Abstract
Introduction
In general populations, short and long sleep duration, poor sleep quality, and sleep disorders have been associated with increased risk of death. We evaluated these associations in individuals with CKD.
Methods
This was a prospective cohort study of 1452 NHANES 2005 to 2008 participants with CKD. CKD was defined by estimated glomerular filtration rate <60 ml/min per 1.73 m2 or urine albumin-to-creatinine ratio ≥30 mg/g. Sleep duration, sleep symptoms (difficulty falling asleep, difficulty staying asleep, daytime sleepiness, and nonrestorative sleep), and sleep disorders (restless legs syndrome and sleep apnea) were self-reported. Vital status was determined using NHANES mortality linkage through December 31, 2011.
Results
In this cohort, the mean age was 61 years, 58% were women, and 75% non-Hispanic white. During 4.4 years of median follow-up, we observed 234 deaths, of which 75 were due to cardiovascular causes. In multivariable analyses, compared with individuals who reported 7 to 8 hours of sleep, HR (95% CI) for all-cause mortality for sleep duration <7 hours and >8 hours were 1.50 (1.08–2.10) and 1.36 (0.89–2.08), respectively. The corresponding HR (95% CI) for cardiovascular mortality were 1.56 (0.72–3.37) and 1.56 (0.66–3.65). Nonrestorative sleep and restless legs syndrome were associated with increased risk for all-cause mortality (HR, 1.63 [95% CI, 1.13–2.35], and HR, 1.69 [95% CI, 1.04–275], respectively).
Discussion
In adults with CKD, short sleep duration, nonrestorative sleep, and restless legs syndrome are associated with increased risk of death. These findings underscore the importance of promoting adequate sleep in patients with CKD, and the need for future studies evaluating the impact of sleep interventions in this population.
Keywords: chronic kidney disease, mortality, sleep
Sleep is an essential state of rest for the well-being of the mind and body, but sleep curtailment has become a common, often voluntary behavior in modern society.1 In general populations, impaired sleep has been found to be associated with poor health outcomes including death.2, 3 In addition, there is increasing evidence for an association between both short and long duration of habitual sleep, as well as impaired sleep quality, with prevalence and severity of major chronic diseases, including hypertension, diabetes, and cardiovascular disease.4, 5, 6, 7
It is estimated that among people with chronic kidney disease (CKD), the prevalence of sleep disturbances can be as high as 80%.8 In an analysis of the National Health and Nutrition Examination Survey (NHANES) 2005 to 2008, Plantinga et al.9 found that the prevalence of inadequate sleep (defined as ≤6 hours per night) was higher in individuals with mild CKD than in those with no CKD. However, the impact of sleep duration and sleep quality on clinical outcomes in individuals with CKD is not well understood. For this reason, we conducted a study to assess the association of sleep duration, sleep symptoms, and disorders with all-cause and cardiovascular mortality in U.S. adults with CKD using data from NHANES 2005 to 2008.
Materials and Methods
Study Population
NHANES is a stratified, clustered, multistage probability sample survey of the civilian, noninstitutionalized U.S. population, conducted by the National Center for Health Statistics (NCHS) of the U.S. Centers for Disease Control and Prevention, with oversampling of non-Hispanic black and Mexican American persons.10 The survey consists of a standardized in-home interview followed by physical examination, as well as blood and urine collection at a mobile examination center. Survey protocol was approved by the NCHS Institutional Review Board and is adherent to the Declaration of Helsinki. All participants provided informed consent. This analysis was limited to NHANES 2005 to 2008 participants who met the inclusion criteria (18 years or older, nonpregnant, and had available serum creatinine and urine albumin and creatinine measurements) and the study definition of CKD.
Measurements and Definitions
Chronic Kidney Disease
Serum and urine creatinine were measured using the modified kinetic Jaffé method. Urine albumin was measured using a solid-phase fluorescent immunoassay. Urine albumin and creatinine concentrations were measured in 1 random urine sample. CKD was defined by either an estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2, using the CKD Epidemiology Collaboration creatinine equation11 or the presence of albuminuria (urine albumin-to-creatinine ratio ≥30 mg/g).
Sleep
During the home interview, through a computer-assisted personal interviewing system, NHANES 2005 to 2008 participants answered questions regarding sleep habits and sleep-related problems from 2 validated instruments: the Sleep Heart Health Study Sleep Habits Questionnaire12 and the Functional Outcomes of Sleep Questionnaire.13, 14 For this study, we used selected questions as described herein. Sleep duration was ascertained using the following question: “How much sleep do you usually get at night on weekdays or workdays?” We classified total hours of sleep as <7, 7 to 8 or >8.2 The items used to ascertain the presence of sleep symptoms were the following: (i) difficulty falling asleep, “In the past month, how often did you have trouble falling asleep?”; (ii) difficulty staying asleep, “In the past month, how often did you wake up during the night and had trouble getting back to sleep?”; (iii) daytime sleepiness, “In the past month, how often did you feel excessively or overly sleepy during the day?”; and (iv) nonrestorative sleep, “In the past month, how often did you feel unrested during the day, no matter how many hours of sleep you had?” Participants were asked to choose from among the following options: Never, rarely (1 time a month); sometimes (2–4 times a month); often (5–15 times a month); almost always (16–30 times a month); refused; or “don’t know.” Sleep symptoms were considered to be present if reported “often” or more (at least 5 times a month). The presence of restless legs was also self-reported using the questions: “Have you ever been told by a doctor or other health professional that you have a sleep disorder?” if yes, “What was the sleep disorder?” The possible answers were “sleep apnea,” “insomnia,” “restless legs,” “other,” “refused,” and “don’t know.”
Covariates
Race or ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican American, or other. In these analyses, income was classified as annual family income <20,000 or ≥20,000 U.S. dollars, and educational attainment as less than high school or high school or beyond. Participants were considered to have health insurance if they self-reported coverage by any health insurance plan. Participants were classified as current or past or never smoker based on responses to the questions “Have you smoked at least 100 cigarettes during your entire life?” and “Do you smoke cigarettes now?” Participants had 3 blood pressure (BP) measurements at the mobile examination center in the sitting position, after 5 minutes of rest, using a standardized protocol.15 The averages of all systolic BP available readings are reported here. Hypertension was defined as systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg or the self-reported use of antihypertensive medications. Diabetes was defined as a history of diabetes, self-reported use of insulin or other medication to treat diabetes, a fasting blood glucose ≥126 mg/dl, or a random blood glucose ≥200 mg/dl. The presence of congestive heart failure was ascertained using the following question: “Has a doctor or other health professional ever told you that you had congestive heart failure?” The use of medications for sleep was ascertained using the following question: “In the past month, how often did you take sleeping pills or other medication to help you sleep?” The possible answers were never, rarely (1 time per month), sometimes (2–4 times per month), often (5–15 times per month), or almost always (16–30 times per month); participants who answered ≥5 times per month were classified as sleeping pills users. Height and weight were measured by trained NHANES staff. Body mass index was calculated as weight in kilograms divided by height in meters squared. The presence of depressive symptoms was defined as a Patient Health Questionnaire (PHQ-9) ≥10.16
Outcome Ascertainment
We used the NHANES Linked Mortality File, which provides follow-up data on vital status from the date of the NHANES 2005 to 2008 survey participation through the date of death or December 31, 2011. Vital status was ascertained by the NCHS through a probabilistic match between NHANES 2005 to 2008 participants and National Death Index death certificate records.17 Participants who were not matched with any death records were considered to be alive through the follow-up period. Cause of death was assigned by the NCHS based on the International Classification of Diseases, 10th Revision. For this study, cardiovascular mortality was defined as death due to diseases of the heart, essential hypertension and hypertensive kidney disease, cerebrovascular disease, atherosclerosis, and other diseases or disorders of the circulatory system (codes I00–I99).18
Statistical Methods
NCHS recommendations were followed to account for stratification and clustering of the survey design, as well as oversampling of ethnic minorities and elderly persons.19 Continuous variables were expressed as means (SE) or medians (interquartile range) if not normally distributed; and categorical variables as weighted percentage. Chi-squared and Student t tests were used to compare categorical and continuous variables, respectively. Cox proportional hazards models were used to determine the association between sleep duration and symptoms and all-cause and cardiovascular mortality adjusting for important covariates (age, sex, race or ethnicity, income, education, diabetes, hypertension, congestive heart failure, sleeping pill use, smoking, eGFR, albuminuria, body mass index, and depression symptoms) that were chosen based on prior publications.3, 20, 21, 22 We evaluated age, sex, and diabetes as potential effect modifiers of the association between sleep variables and mortality by adding an interaction term between the corresponding sleep variable and each of the potential effect modifiers to the fully adjusted model. All tests were 2-sided, and P < 0.05 was considered significant for hypothesis testing. The proportional hazards assumption of the Cox models was examined using Schoenfeld residuals, which showed no significant departure from proportionality over time (P > 0.05).23 All statistical analyses were done using SAS 9.3 (Cary, NC).
Results
Participant Characteristics
Of the 11,791 NHANES 2005 to 2008 adult (age ≥ 18 years) participants who were examined, 1395 did not have data on serum creatinine or urine albumin-to-creatinine ratio. Pregnant women and individuals with eGFR <15 ml/min per 1.73 m2 were excluded. Among the remaining participants, 1820 met our definition of CKD. Of those, we excluded participants due to missing data on sleep duration or symptoms (n = 7), income (n = 64), diabetes (n = 31), smoking (n = 69), body mass index (n = 35), congestive heart failure (n = 12), or other covariate (n = 150). Therefore, our final analytic sample included 1452 individuals. Compared with participants who were included in analyses, those who were excluded due to missing covariate data were more likely to be younger (mean age 56.1 vs. 60.5 years, P = 0.005); of “other” racial or ethnic background (15.6 vs. 7.5%, P < 0.001) and to have higher eGFR (81.7 vs. 74.3 ml/min per 1.73 m2, P < 0.001). There were no differences in sex or urine albumin-to-creatinine ratio distribution.
The mean sleep duration was 7 hours. Approximately 35.4% of individuals reported sleeping <7 hours, 54.7% 7 to 8 hours, and 9.9% >8 hours. Demographic and clinical characteristics are presented overall and by sleep duration category in Table 1. Mean age was 60.4 years; 58.6% of participants were women; and 74.6% were non-Hispanic whites. Compared with individuals sleeping 7 to 8 hours, individuals reporting <7 hours of sleep were more likely to be younger (58.5 vs. 60.5 years), non-Hispanic black (18.3% vs. 7.6%), and have less than a high school education (26.9% vs. 22.7%). In addition, individuals reporting <7 hours sleep were more likely to have a body mass index of ≥30 kg/m2 (46.2% vs. 39.0%), have diabetes (32.5% vs. 24.1%), depressive symptoms (PHQ-9 score ≥10 9.5% vs. 4.4%), and albuminuria >300 mg/g (13.1% vs. 6.9%); they were also more likely to report sleeping medication use (17.8% vs. 10.0%). Compared with individuals reporting 7 to 8 hours of sleep, individuals who reported >8 hours of sleep were older (66.7 vs. 60.5 years), more likely to have less than a high school education (38.9% vs. 22.7%), have health insurance (92.8% vs. 86.4%), diabetes (29.7% vs. 24.1%), congestive heart failure (11.0% vs. 5.9%), lower eGFR (68.0 vs. 73.1 ml/min per 1.73 m2), and albuminuria >300 mg/g (9.6% vs. 6.9%).
Table 1.
Characteristics of individuals with CKD overall and stratified by sleep duration
| Variables | Overall (N = 1452) |
<7 h (n = 543) |
7–8 h (n = 752) |
>8 h (n = 157) |
|---|---|---|---|---|
| Age (yr) | 60.4 ± 0.8 | 58.5 ± 1.0 | 60.5 ± 1.1 | 66.7 ± 1.9a |
| 18–44 | 22.2 | 25.8 | 21.3 | 14.3a |
| 45–64 | 28.5 | 30.9 | 29.3 | 16.0 |
| >65 | 49.2 | 43.3 | 49.4 | 69.6 |
| Female sex | 58.6 | 56.1 | 59.7 | 61.4 |
| Race | ||||
| Non-Hispanic white | 74.6 | 66.5 | 78.6 | 81.1a |
| Non-Hispanic black | 11.3 | 18.3 | 7.6 | 6.6 |
| Mexican American | 6.7 | 5.9 | 7.3 | 5.9 |
| Other | 7.5 | 9.3 | 6.6 | 6.4 |
| Household income < $20,000/yr | 23.3 | 25.9 | 21.4 | 24.6 |
| Education < high school | 25.8 | 26.9 | 22.7 | 38.9a |
| No health insurance | 11.6 | 9.6 | 13.6 | 7.2a |
| Current smoker | 17.1 | 20.7 | 15.2 | 14.3 |
| Hypertension | 56.8 | 60.9 | 53.8 | 58.9 |
| Diabetes | 27.6 | 32.5 | 24.1 | 29.7a |
| Congestive heart failure | 7.2 | 8.3 | 5.9 | 11a |
| Sleeping pill use | 12.5 | 17.8 | 10.0 | 7.8 |
| Depression (PHQ-9 Score ≥10) | 6.2 | 9.5 | 4.4 | 3.8a |
| Systolic BP (mm Hg) | 132 ± 1 | 133 ± 1 | 132 ± 1 | 131 ± 2 |
| Diastolic BP (mm Hg) | 69 ± 1 | 70 ± 1 | 69 ± 1 | 66 ± 2 |
| BMI ≥30 kg/m2 | 40.9 | 46.2 | 39.0 | 32.2a |
| HbA1c (%) | 6.0 ± 0.1 | 6.1 ± 0.1 | 5.9 ± 0.1 | 6.0 ± 0.2 |
| TC ≥200 mg/dl | 44.6 | 44.7 | 45.8 | 37.9 |
| LDL-C ≥100 mg/dl | 56.5 | 60.8 | 55.7 | 46.9 |
| eGFR (ml/min per 1.73 m2) | 74.3 ± 1.2 | 78.0 ±1.7 | 73.1 ±1.6 | 68.0 ± 2.4a |
| Urine ACR | ||||
| <30 mg/g | 33.3 | 25.2 | 36.9 | 42.4a |
| 30–300 mg/g | 57.4 | 61.7 | 56.3 | 47.9 |
| >300 mg/g | 9.3 | 13.1 | 6.9 | 9.6 |
ACR, albumin-to-creatinine ratio; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; LDL-C, low-density lipoprotein cholesterol; PHQ-9, Patient Health Questionnaire for depression screening; TC, total cholesterol.
Values are expressed as weighted means ± SE or weighted percentages.
P < 0.05.
In Table 2, demographic and clinical characteristics are presented by presence or absence of sleep symptoms and sleep disorders. Individuals who reported sleep symptoms were more likely to be women, current smokers, and to have a PHQ-9 score ≥10.
Table 2.
Characteristics of individuals with CKD overall and stratified by sleep symptoms and disorders
| Characteristic | Difficulty falling asleep |
Difficulty staying asleep |
Daytime sleepiness |
Nonrestorative sleep |
Restless legs syndrome |
Sleep apnea |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 233) |
No (n = 1219) |
Yes (n = 302) |
No (n = 1150) |
Yes (n = 242) |
No (n = 1220) |
Yes (n = 302) |
No (n = 1150) |
Yes (n = 195) |
No (n = 1257) |
Yes (n = 93) |
No (n = 1359) |
|
| Weighted (%) | 16.9 | 83.1 | 21.5 | 79.5 | 19.4 | 80.6 | 25.3 | 74.7 | 12.5 | 87.5 | 7.1 | 92.9 |
| Age (yr) | 59.8 ± 1.4 | 60.5 ± 0.9 | 62.2 ± 1.3 | 59.9 ± 0.9 | 59.3 ± 1.4 | 60.7 ± 1.0 | 56.8 ± 1.2 | 61.6 ± 1.0* | 62.7 ± 1.4 | 60.1 ± 0.8 | 58.9 ± 1.8 | 60.5 ± 0.8a |
| 18–44 | 22.3 | 22.2 | 17.7 | 23.5 | 24.2 | 21.7 | 27.0 | 20.6 | 18.7 | 22.7 | 22.0 | 22.2a |
| 45–64 | 27.5 | 28.7 | 29.9 | 28.2 | 28.9 | 28.4 | 32.3 | 27.3 | 31.9 | 28.1 | 37.9 | 27.8 |
| >65 | 50.2 | 49.0 | 52.5 | 48.4 | 46.9 | 50.0 | 40.7 | 52.1a | 49.4 | 49.2 | 40.1 | 49.9 |
| Female sex | 74.1 | 55.4a | 66.5 | 56.4a | 70.0 | 55.8a | 68.2 | 55.3a | 65.0 | 57.6 | 48.1 | 59.1 |
| Race or ethnicity | ||||||||||||
| Non-Hispanic white | 75.7 | 74.3 | 81.8 | 72.6a | 81.4 | 73.0a | 79.3 | 72.9a | 77.9 | 74.1 | 77.8 | 74.3 |
| Non-Hispanic black | 10.2 | 11.5 | 9.1 | 11.9 | 8.6 | 11.9a | 8.2 | 12.3a | 8.2 | 11.7 | 12.5 | 11.2 |
| Mexican American | 3.6 | 7.3a | 5.3 | 7.0 | 4.6 | 7.2 | 4.5 | 7.4a | 7.4 | 6.6 | 2.8 | 7.0 |
| Other | 10.5 | 6.9 | 3.8 | 8.5a | 5.4 | 8.0 | 7.9 | 7.4 | 6.5 | 7.6 | 6.9 | 7.5 |
| Annual income <$20,000 | 27.5 | 22.4 | 25.3 | 22.7 | 28.3 | 22.1a | 25.6 | 22.5 | 32.8 | 21.9a | 17.5 | 23.7a |
| Education <high school | 29.2 | 25.1 | 27.8 | 25.2 | 29.2 | 25.0 | 22.6 | 26.9 | 32.2 | 24.9a | 17.8 | 26.4a |
| No health insurance | 12.6 | 11.4 | 10.7 | 11.8 | 10.1 | 11.9 | 11.9 | 11.5 | 12.2 | 11.5 | 4.4 | 12.1 |
| Current smoker | 26.2 | 15.2a | 24.8 | 15.0a | 23.3 | 15.6a | 25.6 | 14.2a | 26.3 | 15.8a | 16.7 | 17.1 |
| Hypertension | 60.6 | 56.1 | 62.5 | 55.3a | 64.5 | 55.0a | 60.4 | 55.6 | 75.2 | 54.2a | 78.7 | 55.2a |
| Diabetes | 29.6 | 27.2 | 28.0 | 27.5 | 34.2 | 26.0a | 33.4 | 25.7 | 35.7 | 26.6a | 49.0 | 26.0a |
| Congestive heart failure | 7.2 | 7.2 | 10.2 | 6.4a | 9.3 | 6.7 | 8.5 | 6.8 | 12.1 | 6.5a | 16.1 | 6.6a |
| Sleeping pill use | 25.9 | 8.9a | 20.3 | 9.5a | 21.4 | 9.4a | 22.2 | 8.2a | 24.8 | 9.9a | 16.2 | 11.4 |
| Depression (PHQ-9 ≥10) | 17.8 | 3.8a | 13.8 | 4.0a | 17.6 | 3.4a | 14.4 | 3.4a | 17.0 | 4.6a | 20.8 | 5.0a |
| Systolic BP (mm Hg) | 134 ± 2 | 132 ± 1 | 135 ± 1 | 131.4 ± 0.7a | 132 ± 1 | 133 ± 1 | 132 ± 1 | 132 ± 1 | 134 ± 2 | 132 ± 1 | 130 ± 2 | 133 ± 1a |
| Diastolic BP (mm Hg) | 70 ± 2 | 69 ± 1 | 70 ± 1 | 69.1 ± 0.6 | 71 ± 1 | 68.9 ± 0.7 | 71 ± 1 | 69 ± 1 | 68 ± 1 | 70 ± 1 | 71 ± 1 | 69 ± 1 |
| BMI ≥30 kg/m2 | 42.1 | 40.6 | 40.4 | 41.1 | 44.8 | 39.9 | 45.4 | 39.3 | 51.2 | 39.4a | 84.7 | 37.5a |
| HbA1C (%) | 6.0 ± 0.1 | 6.0 ± 0.1 | 5.9 ± 0.1 | 6.0 ± 0.1 | 6.1 ± 0.1 | 6.0 ± 0.1 | 6.1 ± 0.1 | 5.9 ± 0.0 | 6.2 ± 0.1 | 6.0 ± 0.0a | 6.1 ± 0.1 | 6.0 ± 0.0 |
| TC ≥200 mg/dl | 51.7 | 43.2a | 46.9 | 44.0 | 48.2 | 43.8 | 48.3 | 43.3 | 42.6 | 44.9 | 27.7 | 45.8 |
| LDL-C ≥100 mg/dl | 63.5 | 55.3 | 56.1 | 56.7 | 56.1 | 56.7 | 57.6 | 56.2 | 53.5 | 57.0 | 33.3 | 58.8 |
| eGFR (ml/min per 1.73 m2) | 75.7 ± 2.4 | 74.0 ± 1.4 | 71.6 ± 2.3 | 75.1 ± 1.3 | 75.1 ± 2.5 | 74.1 ± 1.6 | 78.3 ± 2.0 | 73.0 ± 1.5* | 70.2 ± 2.9 | 74.9 ± 1.2 | 71.0 ± 3.7 | 74.6 ± 1.3 |
| Urine ACR | ||||||||||||
| <30 mg/g | 29.7 | 34.0 | 34.8 | 32.9 | 31.1 | 33.8 | 30.0 | 34.4 | 37.8 | 32.6 | 25.3 | 33.9a |
| 30–300 mg/g | 58.5 | 57.1 | 54.7 | 58.1 | 55.9 | 57.7 | 56.1 | 57.8 | 51.6 | 58.2 | 59.4 | 57.2 |
| >300 mg/g | 11.8 | 8.9 | 10.6 | 9.0 | 13.1 | 8.5 | 13.9 | 7.8a | 10.6 | 9.2 | 15.3 | 8.9 |
ACR, albumin-to-creatinine ratio; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; LDL-C, low-density lipoprotein cholesterol; PHQ-9, Patient Health Questionnaire for depression screening; TC, total cholesterol.
Values are expressed as weighted means ± SEs or weighted percentages.
P < 0.05.
Association of Sleep Duration With Mortality
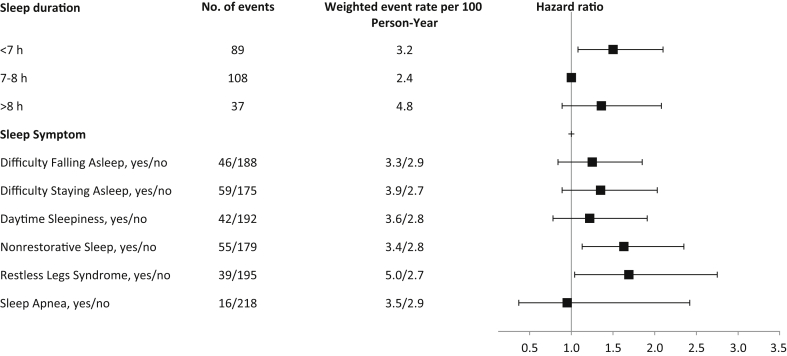
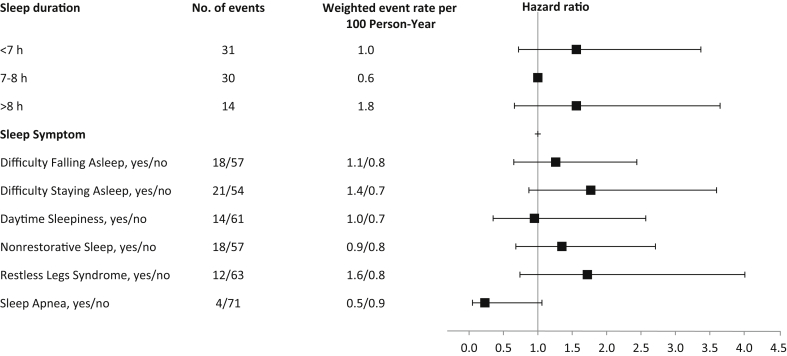
During a median follow-up of 4.4 years, 234 deaths occurred, of which 75 were due to a cardiovascular cause. All-cause mortality rates and hazard ratios (HRs) with 95% confidence intervals (CIs) by category of sleep duration are summarized in Figure 1. After adjustment for sociodemographic and clinical factors, self-reported sleep duration <7 hours was associated with a 50% increased risk for all-cause mortality as compared to individuals reporting 7 to 8 hours of sleep (HR, 1.50 [95% CI, 1.08–2.10]). Self-reported sleep duration >8 hours was associated with a nonsignificant increased risk for all-cause mortality compared with 7 to 8 hours (HR, 1.36 [95% CI, 0.89–2.08]). Similarly, compared with self-reported sleep duration of 7 to 8 hours, sleep duration <7 hours and >8 hours were associated with nonstatistically significant increased risk for cardiovascular death (HR, 1.56 [95% CI, 0.72–3.37], and HR, 1.56 [95% CI, 0.66–3.65]), respectively) (Figure 2). We found no evidence of effect modification by age, sex, or diabetes.
Figure 1.
Event rates per 100 person-years and adjusted hazard ratios (95% confidence intervals) for all-cause mortality according to sleep duration, symptoms, and disorders. Hazard ratios are adjusted for age, sex, race or ethnicity, income, education, diabetes, body mass index, hypertension, smoking, depressive symptoms, estimated glomerular filtration, and albuminuria.
Figure 2.
Event rates per 100 person-years and adjusted hazard ratio (95% confidence intervals) for cardiovascular mortality according to sleep duration, symptoms, and disorders. Hazard ratios are adjusted for age, sex, race or ethnicity, income, education, diabetes, body mass index, hypertension, smoking, depressive symptoms, estimated glomerular filtration, and albuminuria.
Association of Sleep-Related Symptoms With Mortality
Nonrestorative sleep and restless legs syndrome were each associated with an increased risk for all-cause death (HR, 1.63 [95% CI, 1.13–2.35], and HR, 1.69 [95% CI, 1.04–2.75] respectively). There was no significant association between the other sleep symptoms or sleep apnea and all-cause death (Figure 1). Furthermore, there was no significant association between sleep symptoms and cardiovascular death (Figure 2). We found no evidence of effect modification by age, sex, or diabetes.
Discussion
In U.S. adults with CKD, self-reported sleep duration of <7 hours was associated with increased risk of all-cause and cardiovascular death compared with individuals reporting sleep duration of 7 to 8 hours. Additionally, nonrestorative sleep and diagnosed restless legs syndrome were associated with increased risk of all-cause death. To our knowledge, this is the first study to examine the association of sleep duration and sleep symptoms with mortality in a representative sample of U.S. adults with non-dialysis-dependent CKD.
Several population-based cohort studies have demonstrated elevated risk of all-cause and cardiovascular mortality for individuals reporting short sleep duration.20, 21, 22, 24, 25 There is a paucity of data regarding the impact of short sleep on clinical outcomes in CKD. A recent analysis from the Nurses’ Health Study found that shorter sleep was associated with faster decline in kidney function but mortality was not evaluated.26 Our findings suggest that the association between short sleep duration and mortality found in the general population may extend to patients with non-dialysis-dependent CKD.
A number of mechanisms have been proposed to explain the association between poor sleep duration and adverse outcomes in the general population, including alterations of sympathetic nervous system activity, cortisol release, glucose intolerance, and inflammation.8, 27, 28, 29 The associations of short sleep duration with mortality in CKD may also be reflective of disturbances in circadian rhythm. Circadian misalignment can arise when an individual’s sleep is not in synchrony with their endogenous clocks.30 There is growing evidence from animal and human studies suggesting that dysregulation of renal circadian rhythms may be associated with worsening BP and glucose metabolism.31 For example, activation of the circadian clock activity in mice leads to salt-sensitive activation, whereas its suppression leads to low BP.32 Future studies are needed to better understand the role of circadian rhythm in CKD outcomes.
We also found significant associations between nonrestorative sleep and restless legs syndrome and all-cause mortality that have been observed in general population studies.33, 34 In the Health Professionals Follow-Up Study, nonrestorative sleep was associated with HR, 1.24 (95% CI, 1.05–1.46) for all-cause mortality. The potential mechanisms linking nonrestorative sleep with mortality are similar to those mentioned for short sleep. Restless legs syndrome is common in patients with CKD.35 Reasons for this increased prevalence are not understood, but it has been postulated that disordered iron metabolism may be an important factor.36 Multiple observational cohort studies of individuals with end-stage renal disease have shown a significant increase in the risk of death among individuals with restless leg syndrome compared with those without the disease.37, 38, 39, 40 However, less is known about this association in patients with CKD who are not on dialysis.
Our findings of no association between sleep apnea and increased risk of death in individuals with CKD are in contrast with general populations studies that have shown that sleep apnea diagnosed by polysomnography or other objective measure is associated with increased mortality risk.41, 42, 43 The reason for this discordance might be the self-reported method of sleep apnea ascertainment in our study. It is well established that sleep apnea is often underdiagnosed in the clinical setting,44 therefore, underestimation of its prevalence in our study might have limited the power to detect an association between sleep apnea and mortality.
Strengths of our study include the large sample size and prospective study design with median follow-up of 4.4 years and the comprehensive assessment of sleep symptoms. However, several limitations of this study should be taken into account. First, the use of self-reported questionnaires to capture sleep duration may provide an inflated estimate of sleep duration;45 in addition, it might underestimate the prevalence of sleep disorders such as sleep apnea that are known to be common in patients with CKD undergoing objective sleep measurements.46 Second, misclassification of CKD due to single-measurement of eGFR and urine albumin-to-creatinine ratio is possible, which may lead to misclassification of the selected study sample. Third, our study was underpowered to detect associations with cardiovascular mortality. Finally, individuals with missing serum creatinine or urine albumin-to-creatinine ratio data were excluded from analysis. Therefore, our findings may not be representative of the entire CKD population.
In conclusion, short sleep was associated with an increased risk for all-cause mortality in individuals with CKD. Nonrestorative sleep and restless leg symptoms were also associated with an increased risk for all-cause mortality. These findings reinforce the importance of promoting adequate sleep in patients with CKD. Future longitudinal studies with objective measures of sleep duration and symptoms as well as randomized controlled trials investigating the effects of improving sleep duration and symptoms are needed. A better understanding of the effect of sleep duration and sleep symptoms on adverse outcomes among the CKD population could help inform CKD management and lead to improvement of health outcomes.
Disclosure
All the authors declared no competing interests.
Acknowledgments
ACR is funded by a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) award (K23DK094829). ECC is funded by a NIDDK Research Supplement to Promote Diversity in Health-Related Research grant (U01DK060980). JPL is funded by an NIDDK award (K24DK092290). BP is funded by a Department of Veterans Affairs grant (1IK2CX001026-01).
References
- 1.Knutson K.L., Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallicchio L., Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 3.Empana J.-P., Dauvilliers Y., Dartigues J.-F. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the three city study. Stroke J Cereb Circ. 2009;40:1219–1224. doi: 10.1161/STROKEAHA.108.530824. [DOI] [PubMed] [Google Scholar]
- 4.Knutson K.L., Van Cauter E., Rathouz P.J. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169:1055–1061. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knutson K.L., Ryden A.M., Mander B.A., Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 6.Meisinger C., Heier M., Löwel H. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007;30:1121–1127. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cappuccio F.P., D’Elia L., Strazzullo P., Miller M.A. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turek N.F., Ricardo A.C., Lash J.P. Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis. 2012;60:823–833. doi: 10.1053/j.ajkd.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plantinga L., Lee K., Inker L.A., for the CDC CKD Surveillance Team Association of sleep-related problems with CKD in the United States, 2005–2008. Am J Kidney Dis. 2011;58:554–564. doi: 10.1053/j.ajkd.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey Data 2005–2008. 2016 Available at: http://www.cdc.gov/nchs/nhanes. Accessed August 3, 2016.
- 11.Inker L.A., Schmid C.H., Tighiouart H., for the CKD-EPI Investigators Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan S.F., Howard B.V., Iber C. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 13.Chasens E.R., Ratcliffe S.J., Weaver T.E. Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire. Sleep. 2009;32:915–919. doi: 10.1093/sleep/32.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver T.E., Laizner A.M., Evans L.K. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–843. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES—Survey Methods and Analytic Guidelines. Available at: http://www.cdc.gov/nchs/nhanes/survey_methods.htm. Accessed August 17, 2016.
- 16.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. Office of Analysis and Epidemiology, NCHS 2011 Linked Mortality Files Matching Methodology, September, 2013. Hyattsville, Maryland. Available at: http://www.cdc.gov/nchs/data/datalinkage/2011_linked_mortality_file_matching_methodology.pdf. Accessed September 14, 2016.
- 18.Anderson R.N., Miniño A.M., Hoyert D.L., Rosenberg H.M. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2001;49:1–32. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, National Center for Health Statistics. Vital and Health Statistics Reports Series 2, Number 161, September 2013. Available at: http://www.cdc.gov/nchs/data/series/sr_02/sr02_161.pdf. Accessed August 17, 2016.
- 20.Ferrie J.E., Shipley M.J., Cappuccio F.P. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–1666. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hublin C., Partinen M., Koskenvuo M., Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–1253. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikehara S., Iso H., Date C., for the JACC Study Group Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep. 2009;32:295–301. doi: 10.1093/sleep/32.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoenfeld D.A. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 24.Kripke D.F., Langer R.D., Elliott J.A. Mortality related to actigraphic long and short sleep. Sleep Med. 2011;12:28–33. doi: 10.1016/j.sleep.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vgontzas A.N., Liao D., Pejovic S. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMullan C.J., Curhan G.C., Forman J.P. Association of short sleep duration and rapid decline in renal function. Kidney Int. 2016;89:1324–1330. doi: 10.1016/j.kint.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charloux A., Gronfier C., Lonsdorfer-Wolf E. Aldosterone release during the sleep-wake cycle in humans. Am J Physiol. 1999;276:E43–E49. doi: 10.1152/ajpendo.1999.276.1.E43. [DOI] [PubMed] [Google Scholar]
- 28.Knutson K.L., Spiegel K., Penev P., Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briançon-Marjollet A., Weiszenstein M., Henri M. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr. 2015;7:25. doi: 10.1186/s13098-015-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron K.G., Reid K.J. Circadian misalignment and health. Int Rev Psychiatry. 2014;26:139–154. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcheva B., Ramsey K.M., Peek C.B. Circadian clocks and metabolism. Handb Exp Pharmacol. 2013;217:127–155. doi: 10.1007/978-3-642-25950-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuber A.M., Centeno G., Pradervand S. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A. 2009;106:16523–16528. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y., Zhang X., Winkelman J.W. Association between insomnia symptoms and mortality: a prospective study of U.S. men. Circulation. 2014;129:737–746. doi: 10.1161/CIRCULATIONAHA.113.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molnar M.Z., Lu J.L., Kalantar-Zadeh K., Kovesdy C.P. Association of incident restless legs syndrome with outcomes in a large cohort of US veterans. J Sleep Res. 2016;25:47–56. doi: 10.1111/jsr.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novak M., Winkelman J.W., Unruh M. Restless legs syndrome in patients with chronic kidney disease. Semin Nephrol. 2015;35:347–358. doi: 10.1016/j.semnephrol.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Trenkwalder C., Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol. 2010;6:337–346. doi: 10.1038/nrneurol.2010.55. [DOI] [PubMed] [Google Scholar]
- 37.Lin C.-H., Sy H.-N., Chang H.-W. Restless legs syndrome is associated with cardio/cerebrovascular events and mortality in end-stage renal disease. Eur J Neurol. 2015;22:142–149. doi: 10.1111/ene.12545. [DOI] [PubMed] [Google Scholar]
- 38.Winkelman J.W., Chertow G.M., Lazarus J.M. Restless legs syndrome in end-stage renal disease. Am J Kidney Dis. 1996;28:372–378. doi: 10.1016/s0272-6386(96)90494-1. [DOI] [PubMed] [Google Scholar]
- 39.Unruh M.L., Levey A.S., D’Ambrosio C., for the CHOICE Study Restless legs symptoms among incident dialysis patients: association with lower quality of life and shorter survival. Am J Kidney Dis. 2004;43:900–909. doi: 10.1053/j.ajkd.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 40.La Manna G., Pizza F., Persici E. Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment. Nephrol Dial Transplant. 2011;26:1976–1983. doi: 10.1093/ndt/gfq681. [DOI] [PubMed] [Google Scholar]
- 41.Shah N.A., Yaggi H.K., Concato J., Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath. 2010;14:131–136. doi: 10.1007/s11325-009-0298-7. [DOI] [PubMed] [Google Scholar]
- 42.Yaggi H.K., Concato J., Kernan W.N. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 43.Marshall N.S., Wong K.K., Liu P.Y. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 44.Senthilvel E., Auckley D., Dasarathy J. Evaluation of sleep disorders in the primary care setting: history taking compared to questionnaires. J Clin Sleep Med. 2011;7:41–48. [PMC free article] [PubMed] [Google Scholar]
- 45.Lauderdale D.S., Knutson K.L., Yan L.L. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakaguchi Y., Shoji T., Kawabata H. High prevalence of obstructive sleep apnea and its association with renal function among nondialysis chronic kidney disease patients in Japan: a cross-sectional study. Clin J Am Soc Nephrol. 2011;6:995–1000. doi: 10.2215/CJN.08670910. [DOI] [PMC free article] [PubMed] [Google Scholar]