Abstract
Background:
Polymorphism of NFKB1 and NFKB1A are highly associated with cancer. We have assessed polymorphism in the promoter region of NFKB1 -94 del/ins ATTG (rs28362491) and NFKB1A -826 C/T (rs2233406) with the risk of HNSCC in Indian population.
Methods:
Polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) method was used for the genotyping NFKB1 -94 del/ins ATTG and NFKB1A -826 C/T. Sequencing was done to validate the results of PCR-RFLP. Statistical analysis of data was done by Stata/SE-14.0 software.
Results:
ins/ins genotype was observed to be a risk factor of HNSCC as compared del/del genotype of NFKB1 -94 ATTG. Interactive effects of smoking and chewing on ins/ins genotype showed 13.96 and 10.92 fold increased risk of HNSCC. NFKB1A -826 C/T polymorphism, TT genotype showed no association with the risk of HNSCC as compared to wild type CC genotype.
Conclusion:
Our results showed NFKB1 -94 del/ins ATTG with smoking and tobacco chewing may increase the risk of HNSCC while NFKB1A -826 C/T plays a protective role in Indian population.
Keywords: HNSCC, NFKB1, NFKB1A-PCR-RFLP, polymorphism
Introduction
Head and Neck Squamous Cell Carcinoma (HNSCC) is the most prominent cancer in developing countries (Joshi et al., 2014). Over 200,000 cases of HNSCC occur each year in India and 30,000 in the United States (Guru K et al., 2012). HNSCC is a complex process in which multiple genes of the cells are altered and influenced by genetic and environmental factors. Alteration of genes occurred by a single-nucleotide polymorphism (SNP) located within the promoter or other regulatory regions of the gene are found to be associated with the development of various cancers (Shastry, 2002). Cigarette smoking and tobacco chewing are the leading causes of death from HNSCC, and at least 40% of all cancers are estimated to be attributable to tobacco habit in India (Prasad R, 2016). Prolonged environmental exposure like tobacco, alcohol habit and genetic alterations have important relationship with cancer incidence.
Nuclear factor of κB (NF-κB) is a small menagerie of closely related proteins (p50, p52, Rel-A, Rel-B, and c-Rel) that form a different types of heterodimers and bind to common motif sequences known as κB site. Several genes that mediate tumorigenesis and metastasis are regulated by nuclear transcription factor NF-κB complex. In normal cells, NF-κB complex exists is in an inactivated state by IκB, while in a cancer cell, this NF-κB complex is activated by various signals which degrade IκBα inhibitory protein through a cascade of reactions. After activation, NF-κB complex is translocated to the nucleus, binds the κB element (at the promoter region), and activates gene that is responsible for the development of cancer (Karin et al., 2002). Based on other prior research, it is revealed that NF-κB complex is activated by carcinogens, tumor promoters which alter the genetic information and inflammatory cytokines. The activation of NF-κB complex can promotes chemoresistance and tumorigenesis by suppressing apoptosis. It has been reported that the promoter region of the NFKB1 (which encoded protein NF-κB p50) and NFKB1A (which encodes protein IκB) genes are associated with a risk for Hodgkin’s lymphoma (Chang et al., 2009), multiple myeloma (Spink et al., 2007), breast cancer (Curran et al., 2002), prostate cancer (Zhang et al., 2009), gastric cancer (Lo et al., 2009), colorectal cancer (Gao et al., 2007), melanoma (Bu et al., 2007), and oral cancer (Lin et al., 2012).
It has been reported that combined effect of NFKB1 -94 del/ins ATTG and NFKB1A -826 C/Tpolymorphism with betel nut and smoking habit associated with increased risk of oral cancer in Taiwanese population (Lin et al., 2012). NFKB1 -94 del/ins ATTG and NFKB1A -826 C/T may play an important role in the expression of NF-κB p50 and IκB proteins, respectively. Over expression of NF-κB p50 protein found in non-small cell lung carcinomas (Mukhopadhyay et al., 1995), human breast carcinomas, colon, lung, breast tumor cell lines (Dejardin et al., 1995) and in mouse skin carcinogenesis (Budunova et al., 1999). The present study aims to explore the association of NFKB1 -94 del/ins ATTG (rs28362491) and NFKB1A -826 C/T (rs2233406) polymorphism with the risk of HNSCC and correlate the polymorphism with environmental risk factors in Indian population.
Materials and Methods
Study group selection
The participants of this study included 312 HNSCC patients recruited at the time of diagnosis from the Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi and 312 healthy controls resided in the same geographic area. The study was approved by the Institution Ethics Subcommittee of All India Institute of Medical Sciences, New Delhi (Ref. No. IESC/T-60/01.02.2013). Written informed consent was obtained from all participants involved in this study. The demographic data and clinical characteristics of the study group were collected. Controls with any disease or medical history of disease were excluded. Patients with any other chronic diseases and previously exposed to radiotherapy, chemotherapy and surgery were excluded from the study group. Patients were clinically staged at the time of diagnosis, according to the TNM staging system of the American Joint Committee on Cancer (AJCC) Staging Manual.
Genomic DNA extraction
Genomic DNA was extracted from 200 µl EDTA-anticoagulated peripheral blood using mdi Medi G Blood Genomic DNA (gDNA) Miniprep Kit and dissolved in a TE buffer (10 mM Tris, 1 mM EDTA; pH 7.8), which was subsequently quantified by measuring the OD at 260 nm and used for the Polymerase Chain Reaction- Restriction Length Fragment Polymorphism (PCR-RFLP).
Genotyping
Polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) method was used for genotyping of NFKB1 -94 del/ins ATTG (rs28362491) and NFKB1A -826 C/T (rs2233406). A forward primer, TGGGCACAAGTCGTTTATGA, and a reverse primer, CTGGAGCCGGTAGGGAAG, for NFKB1 -94 del/ins ATTG and a forward primer, GGTCCTTAAGGTCCAATCG and a reverse primer, GTT GTGGATACCTTGCACTA, for NFKB1A -826 C/T were used for the experiment (He et al., 2009). PCR program was applied as follows: 95°C for 3 min initial denaturation, followed by 34 cycles of 95°C for 30 sec denaturation, annealing at 56.2°C (NFKB1-94 del/ins ATTG) and 60.5°C (NFKB1A -826 CT) for 30 sec, 72°C for 1 min extension and a final extension at 72°C for 10 min. The PCR product of NFKB1-94 del/ins ATTG was digested with 1 unit of restriction enzyme PfIMI (10 U/μl, Fermentas) and incubated at 37°C for 20 min and subsequently run on 2.5% agarose gel, whereas, the PCR product of NFKB1A -826 C/T was digested with restriction enzyme BfaI (10 U/μl, NEB) at 37°C for 1 h, analysed by 10% polyacrylamide DNA gel.
Statistical evaluation
Statistical analysis of the data was done by using Stata/SE-14.0 software. Chi square test was used to compare the differences in demographic characteristic distributions between the control group and HNSCC patients. Hardy-Weinberg equilibrium (HWE) was tested using a goodness-of-fit χ2 test. The adjusted odds ratios (AORs) and 95% confidence intervals (CIs) of the association of genotype frequencies with risk and clinicopathological characteristics were estimated using multiple logistic regression models after adjusting with covariates (age, sex, smoking, chewing and drinking). A two-sided p-value of less than 0.05 was considered for statistical significance.
Results
Demographic characteristics of the study population
The frequency distribution of demographic and clinical characteristics of the 312 HNSCC patients and 312 controls showed in Table 1. The power calculation analysis showed that the case control pair of 312 samples was sufficient to achieve a 80% power for effective sample size. The majority of the participants were male in both groups, patients (82.69%) and controls (79.17%). Most of the patients in the study cohort were with tobacco habit (73.08%) as compared to control group (29.49%). The sites affected in majority of the patients were oral cavity and oropharynx. The patients with advanced stage (III +IV) were presented in maximum number (84.94%).
Table 1.
Frequency Distribution of Selected Variables of the Controls and HNSCC Patients
| Variable | Controls (n=312) | Patients (n=312) | p value |
|---|---|---|---|
| Age (y) | |||
| 20-40 | 114 (36.54%) | 82 (26.28%) | |
| 41-60 | 115 (36.86%) | 158 (50.64%) | |
| >60 | 83 (26.60%) | 72 (23.08%) | p=0.002 |
| Mean age (year±SD) | 45.00±15.94 | 49.39±11.90 | |
| Sex | |||
| Male | 247 (79.17%) | 258 (82.69%) | |
| Female | 65 (20.83%) | 54 (17.31%) | p=0.262 |
| Smoking Status | |||
| Yes | 92 (29.49%) | 228 (73.08%) | |
| No | 220 (70.51%) | 84 (26.92%) | p=0.0001 |
| Chewing Status | |||
| Yes | 86(27.56%) | 143 (45.83%) | |
| No | 226 (72.44%) | 169 (54.17%) | p=0.0001 |
| Alcoholic Status | |||
| Yes | 110 (35.26%) | 83(26.60%) | |
| No | 202 (64.74%) | 229 (73.40%) | p=0.019 |
| Histopathology | |||
| SCC | - | 66 (21.15%) | |
| WDSCC | - | 55 (17.63%) | |
| MDSCC | - | 178 (57.05%) | |
| PDSCC | - | 13 (4.17%) | |
| Stage | |||
| I+II | - | 47 (15.06%) | |
| III+IV | - | 265 (84.94%) | |
| Tumor Size | |||
| T1+T2 | - | 70 (22.44%) | |
| T3+T4 | - | 242 (77.56%) | |
| Node | |||
| N0 | - | 127 (40.71%) | |
| N+ | - | 185 (59.29%) | |
| Site | |||
| Nasopharynx | - | 11 (3.53%) | |
| Larynx | - | 45 (14.42%) | |
| Oropharynx | - | 107 (34.29%) | |
| Oral Cavity | - | 149 (47.76%) | |
MDSCC, Moderately Differentiated Squamous Cell Carcinoma; WDSCC, Well Differentiated Squamous Cell Carcinoma; PDSCC, Poorly Differentiated Squamous Cell Carcinoma; SCC, Squamous Cell Carcinoma; Chi square test was performed to analyze differences between controls and patients.
PCR-RFLP of NFKB1 and NFKB1A gene
Analysis of the NFKB1 and NFKB1A were performed by the PCR-RFLP method. Genotype of the NFKB1 -94 del/ins ATTG was determined as del/del genotype without PflMI restriction site, the PCR product of 281 bp remained undigested. On the other hand, the insertion variants were cleaved by PflMI (Van91I) restriction enzyme into two fragments of 240bp and 45 bp. Heterozygotes showed all three bands (Figure 1a). The genotypes of NFKB1A -826 C/T polymorphisms were determined as CC (200 bp), TT (180 and 20 bp,) and heterozygote C/T(200 bp, 180 bp and 20 bp) (Figure 1b). The presence or absence of a polymorphism was monitored by UV transilluminator (Bio-Rad Gel Doc XR+) system. The genotype results were confirmed via direct DNA sequencing of the amplified fragments. Ten percent of the total samples were used for sequencing.
Figure 1.
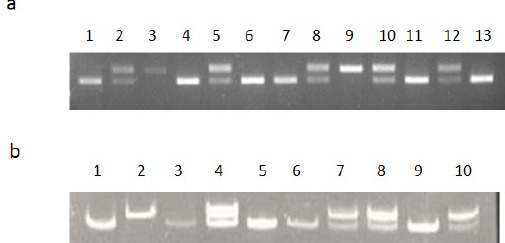
a) The Pattern of PfIMI Enzyme Digestion. Lanes 2, 5, 8, 10 and 12 are heterozygous del/insl ATTG; lanes 1, 4, 6, 7, 11 and 13 are homozygous ins/ins ATTG; and Lane 3 and 9 is homozygous del/del ATTG genotype. b) The BfaI Digestion Profile. Lanes 4, 7, 8 and 10 are heterozygous CT; lanes 1, 3, 5, 6 and 9 are homozygous GG; and Lane 2 is homozygous CC genotype.
Association of NFKB1 and NFKB1A polymorphisms with HNSCC
Genotype distribution of promoter polymorphisms in NFKB1-94 del/ins ATTG and NFKB1A -826 C/T between patients and controls, as well as their associations with HNSCC risk were presented in Table 2. Chi square test showed genotype frequencies of NFKB1-94 del/ins ATTG in controls were (p=0.0641) in accordance to Hardy–Weinberg Equilibrium (HWE). The frequencies of the del/del, del/ins, and ins/ins genotypes were 5.77%, 51.92% and 42.31% among the cases, respectively, and 7.05%, 46.79%, and 46.15% among the controls, respectively. Using the del/del genotypes of NFKB1-94 as reference, 1.12 fold increased risk of HNSCC was observed with ins/ins genotype (OR=1.12, 95% CI =0.57-2.18). We also found that the del/ins genotype was significantly (p=0.034) associated with a increased risk of HNSCC (AOR=2.36, 95% CI=1.06-5.23) after adjusting co-variates sex, age, smoking, chewing and alcoholic status. While no significant association of NFKB1A -826 C/T polymorphism was detected (OR=0.46, 95% CI= 0.23-0.90) with TT genotype as compared to wild type CC genotype.
Table 2.
Distribution Frequencies for NFKB1 Promoter –94 del/ins ATTG and NFKB1A -826 C/T Genotypes in Controls and Patients.
| Variable | Controls (n=312) | Patients (n=312) | OR (95% CI) | p value | AOR (95% CI) | p value |
|---|---|---|---|---|---|---|
| NFKB1 Genotype | ||||||
| del/del | 22 (7.05%) | 18 (5.77%) | 1 (ref) | 1 (ref) | ||
| del/ins | 146 (46.79%) | 162 (51.92%) | 1.35 (0.69-2.62) | 0.367 | 2.36 (1.06-5.23) | 0.034 |
| ins/ins | 144 (46.15%) | 132 (42.31%) | 1.12 (0.57-2.18) | 0.738 | 1.75 (0.79-3.89) | 0.164 |
| del/ins+ins/ins | 290 (92.95%) | 294 (94.23%) | 1.23 (0.65-2.35) | 0.514 | 2.04 (0.94-4.41) | 0.068 |
| NFKB1A Genotype | ||||||
| CC | 16 (5.13%) | 32 (10.26%) | 1 (ref) | 1 (ref) | ||
| CT | 208 (66.67%) | 199 (63.78%) | 0.47 (0.25-0.89) | 0.022 | 0.47 (0.22-0.98) | 0.046 |
| TT | 88 (28.21%) | 81 (25.96%) | 0.46 (0.23-0.90) | 0.024 | 0.42 (0.19-0.92) | 0.031 |
| CT+TT | 296 (94.87%) | 280 (89.94%) | 0.47 (0.25-0.88) | 0.018 | 0.46 (0.22-0.94) | 0.034 |
OR, Odd Ratios; AOR, Adjusted Odd Ratios; ORs with 95% CIs were estimated by logistic regression models; AORs with 95% CIs were estimated by multiple logistic regression models after controlling for sex, age, tobacco chewing, alcohol consumption, and smoking status.
Interactive effects of environmental risk factors on genetic polymorphism
Interactive effects were also analysed between environmental risk factors (smoking, chewing and alcohol status) and polymorphism of NFKB1–94 del/ins ATTG. The ins/ins genotypes was found to be 13.96 fold increased risk of HNSCC (p=0.0001) with smoking habit (OR= 13.96, 95% CI 4.22-46.11) as compared to del/del genotype with non smoking (NS) after adjusting sex, age, chewing and alcohol consumption. Similarly, after adjusting variables, ins/ins genotype showed 10.92 fold higher risk of HNSCC (OR=10.92, 95% CI=3.68-32.40, p=0.0001) with chewing (C) as compared to del/del with non chewing. No significant association of HNSCC risk was observed with alcohol consumption (Table 3).
Table 3.
Interactive Effect of Smoking, Chewing and Alcohol Consumption on NFKB1 –94 del/ins ATTG Polymorphism
| Variable | Controls | Patients | OR (95% C.I.) | p value | AOR (95% C.I.) | p value |
|---|---|---|---|---|---|---|
| Smoking | ||||||
| del/del without NS | 11 (3.65%) | 5 (1.67%) | 1 (ref) | 1 (ref) | ||
| del/ins or ins/ins without S | 209 (69.44%) | 79 (26.42%) | 0.83 (0.28-2.46) | 0.74 | 1.03 (0.32-3.29) | 0.06 |
| del/ins or ins/ins with S | 81 (26.91%) | 215 (71.91%) | 5.83 (1.96-17.32) | 0.001 | 13.96 (4.22-46.11) | 0.0001 |
| Chewing | ||||||
| del/del without NC | 14 (66.67%) | 7 (2.33%) | 1(ref) | 1(ref) | ||
| del/ins or ins/ins without C | 212 (69.74%) | 162 (53.82%) | 1.52 (0.60-3.87) | 0.371 | 2.15 (0.77-6.03) | 0.143 |
| del/ins or ins/ins with C | 78 (25.66%) | 132 (43.85%) | 3.38 (1.30-8.74) | 0.012 | 10.92 (3.68-32.40) | 0.0001 |
| Alcoholic | ||||||
| del/del without NA | 14 (4.61%) | 14 (4.55%) | 1 (ref) | 1 (ref) | ||
| del/ins or ins/ins without A | 188 (61.84%) | 215 (69.81%) | 1.14 (0.53-2.46) | 0.731 | 2.06 (0.81-5.26) | 0.129 |
| del/ins or ins/ins with A | 102 (33.55%) | 79 (25.65%) | 0.77 (0.34-1.71) | 0.53 | 0.81 (0.31-2.13) | 0.679 |
OR, Odd Ratios; AOR, Adjusted Odd Ratios; NS, Non smoking; S, Smoking; NC, Non Chewing; NA, Non alcoholic; A, alcoholic; ORs with 95% CIs were estimated by logistic regression models; AORs with 95% CIs were estimated by multiple logistic regression models after controlling for sex, age, smoketobacco chewing and alcohol consumption status.
Association of NFKB1 –94 del/ins ATTG and NFKB1A -826 C/T with clinicopathologic variables
No significant association was observed between NFKB1 promoter –94 del/ins ATTG and NFKB1A -826 C/T genotypes and the clinopathological variables such as stage, tumor size and lymph node metastasis of to the overall risk of HNSCC (Supplementary Table 1).
Discussion
The primary risk factors for the HNSCC are tobacco chewing, cigarette smoking, alcohol consumption and HPV infection (Vigneswaran and Williams, 2014). Among all, smoking and chewing are the most common etiologic factors in Indian subcontinent. We have observed a higher number of patients affected at oral cavity and oropharynx sites in the study group due to high prevalence of tobacco chewing and smoking habits. Middle age group (40-60 years) is the most susceptible to the disease, because of the accumulation of toxic compounds into the cells due to long term tobacco consumption. The NF-κB signaling pathway plays an important role in cell regulation, expression of genes, influencing a broad range of biological processes which include apoptosis, inflammation, stress responses, infection, etc. and dysregulation of this pathway leads to develop tumor and cause cancer (Karin et al., 2002). Various polymorphic sites of NFKB1 and NFKB1A have been reported in various journals, among them NFKB1-94 del/ins ATTG and NFKB1A -826 C/T are the most significant and have biologic importance, too. To our knowledge this study is the first to identify the association between NFKB1 -94 del/ins ATTG and NFKB1A -826 C/T polymorphism in the promoter region with HNSCC risk in an Indian population. We have observed different frequency of NFKB1 -94 del/del genotype in our population as compared to the Taiwanese population due to the ethinicity differences. The risk of HNSCC was analysed higher in NFKB1 -94 ins/ins as compared to del/del genotype. There is an evidence that the NFKB1 -94 polymorphism containing ins/ins genotype has the higher binding ability to the promotor than del/del genotype (Karban et al., 2004) and consequently enhanced the expression of NF-κB. The frequencies of the smoking and tobacco chewing were observed higher in patients as compared to controls. Carcinogenic compounds of tobacco are accumulated in cells over time and may interfere with DNA replication and damage the DNA of the cell, leads to development of tumor (Hoeijmakers, 2009). Present study analysed the interactive effect of smoking, tobacco chewing and alcohol consumption with NFKB1 -94 ins/ins as higher risk factor of HNSCC. Over time predisposition of toxic compounds into the cell make the individual susceptible for the disease. Hence, ins/ins genotype is known risk factor of HNSCC due to up-regulation of NF-κB protein expression and tobacco smoking further increases the risk of HNSCC in presence of ins/ins genotype in Indian population.
The functional activity of NF-κB complex is regulated by inhibiting through IκB in the cytoplasm and differential expression of IκB leads to dysregulation of NF-κB signaling pathways. Therefore, we also studied polymorphism of NFKB1A -826 C/T which may influence the expression of NF-κB. In this study, NFKB1A -826 C/T polymorphism showed the protective role in population and no association of NFKB1A -826 C/T with risk of HNSCC in Indian population while in other population (Lin et al., 2012), NFKB1A -826 C/T polymorphism was found to be associated with risk of cancer.
Hence, from our research result, it can be summarized that in Indian population, NFKB1-94 ATTG ins/ins genotype along with smoking or chewing habit is more susceptible for developing HNSCC as compared to del/del genotype with non-smoking or non-chewing. NFKB1-94 ATTG ins/ins genotyping may help for prediction of HNSCC risk, while NFKB1A -826 C/T indicates TT genotype plays a protective role in the general population of India.
Supplmentary Table 1
Acknowledgements
Author acknowledges the support from Indian Council of Medical Research, New Delhi, India for providing fellowship of Abhishek Gupta.
References
- Bu H, Rosdahl I, Sun XF, Zhang H. Importance of polymorphisms in NF-kappaB1 and NF-kappaBIalpha genes for melanoma risk, clinicopathological features and tumor progression in Swedish melanoma patients. J Cancer Res Clin Oncol. 2007;133:859–66. doi: 10.1007/s00432-007-0228-7. [DOI] [PubMed] [Google Scholar]
- Budunova IV, Perez P, Vaden VR, et al. Increased expression of p50-NF-kappaB and constitutive activation of NF-kappaB transcription factors during mouse skin carcinogenesis. Oncogene. 1999;18:7423–431. doi: 10.1038/sj.onc.1203104. [DOI] [PubMed] [Google Scholar]
- Chang ET, Birmann BM, Kasperzyk JL, et al. Polymorphic variation in NFKB1 and other aspirin-related genes and risk of Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2009;18:976–86. doi: 10.1158/1055-9965.EPI-08-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran JE, Weinstein SR, Griffiths LR. Polymorphic variants of NFKB1 and its inhibitory protein NFKBIA, and their involvement in sporadic breast cancer. Cancer Lett. 2002;188:103–7. doi: 10.1016/s0304-3835(02)00460-3. [DOI] [PubMed] [Google Scholar]
- Dejardin E, Bonizzi G, Bellahcène A, et al. Highly-expressed p100/p52 (NFKB2) sequesters other NF-kappa B-related proteins in the cytoplasm of human breast cancer cells. Oncogene. 1995;11:1835–41. [PubMed] [Google Scholar]
- Gao J, Pfeifer D, He LJ, et al. Association of NFKBIA polymorphism with colorectal cancer risk and prognosis in Swedish and Chinese populations. Scand J Gastroenterol. 2007;42:345–50. doi: 10.1080/00365520600880856. [DOI] [PubMed] [Google Scholar]
- Guru K, Manoor UK, Supe SS. A comprehensive review of head and neck cancer rehabilitation: physical therapy perspectives. Indian J Palliat Care. 2012;18:87–97. doi: 10.4103/0973-1075.100820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhang H, Yin J, et al. IkappaBalpha gene promoter polymorphisms are associated with hepatocarcinogenesis in patients infected with hepatitis B virus genotype C. Carcinogenesis. 2009;30:1916–22. doi: 10.1093/carcin/bgp226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers HJ. DNA Damage, aging, and cancer. N Engl J Med. 2009;361:1475–85. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- Joshi P, Dutta S, Chaturvedi P, Nair S. Head and neck cancers in developing countries. Rambam Maimonides Med J. 2014;5:e0009. doi: 10.5041/RMMJ.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CW, Hsieh YS, Hsin CH, et al. Effects of NFKB1 and NFKBIA gene polymorphisms on susceptibility to environmental factors and the clinicopathologic development of oral cancer. PLoS One. 2012;7:e35078. doi: 10.1371/journal.pone.0035078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SS, Chen JH, Wu CW, Lui WY. Functional polymorphism of NFKB1 promoter may correlate to the susceptibility of gastric cancer in aged patients. Surgery. 2009;145:280–5. doi: 10.1016/j.surg.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Karban AS, Okazaki T, Panhuysen CI, et al. Functional annotation of a novel NFKB1 promoter polymorphism that increases risk for ulcerative colitis. Hum Mol Genet. 2004;13:35–45. doi: 10.1093/hmg/ddh008. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay T, Roth JA, Maxwell SA. Altered expression of the p50 subunit of the NF-kappa B transcription factor complex in non-small cell lung carcinoma. Oncogene. 1995;11:999–1003. [PubMed] [Google Scholar]
- Prasad R. Tobacco use accounts for 40 per cent of all cancers in India, says report. The Hindu 21st July, 2016. 2016 [Google Scholar]
- Shastry BS. SNP alleles in human disease and evolution. J Hum Genet. 2002;47:561–66. doi: 10.1007/s100380200086. [DOI] [PubMed] [Google Scholar]
- Spink CF, Gray LC, Davies FE, Morgan GJ, Bidwell JL. Haplotypic structure across the IkBαgene (NFKBIA) and association with multiple myeloma. Cancer Lett. 2007;246:92–9. doi: 10.1016/j.canlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123–41. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wei Q, Li X, et al. A functional insertion/deletion polymorphism in the promoter region of the NFKB1 gene increases susceptibility for prostate cancer. Cancer Genet Cytogenet. 2009;191:73–7. doi: 10.1016/j.cancergencyto.2009.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


