Abstract
Background:
Currently, recurrent respiratory papillomatosis (RRP), common laryngeal warts in the upper airway systems of children and adults are on the increase. Human papillomaviruses (HPVs) are suspected as causative agents. This study concerned HPV incidence and genotype distribution in Iranian RRP patients.
Methods:
Specimens were collected from RRP patients referred to hospitals affiliated with Iran University of Medical Sciences, Tehran, Iran, from Dec 2014 to Feb 2016 in a cross sectional study. After DNA extraction with an QIAamp® DNA FFPE Tissue Kit, conventional PCR was performed and products were sequenced. INNO-LiPA HPV Genotyping Extra assays as another method for genotyping were conducted. CLC Main Workbench 5 and MEGA6 software as well as SPSS v.20 were used for further analysis.
Results:
Of the total of 12 patients, 6 (50%) were male. Total mean age (y) ± SD was 9.8±6.3. All RRP patients suffered from HPV infection, with HPV-6 found in 75% (9/12) and HPV-11 in 16.7% (2/12) and one co-infection by both HPV-6 and 11. Statistically, there were no correlations between demographic variables and HPV infection.
Conclusion:
The major cause of RRP is HPV genotypes 6 and 11 increasing the risk of a requirement for medical interventions. Broader studies are needed to clarify the major risk factors in RRP patients.
Keywords: Recurrent respiratory papillomatosis (RRP), Human papillomaviruses (HPVs), genotyping
Introduction
Papillomaviruses (PVs) are a large group of virus consisting of 29 genera (>160 genus). Only 5 genera of PVs are found in humans called human papillomaviruses (HPVs). Alpha-papillomaviruses genera include some significant genus in human diseases such as HPV-6, 11, 16 and 18. PVs virion are naked and have a circular double stranded DNA genome (Jalilvand et al., 2014; Salehi-Vaziri et al., 2015). HPVs in human cause different diseases in genital tract, gastrointestinal, respiratory tract (from mild to severe) and cancer. The role of HPVs in variety of human cancers is well studied (Javanmard et al., 2017; Siegel, et al., 2016; Stewart and Wild, 2016). HPVs are classified into two groups of high risk for malignancy (such as HPV-16, 18 and 31) and low risk (such as HPV-6 and 11). In general, low risk types can cause benign lesions (Bonagura et al., 2010; Javanmard et al., 2017; Yahyapour et al., 2013).
Recurrent respiratory papillomatosis (RRP) is a wart like disease that is commonly caused by HPVs. In RRP, obstruction of airway or voice change occurs with the growth of HPV warts in the upper airway. This illness is mainly found in children younger than 5 years (juvenile-onset RRP [JORRP]) and adults more than 40 years of age (adult-onset RRP [AORRP]) (Bonagura et al., 2010; Farhadi et al., 2016; Țiple, et al., 2015). RRP is mainly caused by common HPV types 6 and 11 that is transmitted during normal vaginal delivery (Farhadi et al., 2016) and could cause malignancy in adults (Țiple et al., 2015). The incidence rate of RRP in children and adult is about 1-4 and 1.8-3.9 per 100,000, respectively (Bonagura et al., 2010; Siegel et al., 2016; Stewart and Wild, 2016).
RRP is manifested by different course of disease; in some cases, there is no recurrence after the first occurrence, others have mild involvement by limited recurrence, while others are affected by severe disease that has multiple recurrences and needs surgical removal even every month. There is surgical de-bulking treatment strategy for RRP involving laser ablation or microdebridment that could be repeated for 100 times to keep airway open and the voice sufficient (Bonagura et al., 2010; Farhadi et al., 2016; Tjon Pian Gi et al., 2016). But these patients are encountered with emotional and economic burdens. Antiviral adjuvant could be utilized as a supplement during non-invasive methods (Farhadi et al., 2016).
For this purpose, the present study aimed at determining the role of papillomavirus in children suffering from recurrent respiratory papillomatosis (RRP) in Iran.
Materials and Methods
Patient selection and sample collection
The present study was conducted in hospitals affiliated to Iran University of Medical Sciences, Tehran, Iran. In this cross sectional study from Dec 2014 to Feb 2016, samples were collected by oriented practitioners. All referred RRP patients that met the inclusion criteria were enrolled. All patients had suffered from laryngeal lesions. The study group comprised 12 patients ranging from 3 to 18 years with confirmed RRP involvement. Ethical approval was obtained from the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran. Informed consent was obtained from each patient. One biopsy of each of the informed patients was taken (except two of them that had 2 biopsies taken before the surgery and one year after surgical therapy) from the larynx and was carried by transport media and stored at -80°C until use.
DNA extraction
From each sample, 20 ng tissue was used for DNA extraction QIAamp® DNA FFPE Tissue Kit (QIAGEN, Hilden, Germany) as previously described (Salehi-Vaziri et al., 2016; Safarnezad Tameshkel et al., 2016). Ca Ski cell and distilled water were used for positive and negative control of extraction. Extracted products were stored at -20°C. Concentration of extracted DNA was evaluated by NanoDrop spectrophotometer (Thermo Scientific, Wilmington, USA).
HPV typing
INNO-LiPA HPV Genotyping Extra assay (Innogenetics NV, Ghent, Belgium) was used for HPV genotyping according to the protocol already described in another study (Salehi-Vaziri et al., 2016). This technique was based on reverse-hybridization and 28 different HPV genotypes were detected by reliable sensitivity and specificity (Salehi-Vaziri et al., 2015; Salehi-Vaziri et al., 2016). Ca Ski cell as a HPV positive control was used.
PCR and Nucleotide sequencing
A Bio-Rad (T100™ Thermal Cycler) thermocycler was utilized for PCR by MY09/MY11 universal primers that described protocol for master mix and heating program (Javanmard et al., 2017; Salehi-Vaziri et al., 2015). A total of 50 µl reaction tube after confirmation by agarose gel electrophoresis and purification by High Pure PCR Product Purification Kit (Roche Diagnostic GmbH, Mannheim, Germany) according to the manufacturer’s instructions was sequenced for each patients. An ABI 3730 XL sequencer was used for bidirectional sequencing of L1 amplified products. Bioinformatics software CLC Main Workbench 5 (CLC bio, Aarhus, Denmark) and MEGA6 (MEGA Inc., Englewood, NJ) were used for comparison and analyses of the sequences.
Statistical analysis
Statistical analysis was performed by SPSS version 20 software (SPSS Inc., Chicago, IL, USA) and descriptive and frequency variables were calculated by proper statistical tests (Fisher exact test, chi-squared test and t test). P-values less than 0.05 were considered to be statistically significant.
Results
From a total of 12 participants, 6 (50%) were male and total mean age (y) ± SD was 9.8±6.3 (Table 1). Majority of our RRP patients had normal delivery (8/12) and were non-alcoholic (10/12). The mean surgical intervention of patients was from 4.5 (ranging from 1 to 9). All of them were candidate for adjuvant therapy with alpha-interferon (dosage data was not available). Not only that among the 12 patients, 6 were under the age of 10 and 4 (50%) were male, but also the other patients had similar male to female ratio (1:1).
Table 1.
Demographic Characteristics of Our RRP Patients
| Variables | No. (%) | mean age±SD1 |
|---|---|---|
| Gender | ||
| Male | 6 (50) | 9 ± 4.5 |
| Female | 6 (50) | 10.7±8.3 |
| Total | 12 (100) | 9.8±6.3 |
| HPV genotype | Male/female | |
| 6 | 9 (75) | 6/3 |
| 11 | 2 (16.7) | 0/2 |
| 6 and 11 | 1 (8.3) | 0/1 |
| Complication | ||
| RD2 | 6 (50) | |
| Hoarseness | 4 (33.3) | |
| RD and Hoarseness | 2 (16.7) | |
SD, Standard deviation; RD, Respiratory disorder
HPV detection was performed by conventional PCR using GP5+/GP6+ primers and visualization on a 2% agarose gel was carried out. All of the 12 patients were positive for HPV genome infection in comparison with proper positive (Ca Ski cell) and negative controls. Purified PCR products of L1 gene amplification were sequenced and then trimmed by CLC Main Workbench 5 and a phylogenetic tree was drawn using MEGA software version 6.06 (Figure 1).
Figure 1.
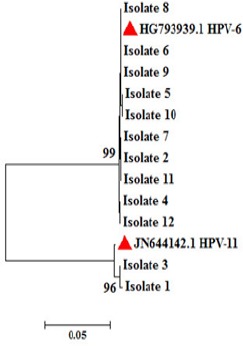
A Neighbor-Joining Tree Constructed with HPV L1 Nucleotide Sequences of the Clones Obtained From the 12 Patients with RRP that Corresponds to HPV Genotypes 6 And 11. Bootstrap values <70 obtained after 1,000 replicates of the data sheet, are shown in the nodes of the tree. Red three angles showed the reference sequences of each genotypes 6 and 11.
Using INNO-LiPA HPV Genotyping Extra assay, all isolates were typed and majority (9/12) belonged to genotype 6, of which 5/9 (66.7%) of them were male (Table 1). HPV genotyping was performed before surgery and one year after for two patients. Results showed that there were no differences in HPV genotype in this period (data not shown in Figure 1). Interestingly, one of the female patients that has a history of cesarean was co-infected with genotype 6 and 11. From that patient using phylogenetic analysis, one clone (isolate 8) matched with HPV-6 was sequenced and neighbor joining tree was drawn. All of the genotype 11 patients were females (Among them, only one has co-infection).
There was no correlation among gender (p: 0.6), age, complications and genotype although there was higher incidence of HPV-6 infection in males.
Discussion
Currently, RRP incidence as a benign neoplasm has been on the increase. RRP is a rare complication of larynx disease where HPV-6 and 11 are two major causative agents with morbidity and occasional mortality (Bonagura et al., 2010). Most of the RRP patients suffered from a local, tissue-specific disease of the larynx when their immunocytes from papillomas are imported into systemic circulation. The burden of the disease could be reduced by interventions (Bonagura et al., 2010; Tjon Pian Gi et al., 2016).
The focus of this study is on Iranian RRP patients with lesion of the larynx diagnosed by sophisticated practitioners and laboratory findings. Patients’ demographic data and history of treatment procedures were analyzed and evaluated by HPV genotypes, respectively. Genotyping was performed by PCR-Sequencing and INNO-LiPA HPV Genotyping Extra assay. In fact, we found that there were 75% (9/12) HPV-6, 16.7% (2/12) HPV-11 and 8.3% (1/12) HPV-6 and 11 co-infection. The limitations of this study were limited sample size (although sampling was done for about 15 months), loss of some accurate data on lesion site, the duration of each patient treatment and the disease severity score or other risk factors. Although, RRP is a rare complication in general population and this samples collected about 15 months, our limitation in project duration prevents us to do this by greater sample size.
RRP male to female ratio is approximately equal in children (McClay, 2004); our study patients were same in children (<10 y) and teenager (≥10 y) gender ratio. Similarly, in the previous study of Iranian RRP patients by Izadi, et al. (Izadi et al., 2012), there were 45% (13/29) male and 55% (16/29) female, although they analyzed 1 to 35 years old patients. However, adults male to female ratio was estimated to be 4:1 (McClay, 2004). Greater sample size of this study could due to including the broader range of patients ages that could in comparison with the present study we try to use children or teenager patients by RRP complication. In this regard our project was elucidate a different understanding of these patients complications.
However, RRP as a wart like disease involved larynx associated with HPV-6 and 11 in 80-100% cases (Draganov et al., 2006; Gerein et al., 2005; Sanchez et al., 2013; Tjon et al., 2015; Tjon Pian Gi et al., 2016; Wiatrak et al., 2004). There are a few studies on the prevalence of HPV in RRP patients in Iran, although Izadi et al., reported that 96.5% of them suffered from HPV infection and almost all of them involved HPV-6 and HPV-11 (Izadi et al., 2012; Jalilvand et al., 2014). Our study had rather similar findings that there was 100% HPV infection in RRP patients and HPV-6 and 11 were predominant genotypes. This result of the present study compared with the broader sample size and more importantly the broader age range of the previous documents could causes non-similar findings.
In the study of 55 RRP patients in Netherlands (Tjon et al., 2015), 76% (42/55) belong to HPV-6 and 24% (13/55) were HPV-11 which is slightly similar to our findings. Their great sample size obtained due to duration of more than 40 years follow up in a cohort study and including aged people but in comparison with our study by limited sample size, their results confirmed our findings.
In a study of Izadi et al., (2012) in Iranian RRP patients, 45% (13/29) HPV-6 and 55% (16/29) HPV-11 were reported and its differences with our findings could be attributed to the broader sample size. Although sample collection was done in the same city, all of our study patients were not from Iran and some were from Iraq. Study of Intakorn and Sonsuwan (Intakorn and Sonsuwan, 2014) in 15 Thai patients showed that in RRP patient, 40% (6/15) were HPV-6 and 60% (9/15) were HPV-11 and no co-infection was reported. Although, their sample collection was carried out for 24 months and 2.65±0.82 aged children were targeted for assays, in the present study we found that RRP is more common in our district if we put away our involved younger children specimens.
Actually, differences of various studies could be attributed to different methods of specimen collection, sensitivity of detection methods in use and other confounding or racial factors (Soheili et al., 2016) and the epidemiology of different diseases improve knowledge about them for using in health care planning and policy makers could adapt their country`s health policy via research-based evidence (Emadzadeh et al., 2017; Samet, 2000). Literature suggested that to reduce the burden of HPV infection especially in RRP patients, the quadrivalent HPV vaccine could be effective in 85% of RRP patients (Chirilă and Bolboacă, 2014; Tjon Pian Gi et al., 2016).
In conclusion, findings of this study demonstrated the higher rate of HPV infection in RRP patients and a significant increase in rate of HPV-6 in RRP patients when compared to previous studies in Iran. We suggest that further studies in this area should be done using broader patients and different age groups to better understanding of each genotype burden of HPV in RRP patients.
Conflict of interest
No.
Acknowledgments
All we thankful for technical assistant of Keyvan laboratory Tehran, Iran, personnel.
References
- Bonagura VR, Hatam LJ, Rosenthal DW, et al. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus-6 and-11. Apmis. 2010;118:455–70. doi: 10.1111/j.1600-0463.2010.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirilă M, Bolboacă SD. Clinical efficiency of quadrivalent HPV (types 6/11/16/18) vaccine in patients with recurrent respiratory papillomatosis. Eur Arch Oto-Rhino-Laryngol. 2014;271:1135–42. doi: 10.1007/s00405-013-2755-y. [DOI] [PubMed] [Google Scholar]
- Draganov P, Todorov S, Todorov I, Karchev T, Kalvatchev Z. Identification of HPV DNA in patients with juvenile-onset recurrent respiratory papillomatosis using SYBR®Green real-time PCR. Int J Pediatr Otorhinolaryngol. 2006;70:469–73. doi: 10.1016/j.ijporl.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Emadzadeh M, Shahidsales S, Bajgiran AM, et al. Head and neck cancers in North-east Iran: A 25 year survey. Iran J Otorhinolaryngol. 2017;29:137. [PMC free article] [PubMed] [Google Scholar]
- Farhadi M, Izadi F, Bahri M, et al. Contovir-a new adjuvant therapy in recurrent respiratory papillomatosis: A case study. Iran Red Crescent Med J. 2016 (Inpress) [Google Scholar]
- Gerein V, Rastorguev E, Gerein J, Draf W, Schirren J. Incidence, age at onset, and potential reasons of malignant transformation in recurrent respiratory papillomatosis patients:20 years experience. J Otolaryngol Head Neck Surg. 2005;132:392–4. doi: 10.1016/j.otohns.2004.09.035. [DOI] [PubMed] [Google Scholar]
- Intakorn P, Sonsuwan N. Human papillomatosis genotyping and severity in patients with recurrent respiratory papillomatosis. J Med Assoc Thai. 2014;97:136–41. [PubMed] [Google Scholar]
- Izadi F, Hamkar R, Ghanbari H, Abdolmotallebi F, Jahandideh H. The role of Human papilloma virus (HPV) genotyping in recurrent respiratory papillomatosis in Rasoul Akram Hospital. Med J Islam Repub Iran. 2012;26:90–3. [PMC free article] [PubMed] [Google Scholar]
- Jalilvand S, Shoja Z, Hamkar R. Human papillomavirus burden in different cancers in Iran: a systematic assessment. Asian Pac J Cancer Prev. 2014;15:7029–35. doi: 10.7314/apjcp.2014.15.17.7029. [DOI] [PubMed] [Google Scholar]
- Javanmard D, Namaei MH, Haghighi F, et al. The frequency and typing of Human Papilloma virus among women with normal and abnormal cytology in southern Khorasan, Eastern Iran. Jundishapur J Microbiol. 2017 (In Press) [Google Scholar]
- McClay JE. Recurrent respiratory papillomatosis. [Accessed June 22nd 2007];E Medicine. 2004 [Google Scholar]
- Salehi-Vaziri M, Sadeghi F, Bokharaei-Salim F, et al. The prevalence and genotype distribution of Human Papillomavirus in the genital tract of males in Iran. Jundishapur J Microbiol. 2015;8 doi: 10.5812/jjm.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi-Vaziri M, Sadeghi F, Hashemi FS, et al. Distribution of Human Papillomavirus genotypes in Iranian women according to the severity of the cervical lesion I. ran Red Crescent Med J. 2016;18:e24458. doi: 10.5812/ircmj.24458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM. Epidemiology and policy: the pump handle meets the new millennium. Epidemiol Rev. 2000;22:145–54. doi: 10.1093/oxfordjournals.epirev.a018013. [DOI] [PubMed] [Google Scholar]
- Sanchez GI, Jaramillo R, Cuello G, et al. Human papillomavirus genotype detection in recurrent respiratory papillomatosis (RRP) in Colombia. Head Neck. 2013;35:229–34. doi: 10.1002/hed.22953. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Soheili F, Heidary N, Rahbar M, et al. Human papillomavirus and its clinical relevance in oesophageal squamous cell carcinoma in a Kurdish population in the west of Iran. Infect Dis (Lond) 2016;48:270–73. doi: 10.3109/23744235.2015.1109134. [DOI] [PubMed] [Google Scholar]
- Stewart B, Wild CP. World cancer report 2014 World. 2016. www.who.int/cancer/publications/WRC_2014/en . ISBN:978-92-832-0429-9.
- Koochak A, Rakhshani N, Karbalaie Niya MH, et al. Mutation analysis of KRAS and BRAF genes in metastatic colorectal cancer: a First large scale study from Iran. Asian Pac J Cancer Prev. 2016;17:603–8. doi: 10.7314/apjcp.2016.17.2.603. [DOI] [PubMed] [Google Scholar]
- Ţiple C, Chirilă M, Dinescu FV, Cosgarea M. A case report of recurrent respiratory papillomatosis progressed into a loco-regionally advanced laryngeal carcinoma. Hum Vet Med. 2015;7 [Google Scholar]
- Tjon Pian Gi RE, San Giorgi M, Slagter-Menkema L, et al. Clinical course of recurrent respiratory papillomatosis: Comparison between aggressiveness of human papillomavirus-6 and human papillomavirus-11. Head Neck. 2015;37:1625–32. doi: 10.1002/hed.23808. [DOI] [PubMed] [Google Scholar]
- Tjon P, Gi R, San GMR, et al. Immunological response to quadrivalent HPV vaccine in treatment of recurrent respiratory papillomatosis. Eur Arch Otorhinolaryngol. 2016;273:3231–6. doi: 10.1007/s00405-016-4085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiatrak BJ, Wiatrak DW, Broker TR, Lewis L. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope. 2004;114:1–23. doi: 10.1097/01.mlg.000148224.83491.0f. [DOI] [PubMed] [Google Scholar]
- Yahyapour Y, Shamsi-Shahrabadi M, Mahmoudi M, et al. High-risk and low-risk human papillomavirus in esophageal squamous cell carcinoma at Mazandaran, Northern Iran. Pathol Oncol Res. 2013;19:385–91. doi: 10.1007/s12253-012-9590-0. [DOI] [PubMed] [Google Scholar]


