Abstract
Purpose
Androgens play a crucial role in prostate cancer progression, and trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid (anti-[18F]FACBC) are used for visualization of prostate cancer. We examined the effect of androgen on the expression of amino acid transporters related to anti-[18F]FACBC transport and uptake of trans-1-amino-3-fluoro-[1-14C]cyclobutanecarboxylic acid (anti-[14C]FACBC).
Procedures
Expression of amino acid transporters and uptake of anti-[14C]FACBC in androgen receptor (AR)-positive LNCaP and AR-negative DU145 human prostate cancer cells cultured with/without 5α-dihydrotestosterone (DHT) and the effect of bicalutamide, an AR antagonist, on DHT-associated changes were investigated.
Results
DHT stimulated the expression of amino acid transporters ASCT2, SNAT5, 4F2 heavy chain, and LAT3 in LNCaP but not in DU145 cells. Anti-[14C]FACBC uptake was enhanced, in a DHT-dependent manner, in LNCaP cells only.
Conclusions
DHT enhanced the expression of ASCT2, the transporter responsible for anti[18F]FACBC uptake, thereby increasing anti-[14C]FACBC uptake in AR-positive LNCaP cells. Androgen-mediated induction may contribute to the distinct anti-[18F]FACBC accumulation pattern in prostate cancer.
Keywords: anti-FACBC, Prostate cancer, Androgen, Amino acid transporter, ASCT2
Introduction
Prostate cancer is the second most frequently diagnosed cancer among men worldwide. Its incidence and mortality rates are rising in several Asian and European countries, including Japan [1]. 2-Deoxy-2-[18F]fluoro-D-glucose ([18F]FDG), 18F- and 11C-labeled choline, and 18F-and 11C-labeled acetate are the most-studied positron emission tomography (PET) radiotracers for use in prostate cancer detection and visualization. These tracers are trapped in prostate cancer cells due to the high glycolytic and metabolic rates of choline and fatty acids, respectively [2]. Recently, these probes have been used clinically for prostate cancer imaging. For example, [18F]FDG PET/computed tomography (CT) has been used to efficiently evaluate the extent and treatment responsiveness of metastatic castration-resistant prostate cancer (CRPC) [3, 4]. However, the present imaging techniques are not ideal for detecting primary prostate cancer or lymph-node metastases, especially in patients with lower prostate-specific antigen (PSA) levels.
Trans-1-amino-3-[18F] fluorocyclobutanecarboxylic acid (anti-[18F]FACBC) is a synthetic amino acid that accumulates in prostate cancer cells [5]. A recent clinical study [6, 7] showed that anti-[18F]FACBC accurately detected prostate cancer within the prostate and in pelvic lymph nodes with greater specificity and sensitivity than [111In]capromab single photon emission computed tomography (SPECT)/CT, which targets prostate-specific membrane antigens. A recent phase I clinical study of anti-[18F]FACBC reported favorable characteristics for prostate cancer imaging, such as slow urinary excretion and high in vivo stability [8]. We reported that two amino acid transporters, system ASC amino acid transporter 2 (ASCT2; encoded by SLC1A5) and system L amino acid transporter 1 (LAT1; encoded by SLC7A5), were important determinants of anti-FACBC uptake by prostate cancer cells [9, 10]. We further demonstrated that their affinities for anti-FACBC were similar to the cognate amino acid substrates (e.g., glutamine) [11]. Therefore, the preferential accumulation of anti-FACBC in prostate cancer may result from cancer cells’ increased requirement for amino acids essential for growth. Because ASCT2 and LAT1 are often upregulated in various human cancer cells and their expression is associated with poor prognosis, they have been considered potential targets for chemotherapy and radiodiagnosis [12, 13].
Male gonadal hormones (androgens) and the androgen receptor (AR) constitute an essential axis for promoting the development and progression of AR-positive prostate cancer [14, 15]. Although serum androgen concentrations significantly decrease after androgen deprivation therapy, low levels of intraprostatic androgens and circulating conjugated androgen are sufficient to activate the AR and stimulate tumor growth [16, 17]. Therefore, even in advanced-stage prostate cancer, AR activity is a key determinant of therapeutic response. Although many androgen-regulated genes have been identified, limited data are available on their mechanisms of regulation. PSA is the protein product of the most-studied androgen-regulated gene [18]. In prostate cancer patients treated with endocrine or other therapies, elevated serum PSA levels are considered evidence for prostate cancer recurrence. In addition, androgen regulates the expression of some amino acid transporters. For example, in human prostate cancer LNCaP cells, androgen exposure upregulated the expression of system L amino acid transporter 3 (LAT3; encoded by SLC43A1) [19], which was first described as a gene overexpressed in human prostate cancer [20, 21]. In addition, in castrated adult mice, androgen exposure upregulated the expression of system N amino acid transporter 2 (SNAT5; encoded by SLC38A5) [22]. A subsequent study reported increased expression of system ASC amino acid transporter 1 (ASCT1; encoded by SLC1A4), ASCT2, and 4F2 heavy chain (4F2hc; encoded by SLC3A2) in the presence of androgen [23]. Therefore, it is hypothesized that androgen induces the expression of amino acid transporters involved in anti-[18F]FACBC transport. If so, prostate cancer cells are expected to show increased uptake of anti-[18F]FACBC. Therefore, anti-[18F]FACBC may be an effective diagnostic tool for prostate cancer.
In the present study, we investigated the effect of androgen on the expression of amino acid transporters that are thought to transport anti-FACBC [9–11] and its effect on anti-FACBC accumulation using AR-positive and AR-negative human prostate cancer cell lines. Furthermore, we examined the effects of androgen on the transport of FDG, choline, and acetate, which are commonly used PET tracers for the diagnosis of various cancers.
Materials and Methods
Materials
14C-labeled compounds were used because of their longer half-lives (T1/2=5,700 years) compared to those of 18F- (T1/2=110 min) or 11C-labeled compounds (T1/2=20 min). Trans-1-amino-3-fluoro-[1-14C]cyclobutanecarboxylic acid (anti-[14C]FACBC, 2.09 GBq/mmol) was radiolabeled by Sekisui Medical Co. Ltd. as described previously [10]. L-[14C (U)]glutamine ([14C]Gln, 10.17 GBq/mmol) was purchased from PerkinElmer. L-[methyl-14C]methionine ([14C]Met, 2.04 GBq/mmol), [14C (U)]2-deoxy-2-fluoro-D-glucose ([14C]FDG, 11.10 GBq/mmol), [methyl-14C]choline chloride ([14C]choline, 2.04 GBq/mmol), [1-14C]acetic acid, and sodium salt ([14C]acetate, 1.85 GBq/mmol) were purchased from American Radiolabeled Chemicals.
The human prostate cancer cell lines LNCaP-FGC (LNCaP) and DU145 and fetal bovine serum (FBS) were purchased from American Type Culture Collection (ATCC). Cell culture medium and antibiotics were purchased from Life Technologies. 5α-Dihydrotestosterone (DHT) and bicalutamide were purchased from Sigma-Aldrich.
Cell Cultures
LNCaP and DU145 cells were maintained in RPMI-1640 medium and Dulbecco’s modified Eagle medium, respectively, and both media were supplemented with 10 % FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were cultured at 37 °C in 5 % CO2/95 % air.
For experiments, LNCaP and DU145 cells were seeded into 6-well and 24-well plates, respectively, with phenol red-free RPMI-1640 and phenol red-free Dulbecco’s modified Eagle medium, respectively. Both media were supplemented with 10 % charcoal-stripped FBS (Thermo Fisher Scientific), 100 units/ml penicillin, and 100 μg/ml streptomycin. On the following day, cells were exposed to 1 nM DHT alone or 1 nM DHT in combination with 10 μM bicalutamide for 3 days. The experimental media containing DHT with or without bicalutamide were changed after 2 days. DHT and bicalutamide were dissolved in ethanol (final concentration of ethanol, 0.1 %). The control cells were treated with the solvent used to prepare DHT or bicalutamide solutions (final concentration of ethanol, 0.1 %).
Cell Proliferation Assay
After exposure to DHT with or without bicalutamide, cells were counted by Trypan blue exclusion using a Countess Automated Cell Counter (Life Technologies).
Quantitative Real-Time Polymerase Chain Reaction
The gene expression of ASCT2, SNAT2, SNAT5, LAT1, 4F2hc, LAT3, B0AT1, B0AT2, and ATB0,+ was analyzed as described previously [9]. Briefly, total RNA was extracted from cells using an RNeasy Plus Mini Kit (QIAGEN) after exposure to DHT with or without bicalutamide. Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using a Transcriptor Universal cDNA Master kit (Roche Applied Science) according to the manufacturer’s protocol. Primers (Supplemental Table 1) were designed using the Roche Applied Science Website-based Universal ProbeLibrary Assay Design Center (http://www.roche-applied-science.com) and were synthesized by Nihon Gene Research Laboratories. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using an Mx3000P QPCR system (Agilent Technologies), FastStart Universal Probe Master Mix (ROX), and the Universal ProbeLibrary (Roche Applied Science). Cycling conditions were as follows: 1 cycle of enzyme activation at 95 °C for 10 min followed by 60 cycles of denaturing at 95 °C for 15 s and extension at 60 °C for 1 min. All reactions were run in triplicate. The PCR products were analyzed by agarose gel electrophoresis, and no nonspecific PCR bands were detected. The PCR products for each gene were subsequently purified using a High Pure PCR Cleanup Micro Kit (Roche Applied Science) and quantified based on concentration and base pairs of the amplicons. The messenger RNA (mRNA) copy number was calculated from standard curves generated by amplifying serial dilutions of known quantities of purified amplicons. Expression data were normalized against the copy number of β-actin.
Uptake Assay
The uptake assay was performed as described previously [9]. Briefly, the sodium-containing incubation medium was prepared using phosphate-buffered saline (PBS, pH 7.4–7.6), 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 5.6 mM D-glucose, 0.9 mM CaCl2, and 0.5 mM MgCl2. After the culture medium was removed, each well was incubated with incubation medium for 5 min at 37 °C. The cells were then incubated with the incubation medium containing the respective 14C-labeled compound (1 μM) for 5 min at 37 °C. At the end of the uptake period, each well was rapidly washed twice with ice-cold incubation medium. The cells were then solubilized in 0.1 N NaOH, and the radioactivity of each aliquot was measured using a liquid scintillation counter (Tri-Carb 2910TR; PerkinElmer) after addition of a scintillation cocktail (Ultima Gold; PerkinElmer). The protein content of the cell lysate was determined by a bicinchoninic acid (BCA) assay, which was performed using a BCA Protein Assay kit (Thermo Fisher Scientific). Data were expressed as the percentage of the control values, which were calculated as follows: percentage of the control=(Vexposed/Vunexposed)×100, where Vexposed and Vunexposed are the uptake levels (pmol/mg protein) in exposed cells and unexposed cells, respectively.
Statistical Analysis
Experiments were repeated at least twice. Data have been presented as mean and standard deviation values. All statistical analyses were performed with SAS software version 5.0 (SAS Institute). Statistical significance between two groups was determined using the F test followed by the Student’s t test for homoscedastic data or the Welch test for non-homoscedastic data. P values less than 0.05 were considered statistically significant.
Results
Androgen Sensitivity of Human Prostate Cancer Cell Lines
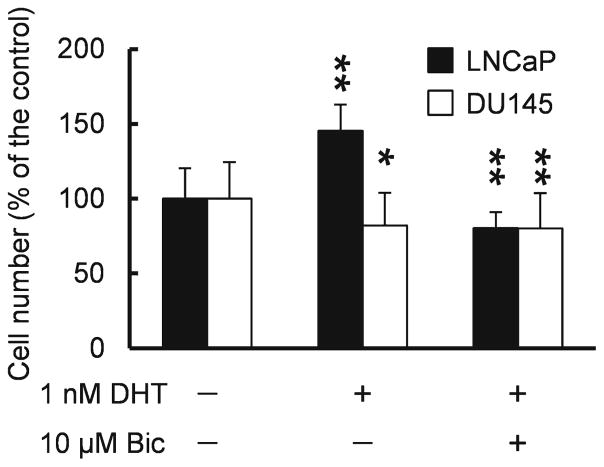
To investigate the androgen sensitivity of AR-positive LNCaP and AR-negative DU145 cells, we evaluated the effect of DHT, the most potent gonadal androgen, on cell proliferation (Fig. 1). When LNCaP cells were exposed to 1 nM DHT, their numbers increased by 45 % relative to that of unexposed cells. In contrast, DHT did not stimulate proliferation in DU145 cells (cell count, 82 % of the control). Next, we evaluated whether these effects occurred through an AR-mediated pathway (Fig. 1). Indeed, an AR antagonist, bicalutamide, blocked the effects of DHT on proliferation of LNCaP cells (cell count, 80 % of the control) but this effect was not observed in DU145 cells (cell count, 80 % of the control).
Fig. 1.
Quantification of LNCaP and DU145 cells exposed to DHT with or without bicalutamide. Each bar represents the mean and standard deviation (n=6). *P<0.05, **P<0.01.
Effect of Androgen on Expression of Amino Acid Transporter Genes
In order to determine whether androgen induces the expression of amino acid transporters involved in anti[18F]FACBC transport, qRT-PCR was performed for nine amino acid transporters. The mRNA expression of ASCT2, SNAT2, and 4F2hc was relatively high in both LNCaP and DU145 cells (Supplemental Table 2). Among system L amino acid transporters, LAT3 was more highly expressed than LAT1 in LNCaP cells, and the inverse was observed in DU145 cells (Supplemental Table 2).
The observed levels of mRNAs encoding SNAT5 and broad-spectrum neutral (0) amino acid transporter 1 (B0AT1; encoded by SLC6A19) were negligible when compared with the mRNA levels of other transporters in both LNCaP and DU145 cells (Supplemental Table 2). mRNAs of broad-spectrum neutral (0) amino acid transporter 2 (B0AT2; encoded by SLC6A15) and amino acid transporter B0,+ (ATB0,+; encoded by SLC6A14) were not detected in LNCaP cells but were detected at low levels in DU145 cells (Supplemental Table 2). These results suggest that SNAT5, system B0 transporters, and ATB0,+ likely make small contributions to anti-[18F]FACBC transport in prostate cancer cells.
Table 1 shows changes in gene expression relative to that in unexposed cells. The levels of LAT3, one of the AR-regulated genes, were monitored as the positive control [19]. In LNCaP cells, DHT exposure significantly increased the levels of mRNAs of ASCT2, SNAT5, LAT3, and 4F2hc, and bicalutamide abolished the DHT-induced increase to the level observed in unexposed cells. DHT combined with bicalutamide significantly increased the expression of LAT3 mRNA; however, compared to DHT alone, bicalutamide significantly decreased the expression of LAT3 mRNA (P<0.05). DHT exposure also increased the expression of LAT1 mRNA, but bicalutamide did not abolish this effect. On the other hand, DHT exposure markedly decreased the expression of B0AT1 mRNA, which was recovered by the addition of bicalutamide. In AR-negative DU145 cells, neither DHT nor bicalutamide affected gene expression of the transporters tested.
Table 1.
Relative amino acid transporter gene expression in LNCaP and DU145 cells exposed to DHT with or without bicalutamide
| Transporters | Exposure | mRNA expression (% of the control) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| DHT | Bic | LNCaP cells | P | DU145 cells | P | |
| ASCT2 | − | − | 100.0±28.2 | 100.0±48.8 | ||
| + | − | 197.4±73.5 | <0.01 | 119.0±51.5 | NS | |
| + | + | 107.1±28.0 | NS | 104.0±47.1 | NS | |
| SNAT2 | − | − | 100.0±20.0 | 100.0±35.3 | ||
| + | − | 112.8±30.2 | NS | 117.5±32.9 | NS | |
| + | + | 91.2±31.9 | NS | 123.0±40.8 | NS | |
| SNAT5 | − | − | 100.0±30.5 | 100.0±77.6 | ||
| + | − | 210.5±74.2 | <0.01 | 85.5±42.6 | NS | |
| + | + | 79.3±48.0 | NS | 101.2±56.2 | NS | |
| LAT1 | − | − | 100.0±34.6 | 100.0±37.7 | ||
| + | − | 157.6±55.4 | <0.01 | 128.5±91.1 | NS | |
| + | + | 149.5±87.3 | <0.05 | 103.6±62.4 | NS | |
| 4F2hc | − | − | 100.0±24.4 | 100.0±15.8 | ||
| + | − | 128.5±26.3 | <0.01 | 94.9±28.9 | NS | |
| + | + | 99.5±23.2 | NS | 89.9±21.7 | NS | |
| LAT3 | − | − | 100.0±25.9 | 100.0±54.8 | ||
| + | − | 201.7±65.5 | <0.01 | 112.3±57.3 | NS | |
| + | + | 147.0±65.8 | <0.01 | 112.4±35.0 | NS | |
| B0AT1 | − | − | 100.0±29.7 | 100.0±53.0 | ||
| + | − | 23.1±5.6 | <0.01 | 158.6±156.2 | NS | |
| + | + | 92.8±44.9 | NS | 102.6±73.7 | NS | |
| B0AT2 | − | − | Not detected | 100.0±27.0 | ||
| + | − | 98.8±36.3 | NS | |||
| + | + | 77.3±44.2 | NS | |||
| ATB0,+ | − | − | Not detected | 100.0±63.0 | ||
| + | − | 144.3±95.8 | NS | |||
| + | + | 102.5±113.8 | NS | |||
Statistical significance was tested by comparing exposed cells and unexposed cells
Bic bicalutamide, NS not statistically significant
Data are expressed as mean ± standard deviation (n=6)
Effect of Androgen on the Uptake of Amino Acids and PET Tracers
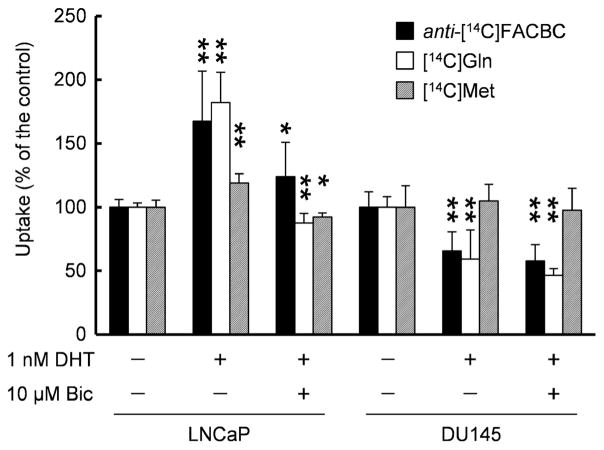
After investigating the increased expression of amino acid transporters, we investigated the effect of androgen on amino acid uptake by measuring cellular uptake of 14C-labeled amino acids (Fig. 2). Anti-[14C]FACBC uptake was the highest among all radiotracers tested in unexposed LNCaP and DU145 cells (Table 2).
Fig. 2.
Uptake of anti-[14C]FACBC, [14C]Gln, and [14C]Met by LNCaP and DU145 cells exposed to DHT with or without bicalutamide. Each bar represents the mean and standard deviation (n=6–12). *P<0.05, **P<0.01.
Table 2.
Radiotracer uptake in LNCaP and DU145 cells that were not exposed to DHT or bicalutamide
| Radiotracers | Uptake amount (pmol/mg of protein) | |
|---|---|---|
|
| ||
| LNCaP cells | DU145 cells | |
| anti-[14C]FACBC | 105.9±15.7 | 110.8±14.5 |
| [14C]Gln | 88.6±14.9 | 59.0±6.2 |
| [14C]Met | 23.0±1.6 | 56.7±10.8 |
| [14C]FDG | 2.8±0.7 | 1.9±0.5 |
| [14C]choline | 45.8±12.4 | 15.6±2.8 |
| [14C]acetate | 14.1±2.4 | 20.8±3.8 |
Data are expressed as mean ± standard deviation (n=6–12)
Note that the uptake amount of [14C]FDG could not be compared with that of other radiotracers because the incubation medium used in this study contained D-glucose
In LNCaP cells, DHT stimulated anti-[14C]FACBC uptake by 68 % relative to that in unexposed cells, and the uptake was reduced to the control levels in the presence of bicalutamide (124 % of the control). On the other hand, DHT or bicalutamide slightly decreased the uptake of anti-[14C]FACBC in DU145 cells (66 % with DHT and 58 % with DHT plus bicalutamide). A similar result was observed with [14C]Gln in LNCaP cells (182 % with DHT and 88 % with DHT plus bicalutamide) and DU145 cells (59 % with DHT and 46 % with DHT plus bicalutamide). Similarly, DHT induced [14C]Met uptake in LNCaP cells (119 % with DHT and 92 % with DHT plus bicalutamide); however, its uptake was unchanged by DHT or bicalutamide in DU145 cells (105 % with DHT and 98 % with DHT plus bicalutamide).
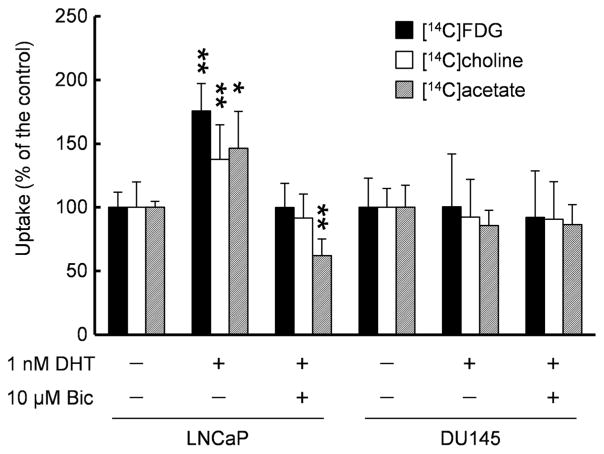
For comparison, we examined the uptake of 14C-labeled FDG, choline, and acetate (Fig. 3). Similar to anti-[14C]FACBC, DHT stimulated the uptake of these tracers (176 % with [14C]FDG, 138 % with [14C]choline, and 146 % with [14C]acetate), and bicalutamide suppressed DHT’s effects (100 % with [14C]FDG, 91 % with [14C]choline, and 62 % with [14C]acetate) in LNCaP cells. Furthermore, neither DHT nor bicalutamide affected the uptake of [14C]FDG (100 % with DHT and 92 % with DHT plus bicalutamide), [14C]choline (92 % with DHT and 91 % with DHT plus bicalutamide), and [14C]acetate (86 % with DHT and 86 % with DHT plus bicalutamide) in DU145 cells.
Fig. 3.
Uptake of [14C]FDG, [14C]choline, and [14C]acetate by LNCaP and DU145 cells exposed to DHT with or without bicalutamide. Each bar represents the mean and standard deviation (n=6–9). *P<0.05, **P<0.01.
Discussion
In the present study, DHT stimulated the expression of amino acid transporters (ASCT2, 4F2hc, and LAT3) that are essential for cell proliferation and that mediate transport of anti[18F]FACBC in LNCaP cells. DHT stimulated the subsequent uptake of anti-[14C]FACBC. Because ASCT2 and system L transporters are upregulated in cancer cells and are thought to stimulate tumor growth by regulating the mammalian target of rapamycin through nutrient pathways [13], accumulation of anti[18F]FACBC in prostate cancer cells may be proportional to cell growth. Therefore, our data reaffirm the utility of anti[18F]FACBC for prostate cancer imaging.
The effects of DHT combined with bicalutamide on anti[14C]FACBC uptake varied with the cell type (AR-positive LNCaP or AR-negative DU145), although DHT combined with bicalutamide inhibited the proliferation of both cells. One possible explanation for this variation is that the optimum effective concentration for bicalutamide could depend on the conditions or the cell type, which could result in one of the effects being slightly predominant. Furthermore, with regard to inhibition of cell proliferation, bicalutamide might inhibit the effect of marginal androgens present in the culture medium and within the LNCaP cells. On the other hand, in DU145 cells, inhibition of proliferation by DHT was similar to that achieved by DHT in combination with bicalutamide. This result is consistent with a previous finding that DHT inhibited the growth of DU145 cells [24]. In addition, inhibition of anti-[14C]FACBC uptake could be partly attributed to the change in the expression of LAT1 and 4F2hc. In LNCaP cells, DHT combined with bicalutamide did not change the amount of 4F2hc mRNA. However, it increased the amount of LAT1 mRNA. This could be one of the reasons for the mild increase in anti-[14C]FACBC uptake. In contrast, both treatments—DHT alone and DHT plus bicalutamide—tended to decrease 4F2hc mRNA levels in DU145 cells; however, the decrease was not significant. Furthermore, while DHT tended to increase LAT1 mRNA levels, albeit nonsignificantly, DHT plus bicalutamide did not change the mRNA levels. Although protein turnover could also contribute to these phenomena, 4F2hc level would be a limiting factor for anti-[14C]FACBC uptake by DU145 cells. In addition, 4F2hc is involved not only in amino acid transport but also in integrin signaling, which is associated with tumor growth and metastasis [25]. Thus, anti-[14C]FACBC uptake would decrease following reduction in the 4F2hc level in DU145 cells.
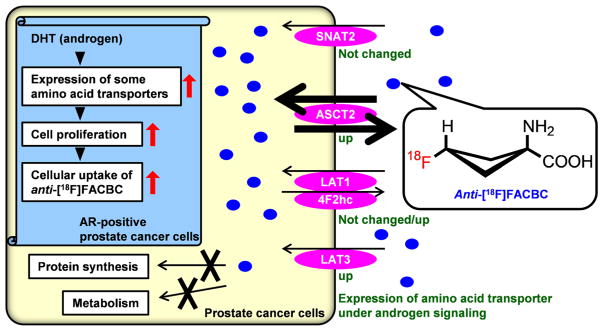
Figure 4 presents a schematic of the transport mechanism for anti-[18F]FACBC in prostate cancer cells whose cellular proliferation is stimulated by androgen. Anti-[18F]FACBC is neither metabolized nor incorporated into proteins [9]. Our present findings showed that transcription of ASCT2, the transporter responsible for anti-[18F]FACBC transport [9, 10], was stimulated by DHT in LNCaP cells, as reported recently [23]. With respect to the expression of system L transporters (LAT1 and LAT3), which may be involved in uptake of anti-[18F]FACBC, LAT3 was also upregulated by DHT in LNCaP cells. However, the increase in LAT1 expression by DHT exposure in LNCaP cells varied widely, and bicalutamide did not abolish DHT’s effects, which suggests the effect of DHT on LAT1 expression could be smaller than that on other transporters. The expression of 4F2hc, which heterodimerizes with LAT1 to form a functional transporter complex, was enhanced by DHT in LNCaP cells. Upregulation of these amino acid transporters could increase anti-[18F]FACBC accumulation in AR-positive prostate cancer cells. However, since the affinity of LAT3 (millimolar range) [21] for its substrate is lower than that of LAT1 (micromolar range) [11, 26], LAT3 may contribute less to anti-[18F]FACBC transport than LAT1. Furthermore, ASCT2 was found to be more highly expressed than LAT1 in LNCaP cells, whereas it was expressed at levels similar to that of LAT1 in DU145 cells (Supplemental Table 2). In addition, the affinity of anti[14C]FACBC for ASCT2 is higher than that for LAT1 [11]; therefore, ASCT2 could be a major transporter of anti[18F]FACBC in prostate cancer cells.
Fig. 4.
Schematic representation of the transport mechanism of anti-[18F]FACBC in AR-positive prostate cancer cells. demonstrate the usefulness of anti-[18F]FACBC relative to [18F]FDG, [18F] and [11C]choline, and [18F] and [11C]acetate for prostate cancer imaging.
Although LAT1 and LAT3 transport Met, the mRNA levels of LAT1 and LAT3 were lower than that of ASCT2 in LNCaP cells. The difference in the expression levels of amino acid transporters may explain why DHT had a lesser effect on the uptake of [14C]Met than on anti-[14C]FACBC. Interestingly, DHT’s effects on the proliferation of LNCaP and DU145 cells were similar to its effects on anti-[14C]FACBC uptake by both cell types. Thus, since the accumulation of anti-[14C]FACBC better reflects androgen-induced cell proliferation than does [14C]Met, anti[18F]FACBC might be more suitable than S-[methyl-11C]-L-methionine for prostate cancer imaging.
In addition to androgen, some steroid hormones (estrogen and aldosterone) and epidermal growth factor activate the expression of ASCT2 [27–29]. The ubiquitous transcription factors, activating transcription factor 4 (ATF4) and c-MYC, also control the expression of ASCT2 [23, 30]. Thus, since a wide variety of factors involved in cancer cell proliferation induce ASCT2 expression and because upregulation of ASCT2 has been observed in several tumors [31], increased accumulation of anti-[18F]FACBC might also occur in other cancer tissues as well as in prostate cancer.
Regarding the representative PET tracers for prostate cancer, DHT stimulated [14C]FDG uptake similar to that of anti[14C]FACBC. However, [18F]FDG PET/CT imaging has limited efficacy because [18F]FDG is internalized by both prostate cancer cells and benign prostatic tissue, such as benign prostatic hyperplasia. Moreover, prostate cancer cells have a relatively lower metabolic rate and lower expression of glucose transporter than the cells of other cancers, suggesting relatively low [18F]FDG uptake by prostate cancer cells [32]. On the other hand, DHT stimulated [14C]choline and [14C]acetate uptake to slightly lower degrees than it did the uptake of anti-[14C]FACBC. Furthermore, anti-[14C]FACBC uptake was higher than that of [14C]choline or [14C]acetate. These findings suggest that anti-[18F]FACBC more accurately detects prostate cancer cells stimulated by androgen than do radiolabeled choline and acetate. Indeed, a recent study reported that anti-[18F]FACBC may better identify prostate cancer recurrence than [11C]choline might [33]. Thus, these data
Androgen-deprivation therapy (usually a combination of orchiectomy, luteinizing hormone-releasing hormone analogs, and anti-androgens) is often the first choice among several therapeutic options for advanced prostate cancer. However, despite an initial response to androgen-deprivation therapy, most prostate cancers eventually become resistant to therapy within several years, which has been termed CRPC. Nevertheless, the AR plays a role in the progression of prostate cancer toward castration resistance, where AR signaling remains intact [15]. Numerous factors contribute to AR reactivation despite castrate serum levels of androgens, including adrenal and intratumoral androgen synthesis, AR gene amplification, AR gene mutations, increased expression of AR coactivators, ligand-independent AR splice variants, and epigenetic modifications [34, 35]. Since AR signaling plays an important role in cell proliferation in both androgen-dependent prostate cancer and CRPC, anti[18F]FACBC, whose uptake is stimulated by androgen, might be useful for the diagnosis of recurrent prostate cancer or for therapy evaluation. Our future studies will investigate anti-FACBC transport in CRPC cells to test this hypothesis.
Conclusions
DHT induced ASCT2 gene expression and enhanced the cellular uptake of anti-[14C]FACBC in AR-positive LNCaP prostate cancer cells but not in AR-negative DU145 cells. Given the role of androgen in prostate cancer cell proliferation, anti-[18F]FACBC may be a valuable PET tracer for the diagnosis of prostate cancer.
Supplementary Material
Acknowledgments
We thank Ms. Sachiko Naito for her excellent technical assistance. We also thank Dr. Atsushi Mizokami for his helpful suggestions and comments regarding these experiments.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11307-014-0756-x) contains supplementary material, which is available to authorized users.
Conflict of Interest. Hiroyuki Okudaira, Shuntaro Oka, Masahiro Ono, and Yoshifumi Shirakami are employees of Nihon Medi-Physics Co., Ltd. Mark M. Goodman and Emory University have patent rights for anti[18F]FACBC and are eligible to receive royalties on anti-[18F]FACBC from Nihon Medi-Physics Co., Ltd. Mark M. Goodman, David M. Schuster, and Keiichi Kawai have ongoing research collaborations with Nihon Medi-Physics Co., Ltd.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52:81–89. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jadvar H, Desai B, Ji L, et al. Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J Nucl Med. 2013;54:1195–1201. doi: 10.2967/jnumed.112.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jadvar H. Molecular imaging of prostate cancer with PET. J Nucl Med. 2013;54:1685–1688. doi: 10.2967/jnumed.113.126094. [DOI] [PubMed] [Google Scholar]
- 5.Schuster DM, Votaw JR, Nieh PT, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48:56–63. [PubMed] [Google Scholar]
- 6.Schuster DM, Savir-Baruch B, Nieh PT, et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology. 2011;259:852–861. doi: 10.1148/radiol.11102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster DM, Nieh PT, Jani AB, et al. Anti-3-[18F]FACBC positron emission tomography-computerized tomography and 111In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191:1446–1453. doi: 10.1016/j.juro.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asano Y, Inoue Y, Ikeda Y, et al. Phase I clinical study of NMK36: a new PET tracer with the synthetic amino acid analogue anti[18F]FACBC. Ann Nucl Med. 2011;25:414–418. doi: 10.1007/s12149-011-0477-z. [DOI] [PubMed] [Google Scholar]
- 9.Okudaira H, Shikano N, Nishii R, et al. Putative transport mechanism and intracellular fate of trans-1-amino-3-18F-fluorocyclobutanecarboxylic acid in human prostate cancer. J Nucl Med. 2011;52:822–829. doi: 10.2967/jnumed.110.086074. [DOI] [PubMed] [Google Scholar]
- 10.Oka S, Okudaira H, Yoshida Y, Schuster DM, Goodman MM, Shirakami Y. Transport mechanisms of trans-1-amino-3-fluoro[1-14C]cyclobutanecarboxylic acid in prostate cancer cells. Nucl Med Biol. 2012;39:109–119. doi: 10.1016/j.nucmedbio.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Okudaira H, Nakanishi T, Oka S, et al. Kinetic analyses of trans-1-amino-3-[18F] fluorocyclobutanecarboxylic acid transport in Xenopus laevis oocytes expressing human ASCT2 and SNAT2. Nucl Med Biol. 2013;40:670–675. doi: 10.1016/j.nucmedbio.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi T, Tamai I. Solute carrier transporters as targets for drug delivery and pharmacological intervention for chemotherapy. J Pharm Sci. 2011;100:3731–3750. doi: 10.1002/jps.22576. [DOI] [PubMed] [Google Scholar]
- 14.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 15.Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91:483–490. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- 16.Dutt SS, Gao AC. Molecular mechanisms of castration-resistant prostate cancer progression. Future Oncol. 2009;5:1403–1413. doi: 10.2217/fon.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arakawa H, Nakanishi T, Yanagihara C, et al. Enhanced expression of organic anion transporting polypeptides (OATPs) in androgen receptor-positive prostate cancer cells: possible role of OATP1A2 in adaptive cell growth under androgen-depleted conditions. Biochem Pharmacol. 2012;84:1070–1077. doi: 10.1016/j.bcp.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Trapman J, Cleutjens KB. Androgen-regulated gene expression in prostate cancer. Semin Cancer Biol. 1997;8:29–36. doi: 10.1006/scbi.1997.0050. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Bailey CG, Ng C, et al. Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression. Cancer Res. 2011;71:7525–7536. doi: 10.1158/0008-5472.CAN-11-1821. [DOI] [PubMed] [Google Scholar]
- 20.Chuaqui RF, Englert CR, Strup SE, et al. Identification of a novel transcript up-regulated in a clinically aggressive prostate carcinoma. Urology. 1997;50:302–307. doi: 10.1016/s0090-4295(97)00194-5. [DOI] [PubMed] [Google Scholar]
- 21.Babu E, Kanai Y, Chairoungdua A, et al. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem. 2003;278:43838–43845. doi: 10.1074/jbc.M305221200. [DOI] [PubMed] [Google Scholar]
- 22.Chauvin TR, Griswold MD. Androgen-regulated genes in the murine epididymis. Biol Reprod. 2004;71:560–569. doi: 10.1095/biolreprod.103.026302. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Tiffen J, Bailey CG, et al. Targeting amino acid transport in metastatic castration-resistant prostate cancer: effects on cell cycle, cell growth, and tumor development. J Natl Cancer Inst. 2013;105:1463–1473. doi: 10.1093/jnci/djt241. [DOI] [PubMed] [Google Scholar]
- 24.Janssen T, Raviv G, Camby I, et al. In-vitro characterization of dihydrotestosterone-induced, epidermal growth factor-induced and basic fibroblastic growth factor-induced modifications in the growth dynamics of the human prostate-cancer cell-line LNCaP, DU145 and PC3. Int J Oncol. 1995;7:1219–1225. doi: 10.3892/ijo.7.5.1219. [DOI] [PubMed] [Google Scholar]
- 25.Cantor JM, Ginsberg MH. CD98 at the crossroads of adaptive immunity and cancer. J Cell Sci. 2012;125:1373–1382. doi: 10.1242/jcs.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanagida O, Kanai Y, Chairoungdua A, et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514:291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 27.Todorova VK, Kaufmann Y, Luo S, Klimberg VS. Tamoxifen and raloxifene suppress the proliferation of estrogen receptor-negative cells through inhibition of glutamine uptake. Cancer Chemother Pharmacol. 2011;67:285–291. doi: 10.1007/s00280-010-1316-y. [DOI] [PubMed] [Google Scholar]
- 28.Amaral JS, Pinho MJ, Soares-da-Silva P. Genomic regulation of intestinal amino acid transporters by aldosterone. Mol Cell Biochem. 2008;313:1–10. doi: 10.1007/s11010-008-9735-3. [DOI] [PubMed] [Google Scholar]
- 29.Wolfgang CL, Lin C, Meng Q, Karinch AM, Vary TC, Pan M. Epidermal growth factor activation of intestinal glutamine transport is mediated by mitogen-activated protein kinases. J Gastrointest Surg. 2003;7:149–156. doi: 10.1016/s1091-255x(02)00130-0. [DOI] [PubMed] [Google Scholar]
- 30.Wise DR, DeBerardinis RJ, Mancuso A, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Schwarzenböck S, Souvatzoglou M, Krause BJ. Choline PET and PET/CT in primary diagnosis and staging of prostate cancer. Theranostics. 2012;2:318–330. doi: 10.7150/thno.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanni C, Schiavina R, Boschi S, et al. Comparison of 18F-FACBC and 11C-choline PET/CT in patients with radically treated prostate cancer and biochemical relapse: preliminary results. Eur J Nucl Med Mol Imaging. 2013;40:S11–S17. doi: 10.1007/s00259-013-2373-3. [DOI] [PubMed] [Google Scholar]
- 34.Galsky MD, Small AC, Tsao CK, Oh WK. Clinical development of novel therapeutics for castration-resistant prostate cancer: historic challenges and recent successes. CA Cancer J Clin. 2012;62:299–308. doi: 10.3322/caac.21141. [DOI] [PubMed] [Google Scholar]
- 35.Shafi AA, Yen AE, Weigel NL. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol Ther. 2013;140:223–238. doi: 10.1016/j.pharmthera.2013.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.