Abstract
BACKGROUND
On the basis of data from a phase 2 trial that compared the checkpoint inhibitor ipilimumab at doses of 0.3 mg, 3 mg, and 10 mg per kilogram of body weight in patients with advanced melanoma, this phase 3 trial evaluated ipilimumab at a dose of 10 mg per kilogram in patients who had undergone complete resection of stage III melanoma.
METHODS
After patients had undergone complete resection of stage III cutaneous melanoma, we randomly assigned them to receive ipilimumab at a dose of 10 mg per kilogram (475 patients) or placebo (476) every 3 weeks for four doses, then every 3 months for up to 3 years or until disease recurrence or an unacceptable level of toxic effects occurred. Recurrence-free survival was the primary end point. Secondary end points included overall survival, distant metastasis–free survival, and safety.
RESULTS
At a median follow-up of 5.3 years, the 5-year rate of recurrence-free survival was 40.8% in the ipilimumab group, as compared with 30.3% in the placebo group (hazard ratio for recurrence or death, 0.76; 95% confidence interval [CI], 0.64 to 0.89; P<0.001). The rate of overall survival at 5 years was 65.4% in the ipilimumab group, as compared with 54.4% in the placebo group (hazard ratio for death, 0.72; 95.1% CI, 0.58 to 0.88; P = 0.001). The rate of distant metastasis–free survival at 5 years was 48.3% in the ipilimumab group, as compared with 38.9% in the placebo group (hazard ratio for death or distant metastasis, 0.76; 95.8% CI, 0.64 to 0.92; P = 0.002). Adverse events of grade 3 or 4 occurred in 54.1% of the patients in the ipilimumab group and in 26.2% of those in the placebo group. Immune-related adverse events of grade 3 or 4 occurred in 41.6% of the patients in the ipilimumab group and in 2.7% of those in the placebo group. In the ipilimumab group, 5 patients (1.1%) died owing to immune-related adverse events.
CONCLUSIONS
As adjuvant therapy for high-risk stage III melanoma, ipilimumab at a dose of 10 mg per kilogram resulted in significantly higher rates of recurrence-free survival, overall survival, and distant metastasis–free survival than placebo. There were more immune-related adverse events with ipilimumab than with placebo. (Funded by Bristol-Myers Squibb; ClinicalTrials.gov number, NCT00636168, and EudraCT number, 2007-001974-10.)
IPILIMUMAB, A FULLY HUMAN MONOCLONAL antibody that blocks cytotoxic T-lymphocyte antigen 4 (CTLA-4) to augment anti-tumor immune responses, was approved by the Food and Drug Administration (FDA) and the European Medicines Agency in 2011 at a dose of 3 mg per kilogram of body weight for the treatment of advanced melanoma.1,2 On the basis of data from a phase 2 trial that indicated the potential for a dose of 10 mg per kilogram to have higher efficacy than the dose of 0.3 mg or 3 mg per kilogram in patients with advanced melanoma, although at a cost of more toxic effects,3,4 we conducted a phase 3 trial (European Organization for Research and Treatment of Cancer [EORTC] 18071) of ipilimumab at a dose of 10 mg per kilogram in patients who had resected regional lymph node–positive (stage III) melanoma with a high risk of recurrence.
The likelihood of systemic metastatic disease among patients with stage III melanoma correlates closely with microscopic versus palpable nodal disease and with the number of positive nodes.5–7 The population of patients with stage III melanoma is heterogeneous, with disease-specific survival rates of 78% among patients with stage IIIA disease, 59% among those with stage IIIB disease, and 40% among those with stage IIIC disease.5–7 Even within the population of patients who have sentinel node–positive cancer, heterogeneity is remarkable and correlates closely with tumor load in the sentinel node (as defined by the Rotterdam criteria; see the Supplementary Appendix, available with the full text of this article at NEJM.org).8–10 Patients with a metastasis of more than 1 mm in the greatest dimension have a significantly higher risk of recurrence or death than those with a metastasis of 1 mm or less in the greatest dimension.8–10
We previously reported the primary results of the EORTC 18071 phase 3 trial in which we compared adjuvant ipilimumab with placebo in patients with resected stage III melanoma.11 At a median follow-up of 2.7 years, adjuvant ipilimumab was associated with significantly prolonged recurrence-free survival, the primary end point, as compared with placebo (hazard ratio, 0.75; P = 0.001). The results on the global health scale, which was the primary health-related quality-of-life end point, were not affected by ipilimumab.12 Approval from the FDA was granted in 2015 on the basis of the results of this trial. The effect of ipilimumab on overall survival and distant metastasis–free survival is important, given that the only other approved systemic therapy in the context of adjuvant therapy, interferon alfa, has a marginal effect on overall survival.13–15 Here, we report, at a median follow-up of 5.3 years, the efficacy of adjuvant therapy with ipilimumab on all survival end points in patients with high-risk stage III melanoma after complete lymph-node dissection.
METHODS
PATIENTS
Eligible patients were 18 years of age or older with histologically confirmed cutaneous melanoma that was metastatic to regional lymph nodes. According to the American Joint Committee on Cancer 2009 classification, patients had stage IIIA melanoma (patients with N1a cancer [i.e., only one node involved with micrometastasis] had to have at least one metastasis measuring >1 mm in the greatest dimension) or stage IIIB or IIIC melanoma with no in-transit metastases (i.e., growing >2 cm away from the primary tumor but before reaching the nearest lymph node).2 Complete regional lymphadenectomy was required within 12 weeks before randomization. Exclusion criteria included an Eastern Cooperative Oncology Group (ECOG) performance-status score of more than 1 (on a scale from 0 to 5, with higher numbers indicating greater disability), autoimmune disease, uncontrolled infection, substantial cardiovascular disease (New York Heart Association functional class III or IV), a lactate dehydrogenase level of more than 2 times the upper limit of the normal range, use of systemic glucocorticoids, and previous systemic therapy for melanoma.
TRIAL DESIGN AND REGIMEN
In this randomized, double-blind, phase 3 trial, patients were enrolled at 99 centers in 19 countries. Registration was done centrally at the EORTC headquarters. A central interactive voice-response system was used for randomization and was based on a minimization technique.16 Randomization was stratified according to disease stage (stage IIIA vs. stage IIIB vs. stage IIIC with one, two, or three positive nodes vs. stage IIIC with four or more positive nodes) and geographic region (North America, Europe, or Australia). Local pharmacists, who were aware of the trial-group assignments, performed the randomization. Clinical investigators and persons collecting or analyzing the data were unaware of the trial-group assignments.
Patients were randomly assigned in a 1:1 ratio to receive an intravenous infusion of ipilimumab at a dose of 10 mg per kilogram or placebo every 3 weeks for four doses, then every 3 months for up to 3 years or until disease recurrence, an unacceptable level of toxic effects, a major protocol violation, or withdrawal of consent (Fig. S1 in the Supplementary Appendix). The rules regarding the withholding of a dose of ipilimumab or placebo and the management of immune-related adverse events are detailed in the full trial protocol, available at NEJM.org.
The primary end point was recurrence-free survival. The secondary end points included overall survival, distant metastasis–free survival, safety, and health-related quality of life.
ASSESSMENTS
Patients in the two trial groups were assessed for recurrence and distant metastases every 3 months during the first 3 years and every 6 months thereafter. Physical examination and radiography of the chest, computed tomography, magnetic resonance imaging, or other imaging techniques were performed if indicated. Recurrence or metastatic lesions had to be histologically confirmed whenever possible. The first date when recurrence was observed was used in the analysis, regardless of the method of assessment.
Recurrence-free survival was defined as the time from randomization until the date of first recurrence (local, regional, or distant metastasis) or death from any cause. Overall survival was defined as the time from randomization until death from any cause. Distant metastasis–free survival was defined as the time from randomization until the date of the first distant metastasis or death from any cause.
Data on adverse events were collected for each group with the use of the Common Terminology Criteria for Adverse Events, version 3.0. Immune-related adverse events were determined programmatically from a prespecified list of terms from the Medical Dictionary for Regulatory Activities (MedDRA), which was updated according to each new version of MedDRA.
Resolution of an immune-related adverse event of grade 3 or 4 was defined as an improvement to grade 1 or less. The grade 3 or 4 event with the longest time to resolution was selected for inclusion in the analysis. If the grade 3 or 4 event did not resolve, follow-up was censored at the last known date that the patient was alive. Similar analyses were repeated for immune-related adverse events of grade 2 through 5.
TRIAL OVERSIGHT
The trial protocol was approved by the EORTC protocol-review committee and by independent ethics committees. The trial was conducted in accordance with the Declaration of Helsinki and with Good Clinical Practice guidelines as defined by the International Conference on Harmonisation. All the patients provided written informed consent.
The trial was funded and sponsored by Bristol-Myers Squibb. The trial was designed by the writing committee (the trial coordinator [the first author], the EORTC headquarters team, and a representative of the sponsor). Data were collected and computerized at the EORTC headquarters and were copied to the sponsor after the database lock. Data were analyzed independently at the EORTC headquarters and by the sponsor. The manuscript was written by two of the academic authors (the first and penultimate authors), all the coauthors commented on it, and editorial assistance was provided by professional medical writers paid by the sponsor. The two specified academic authors made the decision to submit the manuscript for publication, with the consent of all the other authors. The authors vouch for accuracy and completeness of the data and analyses and confirm the adherence of the trial to the protocol.
An independent review committee, whose members were unaware of the trial-group assignments, assessed disease status and date of recurrence. An independent data and safety monitoring board assessed the safety and efficacy data every 6 months, without formal interim analyses. Only at the time of the final analysis of recurrence-free survival were interim analyses of overall survival and distant metastasis–free survival performed by an independent statistician, and the results were forwarded to members of the data and safety monitoring board. On-site source-data verification was provided by a clinical research organization.
STATISTICAL ANALYSIS
We planned for the trial to include 950 patients. In the initial protocol, we calculated that a total of 491 deaths would be required in order to provide the trial with 85% power to detect a difference in the 4.5-year overall survival rates of 42.3% in the placebo group and 52.0% in the ipilimumab group, corresponding to a hazard ratio for death of 0.76. Owing to an improvement in outcomes after recurrence (because of a change in the treatment landscape for patients with melanoma), it was decided, by means of a protocol amendment, to perform the final analyses for overall survival and distant metastasis–free survival at the same time. Given the 506 events of distant metastasis or death and 376 deaths at the clinical cutoff date (January 31, 2016), it was recomputed (with the use of a Lan–DeMets alpha-spending function) that the final analyses of overall survival and distant metastasis–free survival be performed at two-sided alpha levels of 0.049 and 0.042, respectively, so the confidence interval for the hazard ratio of the group comparison regarding these end points was set at 95.1% and 95.8%, respectively; the statistical power was 75.8% and 89.4%, respectively. The statistical analysis plan (available with the trial protocol) indicated that in order to preserve the alpha error, a hierarchical-testing approach would be applied after the analysis of the primary end point of recurrence-free survival. Overall survival was tested first, followed by distant metastasis–free survival. For the subgroup analysis, the estimated hazard ratio was plotted along with its 99% confidence interval.
The main analyses of the efficacy end points included all the patients who had undergone randomization, according to the intention-to-treat principle. The safety profile was assessed in patients who received at least one dose of the randomly assigned regimen. Details of the statistical methods are provided in the Supplementary Appendix.
RESULTS
PATIENTS AND TRIAL REGIMEN
From July 2008 through August 2011, a total of 951 patients underwent randomization: 475 patients were assigned to the ipilimumab group and 476 to the placebo group. The characteristics at baseline were similar between the two randomized groups (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline.*
| Characteristic | Ipilimumab (N = 475) |
Placebo (N = 476) |
|---|---|---|
| Sex — no. (%) | ||
| Male | 296 (62.3) | 293 (61.6) |
| Female | 179 (37.7) | 183 (38.4) |
| Age | ||
| Median (range) — yr | 51 (20–84) | 52 (18–78) |
| Distribution — no. (%) | ||
| <50 yr | 214 (45.1) | 211 (44.3) |
| 51 to <65 yr | 180 (37.9) | 178 (37.4) |
| ≥65 yr | 81 (17.1) | 87 (18.3) |
| Disease stage — no. (%) | ||
| At randomization | ||
| IIIA | 98 (20.6) | 98 (20.6) |
| IIIB | 182 (38.3) | 182 (38.2) |
| IIIC with 1–3 positive lymph nodes | 122 (25.7) | 121 (25.4) |
| IIIC with ≥4 positive lymph nodes | 73 (15.4) | 75 (15.8) |
| According to AJCC 2002 criteria† | ||
| IIIA | 98 (20.6) | 88 (18.5) |
| IIIB | 213 (44.8) | 207 (43.5) |
| IIIC with 1–3 positive lymph nodes | 69 (14.5) | 83 (17.4) |
| IIIC with ≥4 positive lymph nodes | 95 (20.0) | 98 (20.6) |
| Type of lymph-node involvement — no. (%)† | ||
| Microscopic | 210 (44.2) | 193 (40.5) |
| Macroscopic | 265 (55.8) | 283 (59.5) |
| No. of positive lymph nodes on pathological testing — no. (%)† | ||
| 1 | 217 (45.7) | 220 (46.2) |
| 2 or 3 | 163 (34.3) | 158 (33.2) |
| ≥4 | 95 (20.0) | 98 (20.6) |
| Ulceration — no. (%)† | ||
| Yes | 197 (41.5) | 203 (42.6) |
| No | 257 (54.1) | 244 (51.3) |
| Unknown | 21 (4.4) | 29 (6.1) |
There were no significant between-group differences in the characteristics listed here. Percentages may not total 100 because of rounding. AJCC denotes American Joint Committee on Cancer.
Data were from case-report forms.
Six patients (4 patients in the ipilimumab group and 2 in the placebo group) did not start the randomly assigned regimen (Fig. S2 in the Supplementary Appendix). The median number of doses that were received was 4 doses (interquartile range, 3 to 8) in the ipilimumab group and 8 (interquartile range, 4 to 16) in the placebo group. At least 1 maintenance dose (dose 5 and beyond) was received by 198 of 471 patients (42.0%) in the ipilimumab group and by 332 of 474 (70.0%) in the placebo group.
Of 471 patients who started ipilimumab, 251 (53.3%) discontinued treatment owing to an adverse event (including 182 patients [38.6%] who discontinued within 12 weeks after randomization); in 240 patients (51.0%), the event was considered by the investigators to be drug-related. Among 474 patients who received placebo, 22 (4.6%) discontinued treatment owing to an adverse event. A total of 135 patients (28.7%) in the ipilimumab group discontinued treatment because of disease recurrence, as compared with 282 (59.5%) in the placebo group. A total of 63 patients (13.4%) in the ipilimumab group and 143 (30.2%) in the placebo group completed the 3-year treatment period (Fig. S2 in the Supplementary Appendix).
The overall median follow-up was 5.3 years. The median follow-up was 5.3 years in the ipilimumab group and 5.4 years in the placebo group.
EFFICACY AND POSTPROTOCOL TREATMENT
In this updated analysis, the rate of recurrence-free survival at 5 years was 40.8% in the ipilimumab group, as compared with 30.3% in the placebo group (hazard ratio for recurrence or death, 0.76; 95% confidence interval [CI], 0.64 to 0.89; P<0.001) (Fig. 1A). The overall significant prolongation of recurrence-free survival that was due to adjuvant ipilimumab appeared to be consistent across subgroups (Fig. S3A in the Supplementary Appendix), but the trial was not powered to provide robust subgroup analysis. Ipilimumab appeared to be helpful in patients with microscopic involvement (hazard ratio vs. placebo, 0.68) and in patients with macroscopic involvement (hazard ratio, 0.84) (Fig. S3B and S3C in the Supplementary Appendix).
Figure 1. Kaplan–Meier Estimates of Recurrence-free Survival (RFS), Overall Survival, and Distant Metastasis– free Survival (DMFS).
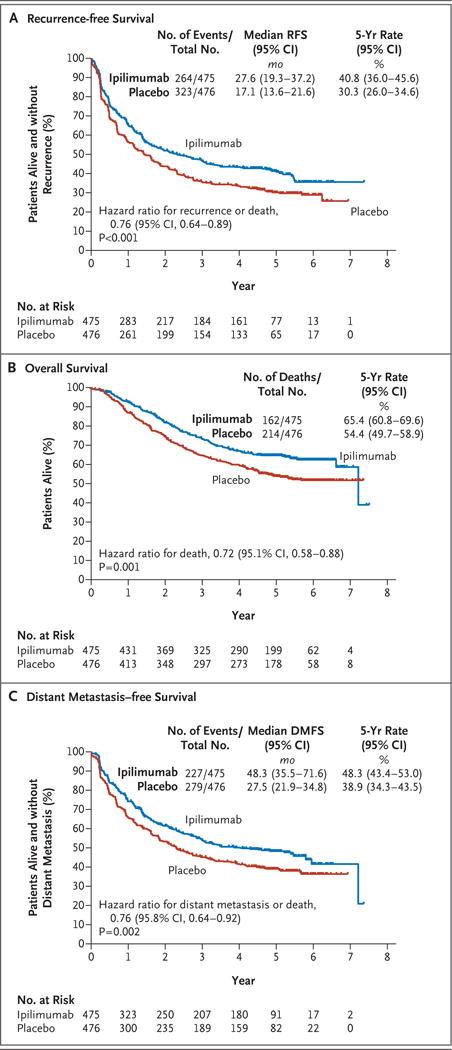
Panel A shows the Kaplan–Meier estimate of recurrence-free survival according to the independent review committee. In the ipilimumab group, local or regional recurrence was reported in 96 patients, distant metastasis or death due to melanoma in 157, and death due to another cause or an unknown cause in 11. In the placebo group, local or regional recurrence was reported in 114 patients, distant metastasis or death due to melanoma in 204, and death due to another cause or an unknown cause in 5. All the statistical comparisons shown here were stratified according to the disease stage as provided at randomization. In the comparison that was stratified according to the disease stage as given on case-report forms, the hazard ratio for recurrence or death was 0.77 (95% CI, 0.65 to 0.90; P = 0.001). In a per-protocol analysis of the comparison that was stratified according to the disease stage as given at randomization, the hazard ratio was 0.77 (95% CI, 0.65 to 0.91; P = 0.002). Panel B shows the Kaplan–Meier estimate of overall survival. Because the number of patients with a follow-up of more than 7 years was too small, the estimated median overall survival was either unreliable or not reached. In the comparison that was stratified according to the disease stage as given on case-report forms, the hazard ratio for death was 0.73 (95.1% CI, 0.60 to 0.90; P = 0.003). In a per-protocol analysis of the comparison stratified according to the disease stage as given at randomization, the hazard ratio was 0.72 (95.1% CI, 0.58 to 0.89; P = 0.002). Panel C shows the Kaplan–Meier estimate of distant metastasis–free survival according to the independent review committee. In the comparison that was stratified according to the disease stage as given on the case-report forms, the hazard ratio for distant metastasis or death was 0.77 (95.8% CI, 0.65 to 0.93; P = 0.004). In a per-protocol analysis of the comparison stratified according to the disease stage as given at randomization, the hazard ratio was 0.76 (95.8% CI, 0.63 to 0.91; P = 0.003).
Among the 264 patients in the ipilimumab group who had recurrence or died, 194 had received at least one postprotocol treatment (Table 2). These treatments included ipilimumab (24 patients), anti–programmed death 1 (PD-1) therapy (24 patients), and a BRAF inhibitor (63 patients). Among the 323 patients in the placebo group who had recurrence or died, 250 received postprotocol treatment: ipilimumab (76 patients), anti–PD-1 therapy (30 patients), and a BRAF inhibitor (88 patients). Overall survival after disease recurrence was similar in the two trial groups (hazard ratio for ipilimumab vs. placebo, 0.89), which suggests that the difference in recurrence-free survival would persist in terms of overall survival.
Table 2.
Postprotocol Treatment in All the Patients Who Underwent Randomization and in Those Who Had Disease Recurrence or Died.*
| Treatment | All Patients
|
Patients Who Had Disease Recurrence or Died
|
||
|---|---|---|---|---|
| Ipilimumab (N = 475) |
Placebo (N = 476) |
Ipilimumab (N = 264) |
Placebo (N = 323) |
|
| number (percent) | ||||
|
| ||||
| First postprotocol treatment | ||||
|
| ||||
| Chemotherapy | 41 (8.6) | 53 (11.1) | 40 (15.2) | 52 (16.1) |
|
| ||||
| Radiotherapy | 19 (4.0) | 19 (4.0) | 19 (7.2) | 19 (5.9) |
|
| ||||
| Surgery | 47 (9.9) | 33 (6.9) | 41 (15.5) | 31 (9.6) |
|
| ||||
| Chemoradiotherapy | 1 (0.2) | 4 (0.8) | 1 (0.4) | 4 (1.2) |
|
| ||||
| Biologic-response modifier | 48 (10.1) | 88 (18.5) | 48 (18.2) | 86 (26.6) |
|
| ||||
| Combination therapy | 13 (2.7) | 22 (4.6) | 12 (4.5) | 22 (6.8) |
|
| ||||
| Other | 37 (7.8) | 36 (7.6) | 33 (12.5) | 36 (11.1) |
|
| ||||
| No treatment reported | 269 (56.6) | 221 (46.4) | 70 (26.5) | 73 (22.6) |
|
| ||||
| Ipilimumab | 24 (5.1) | 76 (16.0) | 24 (9.1) | 76 (23.5) |
|
| ||||
| Anti-PD-1 agent | 25 (5.3) | 30 (6.3) | 24 (9.1) | 30 (9.3) |
|
| ||||
| BRAF inhibitor | 65 (13.7) | 88 (18.5) | 63 (23.9) | 88 (27.2) |
Percentages may not total 100 because of rounding. PD-1 denotes programmed death 1.
The overall survival rate at 5 years was 65.4% (95% CI, 60.8 to 69.6) in the ipilimumab group, as compared with 54.4% (95% CI, 49.7 to 58.9) in the placebo group. Overall survival was significantly longer in the ipilimumab group than in the placebo group (hazard ratio for death from any cause, 0.72; 95.1% CI, 0.58 to 0.88; P = 0.001) (Fig. 1B). The prolongation of overall survival with ipilimumab was generally consistent across subgroups (Fig. 2, and Fig. S4 in the Supplementary Appendix). The rate of distant metastasis– free survival at 5 years was higher in the ipilimumab group than in the placebo group (48.3% vs. 38.9%; hazard ratio for distant metastasis or death, 0.76; 95.8% CI, 0.64 to 0.92; P = 0.002) (Fig. 1C).
Figure 2. Forest Plot for Overall Survival, According to Trial Group.
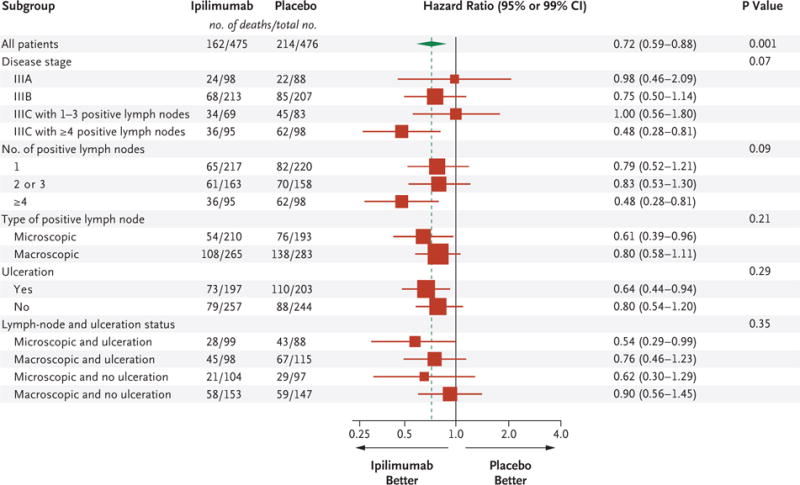
Results are expressed as unstratified hazard ratios for the risk of death in the ipilimumab group as compared with the placebo group with 95% confidence intervals for the analysis of the total group and with 99% confidence intervals for all the subgroup analyses. The size of the box is proportional to the total number of deaths reported in each subgroup, the diamond is centered on the overall hazard ratio for death and covers its 95% confidence interval, and the dashed line represents the overall hazard ratio for death. The P value for the univariate analysis that included all patients was provided by the unstratified log-rank test. The P value for the analysis of heterogeneity between the hazard ratios computed within the subgroups of a given variable was provided by the test of heterogeneity (see the Supplementary Appendix). The disease stage, according to the case-report forms, was determined with the use of the American Joint Committee on Cancer 2002 criteria. The number of positive lymph nodes was determined by means of pathological testing. Additional information is provided in Figure S4A in the Supplementary Appendix.
SAFETY
Among the 471 patients who received ipilimumab, 465 (98.7%) had an adverse event of any grade, with grade 3 or 4 adverse events occurring in 255 patients (54.1%); among the 474 patients who received placebo, 432 (91.1%) had an adverse event of any grade, with grade 3 or 4 adverse events occurring in 124 (26.2%) (Table S1 in the Supplementary Appendix). Immune-related adverse events during the trial were more frequent with ipilimumab than with placebo (Table 3). Immune-related adverse events of grade 3 or 4 occurred in 41.6% of the patients in the ipilimumab group and in 2.7% of those in the placebo group. The most common immune-related adverse events of grade 3 or 4 in the ipilimumab group were gastrointestinal (in 16.1% of the patients), hepatic (in 10.8%), and endocrine (in 7.9%). The median time to the onset of immune-related adverse events of grade 2 through 5 during the trial ranged from 4.0 weeks (skin immune-related adverse events) to 13.1 weeks (neurologic immune-related adverse events) (Table S2 in the Supplementary Appendix). Endocrine immune-related adverse events of grade 2 through 5 resolved in 51.5% of the patients, and the median time to resolution was 54.3 weeks. The majority (82 to 97%) of all other immune-related adverse events of grade 2, 3, or 4 resolved, and the median time to resolution ranged from 4.0 to 8.0 weeks.
Table 3.
Immune-Related Adverse Events.*
| Event | Ipilimumab (N = 471)
|
Placebo (N = 474)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Any Grade | Grade 3 | Grade 4 | Grade 5 | Any Grade | Grade 3 | Grade 4 | Grade 5 | |
| Any immune-related adverse event | 426 (90.4) | 169 (35.9) | 27 (5.7) | 5 (1.1) | 188 (39.7) | 12 (2.5) | 1 (0.2) | 0 |
|
| ||||||||
| Any dermatologic event | 298 (63.3) | 20 (4.2) | 0 | 0 | 99 (20.9) | 0 | 0 | 0 |
| Rash | 161 (34.2) | 5 (1.1) | 0 | 0 | 52 (11.0) | 0 | 0 | 0 |
|
| ||||||||
| Any gastrointestinal event† | 217 (46.1) | 70 (14.9) | 6 (1.3) | 3 (0.6) | 85 (17.9) | 3 (0.6) | 1 (02) | 0 |
| Diarrhea | 194 (41.2) | 46 (9.8) | 0 | 0 | 80 (16.9) | 2 (0.4) | 0 | 0 |
| Colitis | 73 (15.5) | 32 (6.8) | 4 (0.8) | 3 (0.6) | 7 (1.5) | 1 (0.2) | 1 (0.2) | 0 |
|
| ||||||||
| Any endocrine-system event | 178 (37.8) | 34 (7.2) | 3 (0.6) | 0 | 38 (8.0) | 1 (0.2) | 0 | 0 |
| Hypophysitis | 77 (16.3) | 20 (4.2) | 1 (0.2) | 0 | 1 (0.2) | 0 | 0 | 0 |
|
| ||||||||
| Any hepatic event | 115 (24.4) | 38 (8.1) | 13 (2.8) | 0 | 20 (4.2) | 1 (0.2) | 0 | 0 |
| Increase in liver-enzyme levels | 83 (17.6) | 14 (3.0) | 6 (1.3) | 0 | 18 (3.8) | 0 | 0 | 0 |
|
| ||||||||
| Any neurologic event | 21 (4.5) | 5 (1.1) | 4 (0.8) | 0 | 9 (1.9) | 0 | 0 | 0 |
|
| ||||||||
| Other‡ | 111 (23.6) | 34 (7.2) | 2 (0.4) | 2 (0.4) | 23 (4.9) | 8 (1.7) | 0 | 0 |
The safety analysis included all the patients who underwent randomization and received at least one dose of ipilimumab or placebo (945 patients). Immune-related adverse events that occurred in at least 10% of the patients are reported. Patients may have had more than one event. In the ipilimumab group, 5 patients died because of drug-related adverse events; 3 patients died from colitis (2 patients with gastrointestinal perforation), 1 from myocarditis, and 1 from multiorgan failure associated with the Guillain–Barré syndrome.
Gastrointestinal perforation occurred in seven patients (1.5%) in the ipilimumab group (all such events were considered to be related to ipilimumab) and in three patients (0.6%) in the placebo group (none of these events were considered to be related to placebo).
In the ipilimumab group, 26 patients had a grade 3 or 4 lipase level, 4 had a grade 3 or 4 immune-system disorder (hypersensitivity, auto-immune disorder, anaphylactoid reaction, or drug hypersensitivity), 4 had grade 3 lung infiltration, pneumonitis, or interstitial lung disease, 1 had arthritis, and 1 had uveitis.
Five patients (1.1%) died owing to adverse events that were attributed to ipilimumab: three patients died from colitis (two patients with intestinal perforation), one patient from myocarditis, and one patient from multiorgan failure that was associated with the Guillain–Barré syndrome. These deaths occurred before the start of maintenance therapy. Of these patients, four had received glucocorticoids and one anti–tumor necrosis factor antibodies.
DISCUSSION
In this randomized, phase 3 trial involving patients with resected, high-risk stage III melanoma, ipilimumab at a dose of 10 mg per kilogram significantly prolonged overall survival and distant metastasis–free survival as compared with placebo. The risk of death was 28% lower with ipilimumab than with placebo, and the risk of distant metastasis or death was lower by 24%. At 5 years, ipilimumab treatment was associated with rates that were approximately 10 percentage points higher than the rates with placebo for all end points: recurrence-free survival (40.8% vs. 30.3%), overall survival (65.4% vs. 54.4%), and distant metastasis–free survival (48.3% vs. 38.9%). The results show that at the cost of substantial toxic effects, the previously observed prolongation of recurrence-free survival with ipilimumab is confirmed in the current updated analysis and that it translated into a prolongation in overall survival and distant metastasis–free survival.
Despite successful treatment with surgery (followed by adjuvant therapy in patients at high risk for disease recurrence), only approximately 45% of patients with stage III melanoma will be disease-free after 4 years; less than 40% of patients who have surgery alone will be disease-free after 4 years.17 Interferon alfa is currently approved in both the United States and the European Union for the treatment of stage III melanoma after surgery. In a literature-based meta-analysis of 17 randomized, controlled trials involving 8122 patients with high-risk cutaneous melanoma, interferon alfa prolonged the time to recurrence (hazard ratio for disease recurrence with interferon alfa vs. observation, 0.82).15 Owing to the marginal benefit in overall survival (hazard ratio for death, 0.89) and the considerable toxic effects, interferon alfa is not widely used as an adjuvant therapy.18 Although the benefit–risk profile of interferon alfa as compared with ipilimumab remains unclear, a phase 3 trial (ECOG 1609) that directly compares interferon alfa with ipilimumab at a dose of 3 or 10 mg per kilogram in patients with resected stage III or IV melanoma is ongoing (ClinicalTrials.gov number, NCT01274338). In our trial (EORTC 18071), patients were treated for up to 3 years, but only 13.4% of the patients completed this treatment period and 40% had stopped ipilimumab treatment at the end of the first four doses over the first 3 months. Thus, the EORTC 18071 trial cannot address whether maintenance treatment is necessary.
In the current trial, the survival benefit of ipilimumab over placebo was generally consistent across subgroups. This benefit was observed not only in patients with microscopic involvement only (sentinel node–positive) but also in patients with macroscopic or palpable nodes. In contrast, in previous EORTC trials of adjuvant therapy in patients with melanoma,17,19–22 a significant benefit with interferon alfa was observed only in patients with microscopic involvement. Similarly, in contrast to interferon alfa, for which ulceration is the overriding determinant of activity,13,17,20–23 ipilimumab prolonged survival among patients with nonulcerated melanoma and among those with ulcerated melanoma.
The rate of adverse events with ipilimumab in the context of adjuvant therapy was substantial and led to the discontinuation of treatment in approximately 40% of the patients by the end of the initial dosing period (i.e., before maintenance therapy). This frequency is higher than that observed with the same dose in the pooled analysis involving patients with advanced melanoma.2–4 The vast majority of immune-related adverse events of grade 2, 3, or 4 resolved within 4 to 8 weeks with the use of established management guidelines. However, for endocrinopathies, the median time to resolution was 54 weeks, and 48.5% of the patients who had endocrinopathy continue to take hormone-replacement therapies. In this trial, adjuvant therapy with ipilimumab was associated with a higher risk and greater degree of diarrhea, insomnia, and fatigue than placebo during the induction period, but ipilimumab did not have a negative effect on the global health scale of health-related quality of life.12
Of concern are the five patients (1.1%) in the ipilimumab group who died owing to drug-related adverse events. In the context of adjuvant therapy, the benefit–risk profile is particularly important in view of the prognostic heterogeneity observed in patients with stage III melanoma.
In conclusion, adjuvant ipilimumab was associated with clinical improvements and significantly prolonged overall survival and distant metastasis–free survival, as compared with placebo, among patients with high-risk stage III melanoma, thus extending previous findings of a prolongation of recurrence-free survival. Adverse events were common but mostly transient. Some adverse events were serious, and even death from treatment occurred despite the use of established treatment algorithms.
Supplementary Material
Acknowledgments
Dr. Eggermont reports receiving fees for serving on scientific advisory boards from Actelion Pharmaceuticals, Agenus, Bristol-Myers Squibb, GlaxoSmithKline, HalioDx, Incyte, Merck Sharp & Dohme, Novartis, and Pfizer; Dr. Chiarion-Sileni, receiving consulting fees and travel support from Bristol-Myers Squibb, Roche, GlaxoSmithKline, and Merck Sharp & Dohme, and lecture fees from Bristol-Myers Squibb, Roche, and GlaxoSmith-Kline; Dr. Grob, receiving consulting fees from Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Roche, Merck, and Amgen, lecture fees from Bristol-Myers Squibb, GlaxoSmithKline, and Roche, travel support from Roche, and research funding from Bristol-Myers Squibb and Roche; Dr. Dummer, receiving consulting fees, honoraria, and research funding from Bristol-Myers Squibb, Roche, GlaxoSmithKline, Merck Sharp & Dohme, and Novartis; Dr. Wolchok, receiving consulting fees from Bristol-Myers Squibb, Merck, MedImmune, Ziopharm Oncology, Polynoma, Polaris, GlaxoSmithKline, and Jounce Therapeutics, honoraria from EMD Serono and Janssen Oncology, institutional research funding from Bristol-Myers Squibb, Merck, MedImmune, and GlaxoSmithKline, and holding a pending patent related to compositions and methods for the treatment of melanoma (U.S. patent number, US7556805); Dr. Hamid, receiving consulting fees from Amgen, Novartis, Roche, Bristol-Myers Squibb, Merck, Merck Serono, Pfizer, and Genentech, and lecture fees from Novartis, Bristol-Myers Squibb, and Genentech; Dr. Robert, receiving consulting fees from Bristol-Myers Squibb, Merck, Roche, and Amgen, and honoraria from Bristol-Myers Squibb, Merck, GlaxoSmithKline, Roche, Novartis, and Amgen; Dr. Ascierto, receiving consulting fees from Bristol-Myers Squibb, Roche (formerly Genentech), GlaxoSmithKline, Merck Sharp & Dohme, Ventana Medical Systems, Novartis, and Amgen, honoraria from Bristol-Myers Squibb, Roche (formerly Genentech), and GlaxoSmithKline, and grant support to his institution from Bristol-Myers Squibb, Roche (formerly Genentech), and Ventana Medical Systems; Dr. Lebbé, receiving fees for serving on advisory boards from Bristol-Myers Squibb, Merck Sharp & Dohme, Roche, GlaxoSmithKline, and Novartis; Dr. Smylie, receiving consulting fees and honoraria from Bristol-Myers Squibb, Roche, GlaxoSmithKline, and Merck, and lecture fees from Bristol-Myers Squibb and Roche; Dr. Weber, receiving consulting fees and honoraria from Bristol-Myers Squibb, Merck, GlaxoSmithKline, Roche, and Genentech, research funding from Bristol-Myers Squibb, Merck, GlaxoSmithKline, Roche, and Genentech, and MacroGenics, and holding stock in Celldex Therapeutics, Altor BioScience, and cCAM Biotherapeutics; Dr. Maio, receiving consulting fees, honoraria, and travel support from Bristol-Myers Squibb, Merck Sharp & Dohme, Roche, GlaxoSmithKline, and MedImmune, and research funding from Bristol-Myers Squibb and MedImmune; Dr. Bastholt, receiving fees for serving on advisory boards from Bristol-Myers Squibb, Merck Sharp & Dohme, Roche, and GlaxoSmithKline; Dr. Mortier, receiving fees for serving on an advisory board from Bristol-Myers Squibb and clinical-trial support from Bristol-Myers Squibb, GlaxoSmithKline, Merck Sharp & Dohme, and Roche; Dr. Hauschild, receiving consulting fees and honoraria from Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Roche, Med-Immune, Nektar Therapeutics, OncoSec Medical, Philogen, Provectus Biopharmaceuticals, and Regeneron Pharmaceuticals, and grant support from Amgen, Bristol-Myers Squibb, Celgene, Eisai, GlaxoSmithKline, Merck Serono, Merck Sharp & Dohme, Novartis, and Roche; Dr. Hassel, receiving consulting fees from GlaxoSmithKline and Amgen, honoraria and lecture fees from Bristol-Myers Squibb, GlaxoSmithKline, Roche, Merck Sharp & Dohme, and Amgen, and travel support from Bristol-Myers Squibb and Amgen; Dr. Hodi, receiving consulting fees and grant support to his institution from Bristol-Myers Squibb, holding a patent related to therapeutic peptides (U.S. patent number, 20160046716), and holding a pending patent related to methods for treating MHC class I polypeptide-related sequence A (MICA) related disorders (U.S. patent number, 20140037630); Dr. Taitt and Ms. de Pril, being employees of Bristol-Myers Squibb; and Dr. Taitt, holding stock in Bristol-Myers Squibb.
We thank all the team members at the European Organization for Research and Treatment of Cancer (EORTC) headquarters, including Isabelle Blangenois, Sandra Collette, Valérie De-waste, Thierry Gorlia, Sven Janssen, Niels Lema, Larissa Polders, and Simon Vanderschaeghe, for contributions to the trial; the team members at Bristol-Myers Squibb (in particular, Chantal Lejeune); and Ward Pedersen, of StemScientific, for editorial assistance with an earlier version of the manuscript.
APPENDIX
The authors’ full names and academic degrees are as follows: Alexander M.M. Eggermont, M.D., Ph.D., Vanna Chiarion-Sileni, M.D., Jean-Jacques Grob, M.D., Ph.D., Reinhard Dummer, M.D., Jedd D. Wolchok, M.D., Ph.D., Henrik Schmidt, M.D., Omid Hamid, M.D., Caroline Robert, M.D., Ph.D., Paolo A. Ascierto, M.D., Jon M. Richards, M.D., Céleste Lebbé, M.D., Ph.D., Virginia Ferraresi, M.D., Michael Smylie, M.D., Jeffrey S. Weber, M.D., Ph.D., Michele Maio, M.D., Ph.D., Lars Bastholt, M.D., Laurent Mortier, M.D., Ph.D., Luc Thomas, M.D., Ph.D., Saad Tahir, M.D., Axel Hauschild, M.D., Ph.D., Jessica C. Hassel, M.D., F. Stephen Hodi, M.D., Corina Taitt, M.D., Veerle de Pril, M.Sc., Gaetan de Schaetzen, Ph.D., Stefan Suciu, Ph.D., and Alessandro Testori, M.D.
The authors’ affiliations are as follows: Gustave Roussy Cancer Campus Grand Paris, Villejuif (A.M.M.E., C.R.), Aix–Marseille University, Hôpital de La Timone, Marseille (J.-J.G.), Assistance Publique–Hôpitaux de Paris, Hôpital Saint-Louis, Paris (C.L.), University Lille, INSERM Unité-1189, Centre Hospitalier Universitaire (CHU) Lille, Service de Dermatologie, Lille (L.M.), and CHU Lyon, Lyon (L.T.) — all in France; the Oncology Institute of Veneto–Istituto di Ricovero e Cura a Carattere Scientifico, Padua (V.C.-S.), Istituto Nazionale Tumori Fondazione G. Pascale, Naples (P.A.A.), Istituti Fisioterapici Ospitalieri, Rome (V.F.), University Hospital of Siena, Istituto Toscano Tumori, Siena (M.M.), and the European Institute of Oncology, Milan (A.T.) — all in Italy; University of Zurich Hospital, Zurich, Switzerland (R.D.); Memorial Sloan Kettering Cancer Center, New York (J.D.W.); Aarhus University Hospital, Aarhus (H.S.), and Odense University Hospital, Odense (L.B.) — both in Denmark; the Angeles Clinic and Research Institute, Los Angeles (O.H.); Oncology Specialists, Park Ridge, IL (J.M.R.); Cross Cancer Institute, Edmonton, AB, Canada (M.S.); H. Lee Moffitt Cancer Center, Tampa, FL (J.S.W.); Broomfield Hospital, Chelmsford, United Kingdom (S.T.); Universitätsklinikum Schleswig–Holstein, Kiel (A.H.), and University Hospital Heidelberg, Heidelberg (J.C.H.) — both in Germany; Dana–Farber Cancer Institute, Boston (F.S.H.); Bristol-Myers Squibb, Princeton, NJ (C.T., V.P.); and the European Organization for Research and Treatment of Cancer, Brussels (G.S., S.S.).
Footnotes
References
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 4.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–94. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggermont AMM, Spatz A, Robert C. Cutaneous melanoma. Lancet. 2014;383:816–27. doi: 10.1016/S0140-6736(13)60802-8. [DOI] [PubMed] [Google Scholar]
- 6.Balch CM, Gershenwald JE, Soong S-J, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balch CM, Gershenwald JE, Soong S-J, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micro-metastases versus macrometastases. J Clin Oncol. 2010;28:2452–9. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Akkooi ACJ, Nowecki ZI, Voit C, et al. Sentinel node tumor burden according to the Rotterdam criteria is the most important prognostic factor for survival in melanoma patients: a multicenter study in 388 patients with positive sentinel nodes. Ann Surg. 2008;248:949–55. doi: 10.1097/SLA.0b013e31818fefe0. [DOI] [PubMed] [Google Scholar]
- 9.van der Ploeg APT, van Akkooi ACJ, Rutkowski P, et al. Prognosis in patients with sentinel node-positive melanoma is accurately defined by the combined Rotterdam tumor load and Dewar topography criteria. J Clin Oncol. 2011;29:2206–14. doi: 10.1200/JCO.2010.31.6760. [DOI] [PubMed] [Google Scholar]
- 10.van der Ploeg APT, van Akkooi ACJ, Haydu LE, et al. The prognostic significance of sentinel node tumour burden in melanoma patients: an international, multicenter study of 1539 sentinel node-positive melanoma patients. Eur J Cancer. 2014;50:111–20. doi: 10.1016/j.ejca.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–30. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 12.Coens C, Suciu S, Vanna Chiarion-Sileni V, et al. Phase III trial (EORTC 18071/CA184-029) of post-operative adjuvant ipilimumab compared to placebo in patients with resected stage III cutaneous melanoma: health related quality of life (HRQoL) results. Lancet Oncol. in press. [Google Scholar]
- 13.Suciu S, Ives N, Eggermont AM, et al. Predictive importance of ulceration on the efficacy of adjuvant interferon-a (IFN): an individual patient data (IPD) meta-analysis of 15 randomized trials in more than 7,500 melanoma patients (pts) J Clin Oncol. 2014;32(Suppl):5s. abstract. [Google Scholar]
- 14.Wheatley K, Ives N, Hancock B, Gore M, Eggermont A, Suciu S. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev. 2003;29:241–52. doi: 10.1016/s0305-7372(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 15.Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102:493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]
- 16.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–15. [PubMed] [Google Scholar]
- 17.Eggermont AM, Suciu S, Rutkowski P, et al. Long term follow up of the EORTC 18952 trial of adjuvant therapy in resected stage IIB-III cutaneous melanoma patients comparing intermediate doses of interferon-alpha-2b (IFN) with observation: ulceration of primary is key determinant for IFN-sensitivity. Eur J Cancer. 2016;55:111–21. doi: 10.1016/j.ejca.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline — update 2016. Eur J Cancer. 2016;63:201–17. doi: 10.1016/j.ejca.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Eggermont AMM, Suciu S, MacKie R, et al. Post-surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet. 2005;366:1189–96. doi: 10.1016/S0140-6736(05)67482-X. [DOI] [PubMed] [Google Scholar]
- 20.Eggermont AMM, Suciu S, Santinami M, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117–26. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- 21.Eggermont AM, Suciu S, Testori A, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30:3810–8. doi: 10.1200/JCO.2011.41.3799. [DOI] [PubMed] [Google Scholar]
- 22.Eggermont AMM, Suciu S, Testori A, et al. Ulceration and stage are predictive of interferon efficacy in melanoma: results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer. 2012;48:218–25. doi: 10.1016/j.ejca.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Eggermont AM, Spatz A, Lazar V, Robert C. Is ulceration in cutaneous melanoma just a prognostic and predictive factor or is ulcerated melanoma a distinct biologic entity? Curr Opin Oncol. 2012;24:137–40. doi: 10.1097/CCO.0b013e32834fcb0d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


