [Google Scholar]
- 16.Reuter-Lorenz PA, Park DC. How Does it STAC Up? Revisiting the Scaffolding Theory of Aging and Cognition. Neuropsychol Rev. 2014 Aug 21; doi: 10.1007/s11065-014-9270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park DC, Reuter-Lorenz P. The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998 Jan;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- 19.Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000 Jan;12(1):174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- 20.Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FI. The effects of age on the neural correlates of episodic encoding. Cereb Cortex. 1999 Dec;9(8):805–814. doi: 10.1093/cercor/9.8.805. [DOI] [PubMed] [Google Scholar]
- 21.Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997 Jan 1;17(1):391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000 Apr;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- 23.Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magn Reson Med. 2003 May;49(5):953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- 24.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004 Mar;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 25.Wong DF, Young D, Wilson PD, Meltzer CC, Gjedde A. Quantification of neuroreceptors in the living human brain: III. D2-like dopamine receptors: theory, validation, and changes during normal aging. J Cereb Blood Flow Metab. 1997 Mar;17(3):316–330. doi: 10.1097/00004647-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Backman L, Ginovart N, Dixon RA, Wahlin TB, Wahlin A, Halldin C, Farde L. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry. 2000 Apr;157(4):635–637. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigue KM, Kennedy KM, Devous MD, Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, Mathews D, Park DC. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012 Feb 7;78(6):387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. P Natl Acad Sci USA. 2004 Aug 31;101(35):13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006 Feb;18(2):227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- 30.Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial-temporal activity. J Cognitive Neurosci. 2005 Jan;17(1):84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- 31.de Chastelaine M, Wang TH, Minton B, Muftuler LT, Rugg MD. The effects of age, memory performance, and callosal integrity on the neural correlates of successful associative encoding. Cereb Cortex. 2011 Sep;21(9):2166–2176. doi: 10.1093/cercor/bhq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007 Jan-Feb;48(1):57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Benito-Leon J, Mitchell AJ, Hernandez-Gallego J, Bermejo-Pareja F. Obesity and impaired cognitive functioning in the elderly: a population-based cross-sectional study (NEDICES) Eur J Neurol. 2013 Jun;20(6):899–906. e876–897. doi: 10.1111/ene.12083. [DOI] [PubMed] [Google Scholar]
- 34.Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass index over the adult life course and cognition in late midlife: the Whitehall II Cohort Study. Am J Clin Nutr. 2009 Feb 1;89(2):601–607. doi: 10.3945/ajcn.2008.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuo HK, Jones RN, Milberg WP, Tennstedt S, Talbot L, Morris JN, Lipsitz LA. Cognitive function in normal-weight, overweight, and obese older adults: An analysis of the advanced cognitive training for independent and vital elderly cohort. Journal of the American Geriatrics Society. 2006 Jan;54(1):97–103. doi: 10.1111/j.1532-5415.2005.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deschamps V, Astier X, Ferry M, Rainfray M, Emeriau JP, Barberger-Gateau P. Nutritional status of healthy elderly persons living in Dordogne, France, and relation with mortality and cognitive or functional decline. Eur J Clin Nutr. 2002 Apr;56(4):305–312. doi: 10.1038/sj.ejcn.1601311. [DOI] [PubMed] [Google Scholar]
- 37.Alexandre Tda S, Duarte YA, Santos JL, Wong R, Lebrao ML. Prevalence and associated factors of sarcopenia among elderly in Brazil: findings from the SABE study. J Nutr Health Aging. 2014 Mar;18(3):284–290. doi: 10.1007/s12603-013-0413-0. [DOI] [PubMed] [Google Scholar]
- 38.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002 Dec;57(12):M772–777. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 39.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001 Aug;2(3):141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 40.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R. Waist Circumference and Cardiometabolic Risk: a Consensus Statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; the American Diabetes Association. Obesity (Silver Spring) 2007 May;15(5):1061–1067. doi: 10.1038/oby.2007.632. [DOI] [PubMed] [Google Scholar]
- 41.Siervo M, Arnold R, Wells JC, Tagliabue A, Colantuoni A, Albanese E, Brayne C, Stephan BC. Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta-analysis. Obes Rev. 2011 Nov;12(11):968–983. doi: 10.1111/j.1467-789X.2011.00903.x. [DOI] [PubMed] [Google Scholar]
- 42.Singh-Manoux A, Czernichow S, Elbaz A, Dugravot A, Sabia S, Hagger-Johnson G, Kaffashian S, Zins M, Brunner EJ, Nabi H, Kivimaki M. Obesity phenotypes in midlife and cognition in early old age The Whitehall II cohort study. Neurology. 2012 Aug;79(8):755–762. doi: 10.1212/WNL.0b013e3182661f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003 Feb;27(2):260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 44.Almkvist O. Neuropsychological deficits in vascular dementia in relation to Alzheimer's disease: reviewing evidence for functional similarity or divergence. Dementia. 1994 May-Aug;5(3–4):203–209. doi: 10.1159/000106724. [DOI] [PubMed] [Google Scholar]
- 45.van den Berg E, Kloppenborg RP, Kessels RP, Kappelle LJ, Biessels GJ. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochim Biophys Acta. 2009 May;1792(5):470–481. doi: 10.1016/j.bbadis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, Smith S, Schmidt R. Risk factors for progression of brain atrophy in aging - Six-year follow-up of normal subjects. Neurology. 2005 May 24;64(10):1704–1711. doi: 10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed] [Google Scholar]
- 48.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010 Mar;31(3):353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brooks SJ, Benedict C, Burgos J, Kempton MJ, Kullberg J, Nordenskjold R, Kilander L, Nylander R, Larsson EM, Johansson L, Ahlstrom H, Lind L, Schioth HB. Late-life obesity is associated with smaller global and regional gray matter volumes: a voxel-based morphometric study. Int J Obesity. 2013 Feb;37(2):230–236. doi: 10.1038/ijo.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ, Nat R. Body mass index and magnetic resonance markers of brain integrity in adults. Annals of Neurology. 2008 May;63(5):652–657. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bettcher BM, Walsh CM, Watson C, Miller JW, Green R, Patel N, Miller BL, Neuhaus J, Yaffe K, Kramer JH. Body Mass and White Matter Integrity: The Influence of Vascular and Inflammatory Markers. PLoS ONE. 2013 Oct 16;8(10) doi: 10.1371/journal.pone.0077741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005 Oct;62(10):1545–1548. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- 53.Shott ME, Cornier MA, Mittal VA, Pryor TL, Orr JM, Brown MS, Frank GK. Orbitofrontal cortex volume and brain reward response in obesity. Int J Obes (Lond) 2014 Jul 16; doi: 10.1038/ijo.2014.121ijo2014121[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang J, Matheson BE, Kaye WH, Boutelle KN. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int J Obes (Lond) 2014 Apr;38(4):494–506. doi: 10.1038/ijo.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morley JE. The metabolic syndrome and aging. J Gerontol A Biol Sci Med Sci. 2004 Feb;59(2):139–142. doi: 10.1093/gerona/59.2.m139. [DOI] [PubMed] [Google Scholar]
- 56.Bruce-Keller AJ, Keller JN, Morrison CD. Obesity and vulnerability of the CNS. Biochim Biophys Acta. 2009 May;1792(5):395–400. doi: 10.1016/j.bbadis.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Z, Manson KF, Schiller D, Levy I. Impaired associative learning with food rewards in obese women. Curr Biol. 2014 Aug 4;24(15):1731–1736. doi: 10.1016/j.cub.2014.05.075. [DOI] [PubMed] [Google Scholar]
- 58.Grady C. The cognitive neuroscience of ageing. Nature Reviews Neuroscience. 2012;13(7):491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park DC, McDonough IM. The Dynamic Aging Mind: Revelations From Functional Neuroimaging Research. Perspect Psychol Sci. 2013 Jan;8(1):62–67. doi: 10.1177/1745691612469034. [DOI] [PubMed] [Google Scholar]
- 60.Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: A functional magnetic resonance imaging study. Journal of Neuroscience. 1996 Aug 15;16(16):5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001 Sep 28;293(5539):2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 62.Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004 May;7(5):555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- 63.Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997 Jun 1;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carp J, Park J, Polk TA, Park DC. Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. NeuroImage. 2011 May 15;56(2):736–743. doi: 10.1016/j.neuroimage.2010.04.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park J, Carp J, Hebrank A, Park DC, Polk TA. Neural Specificity Predicts Fluid Processing Ability in Older Adults. Journal of Neuroscience. 2010 Jul 7;30(27):9253–9259. doi: 10.1523/JNEUROSCI.0853-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carp J, Park J, Hebrank A, Park DC, Polk TA. Age-Related Neural Dedifferentiation in the Motor System. PLoS ONE. 2011 Dec 22;6(12) doi: 10.1371/journal.pone.0029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dennis NA, Cabeza R. Neuroimaging of Healthy Cognitive Aging. In: Craik F, Salthouse TA, editors. The handbook of aging and cognition. 3. xi. New York, NY: Psychology Press; 2008. pp. 1–54. [Google Scholar]
- 68.Morcom AM, Rugg MD. Retrieval orientation and the control of recollection: An FMRI study. J Cognitive Neurosci. 2005:103–103. doi: 10.1162/jocn_a_00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dickerson BC. Functional MRI in the early detection of dementias. Rev Neurol (Paris) 2006 Oct;162(10):941–944. doi: 10.1016/s0035-3787(06)75103-7. [DOI] [PubMed] [Google Scholar]
- 70.Dickerson BC. Functional magnetic resonance imaging of cholinergic modulation in mild cognitive impairment. Curr Opin Psychiatry. 2006 May;19(3):299–306. doi: 10.1097/01.yco.0000218602.25346.c6. [DOI] [PubMed] [Google Scholar]
- 71.Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010 Jan;35(1):86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004 Jul;56(1):27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005 Aug 9;65(3):404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yassa MA, Mattfeld AT, Stark SM. Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. P Natl Acad Sci USA. 2011 May 24;108(21):8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002 Nov;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 76.Cabeza R, Anderson ND, Kester J, McIntosh AR. Hemispheric Asymmetry Reduction in Old Adults (HAROLD): Evidence for the compensation hypothesis. J Cognitive Neurosci. 2002 Apr;:125–125. [Google Scholar]
- 77.Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: A quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav R. 2010 Jul;34(8):1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Huang CM, Polk TA, Goh JO, Park DC. Both left and right posterior parietal activations contribute to compensatory processes in normal aging. Neuropsychologia. 2012 Jan;50(1):55–66. doi: 10.1016/j.neuropsychologia.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McDonough IM, Wong JT, Gallo DA. Age-related differences in prefrontal cortex activity during retrieval monitoring: testing the compensation and dysfunction accounts. Cereb Cortex. 2013 May;23(5):1049–1060. doi: 10.1093/cercor/bhs064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007 Dec 6;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park DC, Polk TA, Hebrank AC, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Frontiers in Human Neuroscience. 2010 Jan;3 doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grady CL, Grigg O, Ng C. Age differences in default and reward networks during processing of personally relevant information. Neuropsychologia. 2012 Jun;50(7):1682–1697. doi: 10.1016/j.neuropsychologia.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mowinckel AM, Espeseth T, Westlye LT. Network-specific effects of age and in-scanner subject motion: A resting-state fMRI study of 238 healthy adults. NeuroImage. 2012 Nov 15;63(3):1364–1373. doi: 10.1016/j.neuroimage.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 84.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. P Natl Acad Sci USA. 2001 Jan 16;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sheline YI, Raichle ME. Resting state functional connectivity in preclinical Alzheimer's disease. Biol Psychiatry. 2013 Sep 1;74(5):340–347. doi: 10.1016/j.biopsych.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar Mri. Magnet Reson Med. 1995 Oct;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 87.Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008 Feb 12;105(6):2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brooks SJ, Cedernaes J, Schioth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS ONE. 2013;8(4):e60393. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mueller K, Sacher J, Arelin K, Holiga S, Kratzsch J, Villringer A, Schroeter M. Overweight and obesity are associated with neuronal injury in the human cerebellum and hippocampus in young adults: a combined MRI, serum marker and gene expression study. Transl Psychiat. 2012 Dec;2 doi: 10.1038/tp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010 Jan;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers & Dementia. 2011 May;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008 Sep 30;71(14):1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 93.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer's & Dementia. 2013;9(1):63–75.e62. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 94.Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 95.Miller AA, Spencer SJ. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav Immun. 2014 Apr 12; doi: 10.1016/j.bbi.2014.04.001. S0889-1591(14)00088-9 [pii] [DOI] [PubMed] [Google Scholar]
- 96.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003 Jul 14;163(13):1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 97.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Brit Med J. 2005 Jun 11;330(7504):1360–1362B. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004 Nov 23;63(10):1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 99.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of Neurology. 2005 Oct;62(10):1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 100.Whitmer RA, Gunderson EP, Quesenberry CP, Zhou JF, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Current Alzheimer Research. 2007 Apr;4(2):103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 101.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011 May;12(501):e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 102.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008 May;9(3):204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gorospe EC, Dave JK. The risk of dementia with increased body mass index. Age Ageing. 2007 Jan;36(1):23–29. doi: 10.1093/ageing/afl123. [DOI] [PubMed] [Google Scholar]
- 104.Chen YC, Chen TF, Yip PK, Hu CY, Chu YM, Chen JH. Body mass index (BMI) at an early age and the risk of dementia. Arch Gerontol Geriat. 2010 Feb;50:S48–S52. doi: 10.1016/S0167-4943(10)70013-3. [DOI] [PubMed] [Google Scholar]
- 105.Razay G, Vreugdenhil A, Wilcock G. Obesity, abdominal obesity and Alzheimer disease. Dement Geriatr Cogn Disord. 2006;22(2):173–176. doi: 10.1159/000094586. [DOI] [PubMed] [Google Scholar]
- 106.Feng L, Chong MS, Lim WS, Lee TS, Collinson SL, Yap P, Ng TP. Metabolic syndrome and amnestic mild cognitive impairment: Singapore Longitudinal Ageing Study-2 findings. J Alzheimers Dis. 2013;34(3):649–657. doi: 10.3233/JAD-121885. [DOI] [PubMed] [Google Scholar]
- 107.De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. Alzheimer’s Disease. 2012;65:329–352. doi: 10.1007/978-94-007-5416-4_14. [DOI] [PubMed] [Google Scholar]
- 108.Mrak RE. Alzheimer-type neuropathological changes in morbidly obese elderly individuals. Clin Neuropathol. 2009 Jan-Feb;28(1):40–45. doi: 10.5414/npp28040. [DOI] [PubMed] [Google Scholar]
- 109.Shu CJ, Benoist C, Mathis D. The immune system's involvement in obesity-driven type 2 diabetes. Semin Immunol. 2012 Dec;24(6):436–442. doi: 10.1016/j.smim.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, Stranahan AM. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci. 2014 Feb 12;34(7):2618–2631. doi: 10.1523/JNEUROSCI.4200-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Puig KL, Floden AM, Adhikari R, Golovko MY, Combs CK. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS ONE. 2012;7(1):e30378. doi: 10.1371/journal.pone.0030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.El Khoury NB, Gratuze M, Papon MA, Bretteville A, Planel E. Insulin dysfunction and Tau pathology. Front Cell Neurosci. 2014;8:22. doi: 10.3389/fncel.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leahey TM, Myers TA, Gunstad J, Glickman E, Spitznagel MB, Alexander T, Juvancic-Heltzel J. Abeta40 is associated with cognitive function, body fat and physical fitness in healthy older adults. Nutr Neurosci. 2007 Oct-Dec;10(5–6):205–209. doi: 10.1080/10284150701676156. [DOI] [PubMed] [Google Scholar]
- 114.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Sr, Diaz-Arrastia R, Park DC. Risk factors for beta-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurol. 2013 May;70(5):600–606. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ruel E, Reither EN, Robert SA, Lantz PM. Neighborhood effects on BMI trends: examining BMI trajectories for Black and White women. Health & place. 2010 Mar;16(2):191–198. doi: 10.1016/j.healthplace.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mujahid MS, Diez Roux AV, Borrell LN, Nieto FJ. Cross-sectional and longitudinal associations of BMI with socioeconomic characteristics. Obesity research. 2005 Aug;13(8):1412–1421. doi: 10.1038/oby.2005.171. [DOI] [PubMed] [Google Scholar]
- 117.Novak M, Ahlgren C, Hammarstrom A. A life-course approach in explaining social inequity in obesity among young adult men and women. Int J Obes (Lond) 2006 Jan;30(1):191–200. doi: 10.1038/sj.ijo.0803104. [DOI] [PubMed] [Google Scholar]
- 118.Joseph RJ, Alonso-Alonso M, Bond DS, Pascual-Leone A, Blackburn GL. The neurocognitive connection between physical activity and eating behaviour. Obes Rev. 2011 Oct;12(10):800–812. doi: 10.1111/j.1467-789X.2011.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]