Abstract
The aim of the study was to isolate and identify the major cytotoxic principle from plant leaves of Pogostemon quadrifolius (Benth.) and evaluate its antiproliferative potential against human cancer cells. Plant leaves were extracted sequentially with a soxhlet apparatus, using petroleum ether, chloroform and methanol solvents. Petroleum ether and chloroform extracts exhibited antiproliferative properties against Caco-2, HeLa, THP-1, MCF-7 and Jurkat E6-1cancer cell lines tested, but methanol extracts failed to exhibit such activity. The major antiproliferative principle from petroleum ether and chloroform extracts was isolated with the help of bioassay guided column chromatography. This cytotoxic compound was further analysed by UV, TLC, HPLC, LC-MS, GC-MS and NMR analyses and was identified to be novel: (Z)-ethylidene-4,6-dimethoxycoumaran-3-one (Compound 1). The half-maximal inhibitory concentrations for proliferation (IC50) exhibited by compound 1 were 19.4, 23.1, 22.1, 35.9 and 8.32 µM against Caco-2, HeLa, THP-1, MCF-7 and Jurkat E6-1 cancer cell lines, respectively. Further experiments revealed that compound 1 triggered the apoptosis mode of cell death in cancer cell lines. Thus, the present study allowed isolation and identification of a novel cytotoxic natural compound, (Z)-ethylidene-4,6-dimethoxycoumaran-3-one, from plant leaves of P. quadrifolius (Benth.). Our pre-clinical study also indicated that compound 1 is particularly active in the acute T cell leukemia cell line (Jurkat E6-1) with potential for application as a chemotherapeutic agent in the future.
Keywords: Apoptosis; cancer; Pogostemon quadrifolius; (Z)-ethylidene-4,6-dimethoxycoumaran-3-one
Introduction
According to World Health Organization (WHO), cancer is a leading cause of death worldwide, accounting for 8.2 million deaths (Stewart and Wild, 2014). Worryingly, the number of deaths globally due to cancer is projected to rise to 11.5 million in the year 2030 (Strong et al., 2008). Plants are rich source for the bioactive compounds. New natural products have been considered to be the prototype, leads or heads of the series, for the production of new anticancer drugs with high therapeutic potential (Gordaliza, 2007). Most of the chemotherapeutic drugs available today are natural products or their different derivatives or analogues. Taxanes, vinca alkaloids and their analogues are the best examples to prove the importance of plant derived natural products in cancer therapy (Basmadjian et al., 2014)
Pogostemon quadrifolius (Benth.) is a plant mainly distributed in India, Bangladesh and Myanmar. This plant possesses antioxidant, antimicrobial and mosquito larvicidal properties (Cheriyamundath et al., 2015; Thoppil et al., 2003; Trivedi, 2006). Reports show that in India and Bangladesh, the plant is being used as a traditional herbal remedy against chickenpox and worms also (Biswas et al., 2010; Padal and Chandrasekhar, 2013; Padal et al., 2013; Padal and Raju, 2013; Raju et al., 2014). But there were no reports available regarding the antiproliferative or cytotoxic property of the P. quadrifolius (Benth.) extract or the active bioactive compound from this plant against cancer. In this study, we investigated the antiproliferative properties of organic solvent extracts of P. quadrifolius (Benth.) leaves and isolated active principle against cancer cells. The study report the bioactive guided isolation of the active principle (Z)-ethylidene-4,6-dimethoxycoumaran-3-one from the P. quadrifolius (Benth.) leaf, and identification this compound by using Thin layer chromatography (TLC), Ultraviolet-visible (UV-vis) analysis, High Performance Liquid Chromatography electrospray ionization mass spectrometry (HPLC-ESI-MS) and Gas chromatography mass spectrometry (GC-MS) analysis techniques along with its antiproliferative effects on various cancer cell lines.
Materials and Methods
Source of plant material
Fresh leaves of the plant P. quadrifolius (Benth.) were collected from the campus of the University of Calicut, Kerala, India. The plant was identified and authenticated by Dr. A.K. Pradeep, Assistant Professor, Department of Botany, University of Calicut. A voucher specimen (Accession No. 6597, 6598) has been deposited in the herbarium of the Department of Botany, University of Calicut, India.
Preparation of solvent extract
Collected plant leaves were first washed with distilled water, dried in the shade, and crushed into powder prior to extraction. 100g of leaf powder was extracted sequentially using a Soxhlet apparatus with the solvent petroleum ether, chloroform and methanol in increasing order of polarity. Extraction in each solvent was carried out for 24 hours to completely extract all the phytocompound from leaves. After each solvent extraction, the leaf residue was dried and then the next solvent was added. Solvent extracts were further filtered by whatman filter paper and concentrated to dryness by rotary evaporator. Aliquots of the solvent extracts were subsequently dissolved in dimethyl sulfoxide (DMSO) for use in the various assays.
Chromatographic isolation of the cytotoxic constituent
To isolate the active cytotoxic constituents from the solvent extracts, column chromatographic technique was performed over activated aluminum oxide (neutral). An increasing amount of chloroform in petroleum ether solvent mixture was used as mobile phase. Fractions were eluted in the following proportions of petroleum ether and chloroform, respectively (v/v): 100:0, 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90 and 0:100. Cytotoxic fraction was identified by the cell proliferation assay, (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide assay (MTT assay) using various cancer cell lines. The fraction with high cytotoxic effect against cancer cell line was selected.
Identification, characterization and purity determination of compound 1
Preliminary phytochemical analysis of the cytotoxic compound was evaluated using thin layer chromatography (TLC). TLC plates (silica gel G) were made at 1mm thickness and activated in an oven for 1 h at 110 °C prior to use. Equal amount of samples were loaded and plates were developed with two eluents, petroleum ether–ethyl acetate at the ratio of 3:2 (v/v) and ethyl acetate–methanol–water at the ratio of 30:5:4 (v/v). For the detection of alkaloids, TLC plates were sprayed with Dragendorff reagent followed by 10% aqueous sodium nitrite. The presence of phenolic groups was determined using fast blue-B reagent. Amino acids and biogenic amines were detected using ninhydrin. Vanillin–phosphoric acid was used for the detection of terpenoids, lignans and cucurbitacins (Wagner, 1996); while the presence of flavonoids was determined using Shinoda test (Krishnaswamy, 2003).
For Ultraviolet-visible (UV-vis) spectroscopic and High Performance Liquid Chromatography (HPLC) analysis, compound 1 (1 mg) was dissolved in 1 mL of HPLC grade methanol and filtered through a 0.2 µm sterile filter. The UV-vis spectrum was measured in the range 190–900 nm. Analytical HPLC was performed using a Shimadzu HPLC system (system controller, CBM-20Alite; solvent delivery unit, LC-20AD; UV-vis detector, SPD-20A) equipped with a reverse-phase Shimadzu Shim-pack CLC-ODS (M) column (4.6mm i.d.×15cm). The column was loaded with 20 µl of the sample and the compound was detected at 272 nm by eluting isocratically with a flow rate of 1 mL/min using ultrapure water–methanol at the ratio of 1:9 (v/v).
High Performance Liquid Chromatography electrospray ionization mass spectrometry (HPLC-ESI-MS) was conducted on an Agilent 1100 HPLC coupled to an Agilent single-quadrupole mass-selective detector (HP 1101; Agilent Technologies, Waldbronn, Germany) fitted with a reverse-phase C18 Gemini column (250 mm, 4 mm i.d., 5 µm; Phenonenex, Aschaffenberg, Germany). The mobile phase consisted of 2% acetic acid in doubly distilled water (solvent A) and methanol (solvent B) with the following gradient profile: 50% to 100% B over 20 min; and continuing at 100% B until completion of the run (30 min.). Phenolic compounds were detected by their UV absorbance (A) at 272 nm and 340 nm at room temperature. Positive-ion mass spectra were generated under the following conditions: fragmentor voltage, 100 V; capillary voltage, 1500 V; nebulizer pressure, 30 psi; drying gas temperature, 350° C; m/z scan range, 100–1500 D.
Gas chromatography mass spectrometry (GC-MS) analyses were performed using a HP 5973 mass spectrometer coupled to a HP 6890 gas chromatograph. Prior to GC-MS, Compound 1 was derivatized by addition of 100 µl of N, O-Bis(trimethylsilyl) trifluoroacetamide (BSTFA) at room temperature for 30 minutes. Sample volumes of 1 µL were injected into the gas chromatograph. Separation was achieved using a HP 5MS capillary column, (30 m × 0.25 mm I.D., 0.25 µm film thickness). Helium was used as carrier gas with a linear velocity of 0.9 mL/s. The oven temperature program was: initial temperature 100 °C, 100 to 270 °C at 4 °C/min, 270 °C for 57.5 minutes. The GC injector temperature was 250 °C; the transfer line temperature was held at 280 °C. The mass spectrometer parameters for EI mode were: ion source temperature, 230 °C; electron energy, 70 eV; filament current, 34.6 µA; electron multiplier voltage, 1200 V. The isolated compound 1 was further analysed by NMR to confirm the identity of the compound.
Cell culture
Human colorectal (Caco-2), cervix (HeLa), monocytic leukemia (THP-1), breast (MCF-7) and leukemic T cell lymphoblast (Jurkat E6-1) cancer cell lines were obtained from National Centre for Cell Science (NCCS), Pune, India and maintained in recommended medium with fetal bovine serum (FBS), streptomycin (100 µg/mL) and penicillin (50 IU/mL). Caco-2 and HeLa cells were cultured in Minimum Essential Medium Eagle (MEM) with 20% and 10% FBS respectively. THP-1 and Jurkat E6-1 cells were maintained in RPMI-1640 medium with 10% FBS. MCF-7 cell line was maintained in MEM with 10% FBS and 0.01 mg/ml human recombinant insulin. Cells were cultured in a humidified atmosphere containing 5% CO2 at 37 °C.
Cell proliferation assay (MTT assay)
Effect of leaf extracts and isolated compound on cell proliferation was identified using MTT assay. The assay was performed as reported earlier (Raghavan et al., 2014). Briefly, 15,000 cells/well were seeded into a 96 well plate and incubated overnight in CO2 incubator at 37 °C in 5% CO2 atmosphere under humidified condition. After incubation, fresh medium containing varying concentrations of compound 1 was added to each well and incubated for 48 h. After incubation, the media was aspirated and replaced with fresh medium. MTT (0.5 mg/mL) dissolved in phosphate-buffered saline (PBS) was added to each well, and the plates were incubated for further 4 hours at 37 °C in dark. After removing the medium, the formazan crystals formed were dissolved in DMSO and the absorbance was measured at 570 nm. The half maximal inhibitory concentration of proliferation (IC50) values for compound 1 for each cancer cell line were calculated using the ED50 plus v1.0 software program (Walsh et al., 2013) and the results were expressed as mean ± standard deviation of three independant experiments.
DNA fragmentation assay
The assay was performed as described in literature (Brooks and Harris, 2006). Since Jurkat E6-1 has shown more sensitivity to compound 1, this cell line was used for deoxyribonucleic acid (DNA) fragmentation assay. Jurkat E6-1 cells were treated with 10 and 20 µM concentrations of compound 1 for 24 h. DNA was extracted from the cells using 10 mM Tris (pH 8.0), 1 mM ethylenediaminetetraacetic acid (EDTA) and 0.2% Triton X-100. Lysate was centrifuged for 15 min at 13,800 g to separate the fragment DNA (soluble) from intact chromatin (nuclear pellet) and the supernatant was treated with ribonuclease A (RNAse A) for 1 h at 37 °C. Supernatant was then removed for sodium dodecyl sulfate (SDS)-Proteinase K digestion together with sodium chloride (NaCl) solution for 30 min at 37 °C. Supernatant was mixed with an equal amount of saturated phenol solution and centrifuged for 10 min at 13,000 g. It was then mixed with an equal amount of phenol–chloroform–isoamyl alcohol (50:49:1 v/v/v) and centrifuged for 10 min at 13,000 g. The DNA was isolated by overnight precipitation of the supernatant with an equal volume of isopropanol at −20 °C. The isolated DNA was dissolved in Tris-EDTA (TE) buffer. Electrophoresis of the isolated DNA was carried out in 2% agarose gel and visualized under UV light using ethidium bromide dye.
Results
Petroleum ether and Chloroform solvent leaf extracts inhibited the cancer cell proliferation
Yield of extract after sequential extraction with petroleum ether, chloroform and methanol of P. quadrifolius (Benth.) leaves, were 2.1%, 4.4% and 9.8%, respectively. MTT assay was performed to evaluate the antiproliferative properties of the solvent extracts on the Caco-2, HeLa, THP-1, MCF-7 and Jurkat E6-1 cancer cell lines. Methanol extract failed to exhibit cytotoxicity effect against above cancer cells; therefore, no further study was conducted with methanol extract. Petroleum ether and chloroform extracts, on the other hand, significantly inhibited the proliferation of all cell lines in a concentration dependent manner. IC50 values for the petroleum ether, chloroform extract against cancer cell lines are presented in Table 1. Jurkat E6-1 cells were found to be most sensitive to these extracts.
Table 1.
In Vitro Antiproliferative Activity of Petroleum Ether, Chloroform Leaf Extracts of P. Quadrifolius (Benth.) and Isolated Cytotoxic Compound 1 on Cancer Cell Lines
| IC50(µg/ml) | |||
|---|---|---|---|
| Cell line | Petroleum ether | Chloroform | Compound 1 |
| Caco-2 | 18.37 (2.49) | 23.31 (1.75) | 4.27 (0.31) |
| HeLa | 17.75 (0.93) | 21.02 (0.33) | 5.09 (0.07) |
| THP-1 | 13.26 (1.63) | 23.46 (1.01) | 4.86 (0.14) |
| MCF-7 | 24.27 (4.39) | 28.00 (4.49) | 7.91 (0.61) |
| Jurkat E6.1 | 5.96 (0.21) | 7.20 (0.65) | 1.83 (0.11) |
Each value represents the mean (SD) of three independent replicates.
Isolation and identification of (Z)-Ethylidene-4,6-dimethoxycoumaran-3-one
Since petroleum ether and chloroform solvent extracts showed significant antiproliferative effects, attempts were made to isolate the active principle from these extracts. The solvent extracts were fractionated by column chromatography over aluminum oxide, and bioassays of the isolated fractions were conducted using the MTT assay on cancer cell lines. Of the eleven fractions obtained from each of the petroleum ether, and chloroform extracts, the fraction eluted with petroleum ether–chloroform (60:40 v/v) exhibited the maximum antiproliferative activity. Isolated major active principles were further purified for structure determination by precipitating them in diethyl ether and separating by preparative TLC.
High purity of the isolated active principles was initially confirmed by TLC as it developed as a single band on TLC with two very different solvent mixtures. The purified samples showed predominantly single band under fluorescence at 365 nm (Figure 1) with retention factors of 0.27 and 0.90 when petroleum ether–ethyl acetate (3:2 v/v) and ethyl acetate- methanol- water (35:5:4 v/v) were used as eluents, respectively. TLC spraying reaction results were surprising that the compound 1 has exhibited positive result for alkaloids, phenol and terpenoids (Figure 1 and Table 2) detecting spraying reagents. Since retention factors of the isolated compounds from petroleum ether and chloroform leaf extracts were similar in TLC, the major antiproliferative principles in both the extracts were identified to be same (Compound 1). Yield of major active principle (compound1) was 0.73% from the dry leaf.
Figure 1.

Characteristics Features of Compound 1 on TLC, Compound 1 observed under UV 365 nm on TLC plate. Compound 1 displayed the retention factors (Rf) of 0.27 and 0.90 on TLC plate, when petroleum ether- ethyl acetate (3:2 v/v, solvent 2) and ethyl acetate- methanol- water (35:5:4 v/v, solvent 1) were used as eluents, respectively. Compound 1 showed positive results to alkaloids (spray reagent 1), terpenoids (spray reagent 2) and phenol (spray reagent 3) detecting spray reagents on TLC plate.
Table 2.
Preliminary Qualitative Phytochemical Analysis of Compound 1 Isolated from Leaf Extracts of P. Quadrifolius (Benth.). Compound 1 showed positive result to alkaloids (Dragendorff’s), phenol (Fast blue B), and terpenoids (Vanillin-phosphoric acid) detecting TLC spraying reagents.
| Spraying reagents/Test | Compound 1 |
|---|---|
| Dragendorff’s | + |
| Fast blue B | + |
| Ninhydrine | - |
| Vanillin-Phosphoric acid | + |
| Shinoda’s test | - |
“+”, Positive result; “-”, Negetive result
The analytical HPLC profile of compound 1 (Figure 2a) measured at 272 nm indicated high purity as shown by a prominent peak at a retention time of 3.89 min. The main peak area constituted 98.8% and peak height by 99.2%. UV-vis spectrum of compound 1, measured at the range 190–900 nm, revealed absorption maxima at 270 and 316 nm (Figure 2b). Analysis by HPLC-ESI-MS (Figure 2c) in positive-ion mode, using a different column and mobile system from analytical HPLC provided better resolution and separation of isomers of compound 1, so that the Z and E isomers were separated, with retention times of 12.18 and 12.96 min respectively. Both isomers yielded a quasi-molecular ion at m/z [M + H]+ 221.3 (75 and 90%), as well as a mono- [M + Na]+ sodium adduct at m/z 243.3 (25 and 30%) and a double [2M+Na]+ sodium adduct at m/z 463.5 (100 and 100%) respectively. The GC-MS data were in full agreement with the analytical HPLC and HPLC-ESI-MS data. In this case, the major Z isomer had a retention time of 25.40 min, whereas the minor E isomer was detected at 24.58 min (Figure 2d). The molecular ion of both isomers was observed at m/z 220 with very similar fragmentation patterns. The data were entirely consistent with a structural formula of C12H12O4. The 13C and 1H NMR spectrum obtained confirmed that the isolated compound 1 is (Z)-Ethylidene-4,6-dimethoxycoumaran-3-one (Figure 3). These results were fully consistent with our reported structure (Klika et al., 2014).
Figure 2.
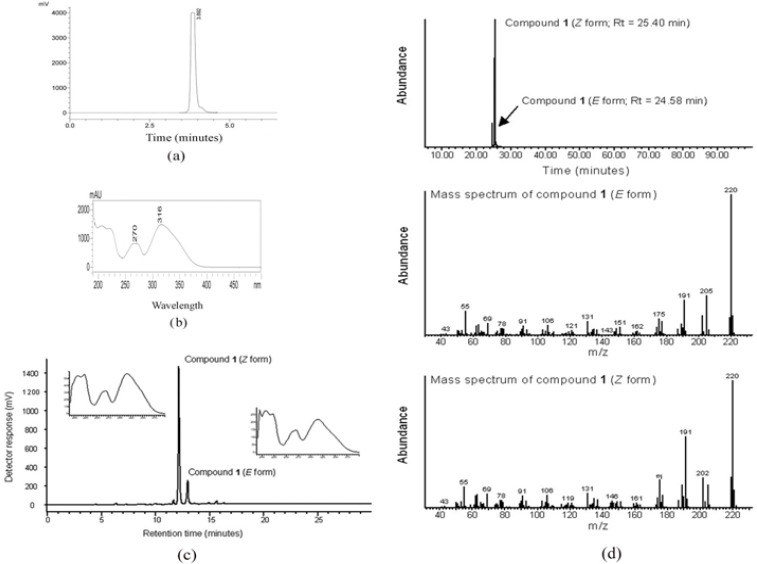
Analytical Properties of Compound 1 or (Z)-Ethylidene-4, 6-Dimethoxycoumaran -3-One. a) HPLC: The analytical HPLC profile of compound 1 measured at 272 nm indicate the high purity of the compound by a prominent peak (peak area of 98.8%) at a retention time of 3.89 min b) UV absorption spectrum: compound 1 revealed the specific UV- absorption maxima of 270 and 316 nm; c) Analytical HPLC-ESI-MS chromatogram indicate the better resolution and separation of isomers (E and Z) of compound 1, Z and E isomers were separated with retention times of 12.18 and 12.96 min respectively. The inlet image indicates the UV absorption spectrums of Z and E isomers of compound 1; d) GC-MS chromatograms: Chromatogram shows the abundance of the isomers of compound 1 and their separation, the major Z isomer showed a retention time of 25.40 min, whereas the minor E isomer showed a retention time of 24.58 min. The molecular ion of E and Z isomers was observed at m/z 220 with very similar fragmentation pattern as shown.
Figure 3.
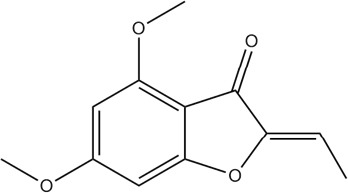
Structure of (Z)-Ethylidene-4, 6-Dimethoxycoumaran-3-One (Compound 1).
Antiproliferative effect of (Z)-Ethylidene-4,6-dimethoxycoumaran-3-one on various cancer cell lines
MTT assay was performed to identify the antiproliferative effect of compound 1 on cancer cell lines. The cell lines were treated for increasing concentration of compound 1 for 48 h. Compound 1 was found to markedly inhibit the proliferation of all cancer cell lines in a concentration dependent manner. Figure 4 shows the percentage of viability of different cancer cell line at various concentrations of compound 1 in comparison with DMSO control. The IC50 values exhibited by compound 1 on various cell lines were of 19.41, 23.14, 22.07, 35.92 and 8.32 µM against Caco-2, HeLa, THP-1, MCF-7 and Jurkat E6-1 cancer cell lines, respectively (Table 1). Results showed that the compound 1 is more sensitive to acute T cell leukemia cell line (Jurkat E6-1). The enlarged microscopic images of cancer cell lines after treatment with compound 1 at IC50 for 48 h are shown in the Figure 5. The images clearly showed the cell shrinkage in all cancer cell lines after treatment with compound 1. Moreover, the adherent cells Caco-2, HeLa and MCF-7 exhibited loss of anchorage after treatment with the compound 1. Most of the cells became detached from the cell culture plate substrate. The suspension cells also have shrunk from the normal structure.
Figure 4.
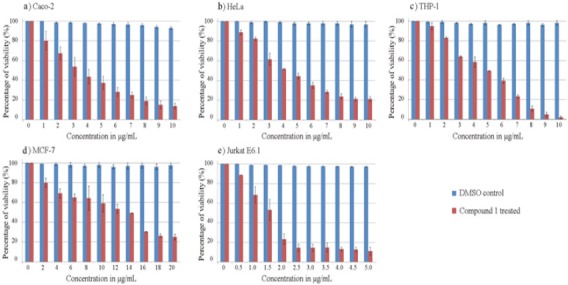
The Antiproliferative Effects of Compound 1 or (Z)-Ethylidene-4, 6-Dimethoxycoumaran-3-One On Human Cancer Cell Lines after 48 H Treatment: Graph Shows the Percentage of Viability of The Cells at Different Concentrations of Compound 1 in Comparison with DMSO Control. a) Caco-2 (colon) b) HeLa (cervix) c) THP-1 (acute monocytic leukemia) d) MCF-7 (breast) e) Jurkat E6-1 (acute T cell leukemia) cell line
Figure 5.
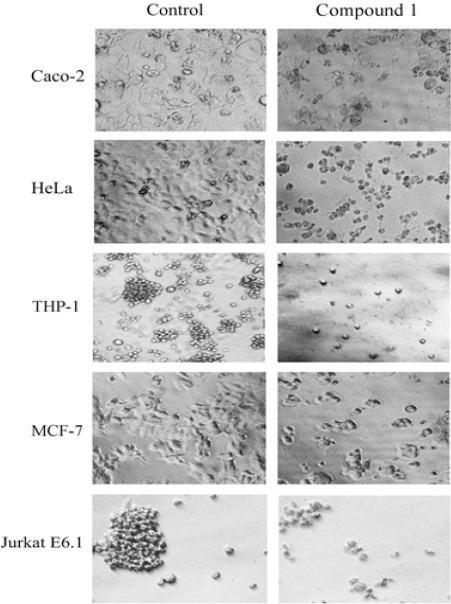
Effects of Compound 1 on Cancer Cell Morphology after 48 Hour Treatment with EDC at IC50. The normal morphology of the cells was lost after treatment with compound 1. The treated cells were shrunk in size compared to control. The attached cells such as Caco-2, HeLa, and MCF-7 have lost their capacity to attach to the cell culture plate and become suspended.
(Z)-Ethylidene-4,6-dimethoxycoumaran-3-one induces apoptosis in Jurkat E6-1cell line
Since the Jurkat E6-1 cells were seen to be more sensitive to the compound 1, this cell line was used for the DNA fragmentation assay. DNA fragmentation is a trademark apoptosis induction. Defects in the apoptosis mechanism are considered to be the major reason for cancer. Induction of apoptosis is the mode of action of most of the chemotherapeutic present in clinical use (Hassan et al., 2014). The results showed that compound 1 (10 and 20 µM) induced apoptosis in Jurkat E6-1 cells within 24 h treatment. The formation of the DNA ladder pattern seen after the compound 1 (Figure 6) treatment confirmed the induction of apoptosis in Jurkat E6-1 cells.
Figure 6.
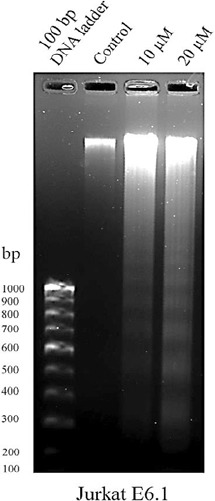
(Z)-Ethylidene-4, 6-Dimethoxycoumaran-3-One Trigger Apoptosis in Cancer Cells: Jurkat E6-1 cells were treated with compound 1 (10 and 20 µM) for 24 h. The isolated DNA from the compound 1 (10 and 20 µM) treated cells (lane 3 and 4) displayed the formation of fragmentation compared to the DMSO control cells (lane 2). Lane 1 indicates the 100 bp DNA ladder marker.
Discussion
Cancer became as the one of the key health problems for human and major threat to life. Cancer related mortality rate is increasing year by year. Reports indicate that the new techniques and chemotherapeutic medicines had helped to improve the health of cancer patients (Quinn et al., 2003; Siegel et al., 2015). Throughout the history, plants were considered to be the source of biologically active compounds. The cancer drug available in the market also confirms the importance of natural products in the cancer treatment. The search for anti-cancer agents from plant sources were started in the 1950s. The anticancer compounds such as vinca alkaloids, vinblastine and vincristine, and podophyllotoxins were isolated from plants and are playing a major role as lifesaving drugs (Newman and Cragg, 2012; Pezzuto, 1997).
P. quadrifolius (Benth.). is a shrub coming under the family Lamiaceae. Researchers have not yet completely elucidated the biological potentials of this plant. In this study, we analysed the antiproliferative potential of P. quadrifolius (Benth.) leaves against cancer cells. Further, major active principle responsible for this activity was isolated and identified. P. quadrifolius (Benth.) leaves were soxhlet extracted using petroleum ether, chloroform and methanol respectively as explained in the section 2.2. Petroleum ether solvent extract exhibited the IC50 between 5.96 and 24.27 µg/mL, whereas chloroform extracts showed the IC50 between 7.20 and 28.00 µg/mL (Table 1). But methanol extract failed to exhibit any antiproliferative property in this study. Our preliminary study reported that methanol leaf extract of P. quadrifolius (Benth.) has anti-oxidant potential and it was mainly due to the high phenol content in methanol extract (Cheriyamundath et al., 2015). Further, the major cytotoxic compounds were isolated from petroleum ether and chloroform leaf extracts as described in the section 2.3. The active principles from these extracts exhibited the same retention factor value under UV 365 on TLC plate. Surprisingly, the active principles showed positive results to three types of natural product category, i.e., terpenoid, alkaloid and phenolic detection spraying reagents. These results indicated that the active principles present in both extracts were same (compound 1). The phytochemical result can mislead the researchers who conclude the phytochemical analysis results just by the preliminary detection methods. Therefore, we further analysed the compound 1 to understand the UV absorption spectrum, and which revealed absorption maxima at 270 and 316 nm and absorption minima at 244 and 284 nm (Figure 2b). The analysis of compound 1 by HPLC-ESI-MS and GC-MS (Figure 2c and 2d) revealed that the active principle is with molecular weight 220. JE Thoppil et al, had conducted the analysis of chemical composition of the plant P.quadrifolius (Benth.), and identified, β-patchoulene, β -elemene, β- caryophyllene and α-patcholulene as major chemical constituents. The molecular weights of these constituents were completely different with the molecular weight of isolated compound 1 (Thoppil and Jose, 1995). Therefore, the isolated compound was considered as a novel compound from P.quadrifolius (Benth.). Pogostemon cablin is the other well-studied plant in Pogostemon family with several bioactive properties. Patchouli alcohol and sesquiterpenes were identified as the major constituents of patchouli oil isolated from P.cablin (Bunrathep et al., 2006). The molecular weights of these compounds were also different than our isolated compound. Since it was a completely new molecular weight compound, we have further analyzed the compound with detailed NMR analysis and identified the compound as (Z)-ethylidene-4,6-dimethoxycoumaran-3-one (Klika et al., 2014). Compound 1 was obtained in a very pure state as evinced by the TLC, HPLC and NMR data. Compound 1 is present in methanol solution as an equilibrating pair of isomers, namely the Z (major) and E (minor) forms (Klika et al., 2014). This inter conversion, readily accounts for the minor trailing peak seen in the analytical HPLC chromatogram under reverse-phase conditions (Figure 2a) which is confirmed by HPLC-ESI-MS, (Figure 2c) and GC-MS (Figure 2d). The trailing of E isomer is also visible in TLC plate as small spot near the band of Z isomer of compound 1 (Figure 1). These results revealed that the observed equilibrium by NMR occurs not only in methanol but also in other solutions (Klika et al., 2014). Yield of (Z)-ethylidene-4,6-dimethoxycoumaran-3-one (compound1) was 0.73% from the dry leaf. This shows the abundance of antiproliferative compound (Z)-ethylidene-4,6-dimethoxycoumaran-3-one in P.quadrifolius (Benth.) leaves.
In the present study, compound 1 exhibited an encouraging IC50 (8- 35 µM) against the cancer cell lines. Cancer cell lines lost their normal morphology. Cells shrunk from the normal morphology after the treatment with the compound 1. The attached cells have lost their anchorage capacity too. These morphological changes are considered to be the sign of apoptosis (Pidgeon et al., 2002). Apoptosis is one of the major modes of action of cytotoxic anticancer agents currently in use. The agents acts on different targets and ultimately lead to apoptosis (Hickman, 1992). Since the cells have showed the similar initial characteristic feature of apoptosis induction, we next tested the DNA fragmentation assay to identify the mechanism of action of compound 1 on Jurkat E6-1 cell line. During apoptosis, the endonucleases get activated and cleave the DNA between nucleosomal units, which leads to the formation of DNA ladder in the assay. DNA fragmentation is a symbol of apoptosis and most of the drugs available for cancer chemotherapy induce apoptosis in cancer cells. The major life saving drugs derived from natural products, such as, paclitaxel and docetaxel (taxane derivatives), vincristine and vinblastine (vinca alkaloids) are inducing apoptosis by targeting microtubule and blocking mitosis (Brooks and Harris, 2006; Hassan et al., 2014). From the results we can see that the isolated (Z)-ethylidene-4,6-dimethoxycoumaran-3-one from P.quadrifolius (Benth.) is inducing apoptosis mode of action of cell death in cancer cells similar to the known chemotherapeutic agents. Our pre-clinical study indicated that the compound 1 is more sensitive to acute T cell leukemia cell line (Jurkat E6-1) comparing with other cancer cell lines and has the potential to become as a chemotherapeutic agent in the future.
In this study, we reported the isolation of the active antiproliferative principle (Z)-ethylidene-4,6-dimethoxycoumaran-3-one from the P. quadrifolius (Benth.) leaf. (Z)-ethylidene-4,6-dimethoxycoumaran-3-one has exhibited an encouraging antiproliferative activity against Caco-2, HeLa, MCF-7, THP-1 and Jurkat E6-1 cancer cell lines. The study also revealed the apoptosis inducing property of (Z)-ethylidene-4,6-dimethoxycoumaran-3-one in cancer cells. Further investigations are required to identify the exact target and mode of action of (Z)-ethylidene-4,6-dimethoxycoumaran-3-one, which leads to apoptosis in cancer cells.
Conflict of interest
We declare that we have no conflict of interest.
Acknowledgments
We acknowledge the University of Calicut and the DBT, Government of India for providing research facilities and financial support. This research is a part of Ph.D thesis of Sanith Cheriyamundath.
References
- Basmadjian C, Zhao Q, Bentouhami E, et al. Cancer wars: natural products strike back. Front Chem. 2014;2 doi: 10.3389/fchem.2014.00020. doi:10.3389/fchem.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas A, Bari M, Roy M, Bhandra S. Inherited folk pharmaceutical knowledge of tribal people in the chittagong hill tracts, Bangladesh. Indian J Tradit Knowl. 2010;9:77–89. [Google Scholar]
- Brooks SA, Harris A. Breast cancer research protocols. Humana Press, New York. 2006:154–5. [Google Scholar]
- Bunrathep S, Lockwood GB, Songsak T, et al. Chemical constituents from leaves and cell cultures of Pogostemon cablin and use of precursor feeding to improve patchouli alcohol level. Sci Asia. 2006;32:293–6. [Google Scholar]
- Cheriyamundath S, Raghavan R, Madassery J. DPPH radical scavenging property of methanol leaf extract from Pogostemon quadrifolius(Benth.) Res J Med Plant. 2015;9:361–7. [Google Scholar]
- Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9:767–76. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- Hassan M, Watari H, Almaaty AA, et al. Apoptosis and molecular targeting therapy in cancer. BioMed Res Int. 2014;2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992;11:121–39. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- Klika KD, Cheriyamundath S, Raghavan R, et al. Ethylidene-4,6-dimethoxycoumaran-3-one: the C2=C8 double bond configuration. Tetrahedron Lett. 2014;55:6550–3. [Google Scholar]
- Krishnaswamy N. Chemistry of natural products: A laboratory handbook. Universities press, India. 2003:26–7. [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padal S, Chandrasekhar P. Ethnomedicinal use of herb species khammam district, andhra Pradesh, India. Int J Innovative Res Dev. 2013;2:1287–98. [Google Scholar]
- Padal S, Chandrasekhar P, Vijayakumar Y. Traditional uses of plants by the tribal communities of salugu panchayati of paderu mandalam, visakhapatnam, district, andhra pradesh, India. Int J Comput Eng Res. 2013;3:98–103. [Google Scholar]
- Padal S, Raju JB. Ethnomedicinal plants used by tribals of rayagadda district, odisha state, India. Int J Innovative Res Dev. 2013;2:1299–1309. [Google Scholar]
- Pezzuto JM. Plant-derived anticancer agents. Biochem Pharm. 1997;53:121–33. doi: 10.1016/s0006-2952(96)00654-5. [DOI] [PubMed] [Google Scholar]
- Pidgeon GP, Kandouz M, Meram A, et al. Mechanisms controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res. 2002;62:2721–7. [PubMed] [Google Scholar]
- Quinn MJ, d'Onofrio A, Møller B, et al. Cancer mortality trends in the EU and acceding countries up to 2015. Ann Oncol. 2003;14:1148–52. doi: 10.1093/annonc/mdg307. [DOI] [PubMed] [Google Scholar]
- Raghavan R, Cheriyamundath S, Madassery J. 14-Deoxy-11, 12-didehydroandrographolide inhibits proliferation and induces GSH-dependent cell death of human promonocytic leukemic cells. J Nat Med. 2014;68:387–94. doi: 10.1007/s11418-014-0815-2. [DOI] [PubMed] [Google Scholar]
- Raju YR, Yugandhar P, Savithramma N. Documentation of ethnomedicinal knowledge of hilly tract areas of east godavri district of andhra pradesh, India. J Pharm Pharm Sci. 2014;6:369–74. [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Stewart BW, Wild C. International agency for research on cancer. World Health Organization; 2014. World cancer report 2014. [Google Scholar]
- Strong K, Mathers C, Epping-Jordan J, et al. Preventing cancer through tobacco and infection control: how many lives can we save in the next 10 years? Eur J Cancer Prev. 2008;17:153–61. doi: 10.1097/CEJ.0b013e3282b6fda8. [DOI] [PubMed] [Google Scholar]
- Thoppil JE, Jose J. Cytological studies and chemical composition in Eusteralis quadrifolia (Benth.). Panigrahi. Proc Indian Natn Sci Acad. 1995;61:209–12. [Google Scholar]
- Thoppil JE, Minija J, Tajo A, et al. Antimicrobial activities of Eusteralis deccanensis and E. quadrifolia essential oils. J Environ Biol. 2003;24:211–12. [PubMed] [Google Scholar]
- Trivedi PC. Medicinal plants: traditional knowledge. Delhi: IK International Pvt Ltd; 2006. pp. 19–22. [Google Scholar]
- Wagner H. Plant drug analysis: a thin layer chromatography atlas. Heidelberg: Springer; 1996. pp. 359–64. [Google Scholar]
- Walsh K, McKinney MS, Love C, et al. PAK1 mediates resistance to PI3K inhibition in lymphomas. Clin Cancer Res. 2013;19:1106–15. doi: 10.1158/1078-0432.CCR-12-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]


