Abstract
Objective:
Exopolysaccharides gained attention as new source for cancer treatment as recent treatments cause side effects and multidrug resistance. Polysaccharides containing sulfur and uronic acids exhibited antioxidant activity, by restoring cell redox regulation, thus inhibiting cell proliferation and cancer formation. Following this context, our study was performed to assess the cytotoxic activity of exopolysaccharides produced by novel Egyptian marine bacterial strains on HepG2 cells.
Methods:
Bacteria were isolated, purified and cultured through routine microbiological techniques. 16S rRNA gene amplification and sequence analyses, Fourier Transform Infra-red (FTIR), Identification of monosugars by HPLC molecular weight estimation, sulfur content determination and neutral red uptake assay were utilized.
Results:
BLAST showed that the isolates were related to the Bacillus sp. FTIR analysis indicated that the four EPSs under study contained sulfur as substituent functional group but with different percentage in each EPS. The highest sulfur percentage (46%) appeared in the EPS-6 that was produced by Bacillus flexus isolated from the Mediterranean Sea. HPLC showed that EPSs contained uronic acids which appeared as glucuronic and galacturonic acid in the low molecular weight EPS-6 (4.296×104 g mol-1). Arabinose appeared besides the glucuronic and galacturonic acid residues. EPS-6 showed the highest cytotoxicity, IC50 (218 µg ml-1) which could be correlated to the presence of sulfure and uronic acids in its structure.
Conclusion:
The novel Firmicutes from the Egyptian saline habitat produced EPSs of cytotoxic activity on hepatocellular carcinoma.
Keywords: Exopolysaccharides, Bacillus sp, cytotoxicity, HepG2 cells, cancer treatment
Introduction
Microbial exopolysaccharides (EPSs) are heterogeneous polymers that are formed of wide range of homo- or hetero-carbohydrates as well as organic and inorganic substituents, the monosaccharides are linked together through glycosidic bonds (Sutherland 1998; Zonag et al., 2012). Bacteria release EPS in the environment in the form of capsules or slime to help these microorganisms cope with adverse environmental conditions as desiccation prevention and adhesions by forming biofilms. (Wijesekara et al., 2011) water soluble Polysaccharides could be ionic or nonionic polymers (Freitas et al., 2011). Polysaccharides can be classified into two groups based on their source: Natural polysaccharides which are obtained from various organisms, such as (algae, plants, microorganisms) and semi-synthetic polysaccharides that are produced by the chemical or enzymatic modification of the parent macromolecules (Caliceti et al., 2010). Several factors and parameters influence the production of EPS among these are the composition of the medium, especially carbon and nitrogen sources, pH, temperature, and incubation time. (Vincent et al., 1994). The highest EPS yield (292 mg ml-1), from Bacillus thuringienisis S13 was observed on using glucose as carbon source (58.5mg ml-1), peptone (49.5mg ml-1) at pH 7.0 this EPS have anti proliferative activity on A549 lung cancer cells (Parthiban et al., 2014).
EPS-producing bacteria are widely present in marine ecosystems and can be isolated from the water column, sediments, animals, etc. Bacteria producing polymers with novel structures and innovative properties have been isolated in atypical environments (Mancuso-Nichols et al., 2005). Among these properties EPS shows efficacy as anti cancer, antioxidant and show immune stimulation activities evidences that made EPS gained increasing attention as source of potential new drugs for Cancer, one of the top ten leading causes of mortality worldwide, as recent treatment strategies shows limitations because severe side effects and multidrug resistance occurred in the clinical application. For example it has been found that MD-b1-derived polysaccharides show significant therapeutic activities against gastric tumors from the endophytic bacteria Bacillus amyloliquefaciens (Chen et al., 2013). Lactobacillus casei 01 EPS exerted anti-proliferation effect on human colon cancer cell, HT-29 which increases as the dosage increased, from 5 to 100µg ml-1. (Liu et al., 2011) and high sulfated EPS B100S selectively triggered strong apoptosis in haematopoietic tumour T cell lines (Ruiz-Ruiz et al., 2010).
Molecular weight, charge density, type of substitution group and the presence of rare sugars as L-arabinose are essential structural features that influence the EPS biological activity as antitumor or antioxidant agent (Wang et al., 2016). Neutral (NPS) and acidic (APS) EPSs are two fractions obtained from Lactobacillus plantarum N14 (LP14) Murofushi et al., (2015) noticed that the acidic fraction(APS) strongly stimulate NF- KB pathway in both transfectant cells and decrease the production of pro-inflammatory cytokines.
Roca et al., (2016) are considered the first to report the production of EPS with high uronic acids content from marine Pseudoalteromonas sp. MD12-642 as Bai et al. 2012 and Poli et al., (2010) report the production of EPS from marine Pseudoalteromonas sp but with high glucose content. The EPS produced by Pseudoalteromonas sp. MD12-642 composed of glucuronic acid (25 – 26 mol %), glucosamine (12 – 16 mol %), galacturonic acid (41 – 42 mol %), and rhamnose(16 -22 mol %) of high molecular weight above 106 Da. The presence of such high content of uronic acids give this EPS interesting properties to be used in regenerative medicine and tissue engineering as heparin. Pseudoalteromonas sp. MD12-642 was isolated from Madeira Archipelago Ocean sediments (Portugal), produced 4.40 g l-1 EPS after 17 h cultivation by pulse feed bioreactor culture method.
Priyanka et al., (2016) showed that the EPS (650 mg l-1) with 7.08% uronic acid containing sugars and sulphate functional group (2.68%); its molecular weight was 90 kDa produced from the Indian south west coast isolated Nitratireductor sp. strain PRIM-31 is a promising drug for brain tumors as it can oppose the the Akt/P13K pathway through this anionic charged EPS (the Presence of uronic acid, sulphate functional groups and phosphate to this EPS assigns an overall anionic charge to the polymer) binding to the epidermal growth factor (EGF) secreted by the tumor and this coincide with the findings of Liu et al., (2014) that anionically charged EPS can prevent EGF receptor phosphorylation. Using MTT assay the EPS (300µg ml-1) produced cytotoxicity against U87MG glioplastoma cells showed IC50 value (234.04 µg ml-1).
The present study aimed to isolate and identify new bacterial strains from the Egyptian sea sediment. EPSs were isolated from these new strains. The cytotoxic effects of these crude EPSs were tested against hepatocellular carcinoma (HepG2) cells.
Materials and Methods
Sample collection
Four sea sediments samples were collected in this study. Samples (Matrouh 1, Matrouh 2) were collected from Marsa Matrouh (Mediterranean Sea). Sample (Matrouh 1) was collected at the water surface interface (0-3cm), while sample (Matrouh 2) below the water surface (50-70 cm). Samples (Ras Ghareb 3, Ras Ghareb 4) from Ras Ghareb (Red sea) were collected respectively to those of Marsa Matrouh. All samples were collected with a flat hand shovel in a sterile 50 ml Falcon tubes and stored in the fridge until processing in the laboratory.
Isolation, purification and cultivation of bacteria
A bacterial stock solution was prepared by homogenizing 10 gm sea sediment from each sample into 100 ml sea water in 250 ml sterile Erlenmeyer flasks. After clumps settle down samples were serially diluted from 10-1 to 10-5 through routine microbiological and serial dilution techniques (Shukla et al., 2011). Media for isolation: Marine agar medium (pH 7.6) containing (gm l-1): peptone (5); yeast extract (5); sucrose (10); agar (15); seawater (500ml). Culture media sterilized by autoclaving at 121 °C for 15 min. (Difco™ and BBL™ Manual, 2009) Plates were inoculated with 100 μl of each dilution and incubated for 24 h at 30 °C. This incubation temperature and period was chosen as it approximates the optimum growth for bacterial strains isolated from the same environment (Zobell et al., 1940; Jing et al., 2013). Highly mucoid colonies were selected and re-streaked several times on Marine agar plates to obtain pure cultures that were stored at -80 °C as glycerol stocks in Marine Broth supplemented with 50% (v/v) glycerol. Colonies were screened for its gram reaction, motility and capsule formation (Holt, 1986).
16S rRNA gene amplification and sequence analyses
Four mucoid bacterial isolates were cultivated in Marin broth for 24 h at 30 °C for DNA extraction, using Genomic DNA purification kit from ThermoFisher Scientific and according to the manufacturer instructions DNA extracted and purified. Amplification of 16S rRNA gene was performed with a thermal cycler using 35 cycles of 95 °C for 1min, 55 °C for 30 sec and 72 °C for 2 min. The PCR reaction (25 µl) contained 2 μl of template DNA, 5 µl of 5U Taq DNA polymerase (Fermentus, Lithuania), 5 µl Taq polymerase reaction buffer, 5 µl of deoxynucleoide triphosphate (dNTP), 2 µl of primer pair (27F, 5’-AGAGTT TGATCMTGGCTCAG-3’; 1492R, 5’- TACGGYTACCTTGTTACGACTT-3’). (da silva et al., 2013) and 4 µl of sterile RNAs free H2O. Purification and Sequencing of the PCR product was performed in Macrogen Sequencing Company Seoul, Korea. The sequences obtained were compared with the NCBI database through BLAST. The four 16S rRNA partial gene sequences have been deposited in the NCBI GenBank under accession numbers KP733900, KP733901, KP733903 and KP733907.
Production, isolation and partial purification of crude exopolysaccharides (EPS)
From each strain glycerol vial 500 µl were transferred to Marine broth flasks, incubated at 30 °C for 24 h. Growth of the twelve strains in batch cultures occurred in 250 ml Erlenmeyer flasks, each containing 50 ml Marine broth which inoculated with 200µl of each strain inoculum flask (O.D 0.5 at 600 nm), and incubated at 30 °C for 48 h (Nielsen et al., 2015).
After centrifugating the culture broth at 3,500 g, 4 °C for 15 min, cell pellets were precipitated and discard. To assure that all cells are removed and no protein content is left in the supernatant, 5% Trichloroacetic acid was then added to the supernatant which is left overnight at 4 °C; this supernatant is then centrifuged at 3,500 g.
The pH of the clear supernatant was adjusted to 7.0 with 0.1 M NaOH. Each supernatant was completed to three- volumes with ethanol 95% and left for overnight at 4 °C; the precipitate (EPS) was separated by centrifugation at 3,500 g at 4 °C for 20 min, washed twice with acetone and dehydrated by ether. Finally, it was dried in a desiccator weighed and stored at room temperature (Vidhyalakshmi et al., 2016).
Structural characterization of the EPSs
Fourier Transform Infra-red (FTIR)
Dried sample (0.5 mg) was ground with 150 mg of KBr crystals and was made into a pellet using a hydraulic press. The pellet was subjected to FTIR analysis using Perkin Elmer IR spectroscope. In the range of 400 – 4,000 cm-1 (Vidhyalakshmi et al., 2016).
Identification of monosugars by HPLC
Sugar analysis was carried out according to (Liu et al., 2002). The polysaccharide (0.01 mg) was hydrolyzed with 88% HCOOH (5 ml) in sealed tube at 100°C for 5 h. After the hydrolysis, the acid was removed by flash evaporated on water bath at a temperature of 40°C. Then, the hydrolyzed monosugars were extracted with ethanol. The purified hydrolyses monosugars were analyzed by HPLC (Agilate Pack, serics1,200), equipped with Aminex carbohydrate HP-87C column (300 mm × 7.8 mm). Deionized water was used as the mobile phase at flow rate 1 ml/min. Chromatographic peaks were identified by comparing the retention times with the respective retention times of known standard reference material. Retention time and peak area were used to calculation of sugar concentration by the data analysis of Agilat Packard.
Molecular weight estimation
Using the Agilent 1,100 HPLC system the molecular weight of the four EPSs were determined. 0.01 g was diffused in solvent (2ml H2O), filtered using the syringe filter 0.45, finally the sample inserted in the gel permeation chromatography (GPC) device (Liu et al., 2009). The Mw/Mn ratio is used to appraise the The polydispersity index through the magnitudes of the RI signal and molecular weight at every elution quantity (You et al., 2013). As described by Hassan et al., (2016) the 1,100 HPLC system is supplied with a Refractive Index Detector, and (5 μm) gel particle size of the FPL, 3 columns of pore type (100, 104, 105 A°) respectively, length 7.5 × 300 mm (1,000- 5,000,000) for DMF solvent Styrogel HR-DMF, 3 μm (7.8 × 300 mm), Water Company Ireland. One column (5,000–600,000) for water solvent (polyethylene oxide/glycol standard) PL aquagel-OH 7.5 mm and 30 μm pore type 8 μm particle size, PL aquagel-OH 7.5 mm, 50 μm pore type, 8um particle size, in series Mw from 100 – 1 250,000 g/mol.
Determination of Sulfur content
Sulphur content was determined using Barium chloride-Tween-80 reagent according to Garido procedure (1964) on 0.01gm EPS previously hydrolyzed with 5ml 5% formic acid at 105 °C. K2SO4 was used as standard. Turbidity is measured at 500 nm.
One ml hydrolyzed EPS was added to 100 µl HCl and100 µl Barium chloride-Tween-80 reagent.
Biological assay
Cell propagation and maintenance
Hepatocellular carcinoma HepG2 cells were purchased from ATCC (American Type Culture Collection) and maintained in the proper conditions. The cells were cultured in Dulbecco’s modified Eagle’s Medium (DMEM) (Lonza, Beligium) supplemented by 10 % fetal bovine serum (FBS), 4 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate at 37 °C in a humidified incubator with 5 % CO2. The cells harvested after trypsinization (0.025 % trypsin and 0.02 % EDTA) and washed twice with Dulbecco’s phosphate-buffered saline (DPBS). When the cell density reached approximately 80%, cells were split for further culture. The experiments were made up when the cells were in the logarithmic growth phase.
Cytotoxicity assay
Cell viability was measured by neutral red uptake assay (Repetto et al., 2008). The neutral red uptake assay provides a quantitative estimation of the number of viable cells in a culture. It is based on the ability of viable cells to incorporate and bind the supravital dye neutral red in the lysosomes. The cells were incubated with various concentrations of the test compounds (125, 250, 500 and 1,000 µg ml-1) for 48 h at a cell density of 104 cells/well of 96 well plate. Using the relation between used concentrations and neutral red intensity value, IC50 of tested compounds was calculated. Four parameter equation logistic curve was used (log concentration vs. % cell growth as compared to control cells). For the untreated cells (negative control), medium was added instead of the test compounds. A positive control doxorubicin (Mr=579.9) was used as cytotoxic natural agent giving 100% inhibition. Dimethyl sulfoxide (DMSO) was the vehicle used for dissolution of tested compound and its final concentration on the cells was less than 0.2%. All tests and analyses were done in triplicate and the results were averaged.
Results
Isolation and cultivation of bacteria
Characterization of the bacterial isolates
The number of isolated strains per sample varied. Ten bacterial strains were isolated from Marsa Matrouh marine sediment samples and two strains were isolated from Ras Ghareb (Red Sea) samples.
All strains showed heavy growth on Marine agar medium after 48 h of incubation at 30 °C. Marsa Matrouh samples showed opaque, off-white with mucoid texture colonies that were Gram positive, motile curved rods. The two bacterial isolates of the Red Sea are both endospore former one was Gram positive non motile Cocci arranged in pairs while the other was Gram negative motile rod shaped.
Crude exopolysaccharides(EPS) production and isolation
According to the cytotoxic activity results represented in (Table 1), the EPS produced by strains 1, 5, 6 and 7 are the most active exopolysaccharides that inhibit growth and proliferation of the HepG2 cell line, these crude EPSs were obtained from bacteria grown under optimum conditions, in the stationary phase of growth, and extracted and purified as described in (Materials and methods). The amounts of the EPSs produced by these bacteria are 8.0, 8.0, 9.0 and 7.8 gm l-1, respectively.
Table 1.
EPSs Production and Its In-Vitro Cytotoxic Activity on the HepG2 Cancer Cell Line
| Bacterial Strain number | EPS | EPS (g l-1) | IC50 (µg ml-1) |
|---|---|---|---|
| 1 | EPS-RS | 8 | 224 |
| 2 | EPS-2 | 4.2 | 398 |
| 3 | EPS-3 | 5 | 371 |
| 4 | EPS-4 | 6.5 | 436 |
| 5 | EPS-5 | 8 | 371 |
| 6 | EPS-6 | 9 | 218 |
| 7 | EPS-7 | 7.8 | 372 |
| 8 | EPS-8 | 6 | 661 |
| 9 | EPS-9 | 5 | 631 |
| 10 | EPS-10 | 7 | 617 |
| 11 | EPS-11 | 6.5 | 832 |
| 12 | EPS-12 | 6 | 978 |
| Doxorubicin | 2.1 |
* IC50, concentration required to inhibit cell viability by 50%; >1,000; inactive
16S rRNA gene amplification and sequence analyses
According to the Blast of the partial sequencing of the 16S rRNA genes, the four isolated bacteria were belonged to the Firmicutes phylogenetic group. Highest similarity showed with Bacillus subtilis subsp. Subtilis (KP733900), Bacillus subtilis subsp. Spizizenii (KP733901), Bacillus megaterium (KP733903) and Bacillus flexus (KP733907) respectively. Numbers in parentheses are accession numbers given by the GenBank.
Determination of the Sulfur content
The major functional groups of the EPS were identified using FTIR spectrophotometer. The four EPSs found to contain sulfur functional group but with different concentrations as shown in (Table 2). EPS-6 produced by Bacillus megaterium found to contain the highest content of sulfur (46%) while EPS-7 produced by Bacillus flexus contain the least content of sulfur (24%). EPS-RS and EPS-5 contain sulfur with 42% and 38% respectively.
Table 2.
Sulfate Content % and Monosaccharides Molar Ratio of the EPS-RS, EPS-5, EPS-6 and EPS-7
| EPS | EPS Sulfate content % | Monosaccharides Molar ratio | ||||
|---|---|---|---|---|---|---|
| Glucoronic acid | Galacturonic acid | Glucose | Mannose | Arabinose | ||
| EPS-RS | 42 | 6.5 | 1.5 | 1.0 | 1.0 | 0 |
| EPS-5 | 38 | 2.3 | 1.0 | 2.0 | 0 | 0 |
| EPS-6 | 46 | 1.0 | 3.6 | 2.3 | 3.6 | 5.3 |
| EPS-7 | 24 | 1.6 | 1.0 | 1.3 | 2.3 | 0 |
Characterization of EPS
The FTIR spectrums of the EPSs (Figure 1-4) showed many peaks specific to the carbonyl compounds. The bands at 3744.12, 3420.14, 3424.96 and 3423.99 cm-1were referred to the stretching vibration of O-H in the EPS-RS, EPS-5, EPS-6 and EPS-7 respectively. (Vidhyalakshmi et al., 2016). The bands at 1,677.77, 1,622.8, 1677.77and 1,653.66 cm-1 were a result of the stretching vibration of the carboxyl group and C=O. CH2 and OH bonding were illustrated at 1,435.74, 1,440.56, 1,441.53 and 1,451.17 cm-1 absorption in the spectrum of the EPSs respectively. The bands at 868.7, 869.7, 873.5 and 862.0 cm-1 indicated the α-pyranose form of the glucosyl residue in the EPS-RS, EPS-5, EPS-6 and EPS-7 respectively. (Miao et al., 2014). The sulfate active group is represented at 1111.76, 1133.9, 1132.9 and 1134.9 cm-1 in the EPS-RS, EPS-5, EPS-6 and EPS-7 respectively. (Haroun-Bouhedja et al., 2000).
Figure 1.

IR Spectrum of EPS-RS from Bacillus Subtilis Subsp. Subtilis (KP733900): band at 3744.12 cm-1 referred to the stretching vibration of O-H, band at 1,677.77 cm-1 referred to the carboxyl group and C=O. CH2 and OH bonding were illustrated at 1435.74 cm-1. The bands at 868.7 cm-1 indicated the α-pyranose form of the glucosyl residue. The sulfate active group is represented at 1,111.76 cm-1
Figure 2.
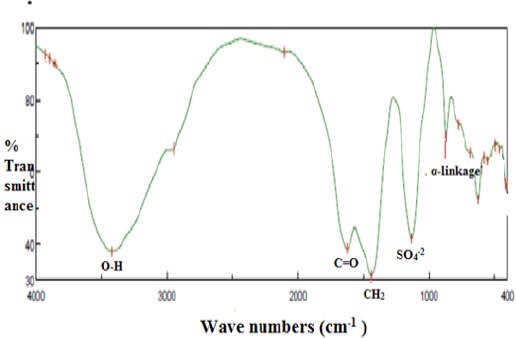
IR Spectrum of EPS-5 from Bacillus Subtilis Subsp. Spizizenii (KP733901) band at 3,420.14 cm-1 referred to the stretching vibration of O-H, band at 1,622.8 cm-1 referred to the carboxyl group and C=O, CH2 and OH bonding were illustrated at 1,440.56 cm-1. The bands at 869.7 cm-1 indicated the α-pyranose form of the glucosyl residue. The sulfate active group is represented at 1,133.9 cm-1
Figure 3.
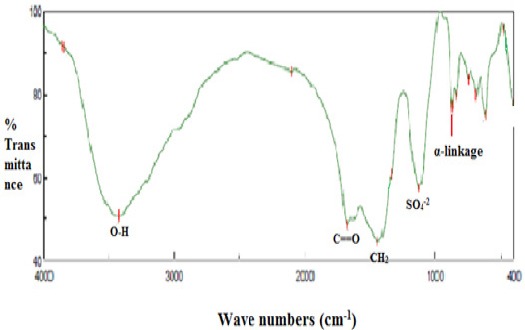
IR Spectrum of EPS-6 from Bacillus Megaterium (KP733903) band at 3,424.96 cm-1 referred to the stretching vibration of O-H, band at 1,677.77 cm-1 referred to the carboxyl group and C=O. CH2 and OH bonding were illustrated at 1,441.53 cm-1. The bands at 873.5 cm-1 indicated the α-pyranose form of the glucosyl residue. The sulfate active group is represented at 1132.9 cm-1
Figure 4.
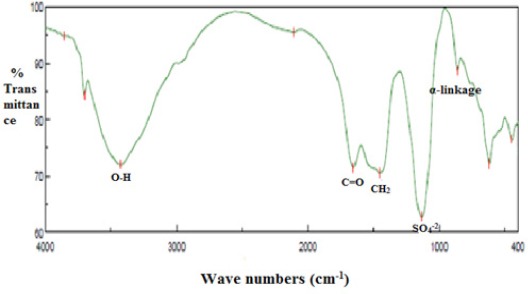
IR spectrum of EPS-7 from Bacillus flexus (KP733907) band at 3,423.99 cm-1 referred to the stretching vibration of O-H, band at 1,653.66 cm-1 referred to the carboxyl group and C=O. CH2 and OH bonding were illustrated at 1,451.17 cm-1. The bands at 862.0 cm-1 indicated the α-pyranose form of the glucosyl residue. The sulfate active group is represented at 1,134.9 cm-1
Identification of monosugars and molecular weight estimation
As shown in (Table 2) the EPS-RS was composed of glucoronic acid, galacturonic acid, glucose and mannose at a molar ratio 6.5:1.5:1.0:1.0 respectively, EPS-5 also found to contain glucoronic acid and galaturonic acid in addition to glucose but at different molar ratio 2.3:1.0:2.0 respectively. The arabinose sugar appeared in the chain of the EPS-6 which was found to be composed of glucoronic acid, galacturonic acid, glucose, mannose and arabinose with molar ratio 1.0:3.6:2.3:3.6:5.3 respectively. The monosugars of the EPS-7 were glucoronic acid, galacturonic acid, glucose and mannose at molar ratio 1.6:1.0:1.3:2.3, respectively.
As shown in (Table 3) calculating the polydispersity index (PI) from the Mw/Mn magnitude relation according to Jun et al., (2009), the weight-average molecular weight (Mw) of the EPS-RS, EPS-5, EPS-6 and EPS-7 were determined as 4.7×104, 5.2×104, 4.3×104 and 3.8×104 g mol-1 respectively. Number-average molecular weight (Mn) of the EPS-RS, EPS-5, EPS-6 and EPS-7 were 4.549×104, 5.052×104, 3.964×104 and 3.376×104 g mol-1, respectively.
Table 3.
EPSs Molecular Weight (Mw), Number-Average Molecular Weight (Mn) and Polydispersity Index (PI)
| EPS | Weight –average molecular weight (Mw) g mol-1 | Number-average molecular weight (Mn) g mol-1 | polydispersity index (PI) (Mw/Mn) |
|---|---|---|---|
| EPS-RS | 4.675×104 | 4.549×104 | 1.0277 |
| EPS-5 | 5.193×104 | 5.052×104 | 1.0279 |
| EPS-6 | 4.296×104 | 3.964×104 | 1.0838 |
| EPS-7 | 3.756×104 | 3.376×104 | 1.1126 |
Biological assay
Cytotoxic activity
According to the ability of viable cells to incorporate and bind the supravital dye neutral red in the lysosomes as stated by the neutral red assay the crude exopolysaccharides at concentrations of 125, 250, 500, and 1,000 µg ml-1 were investigated individually to inhibit the proliferation of the hepatocellular carcinoma (HepG2) cell line as anticancer agents. As shown in (Table 1, Figures 5-7), the most active compounds that inhibit growth and proliferation of the HepG2 cell line were EPS-RS, EPS-5, EPS-6 and EPS-7 with IC50 values 224, 371,218 and 372µg ml-1 respectively, compared to the control cells. EPS- 2, EPS-3, and EPS- 4 showed moderate growth inhibition with IC50 values of 398,371, and 436 µg ml-1 respectively (Table 1, Figures 5–7). However, the rest of the EPSs showed no activity against the HepG2 cancer cell line compared with control cells (Table 1).
Figure 5.

Effect of Crude EPS-RS, EPS-2, EPS- 3 and EPS- 4 on HepG2 Cell Line after 48 h Incubation Time: (•) EPS-RS IC50 224 µg ml-1, (o) EPS-2 IC50 398 µg ml-1, (ʌ) EPS-3 IC50 371 µg ml-1, (Δ) EPS-4 IC50 436 µg ml-1
Figure 6.

Effect of crude EPS-5, EPS-6, EPS-7 and EPS- 8 on HepG2 cell line after 48 h incubation time: (•) EPS-5 IC50 371 µg ml-1, (o) EPS-6 IC50 218 µg ml-1, (ʌ) EPS-7 IC50 372 µg ml-1, (Δ) EPS-8 IC50 661µg ml-1
Figure 7.

Effect of Crude EPS-9, EPS-10, EPS-11 and EPS-12on HepG2 cell line after 48 h incubation time: (•) EPS-9 IC50 631 µg ml-1, (o) EPS-10 IC50 617 µg ml-1, (ʌ) EPS-11 IC50 832µg ml-1, (Δ) EPS-12 IC50 978µg ml-1
Discussion
Exopolysaccharides (EPSs) play an important role in bacterial, fungal and algal defense systems. (Kumar et al., 2007) In recent years, EPSs have great potential as anti tumor drug as recent treatment strategies showed limitations because of severe side effects and multidrug resistance occurred, so we consider it of interest to isolate bacteria producing EPS and screen these EPSs for their anti tumor effects on hepatocellular carcinoma cells (HepG2). In our study the low molecular weight EPS-6 (4.296×104 g mol-1) that was produced by Bacillus megaterium isolated from the Mediterranean Sea, showed the highest cytotoxicity, IC50 (218 µg ml-1) aganist HepG2 cells. Such considerable cytotoxicity was due to the presence of sulfure and uronic acids in the EPS-6 structure. Marine habitats are selected for strains isolation as extreme environments force its microorganisms to produce substances by which they could cope with the adverse environmental conditions present in such habitats (Romanenko et al., 2008). These substances can be useful for biotechnological and pharmaceutical application as they showed cytotoxic and antimicrobial activities. EPS are considered one of these substances (Hussain et al., 2017) The screening results showed that the newly isolated bacteria identified as Bacillus megaterium (SAmt17’), Bacillus subtilis subsp. Spizizenii (SAmt6), Bacillus flexus (SAmt74) and Bacillus subtilis subsp. Subtilis (SAmt3) according to the16SrRNA gene sequence analysis, produced exopolysaccharides (EPS-6, EPS-5, EPS-7 and EPS-1) respectively that contain sulfur and uronic acid. The above EPSs have IC50 values that made them the most promising to be considered as anti cancer drug among the other EPSs screened in this study. Vidhyalakshmi and Vallinachiyar, (2013) also extracted exopolysaccharide from Bacillus that showed cytotoxicity against MCF-7 breast cancer cells without any cytotoxicity against normal cells.
Although the basic carbohydrate structure for most EPSs, does not change significantly, its substituent groups content can vary and thus changing its properties and activity (Wang et al., 2016). Within this context we carried out FTIR analysis to the (EPS-RS, EPS-5, EPS-6 and EPS-7) to explore the influence of the polymer linkage, and substituent functional groups on the biological activity of the polymers. The results of the FTIR analysis showed that the above mentioned EPSs contained sulfur as substituent functional group but with different percentage in each EPS, the highest percentage (46%) appeared in the EPS-6 that was produced by Bacillus megaterium which was isolated from the Mediterranean Sea. Regarding to this result we relate the highest cytotoxic activity of this EPS (IC50 218 µg µl-1) on HepG2 cells to First: the presence of the sulfur functional group, a result that is in line with the findings of (Ruiz-Ruiz et al., 2010) who stated that, sulfated EPS B100S triggered strong apoptosis in hematopoietic tumor T cell lines. The result opposes the opinion of (de Philipps and Vincenzini 1998) who relates the production of sulfated polysaccharides only to cyanobacteria, archaea and eukaryotic algae.
Sulfur is an important mineral required by strict quantity for normal metabolism and anti-oxidant defense systems. Carcinogenic transformation deprives cancer cells from the biodegradation of cysteine to sulfane sulfur metabolite and in turn cellular redox regulation is disrupted. Cellular redox regulation is important for apoptosis induction and the inhibition of cell proliferation. (Iciek et al., 2007).
Toohey (1989) conclude that sulfane sulphure low concentration can be one of the causes of the uncontrolled growth of the cancer cells. In this context Iciek et al., (2012) showed that the polysulfides as sulfane sulfur-containing diallyl trisulfide (DATS) compound is capable of inhibiting HepG2 cell proliferation and induce apoptosis as sulfane sulphur is bioreduced in HepG2 cells relasing H2O2 and thus restore redox regulation.
Roca et al., (2015) and Wang et al., (2016) stated that the presence of rare sugars as D-ribose and L-arabinose besides the Uronic acid in the order of polygalacturonic > glucuronic > galacturonic acid and polymers of low molecular weight are important indicators reflecting the antioxidant activity of the EPSs. Considering the above findings we hypothesized that the abundance of these features could relate to the cytotoxic activity of the EPSs so we carried out HPLC to identify the monosugars of the EPS-RS, EPS-5, EPS-6 and EPS-7. The four EPSs found to contain uronic acids in different molar ratio as stated in the results section. The EPS-6 that showed the highest cytotoxicity found to contain arabinose besides the glucoronic and galacturonic acids. EPS-6 has low molecular weight 4.296×104 g mol-1. HPLC above findings considered to be the second reason through which we relate the highest cytotoxic activity of this EPS (IC50 218 µg ml-1).
Conflict of Interest
The authors declare no conflict of interests.
Acknowledgments
The authors acknowledge the financial support of the National Research Centre, Cairo, Egypt (Grant no: 1159).
References
- Bai Y, Zhang P, Chen G, et al. Macrophage immunomodulatory activity of extracellular polysaccharide (PEP) of Antarctic bacterium Pseudoaltermonas sp.S-5. Int Immunopharmacol. 2012;12:611–17. doi: 10.1016/j.intimp.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Caliceti P, Salmaso S, Bersani S. Polysaccharide-based anticancer prodrugs. In: Reddy L.H, Couvreur P, editors. In Macromolecular anticancer therapeutics. New York, NY: Humana Press Inc; 2010. pp. 163–219. [Google Scholar]
- Chen Y, Yuan T, Shan Q, et al. Antitumor activity of bacterial exopolysaccharides from the endophyte Bacillus amyloliquefaciens sp.isolated from Ophiopogon japonicas. Oncol Lett. 2013;5:1787–92. doi: 10.3892/ol.2013.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva M, Cavalett AC, Spinner A, et al. Phylogenetic identification of marine bacteria isolated from deep-sea sediments of the eastern South Atlantic Ocean. Springerplus. 2013;127:1–10. doi: 10.1186/2193-1801-2-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Philipps R, Vincenzini M. Exocellular polysaccharides from cyanobacteria and their possible application. FEMS Microbiol Rev. 1998;22:151–75. [Google Scholar]
- Zimbro M, Powe J, Miller DA, Wilson SM, Johnson JA, editors. Difco™ and BBL™ Manual. Manual of microbiological culture media. 2nd edition. BD diagnostics –diagnostic systems; 2009. pp. 347–8. [Google Scholar]
- Freitas F, Alves VD, Reis MA. Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol. 2011;29:388–98. doi: 10.1016/j.tibtech.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Garrido ML. Determination of sulphur in plant material. Analyst. 1964;89:61–6. [Google Scholar]
- Haroun-Bouhedja F, Ellouali M, Sinquin C, Boisson-Vidal C. Relationship between sulfate groups and biological activities of fucans. Thromb Res. 2000;100:453–9. doi: 10.1016/s0049-3848(00)00338-8. [DOI] [PubMed] [Google Scholar]
- Hassan AI, Ghoneim MAM, Mahmoud MG, Asker MMS, Mohamed SS. Efficacy of polysaccharide from Alcaligenes xylosoxidans MSA3 administration as protection against γ-radiation in female rats. J Radiat Res. 2016;57:189–200. doi: 10.1093/jrr/rrv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JG. Bergey's manual of systematic bacteriology. 1st Edn. Baltimore U.S.A: Williams and Wilkins Co; 1986. p. 2648. [Google Scholar]
- Hussain A, Zia K, Tabasum M, et al. Blends and composites of exopolysaccharides;properties and applications: A review. Int J Biol Macromol. 2017;94:10–27. doi: 10.1016/j.ijbiomac.2016.09.104. [DOI] [PubMed] [Google Scholar]
- Iciek M, Marcinek J, Mleczko U, Wlodek L. Selective effects of diallyl disulfide, a sulfane sulphur precursor in the liver and Ehrlich ascites tumor cells. Eur J Pharmacol. 2007;569:1–7. doi: 10.1016/j.ejphar.2007.04.055. [DOI] [PubMed] [Google Scholar]
- Iciek M, Kwiecień I, Chwatko G, et al. The effects of garlic-derived sulfur compounds on cell proliferation, caspase 3 activity, thiol levels and anaerobic sulfur metabolism in human hepatoblastoma HepG2 cells. Cell Biochem Funct. 2012;30:198–204. doi: 10.1002/cbf.1835. [DOI] [PubMed] [Google Scholar]
- Jing L, Wei Z, Yun T, Guizhong W, Tianling Z. Optimization of culture conditions and medium composition for the marine algicidal bacterium alteromonas sp. DH46 by uniform design. J Ocean Univ China. 2013;12:385–91. [Google Scholar]
- Kumar AS, Mody K, Jha B. Bacterial exopolysaccharides- a perception. J Basic Microbiol. 2007;47:103–7. doi: 10.1002/jobm.200610203. [DOI] [PubMed] [Google Scholar]
- Liu JR, Chen MJ, Lin CW. Characterization of Polysaccharide and volatile compounds produced by Kefir grains grown in soymilk. J Food Sci. 2002;67:104–8. [Google Scholar]
- Liu J, Luo JL, Ye H, et al. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr Polym. 2009;78:275–81. [Google Scholar]
- Liu CT, Chu FJ, Chou CC, Yu RC. Anti proliferative and anti cytotoxic effects of cell fractions and exopolysaccharides from Lactobacillus casei 01. Mutat Res Genet Toxicol Environ Mutagen. 2011;721:157–62. doi: 10.1016/j.mrgentox.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhou L, Shi S, et al. Oligosaccharide G19 inhibits U-87 MG human glioma cells growth in vitro and in vivo by targeting epidermal growth factor (EGF) and activating p53/p21 signaling. Glycobiology. 2014;24:748–65. doi: 10.1093/glycob/cwu038. [DOI] [PubMed] [Google Scholar]
- Mancuso-Nichols CA, Guezennec J, Bowman JP. Bacterial exopolysac charides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: A review. Mar Biotech. 2005;7:253–71. doi: 10.1007/s10126-004-5118-2. [DOI] [PubMed] [Google Scholar]
- Miao M, Yajun M, Jiang B, et al. Structural investigation of a neutral extracellular glucan from Lactobacillus reuteri SK24.003. Carbohyd Polym. 2014;106:384–92. doi: 10.1016/j.carbpol.2014.01.047. [DOI] [PubMed] [Google Scholar]
- Murofushia Y, Villenaa J, Moriea K, et al. The toll-like receptor family protein RP105/MD1 complex is involved in the immunoregulatory effect of exopolysaccharides from Lactobacillus plantarum N14. Mol Immunol. 2015;64:63–75. doi: 10.1016/j.molimm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- Neely WB. Infrared spectra of carbohydrates. Adv Carbohydr Chem. 1957;12:13–33. [PubMed] [Google Scholar]
- Nielsen TB, Bruhn KW, Pantapalangkoor P, Junus JL, Spellberg B. Cryopreservation of virulent Acinetobacter baumannii to reduce variability of in vivo studies. BMC Microbiol. 2015;15:252–6. doi: 10.1186/s12866-015-0580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthiban K, Vegnish V, Thirumurugan R. Characterization and in vitro studies on anticancer activity of exopolymer of Bacillus thuringiensis S13. Afr J Biotechnol. 2014;13:2137–44. [Google Scholar]
- Poli A, Anzelmo G, Nicolaus B. Bacterial exopolysaccharides from extreme marine habitats: production, characterization and biological activities. Mar Drugs. 2010;8:1779–1802. doi: 10.3390/md8061779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyanka P, Arun AB, Ashwini P, Rekha PD. Functional and cell proliferative properties of an exopolysaccharide produced by Nitratireductor sp. PRIM-31. Int J Biolog Macromol. 2016;85:400–4. doi: 10.1016/j.ijbiomac.2015.12.091. [DOI] [PubMed] [Google Scholar]
- Repetto G, del-Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3:1125–31. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- Roca C, Lehmann M, Torres CAV, et al. Exopolysaccharide production by a marine Pseudoalteromonas sp. strain isolated from Madeira Archipelago ocean sediments. New Biotechnol. 2016;33:460–6. doi: 10.1016/j.nbt.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Romanenko L, Uchino M, Kalinovskaya NI, Mikhailov VV. Isolation, phylogenetic analysis and screening of marine mollusc-associated bacteria for antimicrobial, hemolytic and surface activities. Microbiol. 2008;163:633–44. doi: 10.1016/j.micres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ruiz C, Srivastava GK, Carranza D, et al. An exopolysaccharide produced by the novel halophilic bacterium Halomonas stenophila strain B100 selectively induces apoptosis in human T leukaemia cells. Appl Microb Biotechnol. 2011;89:345–55. doi: 10.1007/s00253-010-2886-7. [DOI] [PubMed] [Google Scholar]
- Shukla P, Patel N, Rao RM. Isolation and characterization of polyhydroxyalkanoate and exopolysaccharide producing Bacillus sp. PS1 isolated from sugarcane field in Bhilai, India. J Microb Biochem Technol. 2011;3:33–5. [Google Scholar]
- Sutherland IW. Novel and established applications of microbial polysaccharides. Trends Biotechnol. 1998;16:41–6. doi: 10.1016/S0167-7799(97)01139-6. [DOI] [PubMed] [Google Scholar]
- Toohey JL. Sulphane sulphur in biological systems: A possible regulatory role. J Biochem. 1989;264:625–32. doi: 10.1042/bj2640625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P, Pignet P, Talmont F, et al. Production and characterization of an exopolysaccharide excreted by a deep-sea hydrothermal vent bacterium isolated from polycheate annelid Alvinella Pompejana. Appl Environ Microbio. 1994;60:4134–41. doi: 10.1128/aem.60.11.4134-4141.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidhyalakshmi R, Vallinachiyar C. Apoptosis of human breast cancer cells (MCF-7) induced by polysacccharides produced by bacteria. J Cancer Sci Ther. 2013;5:31–4. [Google Scholar]
- Vidhyalakshmi R, Valli NC, Kumar GN, Sunkar S. Bacillus circulans exopolysaccharide: Production, characterization and bioactivities. Int J Bilogi Macromol. 2016;87:405–14. doi: 10.1016/j.ijbiomac.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Hu S, Nie S, Yu Q, Xie M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid Med Cell Longev. 2016;2016:1–13. doi: 10.1155/2016/5692852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesekara I, Pangestuti R, Kim SK. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr Polym. 2011;84:14–21. [Google Scholar]
- You L, Gao Q, Feng M, et al. Structural characterisation of polysaccharides from Tricholoma matsutake and their antioxidant and antitumour activities. Food Chem. 2013;138:2242–9. doi: 10.1016/j.foodchem.2012.11.140. [DOI] [PubMed] [Google Scholar]
- Zobell CE, Conn JE. Studies on the thermal sensitivity of marine bacteria. J Bacteriol. 1940;40:223–8. doi: 10.1128/jb.40.2.223-238.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonga A, Cao H, Wang F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr Polym. 2012;90:1395–1410. doi: 10.1016/j.carbpol.2012.07.026. [DOI] [PubMed] [Google Scholar]


