Abstract
Background
Patients with prior malignancies may be at increased risk for non-small cell lung cancer (NSCLC). However, the extent of risk is unknown for many cancer types and it is unclear who may benefit from screening.
Methods
The Surveillance, Epidemiology and End Results dataset from 1992-2012 was used to identify patients with prior malignancies who were diagnosed with NSCLC ≥6 months after their initial cancer. Standardized incidence ratios (SIR) for NSCLC were calculated as a ratio of observed to expected cases adjusted by person-years at risk. Cancers with SIR >1.0 had higher risk for NSCLC than expected. Analyses were stratified by sex, radiation, and histology.
Results
Among cancer survivors, 32,058 developed NSCLC. Smoking-related (lung, head and neck, bladder) and hematologic malignancies regardless of prior radiation therapy had the highest SIR for NSCLC (range 1.97-4.88). Prior colorectal and renal cancer survivors also had increased SIR for NSCLC (1.16 and 1.21, respectively). Women with previous pancreatic cancer treated with radiation, breast cancer with or without radiation, and those with thyroid cancer demonstrated higher SIR for lung adenocarcinoma. Men with prior irradiated prostate cancer also had elevated SIR (1.08, CI 1.01-1.15) for lung adenocarcinoma. Patients with melanoma, prostate or uterine cancer had lower SIR for NSCLC than expected.
Conclusions
Smoking-related malignancies had the highest risk for NSCLC. Radiation conferred elevated risk for NSCLC for certain cancers. Melanoma, prostate, uterine cancer survivors were at low risk for NSCLC. These results may help identify high-risk screening candidates in the growing population of cancer survivors.
Keywords: screening criteria, survivor, smoking, radiation
Introduction
Lung cancer mortality is reduced through screening with annual low-dose computed tomography in high-risk individuals1. Smoking and advanced age are the primary risk factors utilized by major health organizations such as the United States Preventative Services Task Force in selection criteria for lung cancer screening2. In addition, antecedent cancer has been shown to increase the probability of malignancy in patients with solitary pulmonary nodules who are older and have heavy smoking exposure (≥20 pack-years)3. The National Comprehensive Cancer Network (NCCN) and the American Association for Thoracic Surgery (AATS) have reduced the age and pack-year threshold for lung cancer screening candidates with a previous history of malignancy. The NCCN cites an increased risk for new lung cancer in patients with prior lung cancer, lymphoma, head and neck cancer, and other smoking-related cancers4 while the AATS deems any prior cancer history as sufficient for lowering age and smoking history criteria for screening eligibility5.
The literature on non-small cell lung cancer (NSCLC) risk among patients with previous malignancy is variable depending on initial cancer site. Prior NSCLC is a known risk factor for developing second primary NSCLC at estimated rates of 1-2% per year with increasing tobacco exposure conferring higher risk6, 7. Population-based and institutional studies have demonstrated that survivors of head and neck cancer as well as Hodgkin's lymphoma are also at increased risk of developing new primary lung cancer8, 9. However, it is unclear whether the antecedent cancer directly increases risk or whether confounding factors play a role. Smoking has been cited as the likely culprit behind new NSCLC primaries in previous head and neck cancer patients whereas radiation and chemotherapy are associated with increased risk of primary lung cancer in Hodgkin's lymphoma patients. One study using a Markov model has suggested that lung cancer screening in Hodgkin's lymphoma survivors may benefit survival, increase quality of life, and be cost-effective10.
The risk of developing NSCLC is reportedly increased in bladder cancer patients but is incompletely described in patients with other urologic malignancies such as prostate or renal cancers 11, 12 and in those with previous cervical cancer13, 14. Research on breast cancer survivors that develop NSCLC is more abundant; however, the extent to which prior breast cancer confers increased risk for NSCLC independent of age, smoking, and radiation therapy remains unclear15-21.
As the number of cancer survivors increases, understanding their risk of developing new primary NSCLC, the most common cause of cancer death, is important22. Based on current screening guidelines from major health organizations such as the NCCN and AATS, centers may be screening all cancer survivors who meet reduced age and smoking history criteria, regardless of risk for NSCLC. Better understanding of which malignancies are associated with greater risk of developing lung cancer is necessary to identify which high-risk cancer survivors should be screened. Since the risk of NSCLC in patients with prior malignancy is unknown for many cancer types, we used a population-based dataset to study the incidence of primary NSCLC among survivors of twelve common malignancies as stratified by sex, radiation history, and histology. This has important implications for refining lung cancer screening guidelines, some of which include a history of any prior malignancy among screening criteria, which may result in unnecessary screening in cancer survivors at low risk for developing NSCLC.
Materials and Methods
The Surveillance, Epidemiology and End Results 13 (SEER13) dataset represents approximately 13.4% of the United States population and is geographically defined as San Francisco-Oakland, Connecticut, Metropolitan Detroit, Hawaii, Iowa, New Mexico, Seattle/Puget Sound, Utah, Metropolitan Atlanta, San Jose-Monterey, Los Angeles and Rural Georgia. Alaska was excluded from the current study because incidence data were not available23. Using SEER13, we identified a cohort of 31,062 primary NSCLC patients from 1992-2012 who had at least 6 months of event-free follow-up after diagnosis of a single previous malignancy at one of twelve common cancer sites. There were also 942 NSCLC patients that had two previous primaries and 54 that had three or more previous primaries of interest. We included all patients in our analyses (n=32,058). NSCLC cases were identified by International Classification of Diseases for Oncology Third Edition site and morphology codes (Supplemental Table 1).
Previous smoking-related cancer sites included lung and bronchus, bladder, and head and neck, which comprised of all cancers arising from the oral cavity, pharynx, nose, nasal cavity, middle ear, and larynx. Leukemia and lymphomas behaved similarly on analyses and were grouped together. We also studied the most common solid tumors including those of the breast, prostate, colon and rectum, uterus, kidney, thyroid, and pancreas as well as melanoma of the skin. NSCLC patients with history of less common malignancies were excluded from analyses due to small sample size. Analyses were stratified by sex, previous radiation therapy for initial cancer, and NSCLC histology. In the analyses for radiation, 408 patients with unknown radiation treatment status for the first primary were excluded, resulting in 31,543 patients with known radiation treatment status for their initial cancer.
Statistical Methods
Patient characteristics were compared between patients without a history of cancer and those who had a previous malignancy of interest. Categorical data were compared using chi square tests for nominal data and Jonckheere-Terpstra non–parametric tests for ordinal data. Age, as a continuous variable, was compared across groups using the student's T test.
Standardized incidence ratios (SIRs), defined as the ratio of observed over expected (O/E) rates of NSCLC cancers were calculated and adjusted by person-years at risk24, 25. The observed value was derived by looking at those patients who developed NSCLC (numerator) after the first primary of interest and was divided by all patients diagnosed with the same primary but who did not develop NSCLC (denominator). This rate incorporated the amount of time (in person-years) that the patients were at risk. In our case, this would be the amount of time from the first primary diagnosis to the subsequent NSCLC diagnosis for the numerator or the amount of time the patient was followed prior to death or last contact.
The “expected” rate was the number of NSCLC events expected to be experienced by the general population within the accumulated person-time at risk for the NSCLC cohort. The rate associated with the general population was further refined, however, to adjust for potential confounding demographic factors by selecting a subset of the population that represented the same breakdown as the cancer cohort in terms of known demographic confounders such as age, sex, race, Hispanic origin, location of residency, and time period of ascertainment. The reference rates needed for standardization with cancer cases were already incorporated into the SEER*Stat MP-SIR software module as the result of collaboration between SEER and the National Center for Health Statistics and the Census Bureau.
Confidence intervals (CIs) were calculated using the exact method and were considered statistically significant if the 99% CI did not include 1.0 26. Cancers for which the upper boundary of the CI was <1.0 were considered to be significantly lower in risk for NSCLC than expected, whereas cancers for which the lower boundary of the CI was >1.0 were considered to be significantly higher in risk for NSCLC than expected. For sex-specific sites, SIRs applied to only the sex for which the site of first primary was relevant. All analyses were performed using SAS software (SAS Institute, Cary, NC) and SEER*Stat software (version 8.2.1, 04.07.15 build).
Results
Table 1 compares the characteristics of 31,062 NSCLC patients with a single previous malignancy to those of 240,044 NSCLC patients without prior cancer. Three-quarters of prior cancer patients were diagnosed with NSCLC after 2002. Those with previous malignancy were more frequently older and male. Among NSCLC patients with previous malignancy, there were 15,368 (49.5%) adenocarcinomas, 8,856 (28.5%) squamous cell carcinomas, 1,279 (4.1%) large cell carcinomas, and 5,559 (17.9%) other NSCLC histologies. These patients were more likely to be diagnosed with local disease and undergo curative surgery, and less likely to receive radiation for NSCLC compared with patients without previous cancer history. The median time to NSCLC diagnosis after initial primary cancer was 60 months (IQR, 30-105 months).
Table 1.
NSCLC patient characteristics by previous cancer status.
| No Previous Cancer N=240,044 (%) | Single Previous Cancer N=31,062 (%) | p-value | ||
|---|---|---|---|---|
| Diagnosis Time Period | 1992-1996 | 53,254 (22.2) | 2,594 (8.4) | <.0001 |
| 1997-2001 | 53,150 (22.1) | 5,811 (18.7) | ||
| 2002-2006 | 60,576 (25.2) | 9,357 (30.1) | ||
| 2007-2012 | 73,064 (30.4) | 13,300 (42.8) | ||
|
| ||||
| Age | Median (IQR†) | 69 (60-76) | 73 (66-79) | <.0001 |
|
| ||||
| Sex | Men | 133,753 (55.7) | 19,014 (61.2) | <.0001 |
| Women | 106,291 (44.3) | 12,048 (38.8) | ||
|
| ||||
| Race/Ethnicity | White | 188,066 (78.3) | 25,757 (82.9) | <.0001 |
| Black | 28,988 (12.1) | 3,437 (11.1) | ||
| API | 21,575 (9.0) | 1,785 (5.7) | ||
| AI/AK | 831 (0.3) | 75 (0.2) | ||
| Unknown | 584 (0.2) | 8 (0.0) | ||
|
| ||||
| Histology | Squamous | 64,663 (26.9) | 8,856 (28.5) | <.0001 |
| Adenocarcinoma | 118,633 (49.4) | 15,368 (49.5) | ||
| Large Cell | 14,906 (6.2) | 1,279 (4.1) | ||
| NSCLC NOS | 41,842 (17.4) | 5,559 (17.9) | ||
|
| ||||
| Summary Stage | Local | 45,607 (19.0) | 9,952 (32.0) | <.0001 |
| Regional | 62,746 (26.1) | 8,419 (27.1) | ||
| Distant | 121,533 (50.6) | 11,577 (37.3) | ||
| In Situ | 201 (0.1) | 11 (0.0) | ||
| Unknown | 9,957 (4.1) | 1,103 (3.6) | ||
|
| ||||
| Grade | Well | 12,318 (5.1) | 2,314 (7.4) | <.0001 |
| Moderate | 42,229 (17.6) | 6,941 (22.3) | ||
| Poor | 74,064 (30.9) | 9,163 (29.5) | ||
| Undifferentiated | 9,577 (4.0) | 857 (2.8) | ||
| Unknown | 101,856 (42.4) | 11,787 (37.9) | ||
|
| ||||
| Surgery | None | 159,391 (66.4) | 18,219 (58.7) | <.0001 |
| Curative | 55,392 (23.1) | 9,939 (32.0) | ||
| Other | 24,027 (10.0) | 2,780 (8.9) | ||
| Unknown | 1,234 (0.5) | 124 (0.4) | ||
|
| ||||
| Radiation | None | 135,582 (56.5) | 20,649 (66.5) | <.0001 |
| Radiation | 100,564 (41.9) | 10,011 (32.2) | ||
| Unknown | 3,898 (1.6) | 402 (1.3) | ||
IQR=Interquartile range.
Abbr: API=Asian/Pacific Islanders, AI/AN=American Indians/Alaska Natives.
Among 32,058 NSCLC patients who had one or more previous cancers, prostate was the most common initial site (25.4%), followed by breast (15.9%), lung (14.4%), colorectal (10.5%), head and neck (9.5%), bladder (8.8%), leukemia or lymphoma (5.5%), melanoma (4.4%), uterus (2.2%), kidney (2.0%), thyroid (1.0%), and pancreas (0.3%). Patients with former smoking-related cancers had the highest standardized incidence ratios of observed to expected cases of NSCLC. These include patients with prior cancers of lung (SIR 4.88, CI 4.70-5.07), head and neck (SIR 4.00, 99% CI 3.81-4.19), and bladder (SIR 1.97, CI 1.87-2.07). In order of decreasing magnitude, patients with previous pancreatic, hematologic, renal, colorectal, and breast malignancies also had significantly elevated SIRs for NSCLC (SIR range 1.09-1.44, Table 2, Figure 1). In contrast, uterine cancer, prostate cancer, and melanoma survivors had significantly lower than expected NSCLC incidence rates (SIR range 0.84-0.90).
Table 2.
Risk of Primary NSCLC after Previous Malignancy by Site of Initial Primary.
| Previous Primary Site | Observed N=32,058† | Expected N=24,852 | SIR (99% CI) |
|---|---|---|---|
| Head and Neck | 3,047 | 762 | 4 (3.81–4.19)** |
| Colon and Rectum | 3,367 | 2,899 | 1.16 (1.11–1.21)** |
| Pancreas | 97 | 67 | 1.44 (1.09–1.86)** |
| Lung and Bronchus | 4,624 | 947 | 4.88 (4.70–5.07)** |
| Uterus | 713 | 790 | 0.90 (0.82–0.99)* |
| Prostate | 8,139 | 9,491 | 0.86 (0.83–0.88)* |
| Bladder | 2,834 | 1,440 | 1.97 (1.87–2.07)** |
| Kidney | 647 | 534 | 1.21 (1.09–1.34)** |
| Thyroid | 321 | 297 | 1.08 (0.93–1.24) |
| Breast | 5,085 | 4,678 | 1.09 (1.05–1.13)** |
| Melanoma | 1,425 | 1,691 | 0.84 (0.79–0.90)* |
| Leukemia/Lymphoma | 1,759 | 1,256 | 1.4 (1.32–1.49)** |
Total observed cases include 31,062 patients with one previous primary, 942 with two previous primaries, and 54 with three or more previous cancer primaries
SIR = standardized incidence ratio
Incidence rate significantly lower than expected (using exact method and 99% CIs).
Incidence rate significantly higher than expected (using exact method and 99% CIs).
Figure 1. Risk of primary non-small cell lung cancer (NSCLC) in patients with previous malignancy by initial primary site.
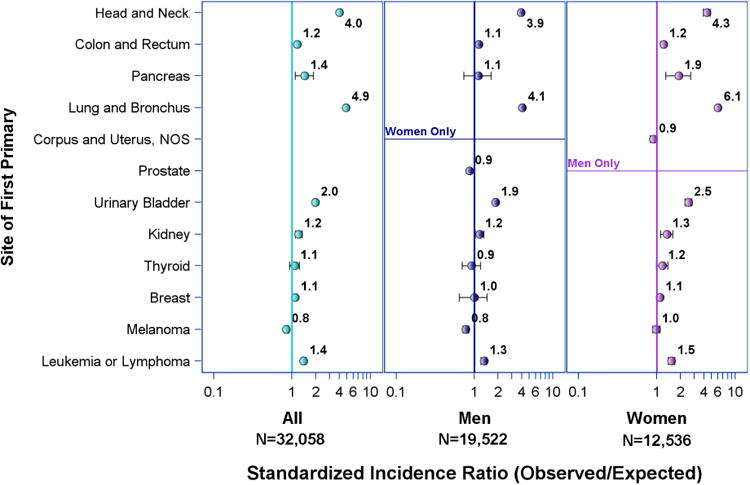
Figure 1 compares the incidence rates for primary NSCLC after previous cancer in men versus women. A higher than expected rate of NSCLC in prior pancreatic cancer patients was only significant in women (SIR 1.90, CI 1.30-2.68). In fact, women consistently had higher observed NSCLC rates than men regardless of previous cancer site. When stratified by race, black women with previous breast cancer demonstrated significantly higher SIR for NSCLC (SIR 1.33, CI 1.02-1.69) whereas white women with previous breast cancer did not (SIR 1.05, CI 0.96-1.14). In Table 3, SIRs for NSCLC in 31,543 patients with previous history of malignancy and known radiation status are displayed according to presence or absence of radiation therapy for the initial cancer. Observed NSCLC incidence rates were significantly higher than expected in survivors of lung, head and neck, bladder, colorectal, and hematologic malignancies regardless of radiation therapy. Additionally, only patients with prior pancreatic and breast cancer treated with radiation had significantly higher incidence rates of NSCLC than expected (SIR 2.54, CI 1.70-3.63 and 1.14 CI 1.08-1.20, respectively). Pancreatic and breast cancer survivors without prior radiation therapy did not have significantly increased SIRs for NSCLC.
Table 3.
Risk of Primary NSCLC in Patients with Previous Malignancy by Site of Initial Primary and Radiation Status (n=31,543)†.
| No Radiation | Radiation | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| First Primary Site | Observed N=21,206 | Expected N=17,076 | SIR (99% CI) | Observed N=10,337 | Expected N=7,333 | SIR (99% CI) |
| Head and Neck | 867 | 310 | 2.8 (2.56–3.05)** | 2,130 | 438 | 4.86 (4.59–5.14)** |
| Colon and Rectum | 2,928 | 2,577 | 1.14 (1.08–1.19)** | 407 | 298 | 1.37 (1.20–1.55)** |
| Pancreas | 48 | 46 | 1.04 (0.69–1.49) | 49 | 19 | 2.54 (1.70–3.63)** |
| Lung and Bronchus | 3,482 | 660 | 5.27 (5.05–5.51)** | 1,096 | 269 | 4.07 (3.76–4.40)** |
| Uterus | 492 | 578 | 0.85 (0.76–0.96)* | 215 | 200 | 1.07 (0.89–1.28) |
| Prostate | 4,526 | 5,777 | 0.78 (0.75–0.81)* | 3,419 | 3,497 | 0.98 (0.94–1.02) |
| Bladder | 2,766 | 1,412 | 1.96 (1.86–2.06)** | 65 | 25 | 2.56 (1.81–3.49)** |
| Kidney | 637 | 526 | 1.21 (1.09–1.34)** | 6 | 7 | 0.85 (0.22–2.22) |
| Thyroid | 157 | 151 | 1.04 (0.84–1.28) | 161 | 143 | 1.13 (0.91–1.38) |
| Breast | 2,409 | 2,327 | 1.04 (0.98–1.09) | 2,526 | 2,218 | 1.14 (1.08–1.20)** |
| Melanoma | 1,401 | 1,663 | 0.84 (0.79–0.90)* | 23 | 25 | 0.94 (0.51–1.57) |
| Leukemia/Lymphoma | 1,493 | 1,049 | 1.42 (1.33–1.52)** | 240 | 194 | 1.24 (1.04–1.46)** |
SIR = standardized incidence ratio
Excluding patients with unknown radiation status, n=31,543
Incidence rate significantly lower than expected (using exact method and 99% CIs).
Incidence rate significantly higher than expected (using exact method and 99% CIs).
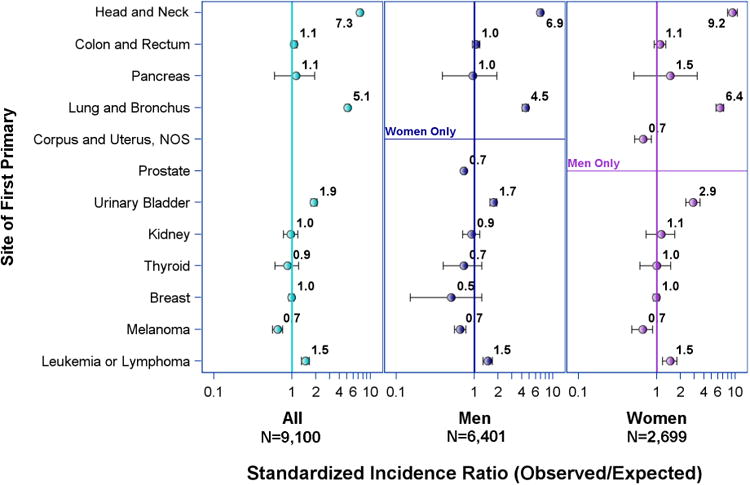
SIRs for lung adenocarcinoma and squamous cell carcinoma in men and women as a second primary following primary cancer of other sites are shown in Figures 2 and 3, respectively. The incidence rates for both types of NSCLC after previous lung, head and neck, bladder, and hematologic malignancy were similar. Former colorectal, pancreatic, renal, thyroid, and breast cancer patients had higher SIR for lung adenocarcinoma (SIR range 1.1-1.9) but not for squamous cell carcinoma (SIR range 0.9-1.1, CI crosses 1.0 for all sites). Specifically, women with prior pancreatic cancer had significantly elevated incidence of lung adenocarcinoma (SIR 2.37, CI 1.48-3.59) while men with prior pancreatic cancer did not (SIR 1.43, CI 0.80-2.34). The same significantly higher SIRs for lung adenocarcinoma were observed in women with previous thyroid and breast cancer (SIR 1.31, CI 1.05-1.62 and 1.14, CI 1.09-1.20, respectively). The latter was observed with or without prior radiation therapy (Supplemental Table 2). Men who had prior prostate cancer treated with radiation had a significantly elevated SIR for lung adenocarcinoma (1.08, CI 1.01-1.15) while those with the same previous cancer not treated with radiation had a significantly lower than expected SIR for lung adenocarcinoma (0.90, CI 0.85-0.95). Radiation did not significantly affect incidence rates of lung squamous cell carcinoma in those with previous malignancy at any site (Supplemental Table 3).
Figure 2. Risk of primary lung adenocarcinoma in patients with previous malignancy by initial primary site.
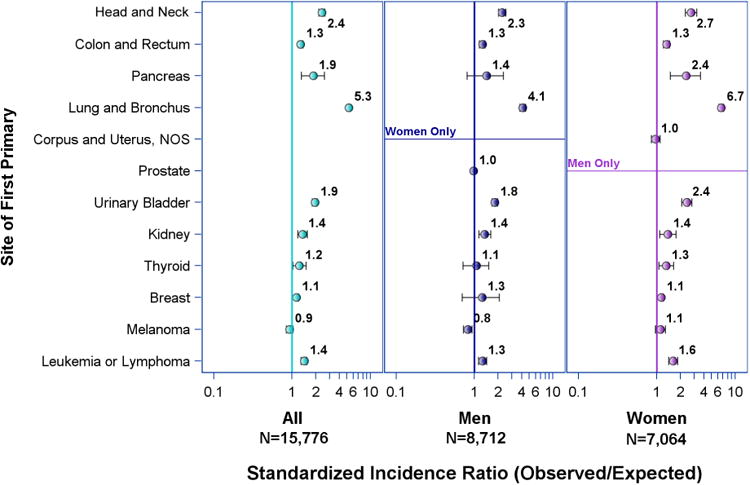
Figure 3. Risk of primary lung squamous cell carcinoma in patients with previous malignancy by initial primary site.
Discussion
Our results support the literature in concluding that previous smoking-related and hematologic malignancies have increased incidence of NSCLC regardless of sex, or prior radiation therapy. The NCCN and AATS nonspecifically incorporate history of malignancy in lung cancer screening guidelines in addition to reducing age criterion to 50 from 55 years and pack-years criterion to 20 from 30. However, the extent to which many extrapulmonary malignancies pose a risk for subsequent NSCLC independent of confounders such as smoking history, prior cancer therapies, and other lung cancer risk factors is incompletely understood. Our study sought to identify which prior cancer sites were associated with higher NSCLC rates and whether sex or prior radiation therapy affected the risk. Similar to previous descriptions, we found that NSCLC patients with cancer history were older and more likely to have localized disease27. This is likely due to active surveillance practices in many cancer survivors. These patients also more often treated with surgery and not radiation compared to NSCLC patients without previous malignancy. This was reported in breast cancer survivors who developed NSCLC and was also postulated to be due to heightened surveillance25.
We observed that patients with prior colorectal, pancreatic, renal, and breast cancer had significantly higher rates of NSCLC, specifically adenocarcinoma. The reasons for this are unclear but likely multifactorial and may be attributed to tumor biology and patient risk factors including smoking history and for postmenopausal women, hormone exposure28. In addition, our data suggested that the increased risk in prior pancreatic cancer patients was limited to women who received prior radiation therapy. We also demonstrated a significantly higher incidence of lung adenocarcinoma in patients with previous breast cancer regardless of radiation therapy as well as those with prior thyroid cancer. However, we discovered that three common previous malignancies (uterine, prostate, melanoma) were not associated with increased rates of new NSCLC and survivors of these cancers may not benefit from specialized screening. Interestingly, when stratified by radiation and histology, men with history of prostate cancer treated with radiation had higher than expected incidence of lung adenocarcinoma, but those without prior radiation therapy had lower than expected incidence. Our data also suggested that women with previous cancers had a higher risk for subsequent NSCLC than men. The effect of hormone exposure on lung cancer risk among women is not well defined but may be a contributing factor in the elevated risk of second primary lung cancer in women with previous malignancy, especially breast cancer29.
In the absence of individual smoking data in SEER, we were unable to determine the extent to which a previous cancer affected the risk for new NSCLC independent of smoking history. This constituted a significant limitation to our study. Other aspects of our study that were limiting include the lack of information on chemotherapy as well as the exclusion of patients with unknown radiation status when comparing NSCLC rates. When our analysis was stratified by histology, sex, and radiation status, the sample sizes for patients with certain previous malignancies became too small to show any significance in NSCLC incidence. Additionally, we included only the twelve most common previous malignancy sites in our analysis and therefore, cannot exclude the hypothesis that less common types of malignancy may increase risk for new NSCLC. Additional research with pooled institutional or larger population databases is necessary to evaluate the risk of NSCLC in survivors of less common malignancies.
To our knowledge, the use of previous malignancy in screening criteria has never been scrutinized. Although our conclusions are limited by lack of individual smoking history, we report NSCLC incidence and risk using standardized incidence ratios among previous cancer types, some of which have never been previously reported. Radiation and sex also factor importantly into the risk for NSCLC in certain cancer survivors. Given the limitations of our study, we seek to inspire further research with the collection of patient-level tobacco exposure data to allow for more evidence-based evaluation of the appropriate smoking threshold at which patients with previous history of malignancy should be screened for lung cancer. We hope our data provides some granularity for understanding who may be at increased risk for NSCLC having survived a previous malignancy and perhaps even more importantly, who may not be at risk and therefore do not need additional screening. Currently, screening in the growing cancer survivor population should be considered on an individual basis with careful consideration of smoking history, prior cancer therapies, time since previous cancer, and other lung cancer risk factors. For active smokers with history of antecedent cancer, every attempt should be made to promote smoking cessation during the screening process to eliminate the modifiable risk in those susceptible to a second primary lung cancer.
Conclusions
Using the SEER database and standardized incidence ratios, we studied the risk of NSCLC in survivors of twelve common malignancies. Our findings concurred with results in the literature, that survivors of smoking-related malignancies as well as those of breast and hematologic malignancies had increased incidence of subsequent NSCLC. Additionally, patients with previous pancreatic, renal, and colorectal malignancies also had elevated incidences of NSCLC. In contrast, those with previous uterine cancer, prostate cancer, and melanoma did not demonstrate increased NSCLC incidence rates. The risk of NSCLC in survivors of previous malignancy was affected by sex and prior radiation therapy and was most significant for development of a second lung adenocarcinoma. This data may have important implications on current lung cancer screening criteria and further research with individual smoking data is warranted to determine what thresholds these patients may benefit from screening.
Supplementary Material
Clinical Practice Points.
Certain health care organizations utilize specialized lung cancer screening criteria that include history of prior malignancy in addition to reduced age and smoking thresholds. Although previous studies have reported increased incidence of NSCLC in survivors of previous lung cancer, head and neck cancer, bladder cancer, breast cancer, and lymphoma, there is less known about the risk of NSCLC in survivors of other common malignancies.
Our study uses a large population database to study the incidence of NSCLC among survivors of thirteen common malignancies. We found that in addition to what has been previously described, survivors of pancreatic, renal, and colorectal cancers also demonstrate increased incidence for NSCLC. However, patients with previous prostate cancer, uterine cancer, and melanoma did not demonstrate higher incidences of NSCLC. Furthermore, we found that radiation therapy affected whether NSCLC was increased in certain malignancies such as pancreatic and breast. The increased incidence for second NSCLC primary was more prominent in cancer survivors who were women and more frequently lung adenocarcinoma than squamous cell carcinoma.
Without smoking data, it is difficult to determine whether increased risk of NSCLC in cancer survivors is due to previous malignancy or to smoking. However, our data may be useful when considering which patients with previous malignancy may or may not benefit from lung cancer screening. Additional research is necessary to determine what thresholds other lung cancer risk factors such as age and smoking history should be used for lung cancer screening criteria in the growing population of cancer survivors.
Acknowledgments
Research reported in this publication was supported by the NCI/NIH (5K12CA001727-20) and work performed by the Biostatistics Core was funded by NCI/NIH (P30CA33572). The content is solely the responsibility of the authors and does not reflect the official views of the NIH. We also acknowledge the Baum Family Foundation for its support of this research.
Abbreviations
- NSCLC
Non-small cell lung cancer
- SIR
Standardized incidence ratio
- NCCN
National comprehensive cancer network
- AATS
American Association for Thoracic Surgery
- SEER
Surveillance, Epidemiology and End Results
- CI
Confidence interval
Footnotes
This research was presented in part at the 2016 annual Society of Thoracic Surgeons meeting in Phoenix, Arizona.
Disclosures: Dr. Raz is a consultant for Cireca, LLC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Lung Screening Trial Research T. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moyer VA Force USPST. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 3.Mery CM, Pappas AN, Bueno R, et al. Relationship between a history of antecedent cancer and the probability of malignancy for a solitary pulmonary nodule. Chest. 2004;125:2175–2181. doi: 10.1378/chest.125.6.2175. [DOI] [PubMed] [Google Scholar]
- 4.Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. Journal of the National Comprehensive Cancer Network: JNCCN. 2012;10:240–265. doi: 10.6004/jnccn.2012.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. The Journal of thoracic and cardiovascular surgery. 2012;144:33–38. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 6.Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. Journal of the National Cancer Institute. 1998;90:1335–1345. doi: 10.1093/jnci/90.18.1335. [DOI] [PubMed] [Google Scholar]
- 7.Boyle JM, Tandberg DJ, Chino JP, D'Amico TA, Ready NE, Kelsey CR. Smoking history predicts for increased risk of second primary lung cancer: a comprehensive analysis. Cancer. 2015;121:598–604. doi: 10.1002/cncr.29095. [DOI] [PubMed] [Google Scholar]
- 8.Milano MT, Peterson CR, 3rd, Zhang H, Singh DP, Chen Y. Second primary lung cancer after head and neck squamous cell cancer: population-based study of risk factors. Head & neck. 2012;34:1782–1788. doi: 10.1002/hed.22006. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim EM, Kazkaz GA, Abouelkhair KM, et al. Increased risk of second lung cancer in Hodgkin's lymphoma survivors: a meta-analysis. Lung. 2013;191:117–134. doi: 10.1007/s00408-012-9418-4. [DOI] [PubMed] [Google Scholar]
- 10.Das P, Ng AK, Earle CC, Mauch PM, Kuntz KM. Computed tomography screening for lung cancer in Hodgkin's lymphoma survivors: decision analysis and cost-effectiveness analysis. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2006;17:785–793. doi: 10.1093/annonc/mdl023. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki K, Satoh H, Kagohashi K, et al. Lung cancer patients with previous or simultaneous urologic cancers. Medical oncology. 2008;25:443–446. doi: 10.1007/s12032-008-9063-1. [DOI] [PubMed] [Google Scholar]
- 12.Rusthoven KE, Flaig TW, Raben D, Kavanagh BD. High incidence of lung cancer after non-muscle-invasive transitional cell carcinoma of the bladder: implications for screening trials. Clinical lung cancer. 2008;9:106–111. doi: 10.3816/CLC.2008.n.016. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi AK, Kleinerman RA, Hildesheim A, et al. Second cancers after squamous cell carcinoma and adenocarcinoma of the cervix. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:967–973. doi: 10.1200/JCO.2008.18.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaturvedi AK, Engels EA, Gilbert ES, et al. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. Journal of the National Cancer Institute. 2007;99:1634–1643. doi: 10.1093/jnci/djm201. [DOI] [PubMed] [Google Scholar]
- 15.Deutsch M, Land SR, Begovic M, Wieand HS, Wolmark N, Fisher B. The incidence of lung carcinoma after surgery for breast carcinoma with and without postoperative radiotherapy. Results of National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trials B-04 and B-06. Cancer. 2003;98:1362–1368. doi: 10.1002/cncr.11655. [DOI] [PubMed] [Google Scholar]
- 16.Grantzau T, Thomsen MS, Vaeth M, Overgaard J. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2014;111:366–373. doi: 10.1016/j.radonc.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman EL, Jacobson JS, Hershman DL, Desai M, Neugut AI. Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:392–398. doi: 10.1200/JCO.2007.13.3033. [DOI] [PubMed] [Google Scholar]
- 18.Lorigan P, Califano R, Faivre-Finn C, Howell A, Thatcher N. Lung cancer after treatment for breast cancer. The Lancet Oncology. 2010;11:1184–1192. doi: 10.1016/S1470-2045(10)70056-5. [DOI] [PubMed] [Google Scholar]
- 19.Prochazka M, Granath F, Ekbom A, Shields PG, Hall P. Lung cancer risks in women with previous breast cancer. European journal of cancer. 2002;38:1520–1525. doi: 10.1016/s0959-8049(02)00089-8. [DOI] [PubMed] [Google Scholar]
- 20.Neugut AI, Murray T, Santos J, et al. Increased risk of lung cancer after breast cancer radiation therapy in cigarette smokers. Cancer. 1994;73:1615–1620. doi: 10.1002/1097-0142(19940315)73:6<1615::aid-cncr2820730612>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Zablotska LB, Neugut AI. Lung carcinoma after radiation therapy in women treated with lumpectomy or mastectomy for primary breast carcinoma. Cancer. 2003;97:1404–1411. doi: 10.1002/cncr.11214. [DOI] [PubMed] [Google Scholar]
- 22.Travis LB. The epidemiology of second primary cancers. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:2020–2026. doi: 10.1158/1055-9965.EPI-06-0414. [DOI] [PubMed] [Google Scholar]
- 23.Surveillance E End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2013 Sub (1973-2011 varying) - Linked To County Attributes - Total U.S., 1969-2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014 (updated 5/7/2014), based on the November 2013 submission www.seer.cancer.gov.
- 24.Davis EJ, Beebe-Dimmer JL, Yee CL, Cooney KA. Risk of second primary tumors in men diagnosed with prostate cancer: a population-based cohort study. Cancer. 2014;120:2735–2741. doi: 10.1002/cncr.28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milano MT, Strawderman RL, Venigalla S, Ng K, Travis LB. Non-small-cell lung cancer after breast cancer: a population-based study of clinicopathologic characteristics and survival outcomes in 3529 women. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014;9:1081–1090. doi: 10.1097/JTO.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 26.Sahai H, K A. SieM, techniques, and applications. Boca Raton: CRC Press; 1996. [Google Scholar]
- 27.Pages PB, Mordant P, Cazes A, et al. Prognosis of lung cancer resection in patients with previous extra-respiratory solid malignancies. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2013;44:534–538. doi: 10.1093/ejcts/ezt031. [DOI] [PubMed] [Google Scholar]
- 28.Baik CS, Strauss GM, Speizer FE, Feskanich D. Reproductive factors, hormone use, and risk for lung cancer in postmenopausal women, the Nurses' Health Study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:2525–2533. doi: 10.1158/1055-9965.EPI-10-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pesatori AC, Carugno M, Consonni D, et al. Hormone use and risk for lung cancer: a pooled analysis from the International Lung Cancer Consortium (ILCCO) British journal of cancer. 2013;109:1954–1964. doi: 10.1038/bjc.2013.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


