Abstract
Objective
Cross-sectional data suggest functional and anatomical disturbances in inferior and orbital frontal regions in bulimia nervosa (BN). Using longitudinal data, we investigated whether reduced cortical thickness (CT) in these regions arises early and persists over adolescence in BN, independent of symptom remission, and whether CT reductions are markers of BN symptoms.
Method
Thirty-three adolescent females with BN symptoms (BN or other specified feeding or eating disorder) and 28 healthy adolescents participated in this study. Anatomical magnetic resonance imaging and clinical data were acquired at three time points within 2-year intervals over adolescence with 31% average attrition between assessments. Using a region-of-interest approach, we assessed group differences in CT at baseline and over time, and tested whether between- and within-subject variations in CT were associated with the frequency of BN symptoms.
Results
Reduced CT in right inferior frontal gyrus persisted over adolescence in BN compared to healthy adolescents, even in those who achieved full or partial remission. Within the BN group, between-subject variations in CT in inferior and orbital frontal regions were inversely associated with specific BN symptoms, suggesting, on average over time, greater CT reductions in those with more frequent BN symptoms.
Conclusion
Reduced CT in inferior frontal regions may contribute to illness persistence into adulthood. Reductions in the thickness of inferior and orbital frontal regions may be markers of specific BN symptoms. Since our sample size precluded correcting for multiple comparisons, these findings should be replicated in a larger sample. Future study of functional changes in associated fronto-striatal circuits could identify potential circuit-based intervention targets.
Keywords: Magnetic Resonance Imaging (MRI), Cortical Thickness, Bulimia Nervosa, Adolescents, Longitudinal design
INTRODUCTION
Bulimia nervosa (BN) typically begins in adolescence and is characterized by binge-eating and purging or other compensatory behaviors to avoid weight gain. Previous cross-sectional findings from adolescents and adults with BN suggest that disturbances in control, reward, and learning processes underlie these symptoms due, in part, to functional1–4 and anatomical5, 6 alterations in fronto-striatal circuits, particularly inferior and orbital frontal regions. Previous cross-sectional findings suggested reduced cortical thickness (CT) and smaller local volumes on the surface of lateral frontal gyri in adolescents and adults with BN relative to their healthy counterparts.5 Further, volume reductions in bilateral inferior frontal gyrus (IFG) were more pronounced in the participants with BN who engaged in more frequent binge-eating and vomiting episodes. Unclear, however, is whether structural abnormalities in frontal regions are stable across development, thereby contributing to diagnostic status, or whether these abnormalities are state-based markers of BN symptoms that fluctuate over time. Thus, we used longitudinal magnetic resonance imaging (MRI) data from adolescents with and without BN symptoms to determine whether reduced CT in inferior frontal regions arises early and persists over adolescence in BN, independent of symptom change. Given the role of the orbital frontal cortex (OFC) in control and reward processes and BN pathophysiology,4, 6–8 we also assessed the trajectory of thickness changes in OFC regions. Specifically, we examined whether CT reductions in inferior and orbital frontal regions constitute trait or state markers of BN and associated binge-eating and purging symptoms.
Many studies have attempted to identify trait and state markers of a range of psychiatric disorders, including anorexia nervosa (AN),9–12 depression,13 bipolar disorder,14 and schizophrenia.15 A trait marker of a disorder is typically conceptualized in these studies as a characteristic that distinguishes an individual with a disorder from healthy controls, is present before and predicts disorder onset, persists following total or partial remission, and is present in individuals with subclinical profiles. In contrast, a state marker of a disorder is defined as a characteristic that is only present, or at least more pronounced, during acute phases of a disorder, with symptoms fluctuating with levels of that marker. Assessing such trait or state markers of an illness that occur before onset and persist following remission would require substantial resources that would render such a study practically unfeasible.
Thus, in the current longitudinal study, we assessed CT early after BN onset and over the course of adolescence. We first tested whether CT in inferior and orbital frontal regions is reduced in BN compared to healthy adolescents and whether these reductions persist over time, even following full or partial remission. Such findings would suggest that reduced CT in these regions is a trait marker of the illness. In addition to illness markers, we can measure trait and state markers of symptoms within a diagnostic group since CT may show both trait- and state-like properties. Average CT over time in an individual could predict average symptom expression relative to others (i.e., trait). In contrast, CT fluctuations over time relative to an individual’s average CT could predict symptom fluctuations over time (i.e., state). Such between- and within-subject variations can be measured reliably with CT.16 Thus, we also assessed whether each participant’s average CT over time predicted the average frequency of their BN symptoms over time, or whether fluctuations in CT over time predicted change in their BN symptoms over time. This question has remained untested in eating disorders, as longitudinal data comprising at least three time points are needed to examine such within-subject fluctuations. Moreover, while multiple biomarkers relate to average individual differences in symptoms across a range of psychiatric disorders,10, 12–15 substantial within-subject fluctuation in symptom expression occurs over time and biomarkers of these within-individual fluctuations remain unidentified.17 Thus, understanding both between-subject and within-subject fluctuations in the thickness of inferior and orbital frontal regions over time may provide a more nuanced understanding of neuroanatomical influences on changes in symptom severity and BN persistence over adolescence.
Although we are unaware of any imaging studies following this approach, the notion that individual characteristics may have both state- and trait-like variance and effects on symptom expression is well-established in other fields. For instance, in the personality-psychopathology literature, it is widely acknowledged that both average levels of a given characteristic and the extent to which it is expressed on a given occasion are important predictors of symptom expression within internalizing disorders.18 In the current study, multilevel modeling makes it possible to identify the symptom correlates of both between-subject and within-subject variability in CT within BN. That is, by decomposing each participant with BN’s CT for a given region at each time point into an average value over time and into deviations from that average at each time point, we can examine whether, on average over time, CT is inversely associated with the frequency of BN symptoms (i.e., a trait effect), whether individual fluctuations in CT are inversely associated with the frequency of BN symptoms at each time point (i.e., a state effect), or both.
Thus, herein we assessed whether CT reductions within inferior and orbital frontal regions are trait markers of BN (i.e., reductions in BN vs. healthy participants that persist over time), or BN symptoms (i.e., average reductions over time in BN participants that predict average symptom expression over time). We also assessed state markers of BN symptoms (i.e., within-subject fluctuations in CT at each time point that predicted BN symptoms at each time point).
METHOD
Participants
Participants were adolescent females with BN symptoms (n = 33) and healthy controls (HC; n = 28) group-matched for age, body mass index (BMI), race, and ethnicity. Participants were recruited through local and online advertisements. Participants with a history of neurological illness, past seizures, head trauma with loss of consciousness, mental retardation, pervasive developmental disorder, or current Axis I disorders (other than depressive or anxiety disorders in the BN group) were excluded. Controls had no lifetime Axis I disorders. Axis I disorders were assessed using the Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version.19 BN symptom severity and prior diagnoses of anorexia nervosa were assessed using the Eating Disorders Examination.20 Participants in the BN group were included if they engaged in an average of one loss-of-control eating episode (including both objectively and subjectively large bulimic episodes) and one compensatory episode (self-induced vomiting, laxative/diuretic misuse, or compulsive exercise) per week within the past 3 months, with at least one loss-of-control eating and compensatory episode occurring in the past month. Two follow-up assessments (FU1 and FU2) were conducted, each within 2-year intervals over adolescence. BN symptom severity and the presence or absence of comorbid illnesses were assessed each time point. The research protocol was approved by the Institutional Review Board of the New York State Psychiatric Institute, and all participants gave informed consent or assent before participating.
Image Acquisition and Processing
Image acquisition and processing procedures are detailed in Supplement 1, available online. Whole-brain structural T1-weighted MRI images were acquired using a standard quadrature GE 8-channel head coil and a GE Signa 3T LX scanner (Milwaukee, WI). Processing, including cortical surface reconstruction and volumetric segmentation, was performed in FreeSurfer image analysis suite (version 5.3.0, http://surfer.nmr.mgh.harvard.edu/) using automated and semi-automated tools21, 22 followed by the longitudinal stream.23 The cerebral cortex of each participant was automatically parcellated into six inferior and orbital frontal regions in each hemisphere (i.e., inferior frontal gyrus pars triangularis, opercularis and orbitalis, medial and lateral orbital gyrus and frontal pole) based on the Desikan-Killiany atlas.24 Mean CT was extracted from each region, and these values were used in region-of-interest (ROI) analyses.
Statistical Analyses
Stability of CT and BN Symptoms Over Time
Pearson correlation coefficients were calculated to examine the stability of CT and BN symptoms from baseline to FU1, FU1 to FU2, and baseline to FU2.
Group Differences in CT Over Time
Growth curve models (GCM) examined a) group (BN versus HC) differences in CT at baseline, b) group differences in the rate of change in CT over time, and c) the persistence of group differences in CT over time. These analyses were then repeated after including only adolescents with BN who showed more than a 50% symptom reduction in the frequency of their core BN symptoms between their baseline and last assessments, calculated as the sum of the frequency of objective bulimic (OBEs) and self-induced vomiting episodes over the past 28 days. This 50% criterion was chosen in an attempt to retain as many participants as possible in our analyses, given that few participants met more stringent criteria for remission.
Trait Versus State Effects of CT on BN Symptoms
The second set of analyses examined whether reduced CT in frontal regions in the BN group constitutes a trait or state marker of binge-eating and purging behaviors. Specifically, between-subject/trait predictors were calculated by averaging participants’ CT scores over time. Within-subject/state CT predictors were calculated by subtracting each participant’s average CT over time from her raw CT value at each time point. These values therefore represented deviations at each time point from each participant’s own average CT. Multilevel models then tested simultaneously whether between- and within-subject variation in CT was associated with the frequency of OBEs and vomiting episodes over the 28 days prior to scanning. Between-subject effects would reveal whether participants who had, on average, reduced CT in a given region over time relative to other participants also engaged in more or fewer BN symptoms over time (i.e., trait effects) relative to other participants. In contrast, within-subject effects test whether changes in CT from each participant’s own average levels of CT from one time point to the next predict fluctuations in symptom frequency at each time point (i.e., state effects). Because significant change in CT over time was detected in several regions, participants with only baseline data (n=6) were excluded from these analyses to avoid biased estimation of their mean CT over time.
Growth curve and multilevel models were conducted using SAS PROC MIXED (version 9.3). Both modeling approaches permit the analysis of unbalanced data (i.e., unequal numbers of data points among participants because of varying numbers of time points completed). These models make use of all available data via maximum likelihood-based estimation.25 Growth curve models regressed CT on time (months from baseline), group status (HC vs. BN), their interaction, and age at baseline (in years), thereby controlling for possible maturational differences in CT due to baseline age. For the multilevel models, a first-order autoregressive covariance structure was used to accommodate expected correlations between unexplained variance from one time point to the next. Between-within degrees of freedom were used. To control for maturational effects on symptoms as well as naturalistic changes in symptoms over time, multilevel models adjusted for the effects of baseline age and months elapsed since baseline, rendering our prediction of BN symptoms very conservative. All models included a random effect for the intercept and fixed effects for predictors.
Effects of Medication and Comorbidities
Both comorbid depression or anxiety and the use of selective serotonin reuptake inhibitors (SSRIs) may influence changes in both CT26, 27 and BN symptoms28, 29 over time. Thus, growth curve and multilevel models were conducted with these additional time-varying covariates (i.e., comorbid depression or anxiety and current SSRI use) at each time point. Since these covariates did not appreciably affect our findings (Tables S1 and S2, available online), they were not included in the models reported below.
RESULTS
Participants
Demographic and clinical characteristics are shown in Table 1. Baseline data was available from thirty-three adolescents with BN and 28 healthy adolescents. Twenty-two of the 33 adolescents with BN met DSM-5 criteria for BN. The remaining 11 met criteria for other specified feeding or eating disorder (OSFED) with subjective or objective loss of control eating episodes30 and compensatory behaviors to avoid weight gain (i.e., OSFED-BN), and were included since adolescents with less-severe BN symptoms tend to engage in more frequent binge-eating and purging behaviors over time.31, 32 Baseline demographic and clinical characteristics across BN subtypes are presented in Table S3 (available online). FU1 MRI and clinical/demographic data were available from 27 adolescents with BN and 22 HC adolescents (Meantime from baseline=15.7 months, SDtime from baseline=6.182), and FU2 data from 18 adolescents with BN and 12 HC adolescents (Meantime difference from FU1=14.8 months, SDtime difference from FU1=3.275). The 31 participants with any missing data did not differ significantly from the 30 with complete data from all time points in terms of baseline demographic characteristics, CT or symptom severity in the BN group (all ps>.05). Thus, data were missing at random and were therefore appropriately estimated via maximum likelihood methods in a multilevel analytic framework.25 Figure S1 (available online) depicts each assessment point and corresponding age of each participant over the course of the study (see Table S4, available online, for statistical details on the time intervals between assessments across groups). Seventeen of the 33 adolescents with BN included in the study showed more than 50% reduction in the frequency of their core BN symptoms between their baseline and last assessments. Baseline demographics and clinical ratings did not differ across those 17 adolescents with BN showing symptom remission over time and the remaining 16.
Table 1.
Demographic and Clinical Characteristics at Each Assessment Point
| Characteristic | Baseline | FU1 | FU2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| BN (n=33) | HC (n=28) | Analysis | BN (n=26) | HC (n=21) | Analysis | BN (n=16) | HC (n=12) | Analysis | ||||
|
|
|
|
|
|
|
|
|
|
||||
| Mean(SD) | Mean(SD) | t(df) | p | Mean(SD) | Mean(SD) | t(df) | p | Mean(SD) | Mean(SD) | t(df) | p | |
| Age | 16.5(1.5) | 16.2(2.1) | −0.73(49) | .47 | 18.1(1.5) | 17.3(2.1) | −1.38(35) | .18 | 19.3(1.5) | 18.8(2.3) | −0.65(18) | .52 |
| BMI (kg/m) | 22.1(2.8) | 21.4(3.5) | −0.86(53) | .39 | 23.2(2.8) | 22.6(3.5) | −0.62(37) | .54 | 23.8(2.7) | 23.9(4.9) | 0.11(16) | .92 |
| WAIS IQ score (Full) | 105.5(16.1) | 104.9(11.4) | 0.16(47) | .87 | ||||||||
| Illness duration (months) | 26.0(19.9) | |||||||||||
| EDE ratings | ||||||||||||
| OBEs | 13.8(17.8) | 7.8(12.1) | 6.7(19.7) | |||||||||
| SBEs | 18.3(24.3) | 3.2(4.0) | 3.6(5.8) | |||||||||
| Vomiting episodes | 32.9(28.7) | 9.4(14.5) | 8.3(19.4) | |||||||||
| LOCs | 25.2(20.1) | 11.0(12.3) | 10.3(24.7) | |||||||||
| Comorbid MDD (%) | 42.4 | 15.4 | 6.3 | |||||||||
| Comorbid Anxiety (%) | 21.2 | 23.1 | 6.3 | |||||||||
| Medication (%) | 27.3 | 46.2 | 31.23 | |||||||||
| Prior AN (%) | 15.2 | |||||||||||
| Treatment (%)a | ||||||||||||
| Inpatient | 39.4 | |||||||||||
| Outpatient | 27.2 | |||||||||||
Note: AN = anorexia nervosa; BMI = body mass index; BN = bulimia nervosa; EDE = Eating Disorder Examination; FU = follow-up; HC = healthy controls; LOC = loss-of-control eating episodes of any size; MDD = major depressive episode; OBE = objective binge-eating episode; SBE = subjective binge-eating episode; WAIS = Weschler Adult Intelligence Scale.
Treatment-seeking participants received inpatient or outpatient treatment in the Eating Disorders Clinic at the New York State Psychiatric Institute only following their baseline assessment/scan.
Stability of CT and BN Symptoms
CT in frontal regions was stable but heterogeneous from baseline to FU1, FU1 to FU2, and baseline to FU2 (BN: mean r=.70, p=.038, range: .16–.95; HC: mean r=.85, p=.001, range: .63–.94; Table S5, available online). In the BN group, the frequency of binge-eating and vomiting episodes was moderately stable over time (OBE: r=.55, p=.136, range: .23–.79; Vomiting: r=.34, p=.304, range: −.04–.63; Table S6, available online).
Group Differences in CT Over Time
Findings from growth curve models including all participants with BN are summarized in Table S6 (available online). All models’ residuals were normally distributed. Group effects (i.e., average group differences in CT at baseline) were detected in bilateral frontal pole (left: p=.020; right: p=.047) and IFG pars opercularis (left: p=.015; marginal effect in right: p=.056), with thinner cortices in the BN relative to control adolescents (Table S7, available online). No significant group-by-time interactions were detected, suggesting that these group differences persisted over time. Findings from the growth curve models including only the adolescents with BN whose symptoms improved by more than 50% between their baseline and last assessments are summarized in Table 2 and Figure 1, with detailed statistical results presented in Table S8 (available online). Significant group effects (i.e., baseline group differences, Table 2 and Figure 1) were detected in right IFG pars opercularis (right: p=.030) and orbitalis (p=.021), with CT reduced in BN relative to HC. No significant group-by-time interactions were detected, suggesting that these reductions persisted following partial remission in BN. CT point slope estimates were computed for each region/year (0, 12, 24, and 36 months) to further assess the persistence of group differences over time. Contrast comparisons of CT in each region at each year revealed group differences that remained in right IFG pars opercularis and orbitalis (Table 3 and Figure 1).
Table 2.
Growth Curve Models Predicting Cortical Thickness in Healthy Controls Vs. Remitted Bulimia Nervosaa
| Cortical Area | Side | Characteristicb | B | SE | t | p |
|---|---|---|---|---|---|---|
| Inferior frontal gyrus (opercularis) | Right | Group | −0.10 | 0.05 | −2.22 | .03 |
| Time | <0.01 | <0.01 | −2.28 | .03 | ||
| Group*Time | <0.01 | <0.01 | 0.32 | .75 | ||
| Age | −0.02 | 0.01 | −1.30 | .20 | ||
|
| ||||||
| Inferior frontal gyrus (orbitalis) | Right | Group | 0.14 | 0.06 | 2.36 | .02 |
| Time | <0.01 | <0.01 | 2.66 | .01 | ||
| Group*Time | <0.01 | <0.01 | 0.13 | .90 | ||
| Age | −0.03 | 0.02 | −2.09 | .04 | ||
Remission is defined as at least 50% reduction in the frequency of objective binge-eating episodes and vomiting episodes between baseline and last assessment.
Group coded as healthy control=0, bulimia nervosa=1; Time coded as months from baseline; Age coded as years at baseline
Figure 1. Group differences in cortical thickness (CT) over time.
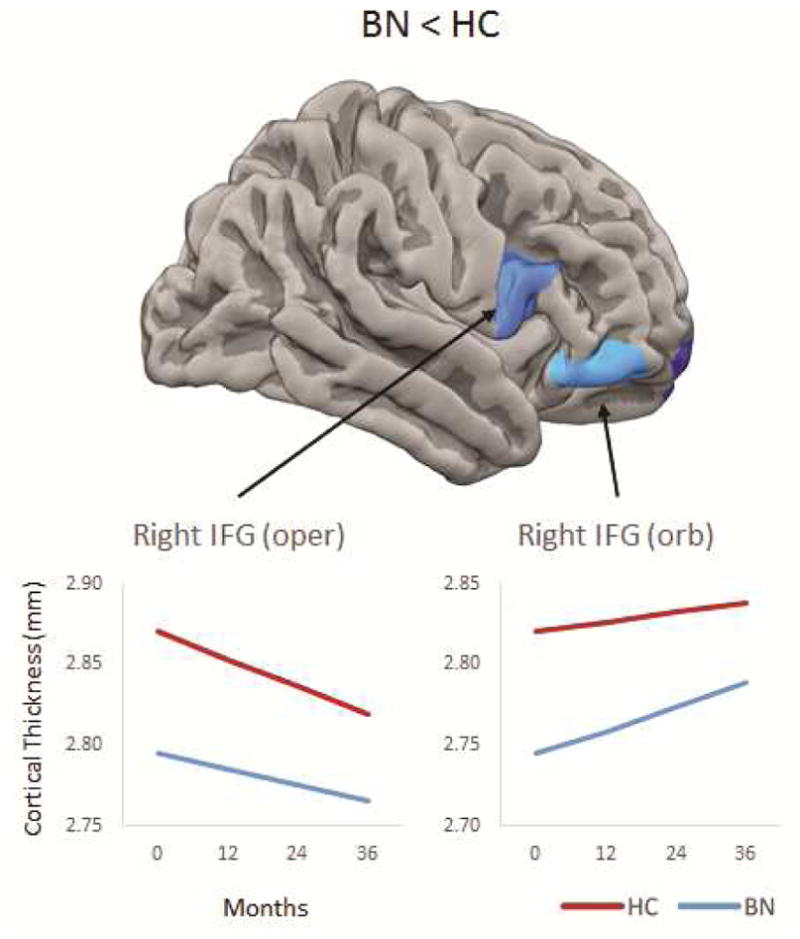
Note: Top images shows group differences in CT at baseline; line graphs show differences yearly based on point slope estimates at 0, 12, 24, and 36 months. Remission is defined as at least 50% reduction in the frequency of objective bulimic episodes (OBEs) and vomiting episodes between baseline and last assessment. BN = bulimia nervosa; HC = healthy control; IFG = inferior frontal gyrus.
Table 3.
Contrast Comparisons of Cortical Thickness (CT) at 0, 12, 24, and 36 Months, Based on CT Point Slope Estimates From Growth Curve Models Reported in Table 2.
| Area | Side | 0 month | 12 months | 24 months | 36 months | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||||||||||
| Ba | SE | t | p | ESb | Ba | SE | t | p | ESb | Ba | SE | t | p | ESb | Ba | SE | t | p | ESb | ||
| IFG oper | Right | −0.10 | 0.05 | −2.22 | .03 | −0.68 | −0.10 | 0.04 | −2.19 | .03 | −0.68 | −0.09 | 0.05 | −2.06 | .04 | −0.61 | −0.09 | 0.05 | −1.86 | .07 | −0.61 |
| IFG orb | Right | 0.14 | 0.06 | 2.36 | .02 | 0.73 | 0.14 | 0.06 | 2.47 | .02 | 0.73 | 0.14 | 0.06 | 2.39 | .02 | 0.73 | 0.14 | 0.07 | 2.18 | .03 | 0.73 |
Note: ES = effect size; IFG, inferior frontal gyrus; oper = pars opercularis; orb = pars orbitalis.
B estimates represent group difference in CT, where group was coded as healthy control = 0 and bulimia nervosa = 1.
ESs were calculated as the (MeanCT BN – MeanCT HC)/residual standard deviation.49
Trait Versus State Effects of CT on BN Symptoms
Results from multilevel models are summarized in Table 4 with detailed statistical results presented in Table S9 (available online). All models’ residuals were normally distributed. Between-subject effects (Table 4) emerged such that the adolescents with BN with reduced CT in left frontal pole (p=.020) reported more frequent binge-eating episodes. Similarly, reduced CT in right IFG pars orbitalis (p=.009) and lateral orbital frontal cortex (OFC; p=.049) was significantly associated with more frequent vomiting episodes. No significant within-subject effects were detected.
Table 4.
Multilevel Models of Cortical Thickness (CT) Predicting Bulimia Nervosa (BN) Symptomsa
| BN symptom | Cortical Area | Side | Between-subjectb | |||
|---|---|---|---|---|---|---|
|
| ||||||
| B | SE | t | p | |||
| OBEs | Frontal pole | Left | −2.63 | 1.05 | −2.5 | .02 |
| Vomiting episodes | Lateral OFC | Right | −3.99 | 1.92 | −2.08 | .05 |
| IFG orb | Right | −3.40 | 1.20 | −2.84 | .01 | |
Note: IFG = inferior frontal gyrus; OBEs = objective binge-eating episodes; OFC = orbital frontal cortex; orb = pars orbitalis.
BN symptoms were the frequency of binge-eating and vomiting episodes over the past 28 days prior to scanning.
Average CT over time is used as the between-subject predictor.
Deviation at each time point from each participant’s own average CT over time is used as the within-subject predictor.
DISCUSSION
This longitudinal study examined whether reductions in the thickness of frontal regions are trait or state markers of BN and/or binge-eating and vomiting behaviors. Growth curve models revealed CT reductions in inferior frontal regions that persisted over time in adolescents with BN relative to their healthy counterparts, even among those whose symptoms fully or partially remitted over the course of the study. Thus, reduced CT in these regions may be a trait marker of BN, potentially contributing to its development and persistence over adolescence and young adulthood. Our findings further suggest that between-subject variations in the thickness of inferior and orbital frontal regions may be trait markers of binge eating and vomiting within BN. While a handful of cross-sectional MRI findings point to potential trait and state markers of AN in remitted patients,9–12 this is the first study to use a longitudinal approach to identify trait and state markers of BN and specific BN symptoms. These findings contribute to our understanding of how these regions supporting control, reward, and learning processes are involved in the pathophysiology and persistence of BN and BN symptoms.
We detected reduced CT in IFG and frontal pole at baseline in BN compared to healthy adolescents, although frontal pole reductions were no longer significant when including only the adolescents with BN who achieved full or partial remission over time. CT reductions in right IFG (pars opercularis and orbitalis) remained stable over time, even following full or partial symptom remission. Reductions in right IFG pars orbitalis remained stable when participants with BN with subclinical symptoms were excluded from growth curve analyses, suggesting that reduced CT in this IFG sub-region may be a more robust marker of BN. These findings are consistent with previous cross-sectional findings of reduced local volumes on the surface of (and reduced CT in) lateral frontal areas in a different sample of BN relative to control participants, with local volume reductions in inferior and middle frontal cortices becoming more prominent with advancing age.5 Previous structural MRI studies of individuals with BN are sparse, with mixed results. Early studies suggest increased cerebrospinal fluid (CSF) in BN compared to control participants,33, 34 perhaps pointing to widespread reductions in grey and/or white matter volumes. More recent findings show enlarged medial orbital frontal cortex in adults with BN,6, 35 although other studies report no alterations in grey matter volume in acutely ill (local volumes36) or remitted patients with BN (total volume37). In contrast, we detected persistent reductions in the thickness of right IFG over time in partially remitted adolescents with BN. These latter cross-sectional studies used voxel-based morphometry (VBM) to assess brain structure in adults with and without BN, whereas we assessed longitudinal structural changes in adolescents, using a methodology that does not share the inherent limitations of VBM approaches.38–41 Thus, discrepancies between the previous findings and ours may be due to such methodological differences across studies or to differences in the demographic and clinical characteristics of the samples studied (i.e., adults vs. adolescents; DSM-5 BN vs. OSFED diagnostic criteria). Nevertheless, our findings should be replicated in a larger sample.
Similar to studies of AN9, 11 and depression,13 this approach of assessing brain structure in individuals ill and remitted from a given disorder permits understanding of whether CT may be a trait versus state marker of that disorder. Examining the effects of between- versus within-subject variations in CT on the frequency of binge-eating and purging behaviors is a novel approach, permitting assessment of CT in frontal regions as trait or state markers of BN symptoms. To our knowledge, this approach has not been previously applied in clinical neuroscience. Multilevel modeling allowed us to determine whether adolescents with BN who, on average, had greater CT reductions over time also had more severe symptoms (i.e., trait effects) and whether individual fluctuations in CT were associated with symptoms over time (i.e., state effects). Within the BN group, significant between-subject effects of frontal CT on symptoms were detected.
Specifically, on average over time, those with the least CT in left frontal pole engaged in more frequent OBEs, and those with the least CT in right IFG pars orbitalis and lateral OFC engaged in more frequent vomiting episodes. Thus, reductions in these regions may be trait markers of symptom expression in adolescents with BN. No significant within-subject effects were detected, suggesting that reductions in the thickness of frontal regions are not state markers of BN symptoms.
An alternative explanation for our findings is that BN symptoms may have a scarring effect on brain structure, thereby contributing to reductions in CT over time. For instance, time point-to-time point fluctuations in symptoms, such as purging and also food restriction, could lead to acute changes in CT, which would be reflected by deviations in CT at each time point. Such possibility is consistent with evidence that episodes of purging and food restriction may lead to reduced brain volumes via the depletion or lack of bodily fluids.42 Similarly, chronically frequent BN symptoms may result in long-term alterations in CT, which would be reflected by reduced mean CT over time. Finally, brain structure and BN symptoms may also show transactional effects such that symptoms contribute to CT reductions that, in turn, contribute to the persistence or worsening of symptoms. While such scarring or transactional effects should be assessed in future longitudinal studies, this is the first to assess neuroanatomical trait and state markers of symptom expression in BN, providing a more nuanced understanding of the relation of such markers to the persistence and expression of binge eating and purging during adolescence.
Associations of inferior and orbital frontal abnormalities with BN symptoms have been documented in anatomical5, 6 and functional2–4 MRI studies of BN. For example, we previously detected in another sample of participants with BN volume reductions in inferior frontal regions that were greatest in those who engaged in the most frequent binge eating and vomiting episodes,5 consistent with the between-subject effects of inferior frontal CT on symptom frequency detected herein. Fronto-striatal circuits support a range of cognitive functions, including control, reward, and learning processes.43 Thus, reduced thickness of inferior and orbital frontal regions may account, in part, for deficits in these processes in BN44, 45 and for deficient activation of these regions during performance of inhibitory control, reward, and learning tasks.2, 3, 46, 47 These functional deficits may, in turn, underlie the binge-eating and purging behaviors that characterize BN, thereby mediating our observed associations between CT and the severity of BN symptoms.
Although this is the first longitudinal anatomical study of individuals with BN, it is limited by the modest sample size combined with attrition. Although some participants dropped out of the study over time, participants with missing data did not differ significantly from those with complete data in terms of demographic characteristics, CT, or symptom severity at baseline. Thus, data were missing at random, and our growth curve and multilevel models, via the use of maximum-likelihood estimation,25 allowed participants with missing data to contribute to model estimation. This analytic approach is one of the gold-standard methods for dealing with attrition,25 an inevitable consequence for longitudinal studies, especially of adolescents.48 Given the low power due to our modest sample size, we did not correct for multiple tests, which were conducted within twelve different brain regions (six per hemisphere), thereby increasing the risk of type I error. We thus underscore the importance of interpreting these findings with caution prior to replication in a larger sample. In addition, 33% of our BN sample met DSM-5 criteria for OSFED-BN at baseline, consistent with the presentation of BN symptoms in early adolescence,30 but our modest sample size precluded testing the differential effects of BN and OSFED-BN on our outcome measures.
To retain as many participants with BN as possible in our analyses, we defined remission as a 50% reduction in symptoms since few met more stringent criteria for remission (i.e., only 12 showed more than 75% reduction, and only 3 fully remitted). Thus, residual ongoing BN symptoms in that subgroup may have contributed to the observed CT reductions over time. Future studies should assess whether such reductions persist following long-term full remission and indeed qualify as a trait marker. Although our stability analyses revealed that CT in inferior and orbital frontal regions that associated with symptoms was highly stable over time in both groups, inclusion of more time points in future studies could ensure more representative estimates of average CT over time. Further, the absence of CT data from participants prior to the onset of BN or BN symptoms precludes testing whether reduced CT in inferior frontal regions precedes and predicts the development of BN. Moreover, a trait effect on BN symptoms was detected in right lateral OFC, but CT in this region did not differ significantly across groups. Future studies should examine whether reduced CT in this region is uniquely associated with BN or BN symptoms, or is instead a non-specific marker that may relate to characteristics common in BN and healthy individuals with subclinical eating, weight, or body image concerns. Finally, the absence of a clinical control group precluded examining whether findings are BN-specific or instead generalize to other eating disorders or psychopathology. Since controlling for comorbid depression and anxiety did not appreciably affect our findings, reduced CT in inferior and orbital frontal regions unlikely constitutes a general marker of psychopathology.
Our results provide the first longitudinal evidence of neuroanatomical trait markers of BN and symptom expression within BN. In particular, reduced CT in right inferior frontal regions may represent a trait marker of the illness that persists over time, even after symptom remission. Moreover, we provide the first evidence that CT reductions in specific inferior and orbital frontal regions may represent trait markers of specific BN symptoms. Future studies should assess functional changes in these regions over adolescence in BN to determine whether, for example, deficient engagement of these regions and of fronto-striatal circuits is also a trait marker of the illness or of specific BN symptoms. Such longitudinal data, together with the present findings, will allow for the development of novel, early interventions for the prevention of persistent BN symptoms that target the enhanced functioning of these regions and circuits.
Supplementary Material
Acknowledgments
The authors wish to thank David C. Zuroff, PhD, of McGill University, and Niall Bolger, PhD, of Columbia University, for their advice on statistical modeling of longitudinal data.
This work was supported by NIMH grant R01MH090062 (R.M.).
Footnotes
Disclosure: Drs. Cyr, Kopala-Sibley, Lee, Berner, Marsh and Mss. Chen, Stefan, Fontaine, Terranova report no biomedical financial interests or potential conflicts of interest.
Supplemental material cited in this article is available online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Marilyn Cyr, Division of Child and Adolescent Psychiatry, New York State Psychiatric Institute and College of Physicians and Surgeons, Columbia University, New York.
Dr. Daniel C. Kopala-Sibley, Stony Brook University, Stony Brook, NY.
Dr. Seonjoo Lee, College of Physicians and Surgeons, Columbia University.
Dr. Chen Chen, College of Physicians and Surgeons, Columbia University.
Ms. Mihaela Stefan, Division of Child and Adolescent Psychiatry, New York State Psychiatric Institute and College of Physicians and Surgeons, Columbia University, New York.
Ms. Martine Fontaine, Division of Child and Adolescent Psychiatry, New York State Psychiatric Institute and College of Physicians and Surgeons, Columbia University, New York.
Ms. Kate Terranova, Division of Child and Adolescent Psychiatry, New York State Psychiatric Institute and College of Physicians and Surgeons, Columbia University, New York.
Dr. Laura A. Berner, University of California, San Diego.
Dr. Rachel Marsh, Division of Child and Adolescent Psychiatry, New York State Psychiatric Institute and College of Physicians and Surgeons, Columbia University, New York.
References
- 1.Cyr M, Wang Z, Tau GZ, et al. Reward-Based Spatial Learning in Teens With Bulimia Nervosa. J Am Acad Child Adolesc Psychiatry. 2016;55(11):962–971. e963. doi: 10.1016/j.jaac.2016.07.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh R, Horga G, Wang Z, et al. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am J Psychiatry. 2011;168(11):1210–1220. doi: 10.1176/appi.ajp.2011.11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh R, Steinglass JE, Gerber AJ, et al. Deficient activity in the neural systems that mediate self-regulatory control in bulimia nervosa. Arch Gen Psychiatry. 2009;66(1):51–63. doi: 10.1001/archgenpsychiatry.2008.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon JJ, Skunde M, Walther S, Bendszus M, Herzog W, Friederich HC. Neural signature of food reward processing in bulimic-type eating disorders. Soc Cogn Affect Neurosci. 2016;11:1393–1401. doi: 10.1093/scan/nsw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsh R, Stefan M, Bansal R, Hao X, Walsh BT, Peterson BS. Anatomical Characteristics of the Cerebral Surface in Bulimia Nervosa. Biological psychiatry. 2015;77(7):616–623. doi: 10.1016/j.biopsych.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schafer A, Vaitl D, Schienle A. Regional grey matter volume abnormalities in bulimia nervosa and binge-eating disorder. Neuroimage. 2010;50(2):639–643. doi: 10.1016/j.neuroimage.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 7.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10(3):284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 8.Torregrossa MM, Quinn JJ, Taylor JR. Impulsivity, compulsivity, and habit: the role of orbitofrontal cortex revisited. Biol Psychiatry. 2008;63(3):253–255. doi: 10.1016/j.biopsych.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardoni F, King JA, Geisler D, et al. Weight restoration therapy rapidly reverses cortical thinning in anorexia nervosa: A longitudinal study. Neuroimage. 2016;130:214–222. doi: 10.1016/j.neuroimage.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Kaye WH, Frank GK, McConaha C. Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharmacology. 1999;21(4):503–506. doi: 10.1016/S0893-133X(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 11.King JA, Geisler D, Ritschel F, et al. Global cortical thinning in acute anorexia nervosa normalizes following long-term weight restoration. Biol Psychiatry. 2015;77:624–632. doi: 10.1016/j.biopsych.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich S, Geisler D, Ritschel F, et al. Elevated cognitive control over reward processing in recovered female patients with anorexia nervosa. J Psychiatry Neurosci. 2015;40:307–315. doi: 10.1503/jpn.140249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Depping MS, Wolf ND, Vasic N, et al. Abnormal cerebellar volume in acute and remitted major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:97–102. doi: 10.1016/j.pnpbp.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Saricicek A, Zorlu N, Yalin N, et al. Abnormal white matter integrity as a structural endophenotype for bipolar disorder. Psychological medicine. 2016;46:1547–1558. doi: 10.1017/S0033291716000180. [DOI] [PubMed] [Google Scholar]
- 15.Beedie SA, Benson PJ, St Clair DM. Atypical scanpaths in schizophrenia: evidence of a trait- or state-dependent phenomenon? J Psychiatry Neurosci. 2011;36:150–164. doi: 10.1503/jpn.090169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Treadway MT, Leonard CV. Isolating biomarkers for symptomatic states: considering symptom-substrate chronometry. Mol Psychiatry. 2016;21:1180–1187. doi: 10.1038/mp.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steyer R, Mayer A, Geiser C, Cole DA. A theory of states and traits--revised. Annu Rev Clin Psychol. 2015;11:71–98. doi: 10.1146/annurev-clinpsy-032813-153719. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman J, Birmaher B, Brent D, et al. The Schedule for Affective Disorders and Schizophrenia for School Aged Children: Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Fairburn CG, Cooper Z, O’Connor M. Eating Disorder Examination (Edition 16.0D) In: Fairburn CG, editor. Cognitive Behavior Therapy and Eating Disorders. New York: Guilford Press; 2008. pp. 265–308. [Google Scholar]
- 21.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 23.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 26.Frick A, Howner K, Fischer H, Eskildsen SF, Kristiansson M, Furmark T. Cortical thickness alterations in social anxiety disorder. Neurosci Lett. 2013;536:52–55. doi: 10.1016/j.neulet.2012.12.060. [DOI] [PubMed] [Google Scholar]
- 27.Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138(1–2):9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 28.Flament MF, Bissada H, Spettigue W. Evidence-based pharmacotherapy of eating disorders. Int J Neuropsychopharmacol. 2012;15(2):189–207. doi: 10.1017/S1461145711000381. [DOI] [PubMed] [Google Scholar]
- 29.Goldschmidt AB, Accurso EC, Crosby RD, et al. Association between objective and subjective binge eating and psychopathology during a psychological treatment trial for bulimic symptoms. Appetite. 2016;107:471–477. doi: 10.1016/j.appet.2016.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzsimmons-Craft EE, Ciao AC, Accurso EC, et al. Subjective and objective binge eating in relation to eating disorder symptomatology, depressive symptoms, and self-esteem among treatment-seeking adolescents with bulimia nervosa. Eur Eat Disord Rev. 2014;22(4):230–236. doi: 10.1002/erv.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotler LA, Cohen P, Davies M, Pine DS, Walsh BT. Longitudinal relationships between childhood, adolescent, and adult eating disorders. J Am Acad Child Adolesc Psychiatry. 2001;40:1434–1440. doi: 10.1097/00004583-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Pearson CM, Wonderlich SA, Smith GT. A risk and maintenance model for bulimia nervosa: From impulsive action to compulsive behavior. Psychol Rev. 2015;122(3):516–535. doi: 10.1037/a0039268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman GW, Ellinwood EH, Jr, Rockwell WJ, Herfkens RJ, Nishita JK, Guthrie LF. Cerebral atrophy in bulimia. Biol Psychiatry. 1989;25(7):894–902. doi: 10.1016/0006-3223(89)90269-2. [DOI] [PubMed] [Google Scholar]
- 34.Krieg JC, Lauer C, Pirke KM. Structural brain abnormalities in patients with bulimia nervosa. Psychiatry Res. 1989;27(1):39–48. doi: 10.1016/0165-1781(89)90007-3. [DOI] [PubMed] [Google Scholar]
- 35.Frank GK, Shott ME, Hagman JO, Mittal VA. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. 2013;170(10):1152–1160. doi: 10.1176/appi.ajp.2013.12101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joos A, Kloppel S, Hartmann A, et al. Voxel-based morphometry in eating disorders: correlation of psychopathology with grey matter volume. Psychiatry Res. 2010;182(2):146–151. doi: 10.1016/j.pscychresns.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Wagner A, Greer P, Bailer UF, et al. Normal brain tissue volumes after long-term recovery in anorexia and bulimia nervosa. Biol Psychiatry. 2006;59(3):291–293. doi: 10.1016/j.biopsych.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 39.Crum WR, Griffin LD, Hill DL, Hawkes DJ. Zen and the art of medical image registration: correspondence, homology, and quality. Neuroimage. 2003;20(3):1425–1437. doi: 10.1016/j.neuroimage.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005;26(2):546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14(6):1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- 42.Blasel S, Pilatus U, Magerkurth J, et al. Metabolic gray matter changes of adolescents with anorexia nervosa in combined MR proton and phosphorus spectroscopy. Neuroradiology. 2012;54:753–764. doi: 10.1007/s00234-011-1001-9. [DOI] [PubMed] [Google Scholar]
- 43.Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Wu M, Hartmann M, Skunde M, Herzog W, Friederich HC. Inhibitory control in bulimic-type eating disorders: a systematic review and meta-analysis. PLoS One. 2013;8(12):e83412. doi: 10.1371/journal.pone.0083412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison A, O’Brien N, Lopez C, Treasure J. Sensitivity to reward and punishment in eating disorders. Psychiatry Res. 2010;177:1–11. doi: 10.1016/j.psychres.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Bohon C, Stice E. Reward abnormalities among women with full and subthreshold bulimia nervosa: a functional magnetic resonance imaging study. Int J Eat Disord. 2011;44:585–595. doi: 10.1002/eat.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frank GK, Reynolds JR, Shott ME, O’Reilly RC. Altered temporal difference learning in bulimia nervosa. Biol Psychiatry. 2011;70:728–735. doi: 10.1016/j.biopsych.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young AF, Powers JR, Bell SL. Attrition in longitudinal studies: who do you lose? Aust N Z J Public Health. 2006;30:353–361. doi: 10.1111/j.1467-842x.2006.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 49.Rouder JN, Morey RD, Speckman PL, Province JM. Default Bayes factors for ANOVA designs. Journal of Mathematical Psychology. 2012;56:356–374. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


