Abstract
Objective:
Human papillomavirus (HPV) is the primary cause of cervical cancer. The purpose of this study was to investigate the prevalence and type distribution of HPV DNA positive in women undergoing routine physical examination.
Methods:
2,452 women were enrolled between March and November 2013. Participants were tested for 23 HPV types using polymerase chain reaction (PCR) and reverse dot blot hybridization. HPV DNA detection prevalence was estimated in different age groups.
Results:
Mean age (SD) of study participants was 47.7 (9.8) years. Overall HPV-positive prevalence was 18.9% (463/2452), and 22 out of 23 known subtypes were detected. Single HPV subtype prevalence of infection was 14.4%, and multiple prevalence of infection was 4.5%. The prevalence of HPV DNA in different age groups (20-29, 30-39, 40-49, 50-59, 60-69, ≥70) were 9.3%, 15.6%, 17.1%, 22.1%, 23.0% and 20.0%, respectively. HPV 52 was the most prevalent HPV type, followed by HPV 58, 53, 16 and 68, in descending order of prevalence. The top five low-risk types of HPV were (in descending order): HPV81, 43, 6, 42 and 11.
Conclusions:
Overall HPV DNA detection prevalence increased significantly with increasing age. Concerning high-risk HPV types, HPV 52, 58, 53, 16 and 51 were the most common in the study.
Keywords: HPV infection, prevalence, physical examination, Shanghai
Introduction
Cervical cancer is the third most common malignancy and the fourth leading cause of cancer-related death among women worldwide. Overall, the mortality incidence ratio is 52%, and cervical cancer caused 275,000 deaths in 2008 alone, about 88% of which occurred in developing countries (de Sanjose et al., 2007; Kroupis et al., 2007; Panotopoulou et al., 2007; Agorastos et al., 2014). Numerous epidemiological and molecular biology studies over the past two decades have shown that human papillomavirus (HPV) plays a central role in the etiology of cervical cancer (Munoz et al., 2003).
HPV is a small, double-stranded DNA virus that contains approximately 7,900 base pairs, and can specifically infect human skin and mucous membrane squamous epithelial cells, causing a variety of benign and malignant lesions (Clifford et al., 2005). More than 100 types of HPV have been identified, and 40 of these are associated with the anogenital area. HPV genotypes of the genital tract have been divided into low-risk and high-risk forms based on pathogenicity. Low-risk HPV types are responsible for 90% of genital warts, while the high-risk types are responsible for 95% of cervical cancers and cervical intraepithelial neoplasia (CIN)(Clifford et al., 2005; de Sanjose et al., 2010). HPV 16 and 18, specifically, have been identified as the cause of 70% of cervical cancers (HPV16 alone is responsible for 50% of cervical cancers) (Bosch et al., 2008).
High-risk HPV infection caused by CIN disorders is a long-term, reversible precancerous lesion, and early intervention can effectively prevent cervical cancer. Therefore, HPV detection through physical examination is an effective means to prevent cervical disease and is important in guiding clinical treatment and disease prognostication, as well as in tracking possible relapse after treatment (de Sanjose et al., 2010). Clarifying the type of HPV involved in infection further enhances such critical clinical decision-making and plan of action. The present study aimed to identify the prevalence of cervical HPV DNA in women undergoing routine physical examination.
Materials and Methods
Clinical data
During the time period between March and December 2013, data were collected from women volunteered for cervical cancer screening at Hua-Dong Sanatorium, a specialized facility for medical examination and health consulting services. The population was comprised of individuals spanning various enterprises and institutions in Shanghai, China. All participants reported experience in vaginal sexual intercourse and voluntarily joined the study free of charge. Informed written consent was obtained from all participants prior to examination. Exclusion criteria included: younger than 20 years of age, Chronic smoker, two or more sex partners, oral contraceptives use for more than 2 years, injection of HPV vaccine, hysterectomy, prior abnormal cytology, prior cervical cancer, pelvic radiotherapy and pregnancy. A total of 2,452 women were enrolled in the study. According to study objective, study participants, aged 20 to 80, were divided into 6 age-defined sub-groups including 20-29, 30-39, 40-49, 50-59, 60-69 and older than 70 years of age. The study was approved by the local ethics committee.
Methods
In accordance with practice protocols, none of the participants had a history of vaginal drug delivery or flushing within 3 days prior to examination. A cervical smear was taken upon receipt of consent. To obtain the smear, the patient’s cervix was exposed with a speculum. A gynecologist with more than 5 years of clinical experience collected samples of exfoliated cervical cells using a standard cytobrush at the transformation zone of the uterine cervix for HPV genotyping.
We performed the HPV Genotyping detection kit (Shenzhen Asia Energy Biotechnology Co, Ltd, in China)to simultaneously identify 23 HPV types, including HPV 6, 11, 16, 18, 31, 33, 35, 39, 42, 43, 45, 51-53, 56, 58, 59, 66, 68, 73, 81-83. According to the manufacturer’s protocol, the kit uses in vitro amplification of PCR and DNA reverse dot blot hybridization combined with DNA chip technology. A positive and negative control was provided within the kit, and was used in the process of testing according to the directions. The process includes HPV DNA Extraction, PCR amplification, hybridization, washing membrane, developing color and results analyses. Specific as follows, HPV-DNA extraction: specimens are collected from cervical via HPV collecting kit (sufficiently elute the samples from the cervical brush), after a centrifuge of 13,000 r/min for 10 min, remove the supernatant and reserve the cell block in tube-bottom; then, add 50 ul of lysate for suspension and precipitate, water bath for 10 min at 100 °C, a centrifuge of 13,000 r/min for 10 min, reserve the supernatant; PCR amplification: add 5 μl of extracted sample in each reaction tube, with the total reaction volume of 25 μl, then add 1 drop mineral oil, a negative and positive control can be set in each test; PCR amplification follows the following condition: 50 °C for 15 min, 95 °C for 10min, 94 °C for 30 sec, 42 °C for 90 sec, 72 °C for 30 sec, 40 cycles, 72 °C for 5min; Hybridization: first, denature PCR amplification products to get single-stranded DNA; then, based on base-pair complementary, add the PCR amplification products in low density gene patches with total 23 gene different HPV subtype probe; then hybridize. Develop the color after the hybridization; Results analysis: HPV genotype hybridization membrane has 23 gene types as well as Biotin and internal control as quality control. HPV genotyping test result can be interpreted as negative when 2 quality control dots are positive while others are negative. If 2 quality control dots are positive, and there is 1 or more HPV genotype dots is positive, the HPV virus type can be determined by the positive point based on the distribution of HPV genotype profile on the membrane.
Any types of HPV detected were defined as HPV-positive, and all others were designated negative. HPV types were grouped into high-risk and low-risk types as follows: high-risk (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, 82, and its subtype IS039) and low-risk (6, 11, 40, 42, 54, 55, 57, 61, 62, 64, 71, 72, 83, 84, and 89) (Munoz et al., 2003). A positive result for any one HPV type was defined as single HPV infection. HPV-positive samples with two or more identified HPV types were defined as multiple HPV infection.
Statistical analysis
Statistical analyses were performed to characterize the prevalence of HPV subtype infection in the different aged groups as well as for the HPV-positive women. Prevalence was calculated by counting single and multiple infections. Chi-square tests were utilized for comparing differences in HPV-positive rates among different aged groups. All statistical analyses were performed with IBM SPSS Statistics 20 (IBM, Armonk, New York City, USA). All p values were two-sided with significance determined when p < 0.05.
Results
HPV DNA prevalence among different age groups
The mean age (Standard deviation, SD) of study participants was 47.7±9.8 years. The prevalence of HPV DNA in different age groups (20-29, 30-39, 40-49, 50-59, 60-69, ≥70) were 9.3%, 15.6%, 17.1%, 22.1%, 23.0% and 20.0%, respectively (Table 1). The prevalence of overall HPV DNA detection increased significantly along with increasing age (Figure 1).
Table 1.
Incidence of HPV in Different Age Groups
| Age group | Cases | HPV+ rate n (%) | Proportion HPV women n (%) | Proportion of all participants n (%) |
|---|---|---|---|---|
| 20-29 | 54 | 5/54 (9.3) | 5/463 (1.1) | 5/2452 (0.2) |
| 30-39 | 463 | 72/463 (15.6) | 72/463 (15.6) | 72/2452 (2.9) |
| 40-49 | 861 | 147/861 (17.1) | 147/463 (31.8) | 147/2452 (6.0) |
| 50-59 | 795 | 176/795 (22.1) | 176/463 (38.0) | 176/2452 (7.2) |
| 60-69 | 239 | 55/239 (23.0) | 55/463 (11.9) | 55/2452 (2.2) |
| ≥70 | 40 | 8/40 (20.0) | 8/463 (1.7) | 8/2452 (0.3) |
| Total | 2452 | 463/2,452 (18.9) | 100 | 463/2,452 (18.9) |
Figure 1.
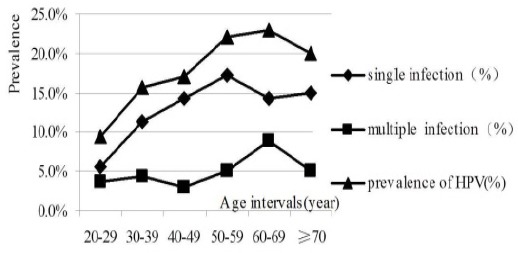
Trend in The Prevalence of HPV by Age Intervals
Overall HPV DNA prevalence
Overall prevalence of any of the 23 HPV types within the study population was 18.9% (463/2452). HPV 52 was the most prevalent type of HPV observed in this study of high-risk HPV infection, followed by HPVs 58, 53, 16 and 68 in descending order of prevalence. HPV 52, 58, 53, 16, 51 were associated with 57.5% (257/447) of high-risk HPV-positive, including single and multiple infections. Among high-risk HPV-positive women, single infections accounted for 72.0% with the other 28.0% exhibiting multiple HPV infection. In this population, the prevalence of HPV 45 and 73 was low, and HPV 82 was not detected. HPV 81 was the most prevalent type of HPV found in cases of low-risk HPV infection, followed by HPV 43, 6, 42 and 11 in descending order of prevalence. HPV 81 and 43 were associated with 59.1% of low-risk HPV infections (Table 2).
Table 2.
Distribution of Detected HPV Infection
| HPV type | Single infection n | Multiple infection n | Overall infection n | Overall Infection rate n/2,452(%) | |
|---|---|---|---|---|---|
| Low-risk | 6 | 16 | 15 | 31 | 1.3 |
| 11 | 6 | 5 | 11 | 0.4 | |
| 42 | 17 | 11 | 28 | 1.1 | |
| 43 | 24 | 9 | 33 | 1.3 | |
| 81 | 36 | 32 | 68 | 2.8 | |
| High-risk | 16 | 20 | 19 | 39 | 1.6 |
| 18 | 12 | 11 | 23 | 0.9 | |
| 31 | 7 | 4 | 11 | 0.4 | |
| 33 | 7 | 12 | 19 | 0.8 | |
| 35 | 6 | 5 | 11 | 0.4 | |
| 39 | 11 | 3 | 14 | 0.6 | |
| 45 | 1 | 1 | 2 | 0.1 | |
| 51 | 18 | 18 | 36 | 1.5 | |
| 52 | 52 | 36 | 88 | 3.6 | |
| 53 | 25 | 18 | 43 | 1.8 | |
| 56 | 19 | 11 | 30 | 1.2 | |
| 58 | 30 | 21 | 51 | 2.1 | |
| 59 | 10 | 9 | 19 | 0.8 | |
| 66 | 8 | 10 | 18 | 0.7 | |
| 68 | 20 | 13 | 33 | 1.3 | |
| 73 | 2 | 1 | 3 | 0.1 | |
| 83 | 6 | 1 | 7 | 0.3 | |
| Total | All types | 353 | 265 | 618 | 24.3 |
Note, Single infection n, Number of Single infection cases; Multiple infection n, Number of Multiple infection cases; Overall infection n, Number of Overall infection cases; Overall Infection rate n/2,452 (%) , Proportion of Overall infection cases in all participants.
Single infection of HPV type
The single HPV DNA prevalence was 14.4% (353/2452), and prevalence of high-risk HPV types was found in 10.4% (254/2452) of the total study population. HPV 52 was found in 2.1% (52/2452) of the women tested and was the most prevalent HPV type. Other HPV types were HPV 58, 53, 16 and 51 in descending order of prevalence.
The prevalence of low-risk HPV types was found in 4.0% (99/2452) of the population. HPV 81 was the most prevalent of the low-risk types and was found in 1.5% (36/2452) of the women tested. Other HPV types were HPV 43, 42, 6 and 11 in descending order of prevalence.
Multiple infections of HPV types
The multiple HPV DNA prevalence was 4.5% (110/2452). The prevalence of high-risk HPV types was observed in 106 participants. HPV 52 was the most prevalent type in this study, which was found in 36 individuals. Other HPV types identified were HPV 58, 16, 53 and 51 in descending order.
The prevalence of low-risk HPV types was observed in 60 cases. HPV 81 was found in 32 individuals, and was the most prevalent of the low-risk types. Other HPV types were HPV 6 and 42 in descending order of prevalence. Among multiple infection cases, one individual was infected with eight types of HPV (HPV6, 11, 16, 51, 52, 53, 58, 66), one was infected with five HPV types (HPV16, 18, 51, 85, 68), and 9 were infected with four HPV types (HPV31, 51, 52, 53).
Discussion
In the present study, the HPV DNA prevalence was 18.9% among all women examined, including 14.4% in single and 4.5% in multiple HPV infection. Overall prevalence of HPV DNA detection increased significantly with increasing age. The characteristics of the participants were of a population that received a healthy check-up, which could represent infection rate in healthy individuals to some extent.
The study participants were 20 years and older, due to delegation of the 2012 consensus conference that proposed less intensive management for other young women with abnormal cytology, according to the American Society for Colposcopy and Cervical Pathology (ASCCP) Consensus Guidelines in 2012 (Massad et al., 2013).
The distribution of HPV types varies among different geographical regions and populations. The HPV DNA prevalence among women in the present study was higher than reported in previous studies in Europe. The worldwide crude HPV prevalence estimate among women with normal cytology was 10.0% (de Sanjose et al., 2007). In Greece, the HPV DNA prevalence was 5.8% (Agorastos et al., 2014). The overall prevalence of HPV in Nigeria was reported at 37% (Akarolo-Anthony et al., 2014). The worldwide HPV DNA prevalence in women without documented cervical abnormalities is approximately 11-12% with higher rates in sub-Saharan Africa (24%), Eastern Europe (21%) and Latin America (16%) (Forman et al., 2012).
In China, some studies have reported HPV prevalence and the distribution of the types of HPV in women in areas including Shanghai. According to one study, human papillomavirus was detected in 5,173 of 17,148 women (30.2%) with the highest prevalence occurring in the ≤20 age group (45.2%) (Xue et al., 2009). Another study reported that the rate of HPV-positive diagnosis was 12.6% in suburban areas of Shanghai, and the five top HPV types were HPV52, 16, 58, 18 and 33 (in descending order) (Zhang et al., 2013).
Our finding that HPV 52 was the most prevalent type is consistent with other Chinese studies(de Sanjose et al., 2007; Xue et al., 2009; Zhang et al., 2013; Agorastos et al., 2014) and similar to findings reported from South Africa and North India (Bhatla et al., 2008; Vinodhini et al., 2012). In contrast, HPV 16 was by far the most common high-risk HPV type in developed countries(de Sanjose et al., 2007; Kroupis et al., 2007; Panotopoulou et al., 2007; Agorastos et al., 2014). In Iranian, the HPV DNA prevalence types were HPV 16, 18, 6/11, 31, and 33, and HPV 16 was the most frequent type in all five different groups (Bhatla et al., 2008; Vinodhini et al., 2012).
At present, there are some different views about the association between HPV infection and age, some research reported that it showed “U” shape of age-specific prevalence of HPV infection occurring in developing countries or economic backward areas (Baseman and Koutsky, 2005). This study suggests that the prevalence of HPV DNA was significantly increased in more than 50 year-old female, with a high incidence of cervical cancer in China in the age of 45 to 60 years old. The observed increase in HPV prevalence in older age groups could be attributed to human estrogen and progesterone level changes affect the cervical epithelial metaplasia process and support the HPV replication; on the other hand, the declining of body’s immune function leads up to decrease the ability of HPV clearance and increase the susceptibility to an HPV infection. Due to the gradual reduction of the sex in elderly women, the HPV DNA prevalence could reflect persistent or potential HPV infection. Therefore, it is important to carry out the detection of HPV infection in the elderly women in order to prevent the occurrence of cervical cancer and precancerous lesions.
The data provide valuable information for HPV based screening and prevention for women in China where robust screening and vaccination programs are not yet established. As the HPV types differ in the Chinese population from those in the Western countries, for optimal population coverage, in addition to HPV 16 and 18, next generation HPV prophylactic vaccines including HPV-52 and -58 may offer higher protection for Chinese women. The unique epidemiological feature of HPV52 and 58 in China should be considered in the design and evaluation of diagnostic assays of cervical screening intended for Chinese women.
Low-risk HPV causes genital warts, but its rate of detection is low, considering the harm is small, so the body is easy to exclude. This study found that HPV81 was the most common type of low-risk types, which was related to regional, ethnic differences, living environment, health habits and subjects. This study reflects the HPV carrying status among healthy people and conforms to the regional characteristics of HPV distribution. Further study is needed to address the prevalence profile of low-risk HPV types in both cytology-normal and cytology-abnormal populations.
In summary, this study covered a considerable proportion of the female population in Shanghai, the most populous city in China according to the latest census. Therefore, the study is somewhat representative of the wider general population.
As a next step, a meta-analysis of studies on genotype-specific prevalence of cervical HPV DNA in the Yangtze River Delta in China is planned and should expand our knowledge base on the overall prevalence and prevalence of specific types of HPV in China.
Statement conflict of Interest
The authors declare that they have no conflict of interest.
References
- Agorastos T, Chatzistamatiou K, Zafrakas M, et al. Epidemiology of HPV infection and current status of cervical cancer prevention in Greece: final results of the LYSISTRATA cross-sectional study. Eur J Cancer Prev. 2014;23:425–31. doi: 10.1097/CEJ.0000000000000060. [DOI] [PubMed] [Google Scholar]
- Akarolo-Anthony SN, Famooto AO, Dareng EO, et al. Age-specific prevalence of human papilloma virus infection among Nigerian women. BMC Public Health. 2014;14:656. doi: 10.1186/1471-2458-14-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32:16–24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Bhatla N, Dar L, Rajkumar PA, et al. Human papillomavirus-type distribution in women with and without cervical neoplasia in north India. Int J Gynecol Pathol. 2008;27:426–30. doi: 10.1097/PGP.0b013e31816085ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26:1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the international agency for research on cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991–8. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- Kroupis C, Thomopoulou G, Papathomas TG, Vourlidis N, Lazaris AC. Population-based study of human papillomavirus infection and cervical neoplasia in Athens, Greece. Epidemiol Infect. 2007;135:943–50. doi: 10.1017/S095026880700876X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17:1–27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- Munoz N, Bosch F.X, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Panotopoulou E, Tserkezoglou A, Kouvousi M, et al. Prevalence of human papillomavirus types 6, 11, 16, 18, 31, and 33 in a cohort of Greek women. J Med Virol. 2007;79:1898–905. doi: 10.1002/jmv.21025. [DOI] [PubMed] [Google Scholar]
- Vinodhini K, Shanmughapriya S, Das BC, Natarajaseenivasan K. Prevalence and risk factors of HPV infection among women from various provinces of the world. Arch Gynecol Obstet. 2012;285:771–7. doi: 10.1007/s00404-011-2155-8. [DOI] [PubMed] [Google Scholar]
- Xue Y, Zhang W, Chen M, Han L, Luo M. “U” shape of age-specific prevalence of high-risk human papillomavirus infection in women attending hospitals in Shanghai, China. Eur J Obstet Gynecol Reprod Biol. 2009;145:214–8. doi: 10.1016/j.ejogrb.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Zhang R, Shi TY, Ren Y, et al. Risk factors for human papillomavirus infection in Shanghai suburbs: a population-based study with 10,000 women. J Clin Virol. 2013;58:144–8. doi: 10.1016/j.jcv.2013.06.012. [DOI] [PubMed] [Google Scholar]


