Abstract
Objective:
The influence of vitamin D receptor (VDR) genetic variation on serum 25-hydroxyvitamin D levels [25(OH)D] after vitamin D3 supplementation remains unclear. We aimed to investigate changes of 25(OH)D in a randomized, double-blind, placebo-controlled clinical trial, according to VDR genotype, after provision of vitamin D3 to breast cancer cases for a 2-month period.
Methods:
Participants were assigned to two treatment arms: placebo (n = 28) and vitamin D3 supplementation (n =28). The supplementation group received 50,000 IU of vitamin D every week for 2 months. Blood samples were collected at baseline and after intervention to measure serum 25(OH)D3. Genotypes were assessed for FokI, BsmI, ApaI, and TaqI polymorphisms.
Results:
After eight weeks supplementation, the intervention group showed a significant increase in the serum concentration of 25 (OH)D3 (28±2.6 to 39±3.5; p=0.004). Subjects were then classified into twelve subgroups according to different VDR genotypes. Subjects with ff/Ff, TT/Tt, and Bb genotypes had significantly higher increases in serum 25(OH)D compared to those with FF, tt, and BB/bb genotypes post-intervention. Serum vitamin D3 levels with the AA genotype were lower than with aa/Aa. No differences were found among other subgroups.
Conclusion:
Vitamin D3 supplementation increases serum 25(OH)D in women with breast cancer. Serum vitamin D3 in TT/Tt, ff/Ff, and Bb carriers was more responsive to vitamin D supplementation than in those with FF/ff and tt genotypes. Other subgroups might gain less from vitamin D3 supplementation.
Keywords: 25-hydroxy vitamin D3, vitamin D3, vitamin D receptor (VDR), polymorphism, breast cancer
Introduction
Breast cancer (BC) is the most common cancer among women. Global statistic data show rising morbidity and mortality from BC annually indicating greater than 1.15 million women are diagnosed with breast cancer every year and around half of them die from the disease . BC is the leading cause of cancer diagnosed in Iranian women. Studies show that BC is 76% of all cancers found in Iranian women leading to about 1200 deaths per year. Iranian women are affected by breast cancer between ages of 47 and 49 years that is 10 years earlier than women in developed countries .
Vitamin D deficiency is prevalent among breast cancer women, low levels of serum vitamin D are associated with recurrence (Vrieling et al., 2011), invasiveness, and mortality (2014). On the other hand, women with higher levels of serum vitamin D are more likely to survive twice than women with low levels (2014). Receptors of vitamin D exist in up to 78% of breast cancers cells. As long as vitamin D receptors exist, tumor growth might stop, and carcinogenic cells may die from increasing blood supply. Function of vitamin D receptors stay until a tumor become very aggressive. This may be the reason for better survival in patients with higher blood levels of vitamin D (Ditsch et al., 2012).
At the present, vitamin D supplementation is a simple, operational and low price approach for treating deficiency and preserving adequacy (Talwar et al., 2007; Aloia et al., 2008; Gallagher et al., 2012; Vande Griend et al., 2012). Although there is not a ‘‘standard’’ definition of vitamin D status, an extensively accepted category is deficiency at <20 ng/mL, insufficiency at 20–31 ng/mL, and an optimal range of ≥32 ng/mL (Malabanan et al., 1998; Calvo et al., 2005). Serum levels of 25 (OH) D are different between individuals, even with a particular intake dose of vitamin D. A number of demographic and biological factors like ethnicity, dietary fat amount and components, genetic variation and some diseases affect 25 (OH) D levels in the population (2012; Mazahery and von Hurst, 2015). VDR SNPs exerting regulatory role on 1, 25(OH) D3. Therefore, they may be evaluated as a predictor of disease response to supplementation. Alterations in 1, 25(OH) D3 levels and VDR polymorphisms have been related to several systemic malignancies (McCullough et al., 2009). FokI RFLP in exon 2, BsmI, and ApaI polymorphisms in intron 8 and an adjacent TaqI RFLP in exon 9 have more correlation with breast cancer (Hutchinson et al., 2000; Newcomb et al., 2002; Lopes et al., 2010). A case–control study investigated the risk of breast cancer with regards to polymorphism of genes correlated to 25 (OH) D in plasma. It was shown the VDR polymorphism, TaqI, was associated with a 26% risk reduction (Reimers et al., 2015). A clinical trial found that the genetic variation in vitamin D receptor protein may affect the 25 (OH)D of the participant taking vitamin D3 supplements (Nimitphong et al., 2013). Despite the epidemiological evidence, there are no published trials regarding the effects of vitamin D supplementation on blood levels of 25 (OH)D in the different variation of VDR polymorphism among breast cancer women. To this purpose, we conducted a pilot, randomized, double-blind, placebo-controlled trial of vitamin D3 supplementation in comparison with placebo over 2 months to estimate the changes of total 25 (OH)D according to a high dose of vitamin D supplement among subgroups of VDR genotype.
Materials and Methods
Study population
This study was conducted on 56 women aged between 30 and 60 years diagnosed with stages I to III breast cancer who were followed up at the oncology ward, University Golestan Medical Center, from April to September 2015. Patients with metastatic breast cancer and those who had histories of other cancers, chemotherapy, radiotherapy, and hormone therapy for any reasons except current cancer, under treatment by corticosteroids, having chronic diarrhea and malabsorption were excluded. Patients with known inflammatory conditions (such as acute bacterial or viral infections), autoimmune diseases (such as rheumatoid arthritis or lupus) were also excluded from the study. The sample size of 23 was calculated for each group considering significance level of p < 0.05 and power of 80% (Hopkins et al., 2011). Applying 20% dropout, a sample size of 28 for the placebo and intervention groups was yielded.
Clinical protocol
A randomized block design method was used to randomly assign the matched participants into placebo (n=28) or weekly 50,000 IU vitamin D3 treatment (n=28) groups. Participants that gave written consent to participate were interviewed and their blood samples were taken in fasting state. The participants were enrolled between April and September 2015. Patients’ diets were assessed within 3 days through 24 hours recall questionnaire at pre- and post-study periods. Body weight was measured using Seca scale, model 769 with the accuracy of 0.1 Kg with no shoes and least clothes and height was measured using a non-stretchable stadiometer. Waist circumference was measured based on standard protocol. Vitamin D and placebo pearls were identical in size, appearance, and taste and were manufactured by Zahravi Pharm. Co, Tabriz, Iran. Pearls were given each month, and obedience was checked by weekly phone calls. Compliance was considered as consumption of at least 90% of the supplied pearls. At the end of two months, blood samples were collected for measurement of serum 25 (OH) D levels and VDR polymorphism in subjects who completed the course. Serum and plasma samples were separated by centrifuging at 2000 RMP for 16 min using a 46H centrifuge (HETTIC, FRANCE). The serum samples were stored at -70°C freezer for further analyses.
Clinical measures
Serum 25 (OH) D levels were determined using ELISA kits (bioactiva diagnostica GmbH, Homburg, Germany); according to the manufacturer’s protocol. SSP-PCR technique was used to determine the different VDR gene polymorphisms including ApaI BsmI FokI, and TaqI of all subject (Dawson-Hughes et al., 2005; Lombard et al., 2006; Søborg et al., 2007). DNA was extracted using commercial DNA extraction Kit (Roach, USA) from blood specimens. PCR reaction in a total volume of 25 µl was performed. Primers sequence are shown in Table 3. Then the product of PCR reaction was electrophoresed in Agarose gel 1.5%. After performing genotyping, allele frequencies and genotype polymorphism of the VDR gene were identified. The four SNPs of VDR are located in exon 2, intron 8, intron 8 and exon 9 of chromosome 12 (12q12-q14), respectively. Mean of 3-day energy and nutrients intakes were analyzed using Nutritionist IV Database Manager Software. A trained nutritionist (HM) performed all data entrance.
Table 1.
Baseline Characteristics of the Participants
| Characteristics | Treatment group | ||
|---|---|---|---|
| Placebo n=24 | Vitamin D n=23 | pa | |
| Demographics | |||
| Age, y | 46.3(9.5) | 47.7(8.0) | 0.6 |
| Ethnicity,% | |||
| Arab | 54 | 43.5 | |
| Fars | 46 | 56.5 | 0.46 |
| Breast cancer Stage, % | |||
| I | 33 | 27 | |
| II | 42 | 43 | |
| III | 25 | 30 | 0.50 |
| Menopaused,% | |||
| Yes | 38 | 65 | |
| No | 62 | 35 | 0.80 |
| Anthropometric | |||
| BMI | 29.2±6.3 | 30.2±5.4 | 0.59 |
| Waist circumference | 103.5±12.5 | 109±11.2 | 0.12 |
| Mean dietary intakes | |||
| Total energy intake, kcal/d | 1,796±528 | 1,848±821 | 0.59 |
| Total fat, g/d | 67±32 | 70 ±32 | 0.59 |
| Dietary calcium, mg/d | 618±308 | 843±526 | 0.41 |
| Dietary fiber, g/d | 15±7 | 18±9 | 0.97 |
Data are given as means±SD unless specified.;
Chi square test was used for categorical variables. Independent t-test was used for continuous variables; NO significant differences were found between the groups.
Table 2.
Genotype Frequencies of the VDR Gene Polymorphisms in Breast Cancer Patients
| Polymorphism | placebo | vitamin D | pa |
|---|---|---|---|
| BsmI | |||
| BB | 5 (20.8) | 2 (8.7) | |
| Bb | 11 (45.8) | 16 (69.6) | |
| bb | 8 (33.4) | 4 (21.7) | 0.2 |
| TaqI | |||
| TT | 5 (21.7) | 13 (56.5) | |
| Tt | 15 (65.3) | 6 (26.1) | |
| tt | 3 (13) | 4 (17.4) | 0.53 |
| ApaI | |||
| AA | 11 (45.8) | 9 (39.1) | |
| Aa | 11 (46.8) | 9 (39.1) | |
| aa | 2 (6.4) | 5 (21.8) | 0.43 |
| FokI | |||
| FF | 2 (8.3) | 2 (8.7) | |
| Ff | 19 (79.2) | 19 (82.6) | |
| ff | 3 (12.5) | 2 (8.7) | 0.9 |
Data are given as number (percent);
, Chi-Square test was used.
Table 3.
Primers Using in VDR Genotyping and Size of PCR Product
| Gene Location | Primers | Amplicon size (bp) | |
|---|---|---|---|
| Exon 2 | FokI F (46559145-46559162) | 5’-TGGCCGCCATTGCCTCCG-3’ | |
| FokI f (46559145-46559162) | 5’-TGGCCGCCATTGCCTCCA-3 | 77 | |
| FokI C (46559204-46559221) | 5’-AGCTGGCCCTGGCACTGA-3’ | ||
| Intron8 | BsmI B(46526083-46526102) | 5’-AGCCTGAGTACTGGGAATGT-3’ | 534 |
| BsmI b(46526083-46526102) | 5’-AGCCTGAGTACTGGGAATGC-3’ | ||
| BsmI C(46526599-46526616) | 5’-GGGAGGGAGTTAGGCACC-3’ | ||
| Intron8 | ApaI A(46525104-46525123) | 5’-TGGGATTGAGCAGTGAGGT-3’ | 229 |
| ApaI a(46525104-46525123) | 5’-TGGGATTGAGCAGTGAGGG-3’ | ||
| ApaI C(46524894-46524912) | 5’-CCTCATTGAGGCTGCGCAG-3’ | ||
| Exon 9 | TaqI T(46525024-46525041) | 5’-CAGGACGCCGCGCTGATT-3’ | 148 |
| TaqI t(46525024-46525041) | 5’-CAGGACGCCGCGCTGATC-3’ | ||
| TaqI C(46524894-46524912) | 5’-CCTCATTGAGGCTGCGCAG-3’ |
Statistical analysis
Both groups were evaluated for comparability of characteristics at baseline and post-intervention using Chi-Square test for categorical variables and independent t-test or paired t-test for continuous variables. The normality checked using KS test. Treatment effects were evaluated by assessing the differences from baseline to 2-month follow-up between the arms using a repeated-measures ANOVA. All statistical analyses were performed using SPSS version 24. P-value less than 0.05 was considered statistically significant.
Results
A total of 125 patients were screened that led to the enrollment of 56 eligible participants. Patients were randomly assigned to receive vitamin D3 (n=28) and placebo (n=28) for 2 months. Nine patients (9/56, 16%) withdrew from the study: 5 patients in the vitamin D3 group and 4 in the control group due to discontinuing (n = 1), unwillingness to continue participation (n = 4), metastasis diagnosis (n =2), and consuming Ca/vitamin D pills (n = 2). Consequently, 47 women (mean age 47 year) were included in the final analysis.
All baseline characteristics of subjects in both placebo and vitamin D3 groups were similar (Table 1). The mean age of participants was 47 years; most subjects were Arab (49%) and Fars (51%). The dietary intake of participants at baseline and 2 months after supplementation indicated no significant differences between the two groups. On average, 95% of supplements were consumed by participants. Table 2 shows the distribution of 12 subgroups VDR between the placebo and vitamin D groups. Baseline distributions of different genotype were identical in the categories.
Thirty-seven out of 47 subjects (78%) had vitamin D deficiency or insufficiency (serum 25(OH) D less than 31 ng/mL). As shown in Figure 1, after supplementation 25 (OH) D3 levels increased significantly (28±2.6 ng/mL vs. 39±3.5 ng/mL; p < 0.004). Furthermore, as seen in Table 4, serum levels of 25(OH)D was divided into three groups according to blood levels of 25(OH)D before intervention [25 (OH) D3< 20 ng/ml, 20 -31 ng/ml and >32ng/ml]. In the deficiency group, six patient had serum levels of vitamin D lower than 20 at baseline. After eight weeks follow-up, two patients (33.4%) improved to higher than 32 ng/ml. In insufficient group (20-31 ng/ml), seven women (78.8%) enhanced to the higher than 32(sufficient). In sufficient group, 5 out of 8 patients (62.5%) stayed in the same group. However, serum levels of one patient (12.5%) reduced to lower than 20. MacNemar test showed no significant differences between serum levels of 25(OH)D ≥32 and <32.
Figure 1.
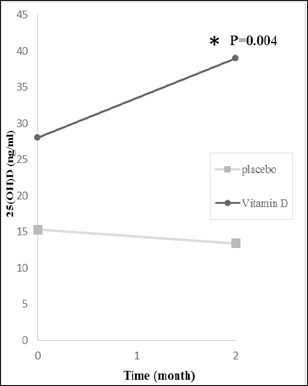
25 (OH)D3 Levels at Baseline, and at 2 Months after bVitamin D3 Supplementation
Table 4.
Changes in Serum 25(OH) D Categories in Vitamin D Supplement Group Pre- and Post- Supplementation
| Baseline | Total n (%) | 8 week follow up | ||
|---|---|---|---|---|
| <20 n (%) | 20-31 n (%) | ≥32 n (%) | ||
| <20 | 6 (100) | 0 | 4 (66.6) | 2 (33.4) |
| 20-31 | 9 (100) | 0 | 2 (22.2) | 7 (77.8) |
| ≥32 | 8 (100) | 1(12.5) | 2(25) | 5 (62.5) |
25(OH)D was defined as <20 ng/ml deficiency, 20-31 ng/ml insufficient and >32 ng/ml sufficient; MacNemar test showed no significant differences between serum levels of 25(OH)D ≥32 and <32.
Table 5 indicates changes in 25(OH) D concentrations based on baseline characteristics of breast cancer women. Among supplemented group, only subjects with BMI 25 to 30 experienced an elevation in serum 25(OH) D after post-intervention (41.5±4.8 ng/mL vs. 28±3.7 ng/mL, p=0.014), while no significant changes were observed in placebo group. Moreover, absolute treatment effect on serum vitamin D was significant in both normal weight (mean change: 21.3±5.5 ng/mL in treatment vs. -2.2±1.3 ng/mL in placebo, p= 0.001) and overweight breast cancer patients (mean change: 13.5±4.3 ng/mL in treatment vs. -1.4±2 ng/mL in placebo, p=0.013).
Table 5.
Changes in 25(OH)D Levels with Regard to Baseline Characteristics of Breast Cancer Patient
| Placebo | Vitamin D | Absolute treatment effect a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | 8 week follow up | pc | n | Baseline | 8 week follow up | pc | placebo | Vitamin D | Pb | |
| BMI | |||||||||||
| <25 | 7 | 16±4.3 | 13.7±3.7 | 0.14 | 3 | 32±3.6 | 54±18.4 | 0.061 | -2.2±1.3 | 21.3±5.5 | 0.001* |
| 25-30 | 7 | 14.8±4.7 | 13.4±4.8 | 0.5 | 9 | 28±3.7 | 41.5±4.8 | 0.014 | -1.4±2 | 13.5±4.3 | 0.013* |
| ≥30 | 10 | 12.7±1.7 | 11.4±2 | 0.4 | 11 | 26.9±3.5 | 32.9±3.7 | 0.26 | -1.3±1.5 | 6±0.5 | 0.19 |
| Ethnicity | |||||||||||
| Arab | 13 | 14.8±2.8 | 12.6±2.6 | 0.13 | 14 | 29.3±4.7 | 45.9±7 | 0.016 | -2.1±1.3 | 17±5.7 | 0.009* |
| fars | 11 | 13.7±2.6 | 12.7±2.7 | 0.46 | 9 | 27±2.8 | 33.5±2.8 | 0.057 | -1±1.3 | 6.4±3 | 0.04* |
| Age | |||||||||||
| ≤50 | 17 | 14.9±2.2 | 12±2.1 | 0.01 | 13 | 23.7±2.3 | 36.2±3.3 | 0.016 | -2.8±0.98 | 12.4±4.45 | 0.005* |
| >50 | 7 | 12.9±3.8 | 14.3±3.8 | 0.43 | 10 | 33.7±4.8 | 42.7±7 | 0.079 | 1.4±1.7 | 9±4.5 | 0.14 |
Values are Mean±SD;
p value< 0.05;
, Absolute treatment effect is the absolute changes from baseline to follow-up in the treatment group minus the absolute change from baseline to follow-up in the placebo group;
, P values for difference between the treatment and placebo groups;
, P values for difference between follow-up and baseline visits.
The mean increment in serum levels of 25 (OH) D among Arab ethnicity, was 35% higher than Fars (p= 0.09). However, increment in both ethnicity was significant post-intervention (Arabs 17±57 ng/mL vs. Fars 6.4±3 ng/mL, 0.04). Supplementation demonstrated significant effect just on age 50 years and less (+12.4±4.45 ng/mL in supplementation vs. -2.8±0.98 ng/mL in placebo group, p=0. 005).
Some differences were found in serum 25(OH)D among subjects after intervention according to their genotypes (Table 6). When comparing serum 25 (OH)D change at 2 months with baseline values, subjects with AA genotype showed 35% increase in 25 (OH)D levels after taking vitamin D3 (P= 0.038) . Patients with TT, Tt, ff, Ff genotype had greater increase in 25 (OH)D levels after taking vitamin D3, i.e., 75%, 46%, 72%, and 57%, p≤ 0.024 respectively. Serum 25 (OH)D levels significantly increased in AA, Ff, ff, Bb, TT, and Tt genotypes (p≤0.04). From six genotype that significantly increased 25(OH)D (p≤ 0.038), The largest increment was in ff (22.5±0.5 ng/mL) compares to TT (14.7±4.3 ng/mL) Bb (11.6±2.9 ng/mL), Tt (11.3±4.2 ng/mL), Ff (11.2±3.3 ng/mL) .
Table 6.
Changes in 25(OH)D Levels with Regards to VDR Polymorphism in Breast Cancer Patients
| Genotype | Placebo | Vitamin D | Absolute treatment effecta | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | 8 week follow up | pc | n | Baseline | 8 week follow up | pc | placebo | Vitamin D | Pb | |
| FokI | |||||||||||
| FF | 2 | 9.4±3.7 | 10±3.2 | 0.18 | 7 | 28.5±15 | 25.0±0 | 0.082 | 0.6±0.6 | -3.5±15.5 | 0.83 |
| Ff | 19 | 14.8±2.3 | 13±2.2 | 0.003 | 19 | 28.1±2.9 | 39.3±4.0 | 0.007 | -1.8±1.1 | 11.2±3.3 | 0.001* |
| ff | 3 | 17.0±0 | 14.0±4 | 0.43 | 2 | 27.0±7.0 | 49.5±6.5 | 0.015 | -3±4 | 22.5±0.5 | 0.024* |
| BsmI | |||||||||||
| BB | 5 | 15.8±5.8 | 12.2±5.7 | 0.82 | 2 | 25.5±14 | 21.0±5.0 | 0.4 | -3.6±1.4 | -4.5±19.5 | 0.93 |
| Bb | 11 | 11.3±2.4 | 11.4±2.4 | 0.003 | 16 | 27.4±3.0 | 39.0±4.3 | 0.003 | 0.09±1.4 | 11.6±2.9 | 0.002* |
| bb | 8 | 17.5±3 | 14.7±3.1 | 0.4 | 4 | 35.5±6.4 | 46.2±9.0 | 0.82 | -2.8±1.6 | 10.7±9.6 | 0.26 |
| ApaI | |||||||||||
| AA | 11 | 15.3±3.4 | 13.5±3.1 | 0.012 | 9 | 35.1±4.4 | 45.8±7 | 0.087 | -1.8±1.2 | 10.7±5 | 0.038* |
| Aa | 11 | 12.5±1.8 | 10.9±1.9 | 0.053 | 9 | 24.5±4.2 | 31.0±3.9 | 0.26 | -1.6±1.6 | 6.5±4.6 | 0.089 |
| aa | 2 | 18.0±12.0 | 17.5±12. | 0.12 | 5 | 21.8±2.3 | 41.0±5.8 | 0.073 | -0.5±0.5 | 19.2±7.5 | 0.07 |
| TaqI | |||||||||||
| TT | 5 | 18.4±5.7 | 15.6±4.6 | 0.11 | 4 | 31±5.6 | 46.2±9.0 | 0.05 | -2.8±2.35 | 14.7±4.3 | 0.007* |
| Tt | 15 | 14.6±2.3 | 14.0±2.3 | 0.001 | 13 | 27±4.1 | 38.3±5.2 | 0.032 | -0.6±15.3 | 11.3±4.2 | 0.017* |
| tt | 3 | 8.6±2.4 | 3.6±0.8 | 0.14 | 6 | 28.1±3.6 | 35.7±5.6 | 0.43 | -5.0±3.0 | 7.6±7.8 | 0.31 |
Values are Mean±SD;
, p value< 0.05;
, Absolute treatment effect is the absolute change from baseline to follow-up in the treatment group minus the absolute change from baseline to follow-up in the placebo group;
, P values for difference between treatment and placebo groups;
, P values for difference between follow-up and baseline visits.
Discussion
Present study demonstrated that vitamin D insufficiency and deficiency, as determined by serum 25-(OH) D levels, were seen in 79 percent of breast cancer women. The overall prevalence of vitamin D insufficiency and deficiency in our study is close to other studies evaluating vitamin D levels in breast cancer patients (Neuhouser et al., 2008; Crew et al., 2009; Khan et al., 2010; Peppone et al., 2011; Prieto-Alhambra et al., 2011) and other cancer types (Fakih et al., 2009; Trump et al., 2009).
Supplementation with 50,000 IU vitamin D3/week increased serum 25(OH)D levels higher than placebo confirming the 25(OH)D increase after vitamin D3 therapy observed in other studies (Khan et al., 2010; Lammersfeld et al., 2010; Peppone et al., 2011). A recent meta-analysis revealed that vitamin D3 is more capable of improving serum 25(OH)D concentration when administered as a high oral dose (300,000 IU single or 50,000 IU monthly) compared with 20,000 IU/d or 400 IU/d and the influence is lost with 1,000-4,000 IU/d supplementation (Macdonald et al., 2012).
The way individuals responded to or metabolized vitamin D differs noticeably base on the individual’s characteristics and biological parameters. Differences in BMI or body fat percentage (Zwart et al., 2011; Gallagher et al., 2012), dietary fat content and composition (Grossmann and Tangpricha, 2010), ethnicity (Aloia et al., 2008; Gallagher et al., 2013), calcium intake (Thomas et al., 2010), as well as genetics (Vuolo et al., 2012; Narvaez et al., 2014) may describe the large discrepancies among individuals regarding vitamin D status. In our study, vitamin D3 supplementation diversely rose serum 25(OH) D. Subjects with insufficient levels of vitamin D showed higher response to vitamin D supplementation. The greatest increase was seen in subjects in the second tertile (20-31 ng/mL), followed by those in the first tertile (5-20 ng/mL) and then those in the third tertile (>32 ng/mL). This result was in contrary to a study which showed that change in 25(OH)D concentrations had significant inverse correlation with its baseline concentrations(Trang et al., 1998). No significant association was found in changes of 25(OH) D between Arab and Fars ethnicity when comparing treatment group and placebo, while Arabs response to vitamin D supplementation was higher. However, due to small sample size, we need to be more cautious to conclude such association.
The current study demonstrated that VDR genetic variation is another factor which can influence the responsiveness to vitamin D supplementation. Circulating levels of 25 (OH) D in AA genotype were significantly higher compared to the aa or Aa genotype after supplementation. This finding is in contrast with a study that showed A alleles increased the breast cancer risk patients by 1.5 fold (Curran et al., 1999). In another survey, AA genotype in Taiwanese patients with sporadic breast cancer was associated with an increased risk of breast cancer.(Hou et al., 2002). We observed six genotypes significantly modified the efficacy of vitamin D3 supplementation for increasing serum 25(OH)D: AA, TT, Tt, ff, Ff and Bb. Expectedly, most VDR genotypes associated with baseline 25(OH)D substantially altered the response to supplementation. De Medeiros showed BB/Bb genotype had higher levels of 25(OH)D post intervention among elderly women (de Medeiros Cavalcante et al., 2015). Shab-Bidar et al suggested BB, Bb, and bb genotype respond positively to vitamin D3 significantly that the greatest increase is in BB subgroup (Shab-Bidar et al., 2015). In present study, ff group had the largest increase of 25(OH)D in respond to vitamin D3 supplementation. On the contrary, Neyestani showed ff genotype is the least increase of 25(OH)D after 12 weeks intake of yogurt drink fortified with 500 IU vitamin D3 in diabetic patients (Neyestani et al., 2013). Our results suggest that VDR signaling pathways might control oral 25(OH)D versus cutaneous synthesis or adipose stores. Few clinical trials have evaluated changes in circulating 25-hydroxyvitamin D in accordance with VDR genotypes after vitamin D3 supplementation. Some studies have attributed 25(OH)D increase to genetic variation in DBP (vitamin D Binding Protein), 25-hydroxylase (CYP2R1), 24-hydroxylase (CYP24A1), and the VDR genes (Fu et al., 2009; Nimitphong et al., 2013; Barry et al., 2014). VDR polymorphisms may explain inter-individual differences in response to vitamin D. Discrepancies in these results among different study populations might be a result of ethnic variation in the incidence of VDR gene polymorphisms (Zmuda et al., 2000; Uitterlinden et al., 2004; Kamel et al., 2014). Potential gene-environment interactions might be related to polymorphisms in the VDR pathway. Interaction of serum vitamin D and VDR phenotypes are complex which deserves further research.
There were some drawbacks in this study. One of the limitations is the type of treatment and changing medically happened during the investigation (chemotherapy to radiotherapy and anti-hormone therapy). It has been demonstrated that Vitamin D levels decline during chemotherapy but improve after ending treatment. Tamoxifen, an anti-hormone drug, may also increase serum vitamin D levels (Kim et al., 2014). Moreover, ultraviolet radiation exposure can influence the circulating levels of 25(OH)D (Bodiwala et al., 2004). Further studies with higher sample size are needed in this area. Despite the limitations of our study, we indicated connections between genetic and nutritional factors, which confirmed by the different responses to supplement therapy, and these outcomes may play a role in the adjustment of more individualized treatments. Also, to our best information, this study is the first RCT to examine the effect of oral vitamin D supplementation on circulating 25(OH)D according to four VDR polymorphisms (i.e. BsmI, ApaI, TaqI, and FokI) in women with breast cancer in Iran.
In conclusion, supplementation with vitamin D3 tends to improve total 25(OH)D levels. Variants VDR polymorphisms influence the responsiveness to vitamin D3. VDR TT genotype may be considered as “high responder” to vitamin D supplementation with regard to responding to circulating 25(OH)D. Nutrigenetic approach is suggested for personalized treatment.
Acknowledgments
This work is a part of Houra Mohseni’s MSc thesis. The authors acknowledge the funding support from the Vice-Chancellor for Research at Ahvaz Jundishapur University of Medical Sciences. Special thanks to staff of Health Research Institute, Nutrition and Metabolic Disease Research Center, Mr. Karbalai and Mrs. Labibzadeh for their contribution in laboratory analyses.
References
- Aloia JF, Patel M, DiMaano R, et al. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr. 2008;87:1952–8. doi: 10.1093/ajcn/87.6.1952. [DOI] [PubMed] [Google Scholar]
- Barry EL, Rees JR, Peacock JL, et al. Genetic variants in CYP2R1, CYP24A1, and VDR modify the efficacy of vitamin D3 supplementation for increasing serum 25-hydroxyvitamin D levels in a randomized controlled trial. J Clin Endocrinol Metab. 2014;99:2133–7. doi: 10.1210/jc.2014-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodiwala D, Luscombe CJ, French ME, et al. Polymorphisms in the vitamin D receptor gene, ultraviolet radiation, and susceptibility to prostate cancer. Environ Mol Mutagen. 2004;43:121–7. doi: 10.1002/em.20000. [DOI] [PubMed] [Google Scholar]
- Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: a global perspective of current status. J Nutr. 2005;135:310–6. doi: 10.1093/jn/135.2.310. [DOI] [PubMed] [Google Scholar]
- Crew KD, Shane E, Cremers S, et al. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J Clin Oncol. 2009;27:2151–6. doi: 10.1200/JCO.2008.19.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran JE, Vaughan T, Lea RA, et al. Association of A vitamin D receptor polymorphism with sporadic breast cancer development. Int J Cancer. 1999;83:723–6. doi: 10.1002/(sici)1097-0215(19991210)83:6<723::aid-ijc4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- de Medeiros Cavalcante IG, Silva AS, Costa MJC, et al. Effect of vitamin D3 supplementation and influence of BsmI polymorphism of the VDR gene of the inflammatory profile and oxidative stress in elderly women with vitamin D insufficiency: Vitamin D3 megadose reduces inflammatory markers. Exp Gerontol. 2015;66:10–6. doi: 10.1016/j.exger.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Ditsch N, Toth B, Mayr D, et al. The association between vitamin D receptor expression and prolonged overall survival in breast cancer. J Histochem Cytochem. 2012;60:121–9. doi: 10.1369/0022155411429155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakih MG, Trump DL, Johnson CS, et al. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis. 2009;24:219–24. doi: 10.1007/s00384-008-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Yun F, Oczak M, et al. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25 (OH) D] to vitamin D supplementation. Clin Biochem. 2009;42:1174–7. doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Peacock M, Yalamanchili V, et al. Effects of vitamin D supplementation in older African American women. J Clin Endocrinol Metab. 2013;98:1137–46. doi: 10.1210/jc.2012-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JC, Sai A, Templin T, et al. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012;156:425–37. doi: 10.7326/0003-4819-156-6-201203200-00005. [DOI] [PubMed] [Google Scholar]
- Grossmann RE, Tangpricha V. Evaluation of vehicle substances on vitamin D bioavailability: A systematic review. Mol Nutr Food Res. 2010;54:1055–61. doi: 10.1002/mnfr.200900578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CM, Frandsen TL, Brünner N, et al. 1α, 25-Dihydroxyvitamin D3 inhibits the invasive potential of human breast cancer cellsin vitro. Clin Exp Metastasis. 1994;12:195–202. doi: 10.1007/BF01753887. [DOI] [PubMed] [Google Scholar]
- Hopkins MH, Owen J, Ahearn T, et al. Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: a randomized, controlled clinical trial. Cancer Prev Res. 2011;4:1645–54. doi: 10.1158/1940-6207.CAPR-11-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M-F, Tien Y-C, Lin G-T, et al. Association of vitamin D receptor gene polymorphism with sporadic breast cancer in Taiwanese patients. Breast Cancer Res Treat. 2002;74:1–7. doi: 10.1023/a:1016048900049. [DOI] [PubMed] [Google Scholar]
- Hutchinson PE, Osborne JE, Lear JT, et al. Vitamin D receptor polymorphisms are associated with altered prognosis in patients with malignant melanoma. Clin Cancer Res. 2000;6:498–504. [PubMed] [Google Scholar]
- Kamel MM, Fouad SA, Salaheldin O, et al. Impact of vitamin D receptor gene polymorphisms in pathogenesis of Type-1 diabetes mellitus. Int J Clin Exp Med. 2014;7 [PMC free article] [PubMed] [Google Scholar]
- Khan QJ, Reddy PS, Kimler BF, et al. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2010;119:111–8. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaee-Pool M, Montazeri A, Majlessi F, et al. Breast cancer-preventive behaviors: exploring Iranian women's experiences. BMC Womens Health. 2014;14:41. doi: 10.1186/1472-6874-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Koh BS, Yu JH, et al. Changes in serum hydroxyvitamin D levels of breast cancer patients during tamoxifen treatment or chemotherapy in premenopausal breast cancer patients. Eur J Cancer. 2014;50:1403–11. doi: 10.1016/j.ejca.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Lammersfeld C, Vashi P, Trukova K, et al. Impact of oral vitamin D supplementation on serum 25-hydroxyvitamin D levels in oncology. ASCO Annual Meeting Proceedings. 2010:e19542. doi: 10.1186/1475-2891-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin GP, Robinson-Cohen C, de Boer IH, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA. 2012;308:1898–905. doi: 10.1001/jama.2012.17304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard Z, Dalton D-L, Venter PA, et al. Association of HLA-DR,-DQ, and vitamin D receptor alleles and haplotypes with tuberculosis in the Venda of South Africa. Hum Immunol. 2006;67:643–54. doi: 10.1016/j.humimm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Lopes N, Sousa B, Martins D, et al. Alterations in Vitamin D signalling and metabolic pathways in breast cancer progression: a study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions Vitamin D pathways unbalanced in breast lesions. BMC Cancer. 2010;10:1. doi: 10.1186/1471-2407-10-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald HM, Wood AD, Tang JC, et al. Comparison of vitamin D2 and vitamin D3 supplementation in increasing serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;96:1152–3. doi: 10.3945/ajcn.112.046110. [DOI] [PubMed] [Google Scholar]
- Malabanan A, Veronikis I, Holick M. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- Mazahery H, von Hurst PR. Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients. 2015;7:5111–42. doi: 10.3390/nu7075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ML, Bostick RM, Mayo TL. Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu Rev Nutr. 2009;29:111–32. doi: 10.1146/annurev-nutr-080508-141248. [DOI] [PubMed] [Google Scholar]
- Mohr SB, Gorham ED, Kim J, et al. Meta-analysis of vitamin D sufficiency for improving survival of patients with breast cancer. Anticancer Res. 2014;34:1163–6. [PubMed] [Google Scholar]
- Narvaez CJ, Matthews D, LaPorta E, et al. The impact of vitamin D in breast cancer: genomics, pathways, metabolism. Genome-wide view on the physiology of vitamin D. Front Physiol. 2014;5:213. doi: 10.3389/fphys.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhouser ML, Sorensen B, Hollis BW, et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr. 2008;88:133–9. doi: 10.1093/ajcn/88.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb PA, Kim H, Trentham-Dietz A, et al. Vitamin D receptor polymorphism and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1503–4. [PubMed] [Google Scholar]
- Neyestani TR, Djazayery A, Shab-Bidar S, et al. Vitamin D receptor fok-I polymorphism modulates diabetic host response to Vitamin D intake. Diabetes Care. 2013;36:550–6. doi: 10.2337/dc12-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitphong H, Saetung S, Chanprasertyotin S, et al. Changes in circulating 25-hydroxyvitamin D according to vitamin D binding protein genotypes after vitamin D 3 or D 2 supplementation. J Nutr. 2013;12:1. doi: 10.1186/1475-2891-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppone LJ, Huston AJ, Reid ME, et al. The effect of various vitamin D supplementation regimens in breast cancer patients. Breast Cancer Res Treat. 2011;127:171–7. doi: 10.1007/s10549-011-1415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Alhambra D, Javaid MK, Servitja S, et al. Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: a prospective cohort study. Breast Cancer Res Treat. 2011;12(5):869–78. doi: 10.1007/s10549-010-1075-9. [DOI] [PubMed] [Google Scholar]
- Reimers LL, Crew KD, Bradshaw PT, et al. Vitamin D-related gene polymorphisms, plasma 25-hydroxyvitamin D, and breast cancer risk. Cancer Causes Control. 2015;26:187–203. doi: 10.1007/s10552-014-0497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöttker B, Jorde R, Peasey A, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:3656. doi: 10.1136/bmj.g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shab-Bidar S, Neyestani TR, Djazayery A. Vitamin D receptor (BsmI) genotypes influence inflammatory and oxidative stress responses to altered vitamin D intake in subjects with Type 2 diabetes: A randomized controlled trial. J Nutr Sci Diet. 2015;1:116–26. [Google Scholar]
- Søborg C, Andersen AB, Range N, et al. Influence of candidate susceptibility genes on tuberculosis in a high endemic region. Mol Immunol. 2007;44:2213–20. doi: 10.1016/j.molimm.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Talwar SA, Aloia JF, Pollack S, et al. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr. 2007;86:1657–62. doi: 10.1093/ajcn/86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazhibi M, Feizi A. Awareness levels about breast cancer risk factors, early warning signs, and screening and therapeutic approaches among Iranian adult women: a large population based study using latent class analysis. Biomed Res Int. 2014;2014 doi: 10.1155/2014/306352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SDC, Need AG, Nordin BC. Suppression of C-terminal telopeptide in hypovitaminosis D requires calcium as well as vitamin D. Calcif Tissue Int. 2010;86:367–74. doi: 10.1007/s00223-010-9354-3. [DOI] [PubMed] [Google Scholar]
- Trang HM, Cole D, Rubin LA, et al. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854–8. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- Trump DL, Chadha MK, Sunga AY, et al. Vitamin D deficiency and insufficiency among patients with prostate cancer. BJU Int. 2009;104:909–14. doi: 10.1111/j.1464-410X.2009.08531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitterlinden AG, Fang Y, van Meurs JB, et al. Vitamin D receptor gene polymorphisms in relation to Vitamin D related disease states. J Steroid Biochem Mol Biol. 2004;89:187–93. doi: 10.1016/j.jsbmb.2004.03.083. [DOI] [PubMed] [Google Scholar]
- Vande Griend JP, McQueen RB, Linnebur SA, et al. Prescription ergocalciferol dosing for vitamin D repletion: a retrospective evaluation. Pharmacotherapy. 2012;32:135–41. doi: 10.1002/PHAR.1052. [DOI] [PubMed] [Google Scholar]
- Vrieling A, Hein R, Abbas S, et al. Serum 25-hydroxyvitamin D and postmenopausal breast cancer survival: a prospective patient cohort study. Breast Cancer Res. 2011;13:1. doi: 10.1186/bcr2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuolo L, Faggiano A, Colao AA. Vitamin D and cancer. Front Endocrinol. 2012;3:58. doi: 10.3389/fendo.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmuda JM, Cauley JA, Ferrell RE. Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev. 2000;22:203–17. doi: 10.1093/oxfordjournals.epirev.a018033. [DOI] [PubMed] [Google Scholar]
- Zwart SR, Mehta SK, Ploutz-Snyder R, et al. Response to vitamin D supplementation during Antarctic winter is related to BMI, and supplementation can mitigate Epstein-Barr virus reactivation. J Nutr. 2011;141:692–7. doi: 10.3945/jn.110.134742. [DOI] [PubMed] [Google Scholar]


