Abstract
Background:
Cervical cancer (CCa) and breast cancer (BCa) are the two leading cancers in women worldwide. Early detection and education to promote early diagnosis and screening of CCa and BCa greatly increases the chances for successful treatment and survival. Screening uptake for CCa and BCa in low and middle - income countries (LMICs) is low, and is consequently failing to prevent these diseases. We conducted a systematic review to identify the key barriers to CCa and BCa screening in women in LMICs.
Methods:
We performed a systematic literature search using Ovid MEDLINE, EMBASE, PsycINFO, SCOPUS, CINHAL Plus, and Google scholar to retrieve all English language studies from inception to 2015. This review was done in accordance with the PRISMA-P guidelines.
Results:
53 eligible studies, 31 CCa screening studies and 22 BCa screening studies, provided information on 81,210 participants. We found fewer studies in low-income and lower - middle - income countries than in upper - middle - income countries. Lack of knowledge about CCa and BCa, and understanding of the role of screening were the key barriers to CCa and BCa screening in LMICs. Factors that are opportunities for knowledge acquisition, such as level of education, urban living, employment outside the home, facilitated CCa and BCa screening uptake in women in LMICs.
Conclusions:
Improvements to CCa and BCa screening uptake in LMICs must be accompanied by educational interventions which aim to improve knowledge and understanding of CCa and BCa and screening to asymptomatic women. It is imperative for governments and health policy makers in LMICs to implement screening programmes, including educational interventions, to ensure the prevention and early detection of women with CCa and BCa. These programmes and policies will be an integral part of a comprehensive population-based CCa and BCa control framework in LMICs.
Keywords: Barriers, breast cancer, cervical cancer, screening, LMICs
Introduction
Cervical cancer (CCa) and breast cancer (BCa) are the two leading cancers in women worldwide. It is estimated that over 270,000 and 508,000 women die from CCa and BCa each year globally of whom approximately 85% and 58%, respectively are women living in low-income and middle-income countries (LMICs) (World Health Organization, 2015c; World Health Organization, 2015b). With the ageing population, the prevalence of both of these female cancers are increasing in these settings (Ferlay et al., 2004; Ferlay et al., 2010).
Early detection and education to promote early diagnosis and screening of CCa and BCa greatly increases the chances for successful treatment and survival (World Health Organization, 2015a). Cervical cancer screening using a cytology-based approach, and BCa screening using mammography, can detect cancer at an early stage and treatment has a high potential for cure and reduced mortality, particularly for CCa (Anderson et al., 2008; World Health Organization, 2015a; World Health Organization, 2015b).
In many LMICs, screening programs do exist in some form, however, they tend to be opportunistic and not organised. Consequently, these programs are failing to achieve a major impact in most settings with low screening uptake (World Health Organization, 2002; Sankaranarayanan et al., 2005; Islam et al., 2015). Numerous studies have reported a broad range of barriers to CCa and BCa screening uptake in LMICs in which socio-cultural, religious and structural barriers are foremost (Rajaram and Rashidi, 1999; Anderson, 2010; Harford, 2011; Story et al., 2012; Garrett and Barrington, 2013; Khazaee-Pool et al., 2014). Many of the barriers are based on speculation rather than on research-derived evidence (Harford, 2011). Given the high disease burden from CCa and BCa, a more detailed understanding of the barriers is urgently needed to help in prevention and the planning of interventions to improve participation in screening. However to date, a systematic review has not been conducted to understand why women are reluctant to take-up CCa and BCa screening in this setting. This review aims to identify the key barriers to CCa and BCa screening in LMICs.
Materials and Methods
Data source and search strategy
We performed a systematic literature search using Ovid MEDLINE, EMBASE, PsycINFO, SCOPUS, CINHAL Plus, and Google scholar in December 2015 to retrieve all English language studies that contained information on barriers to CCa and BCa screening in LMICs. Studies were categorised into ‘low income’, ‘middle income’ and ‘upper middle income’ countries as defined by the World Bank (World Bank, 2015). We also completed a retrospective literature search of published papers to retrieve relevant articles. The subject search and text word search were done separately in all databases and then combined with ‘OR’ and ‘AND’ operators. The MeSH (Medical Subject Headings) terms included cervix, cervical.mp., cancer, neoplasm, breast cancer.mp. or exp breast neoplasms, (cervix* adj3 neoplas*.mp), (breast adj3 neoplasm.mp), screening.mp. or exp mass screening, early detection of cancer.mp., breast self-examination.mp. or exp breast self-examination, pap smear.mp. or exp papanicolaou, mammogra*.mp., HPV.mp. or exp human papillomavirus, barrie*.mp., obstacl*.mp. and challeng*.mp. (Afghanistan* or Albania* or Algeria* or Angola* or Argentina* or Armenia* or Azerbaijan* or Bangladesh* or Belarus* or Beliz* or Benin* or Bhutan* or Bolivia* or Bosnia* or Herzegovin* or Botswan* or Brazil* or Bulgaria* or Burkina* or Burundi* or Cabo Verde* or Cape Verde* or Cambodia* or Cameroon* or Central African or Chad* or China or Chinese or Colombia* or Comor* or Congo* or Costa Rica* or Cote d’Ivoir* or Ivory Coast or Cuba* or Djibouti* or Dominica* or Ecuador* or Egypt* or El Salvador* or Eritrea* or Ethiopia* or Fiji* or Gabon* or Gambia* or Georgia* or Ghana* or Grenad* or Guatemala* or Guinea* or Guyan* or Haiti* or Hondura* or Hungar* or India* or Indonesia* or Iran* or Iraq* or Jamaica* or Jordan* or Kazakhstan* or Kenya* or Kiribati* or Korea* or Kosov* or Kyrgyz Republic or Lao* or Leban* or Lesotho* or Liberia* or Libya* or Macedonia* or Madagascar* or Malawi* or Malaysia* or Maldiv* or Mali* or Marshall Island* or Mauritania* or Mauriti* or Mexic* or Micronesia* or Moldova* or Mongolia* or Montenegr* or Morocc* or Mozambi* or Myanma* or Burmese or Namibia* or Nepal* or Nicaragua* or Niger* or Nigeria* or Pakistan* or Palau* or Panama* or Papua New Guinea* or Paraguay* or Peru* or Philippines or Filipino or Romania* or Rwanda* or Samoa* or Sao Tome* or Senegal* or Serbia* or Seychell* or Sierra Leon* or Solomon Island* or Somalia* or South Africa* or Sudan* or Sri Lanka* or St Lucia* or St Vincent or Grenadines or Surinam* or Swazi* or Syria* or Tajikistan* or Tanzania* or Thai* or Timor* or Togo* or Tonga* or Tunisia* or Turk* or Turkmenistan* or Tuvalu* or Uganda* or Ukrain* or Uzbekistan* or Vanuatu* or Venezuela* or Vietnam* or West Bank or Gaza or Yemen* or Zambia* or Zimbabwe*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]. (africa* or asia* or caribbean or central america* or latin america* or south america* or melanesia* or micronesia* or polynesia*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]. (resource-limit* or resource-poor or low-resource* or limited-resource* or resource-constrain* or constrain* resource* or under-resource* or poor*-resource* or resource-scarce* or scarce*-resource* or low-income or middle-income or lowincome or middleincome or LMIC*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]. developing or underdeveloped or under-developed or less-developed or least-developed) adj world) or third-world* or thirdworld* or 3rd-world*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms].
Our systematic review included both quantitative and qualitative studies. Quantitative studies were examined to identify factors associated with screening uptake, whereas qualitative studies were included to explore barriers to screening of CCa and BCa that were self-reported by women. Qualitative studies were included in the systematic review to triangulate findings from the quantitative studies or offer alternative understandings (Grant and Booth, 2009).
Inclusion criteria
Irrespective of study design, we included studies that included healthy women, were conducted either in community or hospital/clinic settings, and were conducted in LMICs. We included only articles that reported barriers from women’s perspective rather than barriers to delivery side, for instance, barriers to setting up cytology-based screening programs.
Exclusion criteria
We excluded studies that were undertaken in developed countries, included women from LMICs who were currently living in developed countries, included women who presented with CCa and BCa for which they were receiving treatment, included only working women or students, and included the views of people other than women themselves (e.g. studies which presented the views of the parents of girls/women were excluded, particularly in the case of HPV vaccination uptake). Studies that reported barrier scores but did not provide data for specific barrier were also excluded. In addition, editorials, letters and personal views were excluded.
Data extraction
Data were extracted independently by two of the authors (RMI and MNH). Two other authors cross-checked all the final papers selected for this review. If there was disagreement on a particular article, consensus was reached by discussion before the final inclusion of the paper. We performed a narrative review of quantitative studies because there was considerable variation in estimates of barriers to screening as each barrier covered different dimensions, and the methodology varied widely between studies. Data were abstracted into evidence tables and summarised descriptively. Our review was done in accordance with the PRISMA-P guidelines (Moher et al., 2015).
Quality assessment
The quality of quantitative studies was identified for 9 of the 11 quality criterion assessed by the checklist of the critical appraisal skill program (CASP) modified tool: clear study objectives, appropriate methodology, representative sample and power, response rate and validation of instrument, reliability of the results, appropriate tables and graphs, appropriate statistical methods, important variables considered, and the application of results to local settings (Critical Appraisal Skill Program). The quality of the included qualitative studies was assessed using the quality criteria in the qualitative checklist of the CASP tool: clear study objectives, appropriate methodology, appropriate study design, recruitment strategy, data collection, consideration of relationship between researchers and participants, ethical issues, rigorous analysis, clear findings and contribution to knowledge (Critical Appraisal Skill Program). For both tools, each of the quality criterion was given a score from 0 to 4 based on the author’s subjective judgement. These were then summed and an assessment of the overall quality of a particular study was ranked as low, medium or high. The quality score for quantitative studies ranged from 0 to 36, (0-18 = low; 19-28 = medium; 29-36 = High). The quality score for qualitative studies ranged from 0-40 (0-20 = low; 21-30 = medium; 31-40 = high).
Results
Study Characteristics
The flow diagram of included studies is shown in Figure 1. The initial database search yielded 8,167 studies, of which 1,876 were duplicates and 6,167 studies were excluded because they were conducted either in woman with CCa and BCa receiving treatments or were not conducted in LMICs. Of the remaining 124 studies, 53 met the inclusion criteria of which 31 studies focused on barriers to CCa screening, while 22 studies on barriers to BCa screening. 36 were quantitative and 17 qualitative studies. Included studies were published between 1999 and 2015. They included a total of 81,210 participants across the 52 independent studies. The sample size of one qualitative study conducted in five Latin American countries of Ecuador, El Salvador, Mexico, Peru and Venezuela was unclear (Agurto et al., 2004). All included studies were population-based cross-sectional, except one case-control study (Budkaew et al. 2014).
Figure 1.
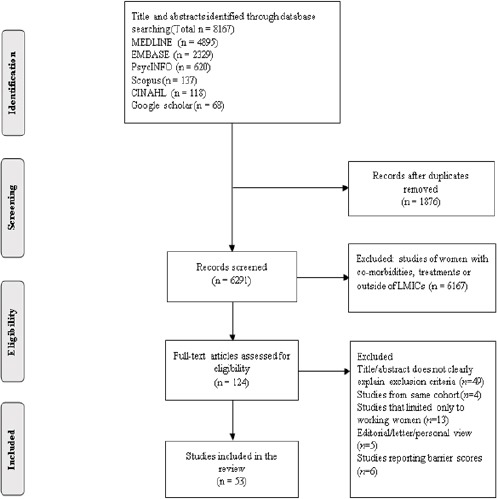
MEDLINE: International Biomedical Bibliographic Database; EMBASE, International biomedical and pharmacological bibliographic database; PsycINFO, Psychological Information Database; CINAHL Plus, Cumulative Index to Nursing and Allied Health Literature; Scopus, A Multidisciplinary Database; LMICs, Low and Middle Income Countries.
Study sample, design and measures
We extracted the following key characteristics of quantitative and qualitative studies: lead author and country, year published, study design and methodology, sampling technique and sampling frame, sample size, age group, screening method used, and barriers themes (Table 1 and 2).
Table 1.
Barriers to CCa Screening Based on Level of Income of the Countries
| Author, Country and Year | Study design and Methodology | Sampling technique and frame | Sample size (n) | Age group (yrs.) | Screening method used | Barriers themes | Quality rating* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low income countries | |||||||||||||
| Quantitative studies | |||||||||||||
| Audet CM et al. | Quantitative | Convenience | 101 | 30-56 | VIA | A | Medium | ||||||
| Mozambique, 2012 | Questionnaire survey | In two clinics | B | ||||||||||
| Cunningham MS et al. | Quantitative | Multistage cluster random | 575 | 18-55 | VIA | A B D E |
High | ||||||
| Tanzania, 2015 | Questionnaire survey | In two districts | |||||||||||
| Mupepi SC et al. | Quantitative | Random | 514 | Dec-84 | VIAC | A | High | ||||||
| Zimbabwe, 2011 | Questionnaire survey | In a rural district | D | ||||||||||
| Perng P et al. | Quantitative | Convenience | 300 | 25+ | VIA | A | High | ||||||
| Tanzania, 2013 | Questionnaire survey | In a rural village | B D |
||||||||||
| Qualitative studies | |||||||||||||
| Fort VK et al. | Qualitative | Convenience | 20 | 20-50 | VIA | A D E |
High | ||||||
| Malawi, 2011 | In-depth interview | In one hospital and catchment area | |||||||||||
| Ports KA et al. | Qualitative | Unclear | 30 | 18-49 | VIA | D | Medium | ||||||
| Malawi, 2015 | In-depth interview | In four villages | |||||||||||
| Lower-middle income countries | |||||||||||||
| Quantitative studies | |||||||||||||
| Quantitative | Random | 469 | 25-65 | VIA | A | High | |||||||
| Basu P et al. | Questionnaire survey | In one area | D | ||||||||||
| India, 2006 | |||||||||||||
| Islam RM et al. | Quantitative | Multistage cluster random | 1590 | 30-59 | VIA | A | High | ||||||
| Bangladesh, 2015 | Questinnaire survey | Nationally représentative | B | ||||||||||
| Montgomery MP et al. | Quantitative | Convenience | 202 | 18-44 | Pap smear | A D F |
Low | ||||||
| India, 2015 | Questinnaire survey | In one hospital | |||||||||||
| Quantitative | Systematic random | 388 | 15-49 | Pap smear | A | Medium | |||||||
| Sudenga SL et al | Questionnaire survey | In 4 health facilities in under one district | B | ||||||||||
| Kenya, 2013 | D | ||||||||||||
| Qualitative studies | |||||||||||||
| Ansink AC et al. | Qualitative | Convenience | 220 | 20-49 | VIA | A | Medium | ||||||
| Bangladesh, 2008 | Focus group | In catchment areas of 2 hospitals | Men, women and Adolescents | D | |||||||||
| Garrett JJ et al. | Qualitative | Convenience | 20 | 18-65 | Pap smear | A C D E |
High | ||||||
| Honduras, 2013 | Focus group & in-depth interviews | In rural settings | |||||||||||
| Kim YM et al. | Qualitative | Convenience | 20 received VIA | 25-50 | Cryotherapy after VIA | A | High | ||||||
| Indonesia, 2012 | Focus group | In 7 health centres | |||||||||||
| Ngugi CW et al. | Qualitative | Convenience | 50 | 18+ | VIA/VILLI | A C D |
Medium | ||||||
| Kenya, 2012 | In-depth interviews | In one district hospital | |||||||||||
| Upper middle income countries | |||||||||||||
| Quantitative studies | |||||||||||||
| Augusto EF et al. | Quantitative | Unclear | 351 | 17-79 | Pap smear | B | Medium | ||||||
| Brazil, 2013 | Questinnaire survey | D | |||||||||||
| Budkaew J at al. | Quantitative | Systematic | 195 | 30-60 | Pap smear | A B D |
Medium | ||||||
| Thailand, 2014 | Questionnaire survey & in-depth interviews | In one medical hospital | |||||||||||
| Gan DEH et al. | Quantitative | Multistage random | 959 | 20-64 | Pap smear | A B E |
High | ||||||
| Malaysia, 2013 | Questinnaire survey | In 5 rural districts | |||||||||||
| Fernandes JV et al. | Quantitative | Stratified | 267 | 15-69 | Pap smear | B D E |
Low | ||||||
| Brazil, 2009 | Questinnaire survey | In a city | |||||||||||
| Jia Y et al. | Quantitative | Convenience | 5929 | 26-65 | VIA/VILLI/Colposcopy | A B D F |
High | ||||||
| China, 2013 | Questinnaire survey | In 3 high incidence towns | |||||||||||
| Kangmennaang J et al. | Quantitative | Stratified random | 6542 | 15-64 | Pap smear | A | High | ||||||
| Namibia, 2015 | Questinnaire survey | Nationally representative | D | ||||||||||
| Marvan M L et al. | Quantitative | Convenience | 384 | 26-64 | Pap smear | A B D |
Medium | ||||||
| Mexico, 2013 | Questinnaire survey | In one urban and 2 rural areas | |||||||||||
| Nwankwo KC et al. | Quantitative | Convenience | 815 | 18-70 | Pap smear | A | Medium | ||||||
| Nigeria, 2011 | Questinnaire survey | In a church-based mandatory annual meeting | D | ||||||||||
| Reis N et al. | Quantitative | Random | 387 | Average age 34.4 years | Pap smear | A | Medium | ||||||
| Turkey, 2012 | Questinnaire survey | In outpatient clinics of 2 cities | B D F |
||||||||||
| Watkins MM et al. | Quantitative | Convenience | 97 | 16-66 | Pap smear | A | Medium | ||||||
| Mexico, 2002 | Questinnaire survey | In a rural village | B D |
||||||||||
| Agurto I et al. | Combination of 5 Qualitative studies | Convenience | Unclear | 25-64 | Pap smear | C | Low | ||||||
| Latin America, 2004 | Focus group and in-depth interviews | In 6 areas in 5 countries | D | ||||||||||
| Duran ET | Qualitative | Convenience | 11 | 15-49 | No specific CCa screening | A | Low | ||||||
| Turkey, 2011 | Case studies | In 2 hospitals in a small city | C D |
||||||||||
| Ersin F et al. | Qualitative | Random | 35 | 40+ | Pap smear | A | Low | ||||||
| Turkey, 2013 | Focus group | In one district | B D |
||||||||||
| Lazcano-ponce EC et al. | Qualitative | Convenience | 4 FG | 25-35 | Pap smear | A | High | ||||||
| Mexico, 1999 | Focus group | One urban and one rural city | (each 7/8) | B D E |
|||||||||
| Markovic M et al. | Qualitative | Convenience | 62 | 35-55 | Pap smear | A | Medium | ||||||
| Serbia, 2005 | Focus group | In 2 cities | C D E |
||||||||||
| McFarland D M | Qualitative | Convenience | 30 | 30+ | Pap smear | A | High | ||||||
| Botswana 2003 | Questionnaire and | In capital city | D | ||||||||||
| semi-structured interview | E | ||||||||||||
| Paz-Soldan VA et al. | Qualitative | Convenience | 177 | 18-40 | Pap smear | A | Medium | ||||||
| Peru, 2010 | Focus group | In 4 cities | C | ||||||||||
Note: CCa, Cervical cancer; VIA, Visual inspection with acetic acid; VIAC, Visual inspection with acetic acid and camera; VILLI, Visual inspection with Lugol’s iodine, colposcopy; A, Barriers related to lack of knowledge and awareness about cervical cancer, and screening methods; B, Demographic factors include age, marital status, occupation; C, Psychological factors include fear, anxiety, depression etc.; D, structural barriers include education, income and cost associated with screening and treatment, distance to the service centres, access and availability to screening; E, Socio-cultural and religious barriers include that family does not allow screening, modesty mostly associated with religion, believing the disease caused by a curse; and F, Perceived barriers, particularly the health belief model.
Table 2.
Barriers to BCa Screening Based on Level of Income of the Countries
| Author, Country and Year | Study design and Methodology | Sampling technique & frame | Sample size (n) | Age group (yrs.) | Screening method used | Barriers themes | Quality rating* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low income countries | |||||||||||||
| No studies have found on barriers to BCa screening in Low income countries | |||||||||||||
| Lower-middle income countries | |||||||||||||
| Quantitative studies | |||||||||||||
| Aboserea M et al. | Quantitative | Multistage cluster random | 390 | Unclear | BSE, CBE, Mammography | A | Medium | ||||||
| Egypt, 2011 | Questionnaire survey | In one district | B D |
||||||||||
| Amoran OE et al. | Quantitative | Multistage cluster stratified | 495 | Unclear | BSE | A | Low | ||||||
| Nigeria, 2015 | Questionnaire survey | In one state | D | ||||||||||
| Frie KG et al. | Quantitative | Among intervention group of RCT | 52, 011 | 30-69 | BSE, CBE | A | High | ||||||
| India, 2013 | Questionnaire survey | B | |||||||||||
| Islam RM et al. | Quantitative | Multistage cluster random | 1590 | 30-59 | BSE, CBE, Mammography | A | High | ||||||
| Bangladesh, 2015 | Questinnaire survey | Nationally représentative | B | ||||||||||
| Rasu RS et al. | Quantitative | Convenience | 152 | 40+ | BSE, Mammography | A | Medium | ||||||
| Bangladesh, 2011 | Questionnaire survey | In one district | Women from uni and college | ||||||||||
| Sreedevi A et al. | Quantitative | Multistage random | 809 | 15-50 | BSE, CBE, Mammography | A | Medium | ||||||
| India, 2014 | Questionnaire survey | In one district | B D |
||||||||||
| Upper middle income countries | |||||||||||||
| Quantitative studies | |||||||||||||
| Ahmadian M et al. | Quantitative | Multistage cluster random | 400 | 35-69 | Mammography | A | |||||||
| Iran, 2012 | Questionnaire survey | In 4 outpatients clinic | B D |
Medium | |||||||||
| Al-Naggar RA et al. | Quantitative | Random | 200 | 40+ | Mammography | A | |||||||
| Malaysia, 2012 | Questionnaire survey | In one area | B D |
Low | |||||||||
| Avci IA et al. | Quantitative | Unclear | 387 | 35+ | Mammography | F | Medium | ||||||
| Turkey, 2008 | Questionnaire survey | In one health center | |||||||||||
| Cam O et al. | Quantitative | Stratified random | 382 | 40+ | BSE, CBE, Mammography | A | Medium | ||||||
| Turkey, 2009 | Questionnaire survey | In 3 health clinics in one area | B | ||||||||||
| Dunder PE et al. | Quantitative | Systematic random | 446 | 50-69 | Mammography | D | Medium | ||||||
| Turkey, 2012 | Questionnaire survey | In 2 districts | F | ||||||||||
| Gang M et al. | Quantitative | Convenience | 406 | 20+ | Mammography | B | |||||||
| China, 2013 | Questionnaire survey | In one city | D F |
High | |||||||||
| Gürsoy AA et al. | Quantitative | Cluster | 1342 | 18+ | BSE, CBE, Mammography | A | |||||||
| Turkey, 2011 | Questionnaire survey | In catchment area of 2 urban clinics | B E F |
Medium | |||||||||
| Hasan N et al. | Quantitative | Unclear | 1317 | 40-74 | Mammography | A | High | ||||||
| Malaysia, 2015 | Questionnaire survey | In one private hospital | C | ||||||||||
| Monatazeri A et al. | Quantitative | Convenience | 410 | 19-58 | BSE, CBE | A | |||||||
| Iran, 2003 | Questionnaire survey | In 7 health centres | B D |
Low | |||||||||
| Secginli S et al. | Quantitative | Convenience | 656 | 20+ | BSE | A | Low | ||||||
| Turkey, 2006 | Questionnaire survey | In 3 heath centres | Mammography | D | |||||||||
| Qualitative studies | |||||||||||||
| Khazaee-Pool M et al. | Qualitative | Convenience | 24 | 30+ | BSE, CBE, Mammography | A | |||||||
| Iran, 2014 | Focus group | In one health care centre | C D |
Medium | |||||||||
| Kissal A et al. | Qualitative | Convenience | 46 | 60-75 | BSE, CBE, Mammography | A | Medium | ||||||
| Turkey, 2011 | Focus group | In one district | C | ||||||||||
| Lamyian M et al. | Qualitative | Convenience | 31 | 40+ | Unknown | C | |||||||
| Iran, 2007 | In-depth interviews | Unclear | D E |
High | |||||||||
| Tuzco A et al. | Qualitative | Convenience | 39 | 20+ | BSE, CBE, Mammography | A | |||||||
| Turkey, 2015 | Focus group | In one area among migrants women | B D |
Medium | |||||||||
| *Not an independent sovereign country | |||||||||||||
| Azaiza F et al. | Quantitative | Stratified | 397 | 30-65 | BSE, CBE, Mammography | A | |||||||
| *Palestine, 2010 | Questionnaire survey | In 4 districts | B D E |
High | |||||||||
| Shaheen R et al. | Quantitative Questionnaire/telephone interviews | Convenience | 100 | 35+ | Diagnostic and Mammography | D | Low | ||||||
| *Palestine, 2011 | Unclear | ||||||||||||
Note: BCa, Breast cancer; BSE, Breast self-examination; CBE, Clinical breast examination; RCT, Randomised controlled trial; A, Barriers related to lack of knowledge and awareness about cervical cancer, and screening methods; B, Demographic factors include age, marital status, occupation; C, Psychological factors include fear, anxiety, depression etc.; D, structural barriers include education, income and cost associated with screening and treatment, distance to the service centres, access and availability to screening; E, Socio-cultural and religious barriers include that family does not allow screening, modesty mostly associated with religion, believing the disease caused by a curse; and F, Perceived barriers, particularly the health belief model.
Quantitative studies
The 36 quantitative studies were from Africa (8 studies), Turkey (6), India (4), Bangladesh (3), Malaysia (3), Brazil (2), China (2), Mexico (2), Iran (2), Palestine (2), Thailand (1) and Egypt (1). All 36 quantitative studies were cross-sectional. All studies used questionnaire survey methodology. One used in-depth interviews and 1 used telephone interviews in addition. 27 studies used convenience sampling, while 22 studies used random sampling. Sampling procedures were not clearly discussed in 4 studies. The sample size of the quantitative studies ranged from 97 (Watkins et al., 2002) to 52,011 (Frie et al., 2013) participants. The age of the study participants was from 12 years and older as nine quantitative studies did not have an upper age limit (Perng et al., 2013; Rasu et al., 2011; Al-Naggar and Bobryshev, 2012; Avci and Kurt, 2008; Çam and Gümüs, 2009; Gang et al., 2013; Gürsoy et al., 2011; Secginli and Nahcivan, 2006; Shaheen et al., 2011).
Qualitative studies
The 17 qualitative studies were from Africa (4), Turkey (4), Iran (2), Bangladesh (1), Honduras (1), Indonesia (1), Latin America (1), Mexico (1), Serbia (1) and Peru (1). Nine used focus groups discussion (FGD) as the method of data collection, 4 in-depth interviews, 2 FGD and in-depth interview, 1 used case studies and 1 used questionnaire and semi-structured interviews. All qualitative studies used convenience sampling except 1 which used random sampling and 1 in which the sampling was unclear. The age of the study participants was 15 and older as six qualitative studies did not have an upper age limit (McFarland, 2003; Lamyian et al., 2007; Ngugi et al., 2012; Ersin and Bahar, 2013; Khazaee-Pool et al., 2014; Tuzcu and Bahar, 2015).
Reporting of barriers to cervical cancer screening Low income countries
Quantitative studies
Four quantitative studies that investigated enablers and barriers for CCa screening in low income countries were from three African countries namely Mozambique, Tanzania and Zimbabwe (Table 1) (Audet et al., 2012; Cunningham et al., 2015; Mupepi et al., 2011; Perng et al., 2013). These studies reported lack of awareness of, and knowledge about, CCa and CCa screening as a common barrier to screening uptake. Screening uptake was also lower among multiparous Mozambican women and in women who believe that CCa is caused by a curse/witchcraft (Audet et al., 2012). Zimbabwean women who were employed and financially independent were more likely to undergo screening (Mupepi et al., 2011). The Tanzanian studies reported that women who attended screening service were older, listened regularly to the radio, had a poorer quality of life, had health insurance or faced cost barriers to obtaining health care in the preceding year, and held a more positive attitude towards CCa screening compared with women who did not attend (Perng et al., 2013; Cunningham et al., 2015).
Qualitative studies
Two qualitative studies from Malawi in women aged 18-50 years found that demographic factors include age, marital status, occupation were key barriers to uptake of CCa screening(Fort et al., 2011) (Ports et al., 2015). One study also found that lack of awareness of, and knowledge about, CCa and socio-cultural and religious barriers include that family does not allow screening, modesty mostly associated with religion (Fort et al., 2011).
Lower-middle income countries
Quantitative studies
Four studies in lower-middle income countries explored enablers and barriers for CCa screening (Table 1) (Basu et al., 2006; Islam et al., 2015; Montgomery et al., 2015; Sudenga et al., 2013). These studies reported lack of awareness of, and knowledge about, CCa and CCa screening as a common barrier to screening uptake. Studies undertaken in Kenya and India also identified socio-demographic, structural barriers to screening uptake (Basu et al., 2006; Montgomery et al., 2015; Sudenga et al., 2013). One Indian study also reported that health beliefs, particularly the health belief model, was a barrier (Montgomery et al., 2015).
Qualitative studies
Four qualitative studies investigated barrier to CCa screening in LMICs (Ansink et al., 2008; Garrett and Barrington, 2013; Kim et al., 2012; Ngugi et al., 2012). These studies reported lack of awareness of, and knowledge about, CCa and CCa screening as a common barrier to screening uptake. Three studies, one each from Bangladesh, Honduras and Kenya, reported that structural barriers such as education, income and cost associated with screening and treatment, distance to the service centres, access and availability to screening were barriers to screening (Ansink et al., 2008; Garrett and Barrington, 2013; Ngugi et al., 2012). In relation to the Bangladesh study (Ansink et al., 2008), an opportunistic screening program had not been initiated in the country when this study was undertaken. However, a recent nationally representative study reported that simple lack of knowledge of CCa and of understanding of the role of screening are the key barriers to screening uptake in Bangladesh (Islam et al., 2015).
The studies from Honduras and Kenya both found psychological factors include fear, anxiety, and depression were barriers (Garrett and Barrington, 2013; Ngugi et al., 2012). The study in Honduras reported structural, psychological and religious barriers that included cost, distance, access, fear, lack of knowledge and male partners’ attitude towards screening (Garrett and Barrington, 2013). The issue of the partner’s attitude is that the procedure violates his expectations for his wife’s modesty. A study in Indonesia revealed that knowledge and perceptions were the most important barriers to screening as women were not aware of CCa and were reluctant to go for screening because they were afraid of the procedure or felt shy about exposing themselves to providers (Kim et al., 2012)
Upper-middle income countries
Quantitative studies
Ten quantitative studies in upper-middle income countries were from Brazil (Fernández et al., 2009; Augusto et al., 2013), China (Jia et al., 2013), Malaysia (Gan and Dahlui, 2013), Namibia (Kangmennaang et al., 2015), Nigeria (Nwankwo et al., 2011), Thailand (Budkaew and Chumworathayi, 2013), Turkey (Reis et al., 2012) that explored enablers and barriers for CCa screening. Structural barriers include education, income and cost associated with screening and treatment, distance to the service centres, access and availability to screening was the most common barrier identified in nine studies (Augusto et al., 2013; Budkaew and Chumworathayi, 2013; Fernández et al., 2009; Jia et al., 2013; Kangmennaang et al., 2015; Marván et al., 2013; Nwankwo et al., 2011; Reis et al., 2012; Watkins et al., 2002). This was followed by lack of awareness of, and knowledge about, CCa and CCa screening in eight studies (Budkaew and Chumworathayi, 2013; Gan and Dahlui, 2013; Jia et al., 2013; Kangmennaang et al., 2015; Marván et al., 2013; Nwankwo et al., 2011; Reis et al., 2012; Watkins et al., 2002) and demographic factors include age, marital status, occupation in another eight studies (Augusto et al., 2013; Budkaew and Chumworathayi, 2013; Gan and Dahlui, 2013; Fernández et al., 2009; Jia et al., 2013; Marván et al., 2013; Reis et al., 2012; Watkins et al., 2002).
Qualitative studies
Seven qualitative studies in upper-middle income countries explored enablers and barriers for CCa screening (Agurto et al., 2004; Duran, 2011; Ersin and Bahar, 2013; Lazcano-Ponce et al., 1999; Markovic et al., 2005; McFarland, 2003; Paz-Soldán et al., 2011). They were from Latin America (Agurto et al., 2004), Turkey (Duran, 2011; Ersin and Bahar, 2013), Mexico (Lazcano-Ponce et al., 1999), Serbia (Markovic et al., 2005), Botswana (McFarland, 2003), and Peru (Paz-Soldán et al., 2011). Six of these studies reported that lack of awareness of, and knowledge about, CCa and CCa screening were barriers (Duran, 2011; Ersin and Bahar, 2013; Lazcano-Ponce et al., 1999; Markovic et al., 2005; McFarland, 2003; Paz-Soldán et al., 2011). Six studies also reported structural barriers include education, income and cost associated with screening and treatment, distance to the service centres, access and availability to screening (Agurto et al., 2004; Duran, 2011; Ersin and Bahar, 2013; Lazcano-Ponce et al., 1999; Markovic et al., 2005; McFarland, 2003). Four studies reported psychological factors include fear, anxiety, and depression were barriers (Agurto et al., 2004; Duran, 2011; Markovic et al., 2005; McFarland, 2003). Three studies reported socio-cultural and religion were barriers (Lazcano-Ponce et al., 1999; Markovic et al., 2005; McFarland, 2003).
Reporting of barriers to breast cancer screening
Low income countries
No studies were found to have investigated barriers to BCa screening in low income countries (Table 2).
Lower-middle income countries
Quantitative studies
Six studies were found in lower-middle income countries which investigated enablers and barriers for BCa screening (Aboserea et al., 2011; Rasu et al., 2011; Frie et al., 2013; Sreedevi et al., 2013; Amoran and Toyobo, 2015; Islam et al., 2016). All studies reported that lack of knowledge and awareness about breast cancer were key barriers. Four studies conducted in India (Frie et al., 2013; Sreedevi et al., 2013) Egypt (Aboserea et al., 2011) and Bangladesh (Islam et al., 2016) reported demographic and personal factors such as not being married, fear and anxiety were also barriers. Three studies conducted in Egypt (Aboserea et al., 2011), Nigeria (Amoran and Toyobo, 2015), and India (Sreedevi et al., 2013) presented all the barriers mentioned above as well as structural barriers which included access, availability and cost. One Nigerian study reported that women did not check their breasts as they had no knowledge about breast self-examination (BSE) and perceived that they were not at risk (Amoran and Toyobo, 2015).
No qualitative studies were found to have investigated barriers to BCa screening in lower-middle income countries (Table 2).
Upper-middle income countries
Quantitative studies
Ten quantitative studies were found in upper-middle income countries (Ahmadian et al., 2012; Al-Naggar and Bobryshev, 2012; Avci and Kurt, 2008; Çam and Gümüs, 2009; Dundar et al., 2012; Gang et al., 2013; Gürsoy et al., 2011; Hassan et al., 2015; Montazeri et al., 2003; Secginli and Nahcivan, 2006), of which five were from Turkey (Avci and Kurt, 2008; Çam and Gümüs, 2009; Dundar et al., 2012; Gürsoy et al., 2011; Secginli and Nahcivan, 2006). Seven studies identified lack of knowledge and awareness about breast cancer as common barriers (Ahmadian et al., 2012; Al-Naggar and Bobryshev, 2012; Çam and Gümüs, 2009; Gürsoy et al., 2011; Hassan et al., 2015; Montazeri et al., 2003; Secginli and Nahcivan, 2006), and six reported demographic and personal factors such as being single and psychological factors such as fear and anxiety as being key barriers (Ahmadian et al., 2012; Al-Naggar and Bobryshev, 2012; Çam and Gümüs, 2009; Gang et al., 2013; Gürsoy et al., 2011; Montazeri et al., 2003). Six studies also identified structural barriers include education, income and cost associated with screening and treatment, distance to the service centres, access and availability to screening as key barriers (Ahmadian et al., 2012; Al-Naggar and Bobryshev, 2012; Dundar et al., 2012; Gang et al., 2013; Montazeri et al., 2003; Secginli and Nahcivan, 2006). The Malaysian studies (Al-Naggar and Bobryshev, 2012; Hassan et al., 2015) reported the most barrier was the perception of not being at risk and fear of painful mammography. The Chinese study reported socio-demographic, cultural, religious, psychological and structural barriers to screening mammography among Chinese and Korean Chinese women (Gang et al., 2013).
Two quantitative studies were conducted in Palestine (Azaiza et al., 2010; Shaheen et al., 2011), however, the list provided by the World Bank does not include Palestine as an independent sovereign country. One of these studies (Azaiza et al., 2010) stated knowledge, socio-demographic, cultural, religious, and structural factors as barriers of BCa screening uptake while the other Palestinian study (Shaheen et al., 2011) reported structural barriers as an important factors that influence women’s decision of not undergoing for BCa screening.
Qualitative studies
Four qualitative studies investigated key barriers to breast cancer screening in upper-middle income countries. Two were from Iran (Khazaee-Pool et al., 2014; Lamyian et al., 2007) and two from Turkey (Kissal and Beser, 2011; Tuzcu and Bahar, 2015). All but one Iranian study (Lamyian et al., 2007) identified knowledge as a key barrier to uptake of BCa screening. Three studies also identified psychological factors include fear, anxiety, and depression as key barriers (Khazaee-Pool et al., 2014; Lamyian et al., 2007; Kissal and Beser, 2011).
Quality of the included studies
The quality for each study is shown in Tables 1 and 2. Of 36 quantitative studies, 13 (36%) studies were identified as high, 16 (44%) were medium while 7 (20%) were rated as low quality. Of 17 qualitative studies, 6 (35%) studies were rated as high, 8 (47%) were medium and 3 (18%) study was identified as low quality. High quality quantitative studies had clearly stated aims (11/13 studies), appropriate methods to address the study questions (12/13 studies), and were relevant to local settings (13/13 studies). High quality qualitative studies also had clearly stated aims (4/6 studies), used appropriate methods seeking to illuminate subjective experiences (4/6 studies), and had clear descriptions of the value of the research (6/6 studies).
Discussion
This systematic review investigated the key barriers to CCa and BCa screening uptake in LMICs. Our main finding was that, irrespective of the economic level of the countries, and study design and methodology of the studies, lack of knowledge about CCa and BCa, and a poor understanding of the role of screening were the key barriers of women’s preparedness to be screened in LMICs. Previous mini-reviews on BCa argued that psycho-social, cultural or cognitive factors such as belief, attitude, self-efficacy, social influence, modesty and perceived barriers were dominant in relation to BCa screening uptake in Asian, predominantly Muslim countries (Parsa et al., 2006; Ahmadian and Samah, 2013). In addition, several studies conducted in Muslim migrants women in the United States also reported that cultural and religious beliefs, as well as access to screening facilities are functioning as barriers to CCa and BCa screening uptake (Abdullahi et al., 2009; Fang and Baker, 2013; Guimond and Salman, 2013; Padela et al., 2014; Patel, 2014). Our review extends these findings by investigating barriers to CCa and BCa in a wide range of LMICs, not only Muslim countries. Our findings suggest that even though each country described barriers in slightly different ways depending on the mix of cultures, religions, perceptions, education and accessibility of screening services, lack of knowledge about the diseases and screening is the primary barrier to CCa and BCa screening in women in LMICs.
We found more than half (53%) of the included studies (Khazaee-Pool et al., 2014; Islam et al., 2015; Islam et al., 2016; Audet et al., 2012; Fort et al., 2011; Mupepi et al., 2011; Basu et al., 2006; Kim et al., 2012; Ngugi et al., 2012; Budkaew and Chumworathayi, 2013; Ersin and Bahar, 2013; Jia et al., 2013; Lazcano-Ponce et al., 1999; Markovic et al., 2005; Marván et al., 2013; McFarland, 2003; Nwankwo et al., 2011; Paz-Soldán et al., 2011; Reis et al., 2012; Rasu et al., 2011; Sreedevi et al., 2013; Amoran and Toyobo, 2015; Çam and Gümüs, 2009; Kissal and Beser, 2011; Montazeri et al., 2003; Tuzcu and Bahar, 2015; Hassan et al., 2015; Azaiza et al., 2010) reported that lack of knowledge and a poor understanding of the role of screening for CCa and BCa, as the key barriers for screening in LMICs. Of these, eleven (36%) were assessed as being high quality (Fort et al., 2011; Islam et al., 2015; Islam et al., 2016; Mupepi et al., 2011; Basu et al., 2006; Kim et al., 2012; Jia et al., 2013; Lazcano-Ponce et al., 1999; McFarland, 2003; Hassan et al., 2015; Azaiza et al., 2010). Typical of these were two recent, large nationally representative quantitative studies both conducted in Bangladesh which demonstrated that lack of awareness and understanding of CCa and BCa and screening were considered as the leading barriers to screening uptake in women at midlife (Islam et al., 2015; Islam et al., 2016) . However, not all studies found knowledge was a key barrier. Eighteen studies (Garrett and Barrington, 2013; Perng et al., 2013; Ports et al., 2015; Cunningham et al., 2015; Ansink et al., 2008; Sudenga et al., 2013; Montgomery et al., 2015; Duran, 2011; Gan and Dahlui, 2013; Watkins et al., 2002; Kangmennaang et al., 2015; Frie et al., 2013; Aboserea et al., 2011; Ahmadian et al., 2011; Ahmadian et al., 2012; Al-Naggar and Bobryshev, 2012; Gürsoy et al., 2011; Secginli and Nahcivan, 2006) reported lack of knowledge as an influence on screening, but did not identify it as a key barrier, and only 15% studies (Agurto et al., 2004; Augusto et al., 2013; Fernández et al., 2009; Avci and Kurt, 2008; Dundar et al., 2012; Gang et al., 2013; Lamyian et al., 2007; Shaheen et al., 2011) did not report knowledge as a barrier at all. This may reflect discrepancies in study designs, modes of recruitment, sampling procedures and sample size or study quality. About two-third of these studies had low or medium level of quality due to such factors as use of convenience sampling or the sampling process was not specified, suggesting bias cannot be excluded from these studies. It may also be because different domains of knowledge were examined across these studies (such as background education compared with health literacy and knowledge of medical treatments). The lack of knowledge we identified included women who did not take up screening because they reported that either they were asymptomatic, did not know screening was needed, did not know where to go for the screening/test, did not know how screening, especially BSE is done, had poor knowledge about the screening methods and lack of general education and health literacy including risk factors and early signs and symptoms of the diseases. There are many factors that influence health seeking behaviour in LMICs, in addition to lack of knowledge. More research is needed to examine how lack of knowledge affects health seeking behaviour and health outcomes in these settings.
There was concordance between findings from quantitative and qualitative studies. Of the 28 studies suggesting lack of knowledge were barriers for screening, 17 were quantitative studies (Islam et al., 2015; Islam et al., 2016; Audet et al., 2012; Mupepi et al., 2011; Basu et al., 2006; Budkaew and Chumworathayi, 2013; Jia et al., 2013; Marván et al., 2013; Montazeri et al., 2003; Çam and Gümüs, 2009; Nwankwo et al., 2011; Rasu et al., 2011; Reis et al., 2012; Sreedevi et al., 2013; Amoran and Toyobo, 2015; Hassan et al., 2015; Azaiza et al., 2010), and eleven were qualitative (Lazcano-Ponce et al., 1999; McFarland, 2003; Markovic et al., 2005; Fort et al., 2011; Kissal and Beser, 2011; Paz-Soldán et al., 2011; Kim et al., 2012; Ngugi et al., 2012; Ersin and Bahar, 2013; Khazaee-Pool et al., 2014; Tuzcu and Bahar, 2015). For example, one quantitative study from Africa (Zimbabwe) showed of 514 women aged 12-84 years surveyed, 91% had never had cervical screening and 81% had no previous knowledge of cervical screening tests (Mupepi et al., 2011). This is supported by qualitative data also from Africa (Malawi) in which it was found that knowledge of cervical cancer was very low such that the majority of asymptomatic women interviewed could not describe anything about cervical cancer or what screening was for (Fort et al., 2011). Interestingly, those that had heard of screening had done so while attending hospital for another service (Fort et al., 2011). Although from different African countries, this illustrates how quantitative and qualitative studies can provide complementary data. The qualitative studies also tended to identify themes on sensitive issues, in addition to knowledge. These included psychological factors such as fear, anxiety, and depression in 5 of the qual studies; structural barriers including education, income and cost associated with screening and treatment in 6 of the qual studies; and socio-cultural and religious barriers in 3 of the qual studies. Future reviews may also benefit from using mixed methodology to triangulate data.
Our review also found that there were some variables that facilitated CCa and BCa screening uptake in women in LMICs such as level of education, urban living, employment outside the home, and age. These are all indices of opportunity for knowledge acquisition. This suggests that in the future there may be a greater uptake of screening still, as higher levels of maternal education are expected as a result of implementation of the Sustainable Development Goals and the increasing urbanisation of the world’s population, particularly in LMICs (Division, 2002). Further research into the mechanisms by which these factors increase screening uptake is needed so that they can inform policy in LMICs.
We found fewer studies in low-income and lower-middle income countries than in upper-middle income countries. This may be because CCa and BCa are not a health priorities in low-income and lower-middle income countries, resulting in screening programmes being either opportunistic or not present here. This suggestion is supported by a recent systematic review of interventions to increase CCa and BCa screening in Asian women which found most programmes located in upper-middle and high income countries (Lu et al., 2012). It underlines the urgency of the development of a comprehensive population-based CCa and BCa control framework in LMICs.
Strength and limitations
We performed a systematic search of the literature between 1999 and 2015, which included both qualitative and quantitative studies, investigating screening barriers for both BCa and CCa, and all LMICs, is a strength. This contrasts with previous reviews which have been restricted to barriers to BCa in Asian countries only with high Muslim populations. Our study is limited by the variation in methodology between quantitative studies which precluded a meta-analysis of the association of factors with screening practices for BCa and CCa in LMICs.
Conclusion and policy implication
Lack of knowledge and lack of understanding the role of screening are the key barriers to CCa and BCa screening uptake amongst women in LMICs irrespective of the economic level of the countries. Improvements to screening uptake in LMICs must be accompanied by educational interventions which aim to raise awareness of CCa and BCa and screening to asymptomatic women evidenced by studies (Shankar et al. 2015). It is imperative for governments and health policy makers in LMICs to give equal importance to the prevention and early detection of CCa and BCa as is given to the diagnosis, treatment and rehabilitation of women with these diseases.
The correct and effective advocacy of programmes and policies prove to be an integral in the development of a comprehensive population-based CCa and BCa control framework in LMICs. In addition, the success of CCa and BCa screening programmes implementation in LMICs requires meticulous planning, sufficient organisational resources, sustainability and professional coordination, and effective population targeting to ensure the quality and continuity of the screening programme.
Contributors
RMI, BB, MNS and JO contributed to the generation of ideas for systematic review. RMI, MNS contributed to the development and completed search strategy for the review. All the authors contributed to review, revise and finalise of the search strategy. RMI prepared the first draft of the protocol. JO, BB, and MNH reviewed and provided subsequent feedback on the revision of the manuscript and its finalisation.
Role of funding
This study is not supported by any funding body. Thus, no funding bodies had any role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflicts of interest
The authors have no competing interests to declare.
Acknowledgements
We are grateful to Professor Susan Davis and Professor Robin Bell for critically revising the first draft for content and tables, and contributed to the final draft. We are also thankful to Lorena Romero, a librarian at The Ian Potter Library, The Alfred Hospital, Melbourne, for her assistance in searching the literature
References
- Abdullahi A, Copping J, Kessel A, et al. Cervical screening: perceptions and barriers to uptake among Somali women in Camden. Public Health. 2009;123:680–5. doi: 10.1016/j.puhe.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Aboserea M, Abdelgawad M, Wafik W. Early detection of breast cancer among females at Fakous district Sharqia Governorate Egypt. Life Sci J. 2011;8:196–203. [Google Scholar]
- Agurto I, Bishop A, Sanchez G, et al. Perceived barriers and benefits to cervical cancer screening in Latin America. Prev Med. 2004;39:91–8. doi: 10.1016/j.ypmed.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Ahmadian M, Samah AA. Application of health behavior theories to breast cancer screening among Asian women. Asian Pac J Cancer Prev. 2013;14:4005–13. doi: 10.7314/apjcp.2013.14.7.4005. [DOI] [PubMed] [Google Scholar]
- Ahmadian M, Samah AA, Redzuan Mr, et al. Barriers to mammography among women attending gynecologic outpatient clinics in Tehran, Iran. Sci Res Essays. 2011;6:5803–11. [Google Scholar]
- Ahmadian M, Samah AA, Redzuan M, et al. Predictors of mammography screening among Iranian women attending outpatient clinics in Tehran, Iran. Asian Pac J Cancer Prev. 2012;13:969–74. doi: 10.7314/apjcp.2012.13.3.969. [DOI] [PubMed] [Google Scholar]
- Al-Naggar RA, Bobryshev YV. Practice and barriers of mammography among Malaysian women in the general population. Asian Pac J Cancer Prev. 2012;13:3595–600. doi: 10.7314/apjcp.2012.13.8.3595. [DOI] [PubMed] [Google Scholar]
- Amoran OE, Toyobo OO. Predictors of breast self-examination as cancer prevention practice among women of reproductive age-group in a rural town in Nigeria. Niger Med J. 2015;56:185. doi: 10.4103/0300-1652.160362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BO. Understanding social obstacles to early breast cancer detection is critical to improving breast cancer outcome in low-and middle-resource countries. Cancer. 2010;116:4436–9. doi: 10.1002/cncr.25361. [DOI] [PubMed] [Google Scholar]
- Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries. Cancer. 2008;113:2221–43. doi: 10.1002/cncr.23844. [DOI] [PubMed] [Google Scholar]
- Ansink A, Tolhurst R, Haque R, et al. Cervical cancer in Bangladesh: community perceptions of cervical cancer and cervical cancer screening. Trans R Soc Trop Med Hyg. 2008;102:499–505. doi: 10.1016/j.trstmh.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Audet CM, Matos CS, Blevins M, et al. Acceptability of cervical cancer screening in rural Mozambique. Health Edu Res. 2012;27:544–51. doi: 10.1093/her/cys008. [DOI] [PubMed] [Google Scholar]
- Augusto EF, Rosa ML, Cavalcanti SM, et al. Barriers to cervical cancer screening in women attending the Family Medical Program in Niteroi, Rio de Janeiro. Arch Gynecol Obstet. 2013;287:53–8. doi: 10.1007/s00404-012-2511-3. [DOI] [PubMed] [Google Scholar]
- Avci IA, Kurt H. Health beliefs and mammography rates of Turkish women living in rural areas. J Nurs Scholarsh. 2008;40:170–5. doi: 10.1111/j.1547-5069.2008.00222.x. [DOI] [PubMed] [Google Scholar]
- Azaiza F, Cohen M, Awad M, et al. Factors associated with low screening for breast cancer in the Palestinian authority. Cancer. 2010;116:4646–55. doi: 10.1002/cncr.25378. [DOI] [PubMed] [Google Scholar]
- Basu P, Sarkar S, Mukherjee S, et al. Women's perceptions and social barriers determine compliance to cervical screening: results from a population based study in India. Cancer Detect Prev. 2006;30:369–74. doi: 10.1016/j.cdp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Budkaew J, Chumworathayi B. Factors associated with decisions to attend cervical cancer screening among women aged 30-60 years in chatapadung contracting medical unit, Thailand. Asian Pac J Cancer Prev. 2013;15:4903–7. doi: 10.7314/apjcp.2014.15.12.4903. [DOI] [PubMed] [Google Scholar]
- Çam O, Gümüs AB. Breast cancer screening behavior in Turkish women: Relationships with health beliefs and self-esteem, body perception and hopelessness. Asian Pac J Cancer Prev. 2009;10:49–56. [PubMed] [Google Scholar]
- Critical appraisal skill program. CASP qualitative checklist. 2015. [Accessed on 19 October, 2015 [Online]]. Available from: http://www.casp-uk.net/#!casp-tools-checklists/c18f8 .
- Critical appraisal skill program. Health evidence bulletins - Wales: Questions to assist with the critical appraisal of an observational study eg cohort, cross-sectional, longitudinal, case-control. (Type IV evidence) 2015. [Accessed on 19 October, 2015]. Avaialable from: http://hebw.cf.ac.uk/methodology/appendix8.htm. [Online]
- Cunningham MS, Skrastins E, Fitzpatrick R, et al. Cervical cancer screening and HPV vaccine acceptability among rural and urban women in Kilimanjaro Region, Tanzania. BMJ Open. 2015;5:e005828. doi: 10.1136/bmjopen-2014-005828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Division SDP. World urbanization prospects: the 2001 revision. United nations publications; 2002. [Google Scholar]
- Dundar PE, Ozyurt BC, Erdurak K. Sociodemographic determinants of nonattendance in a population-based mammography screening program in the city of Manisa, Turkey. Scientific World J. 2012;2012 doi: 10.1100/2012/816903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran ET. Examination with the health belief model of women's attitudes to cervical cancer and early diagnosis in Turkey: a qualitative study. Asian Pac J Cancer Prev. 2011;12:1179–84. [PubMed] [Google Scholar]
- Ersin F, Bahar Z. Barriers and facilitating factors perceived in Turkish women's behaviors towards early cervical cancer detection: A qualitative approach. Asian Pac J Cancer Prev. 2013;14:4977–82. doi: 10.7314/apjcp.2013.14.9.4977. [DOI] [PubMed] [Google Scholar]
- Fang DM, Baker DL. Barriers and facilitators of cervical cancer screening among women of Hmong origin. J Health Care Poor Underserved. 2013;24:540–55. doi: 10.1353/hpu.2013.0067. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Fernández J, Rodrigues S, Silva da Costa Y, et al. Knowledge, attitudes, and practices related to Pap test in Northeastern Brazil. Rev Saúde Pública. 2009;43:851–8. doi: 10.1590/s0034-89102009005000055. [DOI] [PubMed] [Google Scholar]
- Fort VK, Makin MS, Siegler AJ, et al. Barriers to cervical cancer screening in Mulanje, Malawi: a qualitative study. Patient Prefer Adherence. 2011;5:125. doi: 10.2147/PPA.S17317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frie KG, Ramadas K, Anju G, et al. Determinants of participation in a breast cancer screening trial in Trivandrum district, India. Asian Pac J Cancer Prev. 2013;14:7301–7. doi: 10.7314/apjcp.2013.14.12.7301. [DOI] [PubMed] [Google Scholar]
- Gan DEH, Dahlui M. Cervical screening uptake and its predictors among rural women in Malaysia. Singapore Med J. 2013;54:163–8. doi: 10.11622/smedj.2013047. [DOI] [PubMed] [Google Scholar]
- Gang M, Kim JI, Oh KO, et al. Factors associated with mammography adherence among married Chinese women in Yanbian, China. Asian Pac J Cancer Prev. 2013;14:7207–13. doi: 10.7314/apjcp.2013.14.12.7207. [DOI] [PubMed] [Google Scholar]
- Garrett JJ, Barrington C. 'We do the impossible': women overcoming barriers to cervical cancer screening in rural Honduras–a positive deviance analysis. Cult Health Sex. 2013;15:637–51. doi: 10.1080/13691058.2012.760206. [DOI] [PubMed] [Google Scholar]
- Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Guimond ME, Salman K. Modesty matters: cultural sensitivity and cervical cancer prevention in Muslim women in the United States. Nurs Womens Health. 2013;17:210–7. doi: 10.1111/1751-486X.12034. [DOI] [PubMed] [Google Scholar]
- Gürsoy AA, Mumcu HK, Çalık KY, et al. Attitudes and health beliefs associated with breast cancer screening behaviors among Turkish women. J Transcult Nurs. 2011;22:368–75. doi: 10.1177/1043659611414137. [DOI] [PubMed] [Google Scholar]
- Harford JB. Breast-cancer early detection in low-income and middle-income countries: do what you can versus one size fits all. Lancet Oncol. 2011;12:306–12. doi: 10.1016/S1470-2045(10)70273-4. [DOI] [PubMed] [Google Scholar]
- Hassan N, Ho WK, Mariapun S, et al. A cross sectional study on the motivators for Asian women to attend opportunistic mammography screening in a private hospital in Malaysia: the MyMammo study. BMC Public Health. 2015;15:1. doi: 10.1186/s12889-015-1892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam RM, Bell RJ, Billah B, et al. Lack of understanding of cervical cancer and screening is the leading barrier to screening uptake in women at midlife in Bangladesh: Population-based cross-sectional survey. Oncologist. 2015;20:1386–92. doi: 10.1634/theoncologist.2015-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam RM, Bell RJ, Billah B, et al. Awareness of breast cancer and barriers to breast screening uptake in Bangladesh: A population based survey. Maturitas. 2016;84:68–74. doi: 10.1016/j.maturitas.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Jia Y, Li S, Yang R, et al. Knowledge about cervical cancer and barriers of screening program among women in Wufeng County, a high-incidence region of cervical cancer in China. PLoS One. 2013;8:e67005. doi: 10.1371/journal.pone.0067005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangmennaang J, Thogarapalli N, Mkandawire P, et al. Investigating the disparities in cervical cancer screening among Namibian women. Gynecol Oncol. 2015;138:411–6. doi: 10.1016/j.ygyno.2015.05.036. [DOI] [PubMed] [Google Scholar]
- Khazaee-Pool M, Montazeri A, Majlessi F, et al. Breast cancer-preventive behaviors: exploring Iranian women's experiences. BMC Womens Health. 2014;14:41. doi: 10.1186/1472-6874-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-M, Ati A, Kols A, et al. Influencing women's actions on cervical cancer screening and treatment in Karawang District, Indonesia. Asian Pac J Cancer Prev. 2012;13:2913–21. doi: 10.7314/apjcp.2012.13.6.2913. [DOI] [PubMed] [Google Scholar]
- Kissal A, Beser A. Knowledge, facilitators and perceived barriers for early detection of breast cancer among elderly Turkish women. Asian Pac J Cancer Prev. 2011;12:975–84. [PubMed] [Google Scholar]
- Lamyian M, Hydarnia A, Ahmadi F, et al. Barriers to and factors facilitating breast cancer screening among Iranian women: a qualitative study. East Mediterr Health J. 2007;13:1160–9. doi: 10.26719/2007.13.5.1160. [DOI] [PubMed] [Google Scholar]
- Lazcano-Ponce EC, Castro R, Allen B, et al. Barriers to early detection of cervical-uterine cancer in Mexico. J Womens Health. 1999;8:399–408. doi: 10.1089/jwh.1999.8.399. [DOI] [PubMed] [Google Scholar]
- Lu M, Moritz S, Lorenzetti D, et al. A systematic review of interventions to increase breast and cervical cancer screening uptake among Asian women. BMC Public Health. 2012;12:413. doi: 10.1186/1471-2458-12-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic M, Kesic V, Topic L, et al. Barriers to cervical cancer screening: a qualitative study with women in Serbia. Soc Sci Med. 2005;61:2528–35. doi: 10.1016/j.socscimed.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Marván ML, Ehrenzweig Y, Catillo-López RL. Knowledge about cervical cancer prevention and psychosocial barriers to screening among Mexican women. J Psychosom Obstet Gynaecol. 2013;34:163–9. doi: 10.3109/0167482X.2013.846904. [DOI] [PubMed] [Google Scholar]
- McFarland DM. Cervical cancer and Pap smear screening in Botswana: knowledge and perceptions. Int Nurs Rev. 2003;50:167–75. doi: 10.1046/j.1466-7657.2003.00195.x. [DOI] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Database Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri A, Haji-Mahmoodi M, Jarvandi S. Breast self-examination: do religious beliefs matter?A descriptive study. J Public Health Med. 2003;25:154–5. doi: 10.1093/pubmed/fdg031. [DOI] [PubMed] [Google Scholar]
- Montgomery MP, Dune T, Shetty PK, et al. Knowledge and acceptability of human papillomavirus vaccination and cervical cancer screening among women in Karnataka, India. J Cancer Educ. 2015;30:130–7. doi: 10.1007/s13187-014-0745-4. [DOI] [PubMed] [Google Scholar]
- Mupepi SC, Sampselle CM, Johnson TR. Knowledge, attitudes, and demographic factors influencing cervical cancer screening behavior of Zimbabwean women. J Womens Health. 2011;20:943–52. doi: 10.1089/jwh.2010.2062. [DOI] [PubMed] [Google Scholar]
- Ngugi CW, Boga H, Muigai AW, et al. Factors affecting uptake of cervical cancer early detection measures among women in Thika, Kenya. Health Care Women Int. 2012;33:595–613. doi: 10.1080/07399332.2011.646367. [DOI] [PubMed] [Google Scholar]
- Nwankwo K, Aniebue U, Aguwa E, et al. Knowledge attitudes and practices of cervical cancer screening among urban and rural Nigerian women: a call for education and mass screening. Eur J Cancer Care. 2011;20:362–7. doi: 10.1111/j.1365-2354.2009.01175.x. [DOI] [PubMed] [Google Scholar]
- Padela AI, Peek M, Johnson-Agbakwu CE, et al. Associations Between Religion-Related Factors and Cervical Cancer Screening Among Muslims in Greater Chicago. J Low Genit Tract Dis. 2014;18:326–32. doi: 10.1097/LGT.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa P, Kandiah M, Abdul Rahman H, et al. Barriers for breast cancer screening among Asian women: a mini literature review. Asian Pac J Cancer Prev. 2006;7:509. [PubMed] [Google Scholar]
- Patel S. Muslim Americans reaching for health and building alliances (MARHABA): A study of breast and cervical cancer screening barriers and facilitators among Muslim women in New York City. 142nd APHA annual meeting and exposition (November 15-November 19, 2014), 2014. APHA. 2014 [Google Scholar]
- Paz-Soldán VA, Nussbaum L, Bayer AM, et al. Low knowledge of cervical cancer and cervical Pap smears among women in Peru, and their ideas of how this could be improved. Int Q Community Health Edu. 2011;31:245–63. doi: 10.2190/IQ.31.3.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng P, Perng W, Ngoma T, et al. Promoters of and barriers to cervical cancer screening in a rural setting in Tanzania. Int J Gynaecol Obstet. 2013;123:221–5. doi: 10.1016/j.ijgo.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ports KA, Reddy DM, Rameshbabu A. Cervical cancer prevention in Malawi: A qualitative study of women's perspectives. J Health Commun. 2015;20:97–104. doi: 10.1080/10810730.2014.908986. [DOI] [PubMed] [Google Scholar]
- Rajaram SS, Rashidi A. Asian-Islamic women and breast cancer screening: a socio-cultural analysis. Women Health. 1999;28:45–58. doi: 10.1300/J013v28n03_04. [DOI] [PubMed] [Google Scholar]
- Rasu RS, Rianon NJ, Shahidullah SM, et al. Effect of educational level on knowledge and use of breast cancer screening practices in Bangladeshi women. Health Care Women Int. 2011;32:177–89. doi: 10.1080/07399332.2010.529213. [DOI] [PubMed] [Google Scholar]
- Reis N, Bebis H, Kose S, et al. Knowledge, behavior and beliefs related to cervical cancer and screening among Turkish women. Asian Pac J Cancer Prev. 2012;13:1463–70. doi: 10.7314/apjcp.2012.13.4.1463. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Gaffikin L, Jacob M, et al. A critical assessment of screening methods for cervical neoplasia. Int J Gynaecol Obstet. 2005;89:4–12. doi: 10.1016/j.ijgo.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Secginli S, Nahcivan NO. Factors associated with breast cancer screening behaviours in a sample of Turkish women: a questionnaire survey. Int J Nurs Stud. 2006;43:161–71. doi: 10.1016/j.ijnurstu.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Shaheen R, Slanetz P, Raza S, et al. Barriers and opportunities for early detection of breast cancer in Gaza women. Breast. 2011;20:30–4. doi: 10.1016/j.breast.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Shankar A, Rath G, Roy S, et al. Level of awareness of cervical and breast cancer risk factors and safe practices among college teachers of different states in India: do awareness programmes have an impact on adoption of safe practices? Asian Pac J Cancer Prev. 2015;16:927–32. doi: 10.7314/apjcp.2015.16.3.927. [DOI] [PubMed] [Google Scholar]
- Sreedevi A, Quereshi MA, Kurian B, et al. Screening for breast cancer in a low middle income country: predictors in a rural area of Kerala, India. Asian Pac J Cancer Prev. 2013;15:1919–24. doi: 10.7314/apjcp.2014.15.5.1919. [DOI] [PubMed] [Google Scholar]
- Story HL, Love RR, Salim R, et al. Improving outcomes from breast cancer in a low-income country: lessons from Bangladesh. Int J Breast Cancer. 2012;2012:423562. doi: 10.1155/2012/423562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudenga SL, Rositch AF, Otieno WA, et al. Brief Report: Knowledge, attitudes, practices and perceived risk of cervical cancer among Kenyan women. Int J Gynecol Cancer. 2013;23:895. doi: 10.1097/IGC.0b013e31828e425c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzcu A, Bahar Z. Barriers and facilitators to breast cancer screening among migrant women within Turkey. J Transcult Nurs. 2015;26:47–56. doi: 10.1177/1043659614526245. [DOI] [PubMed] [Google Scholar]
- Watkins M, Gabali C, Winkleby M, et al. Barriers to cervical cancer screening in rural Mexico. Int J Gynecol Cancer. 2002;12:475–9. doi: 10.1046/j.1525-1438.2002.01170.x. [DOI] [PubMed] [Google Scholar]
- World Bank. World bank country classification. 2015. [Accessed on 29 June 2015]. http://data.worldbank.org/about/country-and-lending-groups . [Online]


