Abstract
Background
Relatively little is known regarding the perception of medication-assisted treatments (MATs) and other treatment options amongst individuals that engage in non-medical prescription opioid use. This study surveyed out-of-treatment individuals that misuse opioids to better understand how perceived access to treatment shapes treatment preference.
Methods
Participants (n=357) were out-of-treatment adults registered as workers on the Amazon Mechanical Turk platform who reported current non-medical prescription opioid use. Participants were surveyed regarding demographics, insurance status, attitudes toward opioid use disorder (OUD) treatments, and self-reported symptoms of OUD.
Results
Participants who were male, did not have health insurance, and knew that counseling-type services were locally available were most likely to first attempt counseling/detox treatments (χ2(6) = 30.19, p<.001). Participants who met criteria for severe OUD, used heroin in the last 30 days, knew their insurance covered MAT, and knew of locally available MAT providers were most likely to first attempt MAT (χ2(4) = 26.85, p<.001). Participants with insurance and who knew of locally available physicians were most likely to attempt physician visits without the expressed purpose of MAT (χ2(3) = 24.75, p<.001).
Conclusion
Out-of-treatment opioid users were particularly interested in counseling-based services and medical care that could be attained from a primary-care physician. Results suggest that insurance coverage and perceived access to OUD treatment modalities influences where out-of-treatment opioid users might first seek treatment; understanding the factors that shape treatment preference is critical in designing early interventions to effectively reach this population.
Keywords: opioid use disorder (OUD), medication-assisted treatment (MAT), prescription opioid, treatment accessibility, insurance
1. Introduction
More than 12 million Americans misused prescription opioids in 2015 (Center for Behavioral Health Statistics and Quality, 2016). Misuse of prescription opioids has led to increased prevalence of opioid use disorder (OUD) (Dart et al., 2015; Jones, 2017) and opioid-related deaths (National Center for Health Statistics et al., 2015; Compton et al., 2016; Braden et al., 2017). In response to the opioid epidemic, scientific and medical communities have advocated for increased availability of evidence-based, pharmacotherapeutic approaches that have been empirically shown to mitigate the incidence of opioid related death and disease transmission (Volkow et al., 2014; Blum et al., 2016) (Volkow et al., 2014; Blum et al., 2016). Pharmacotherapeutic options for OUD treatment, often referred to as medication-assisted treatments (MATs), include opioid agonist/partial agonist maintenance treatments to manage opioid withdrawal and cravings such as buprenorphine (Ling et al., 1998) and methadone (Sees et al., 2000), or opioid antagonist treatment to decrease relapse potential such as oral or extended-release (XR) injectable naltrexone (Krupitsky et al., 2011). MATs are often, but not always, layered with other treatment options such as counseling and 12-step programs. Alternatively, many treatment-seeking individuals with OUD elect not to utilize MATs, relying solely on “abstinence-based” approaches or nonspecific forms of substance use treatment (e.g., counseling).
The factors impacting individual preferences for various OUD treatment options are not fully understood. Initiation of MAT might depend on the point of first contact, as individuals with OUD are most often inducted onto MAT in outpatient settings (Polydorou et al., 2016; Sullivan et al., 2017). However, there have been conflicting reports regarding patient preference for MAT, as one study noted that 63% of patients in a residential setting prefer sustained MAT (particularly XR naltrexone) (Bailey et al., 2013), while another study reported that more than half of OUD patients undergoing detoxification prefer continued residential, drug-free counseling, or 12-step based recovery over agonist maintenance (Stein et al., 2015b).
Non-MAT based OUD treatment options are frequently provided as part of a general substance use disorder treatment center that is not solely focused on OUD treatment; this treatment path usually includes some combination of residential treatment (including detoxification), individual counseling, and/or 12-step groups (Zijlstra et al., 2009). Non-MAT approaches have varying degrees of success for OUD treatment. For example, a large clinical trial examining buprenorphine in persons with prescription OUD found that individualized manual-based counseling had no additional effect on treatment outcome relative to standard medical management (Weiss et al., 2011). Alternatively, a study with young adults reported that patients meeting criteria for opioid dependence provided fewer positive urine drug screens following a 12-step based residential treatment compared to those without opioid dependence (Schuman-Olivier et al., 2014). Finally, a retrospective study of physicians with OUD reported that a combination of residential treatment, intensive outpatient (IOP), counseling, and long-term 12-step participation without agonist therapy promoted extended (>4 year) opioid abstinence in 77% of participants (Merlo et al., 2016).
There is a major gap in our knowledge of treatment preference among individuals who are actively engaged in non-medical prescription opioid use, but have yet to initiate treatment for OUD. Practical factors such as treatment affordability, geographic location, and OUD severity, likely affect individual treatment preferences (Peterson et al., 2010; Stein et al., 2015a). Understanding the point of first contact for OUD treatment could help inform targeted efforts to identify persons with OUD in different treatment modalities, and improve efforts to educate OUD users about unfamiliar treatment options. Together, this would help expand treatment access for OUD patients and combat the opioid epidemic. To address these gaps in knowledge, we surveyed individuals who reported current non-medical use of prescription opioids but were not currently in treatment to better understand their perceptions of treatment options and barriers to treatment. This study hypothesized that demographics, perceived treatment accessibility, and opioid use severity would affect preference for various types of OUD treatment, and that perceived access to treatment (e.g., insurance coverage, local availability, and price) would inform which treatment modalities this population would use first to seek help for OUD.
2. Methods
2.1 Participants
The sample was recruited between November 2016 and January 2017. Participants (N=357) were registered as workers on the Amazon Mechanical Turk (AMT) platform. AMT is regularly used in biomedical research studies to target nationally representative samples (Paolacci et al., 2010; Mason and Suri, 2012; Tompkins et al., 2016). Requesters in AMT make human intelligence tasks (HITs) available for workers; in turn, requestors can then rate workers based on completion and data quality. For the current study, ≥ 90% worker approval rating was required to access the study.
Eligibility was reserved for individuals 18 years or older, who were United States residents and endorsed non-medical prescription opioid use in the last 30 days. Non-medical prescription opioid use was defined for participants as “use of prescription opioids more than once in the last 30 days to “get high” or for purposes other than prescribed”. Prescription opioids were operationalized for participants as prescription medications that include: “Opioids (examples include Vicodin, Percocet, oxycodone, Dilaudid, Suboxone, etc.)”. Eligibility questions were intermixed with distractor items to obscure the criteria under investigation. Only participants who met eligibility criteria were invited to complete the survey and distractor questions were embedded throughout the survey as a measure of quality control. Participants were also asked whether they had experienced computer problems or had other reasons their data were inaccurate and should not be analyzed. The survey was hosted on Qualtrics (Provo, UT).
2.2 Measures
Questions included items to characterize the sample (demographics and health insurance) and to assess perception of OUD treatment options and current OUD status (Table 1). Health insurance status was defined as a binary variable (yes/no). For individuals with insurance, the source of insurance (e.g., healthcare exchange, Medicaid/Medicare) was also queried. Participants were asked how much they were willing to pay out of pocket for one month of residential treatment or one month of MAT. As a proxy of opioid use severity, OUD status was defined by the number of self-reported symptoms endorsed on a DSM-5 checklist for OUD (range 0–11); participants were classified as meeting criteria for mild (2–3), moderate (4–5), or severe (6+) OUD based upon established cut-offs (Table 1). Respondents who did not meet criteria for OUD were retained in the analyses because their endorsement of past 30-day misuse suggested they are at risk of developing OUD and/or seeking treatment in the future.
Table 1.
Demographics (n=357)
| Participant Characteristics | ||
|---|---|---|
| Male (%) | 59.1 | |
| Age [Mean yrs, (SD)] | 32.6 (8.5) | |
| White/Caucasian (%) | 83.5 | |
| Income (Median) | $37,500 | |
| Setting (%) | ||
| Urban | 32.2 | |
| Suburban | 52.4 | |
| Rural | 15.4 | |
| OUD Category (%) | ||
| None | 25.5 | |
| Mild | 12.6 | |
| Moderate | 13.0 | |
| Severe | 48.8 | |
| Insurance Coverage (%) | ||
| Provided by employer | 42.3 | |
| None | 22.7 | |
| Healthcare Exchange/private pay | 11.4 | |
| Medicaid | 11.2 | |
| Source unknown | 6.2 | |
| Medicare | 5.3 | |
SD= Standard Deviation, OUD= Opioid Use Disorder
OUD category based upon self-reported responses to DSM-5 checklist
2.2.1 Perception of OUD Treatments
Participants answered several questions pertaining to the following OUD treatment options: residential treatment (28 days or longer), one-on-one counseling, cognitive behavioral therapy, group counseling, intensive outpatient (IOP), inpatient detox (less than 28 days), outpatient detox, buprenorphine (Suboxone®, Subutex®, Zubsolv®), methadone, naltrexone/Vivitrol® (extended release naltrexone), physician visit, sober living environment (e.g., halfway house), and 12-step group. Some multiple-choice questions permitted a single answer (Table 2), such as what is the first treatment you would try to help stop abusing opioids? Additional questions allowed multiple responses, such as which of these treatment options would help YOU with opioid abuse or addiction (i.e., treatment preference), which treatment options are available in your area, which treatment options are not effective/you would not use, and which treatment options does your insurance cover? Visual analogue scale (VAS) items asked participants to gauge (1) their familiarity with each treatment option (e.g., how familiar are you with each of the substance abuse treatment options listed) using the anchors: “No knowledge of this treatment option” at 0 and “Extensive knowledge of this treatment option” at 100, (2) how price affects their ability to use each treatment option (i.e., price affects access) with the anchors: “No effect” at 0 and “Major effect” at 100, and (3) treatment effectiveness for individuals with opioid addiction, using the anchors: “Not effective” at 0 and “Extremely effective” at 100 (Supplemental Table1). As a control, participants were only asked to rate the impact of price on accessibility as well as effectiveness if they rated their familiarity with the treatment option at ≥ 10; this was meant to ensure that participants would not rate treatment options for which they endorsed having no knowledge.
Table 2.
Perception of Treatment Options
| Treatment Modality | Personal Preference (%) |
Locally Available (%) |
Would Try First (%)a |
Would Not Be Effective (%) |
Believe Insurance Will Cover (%) |
|---|---|---|---|---|---|
| Residential Treatment | 31.9 | 46.5 | 9.8 | 14.8 | 9.8 |
| One-on-One Counseling | 53.8 | 60.8 | 17.1 | 5.6 | 21.0 |
| Cognitive Behavioral Therapy | 31.4 | 36.4 | 5.1 | 9.2 | 13.4 |
| Group Counseling | 27.5 | 54.9 | 4.2 | 13.7 | 14.0 |
| Intensive Outpatient | 17.1 | 24.6 | 0.6 | 7.6 | 7.0 |
| Detox - Inpatient | 25.5 | 33.9 | 5.9 | 12.9 | 7.3 |
| Detox - Outpatient | 25.2 | 31.7 | 5.1 | 11.5 | 6.4 |
| Factor 1 (counseling/detox) | 80.7 | 74.5 | 47.6 | 43.7 | 28.9 |
| Buprenorphine | 26.6 | 33.3 | 9.0 | 24.4 | 12.0 |
| Methadone | 16.5 | 35.6 | 3.9 | 32.2 | 7.8 |
| Oral/XR Naltrexone | 10.4 | 15.4 | 0.3 | 16.8 | 6.4 |
| Factor 2 (MAT) | 31.9 | 46.5 | 13.2 | 46.2 | 14.0 |
| Physician Visit | 32.8 | 66.4 | 21.9 | 10.6 | 28.6 |
| Sober Living Environment | 14.8 | 39.5 | 2.1 | 33.1 | 4.5 |
| 12 Step Group | 41.2 | 69.7 | 13.8 | 26.6 | na |
| None | 5.3 | 0.8 | na | 0 | 22.7 |
| Other | 2.2 | na | 2 | 1.4 | na |
| Don't know | na | 19.0 | na | na | 51.8 |
Respondents (n=357) were asked about their perception of several treatment options for opioid use disorder. All responses indicate the percent of respondents who aswered "yes" to that form of treatment; respondents could respond to multiple treatment domains per category unless noted. Factor 1 and Factor 2 were determined via principle component analysis and represent at least one endorsed answer in the treatment options listed directly above the factor. na= non-applicable for that category; Oral/XR Naltrexone = oral or injectable extended release naltrexone.
Respondents selected top-rated treatment domain only (as opposed to multiple choice)
2.3 Statistical Analyses
Frequencies of endorsed answers were calculated for the entire sample and analyzed for all treatment options as a function of insurance, geographic setting (e.g., rural, suburban, and urban), and OUD severity level (none, mild, moderate, severe) using Chi-Square analyses. Continuous values that were not normally distributed were assessed using Mann-Whitney U tests and those that were normally distributed were assessed via t-tests and one-way ANOVAs with Bonferroni post-hoc analyses. Linear regression was used to analyze relationships between continuous variables.
Given the wide range of treatment options, it was important to establish whether treatments clustered into meaningful categories. Principle component analysis (PCA) with varimax rotation, Kaiser normalization, and a cutoff of 0.6 was calculated based upon participant response to the continuous variable “treatment effectiveness” (Table 2). 12 step-based groups are not professional services so were treated as their own entity and not included in the PCA. The factors defined by the PCA were then used as discrete outcome variables for logistic regression analyses to identify which factors might predict participant preference for initial treatment contact. Sober living environments were not included in logistic analyses because the number of participants endorsing them was too low to infer meaningful results (1.4%). A hypothesis-driven approach was used to select the following socioeconomic variables for logistic regression models (Becker et al., 2008; Wu et al., 2016): gender (binary), age (continuous), income (continuous), personal insurance (binary), knowledge of insurance coverage for a specific treatment (binary), severe OUD self-diagnosis (binary), current heroin use (binary), whether the treatment was rated as available in the participant’s area (binary), and the degree to which price affects accessibility (continuous). Variables that did not show marginal correlation (p≤.20) within the model were discarded and the logistic regression was run a second time. Alpha levels for significant findings were set at p<.05 and analyses were conducted using SPSS version 24.0.
3. Results
3.1 Participant Characteristics
Participants were 59.1% male, 83.5% Caucasian, and lived in urban (32.2%), suburban (52.4%), and rural (15.4%) settings (Table 1). Past 30-day heroin use was endorsed by 8.4% of the sample, and 22.7% reported not having health insurance. OUD severity was widely distributed, with approximately half (48.8%) of participants meeting criteria for severe OUD and 25.5% of participants not meeting criteria for any level of OUD despite endorsing non-medical use of prescription opioids at least twice in the last 30 days. Current non-medical opioid use (number of days misusing opioids in the last 30 days) was significantly different for individuals depending on the number of OUD symptoms endorsed (F(2,353)=36.49, p<.001). Bonferroni post-hoc analyses revealed no significant differences in current opioid use between participants endorsing no-OUD (M=8.75, SD=8.8) and those endorsing mild/moderate OUD (M=10.23, SD=8.3; p=.88). However, participants meeting criteria for severe OUD differed significantly from both the no-OUD (p<.001) and the mild/moderate OUD (M=18.03, SD=10.4; p<.001) groups.
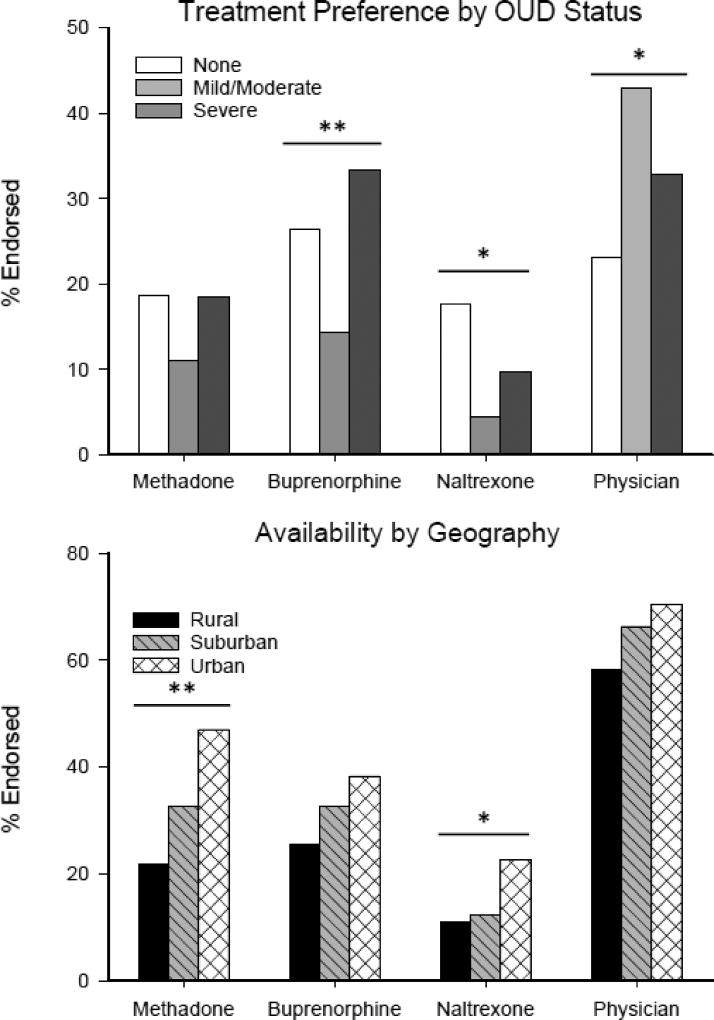
The treatment options participants were most likely to report willingness to use were one-on-one counseling (53.8%), 12-step groups (41.2%), and physician visits (32.8%); the option that was least likely to be endorsed was oral/XR naltrexone (10.4%) (Table 2). Similarly, when participants were asked to rate how familiar they were with each treatment on a 0–100 VAS, the most familiar options were physician visits (M=65.0, SD=31.0), one-on-one counseling (M=63.8, SD=28.8), and 12-step groups (M=62.8, SD=29.5), and the least familiar option was oral/XR naltrexone (M=21.6, 27.8) (Supplemental Table2). When asked which treatments they refused to use/believed to be not effective, the top three responses were sober living environment/half-way house (33.1%), methadone (32.2%), and 12-step groups (26.6%); the least endorsed option was one-on-one counseling (5.6%) (Table 2). Group differences for availability and preference for MATs based on possible OUD status and geographic locations (e.g., urban, suburban, and rural) are shown in Figure 1. Since buprenorphine and oral/XR naltrexone can both be prescribed by a physician in an office-based practice, Figure 1 also shows differences in availability and preference for physician visits.
Figure 1.
(Top) The preference for MATs and physician visits (without expressed purpose of MAT) was examined based on self-reported opioid use severity. Mild and moderate OUD was combined to balance group size. This led to three groups including no OUD (0–1 symptoms; n=91), mild/moderate OUD (2–5 symptoms, n=91), and severe OUD (6+ symptoms; n=174). Participants with severe OUD were more likely to endorse buprenorphine as a preferred treatment option when compared to participants with no-OUD or mild/moderate OUD (χ2(2)=11.09, p=.004). In addition, participants with no-OUD and severe OUD were more likely to endorse oral/XR naltrexone as a preferred treatment option relative to participants with mild/moderate OUD (χ2(2)=8.64, p=.013). There were no group differences in preference for methadone. Participants with mild/moderate and severe OUD were more likely to endorse preference for a physician visit as a treatment option compared to participants with no-OUD (χ2(2)=8.07, p=.018). (Bottom) Participants rated the perceived availability of methadone (χ2(2)=11.75, p=.003) and naltrexone (χ2(2) =6.82, p=.033) as less available in rural and suburban areas compared to urban areas; buprenorphine did not display a significant difference in perceived availability. *=p<.05; **=p<.01.
3.2 Treatment Perception based on Insurance Status and Annual Income
Participants were dichotomized into groups with (N=276) and without (N=81) insurance, to examine factors that might impede participants without insurance from seeking OUD treatment. The variable “price affects access to treatment” displayed large differences in all 13 treatment options among participants with and without insurance; therefore, the mean VAS score for the variable “price affects access to treatment” was collapsed across all treatment options and compared between insurance groups (without insurance: M=64.1, SD=18.7; with insurance: M=49.4, SD=20.5; t(350)=5.72, p<.001). Participants without insurance endorsed less perceived availability for the following treatment options compared to participants with insurance: residential treatment (χ2(1)=10.29, p=.001), sober living environments (χ2(1)=6.67, p=.010), IOPs (χ2(1)=5.46, p=.019), and physician visits (χ2(1)=4.32, p=.038).
Participants without insurance also had significantly lower annual income (Mdn=$37,500,) compared to those with insurance (Mdn=$52,500; U=6950, p<.001). When asked about out-of-pocket expenses, participants were willing to pay a median price of $250 (M=$735, SD=$1769, range: $0–22,500) for one month of residential treatment and a median price of $75 (M=$266, SD=$857, range: $0–12,500) for one month of MAT. Linear regression analysis revealed a significant association between personal income and the amount an individual was willing to pay for one month of residential treatment (F(1,353)=87.07, p<.001) and for one month of MAT (F(1,349)=11.64, p=.001). Further, a significant association between income and willingness to pay for medication (F(1,302)=8.77, p=.003), was observed among participants who reported not having insurance and/or no knowledge of whether insurance covered MAT, whereas the association between income and willingness to pay for medication amongst those who knew their insurance covered MAT was not significant (F(1,49)=1.37, p=.25).
3.3 Initial Treatment Choice
Standard criteria for the factorability of treatment options in a PCA were examined as a function of participant endorsement of the “perceived effectiveness” of the treatment. Kaiser-Meyer-Olkin measure of sampling adequacy was 0.85 and Bartlett’s test of sphericity (χ2 (66) = 886.5, p < .001) suggested that data were appropriate to factor. Based on this analysis, 2 factors emerged. Factor 1 was named “counseling/detox” and consisted of predominately non-MAT treatments such as residential treatment, detoxification, intensive outpatient, group and personal counseling. Factor 2 was named “MAT” and consisted of buprenorphine, methadone, and oral/XR naltrexone. Full information regarding factors is presented in Table 2. Physician visits and sober living environments (e.g., half-way houses) did not fit into either factor.
Participants were asked which of the 13 treatments they would try first; several of these options clustered based the aforementioned PCA and were amalgamated for the purpose of predictive modeling. Logistic regression analyses were significant for the following three categories: Factor 1 (counseling/detox) treatments (χ2(6) = 30.19, p<.001, Nagelkerke R2 = .11, correct classification = 62.1%); Factor 2 (MAT) (χ2(5) = 32.54, p<.001, Nagelkerke R2 = .20, correct classification = 85.8%); and physician visits (χ2(3) = 24.75, p<.001, Nagelkerke R2 = .10, correct classification = 71.1%). Results are summarized in Table 3. Briefly, participants interested in Factor 1 services were more likely to be male, did not have insurance, and believed those services were locally available (Table 3). Participants interested in Factor 2 services were more likely to have severe OUD, know their insurance covered pharmacotherapies, have used heroin in the last 30 days, and perceived MATs to be locally available (Table 3). Similarly, participants with insurance who knew of locally available physicians were more likely to consult with a physician as a first attempt at treatment (Table 3). None of the variables in these analyses were predictive of individuals utilizing 12-step programs as their first-try in OUD treatment.
Table 3.
Logistic Regression Predicting Initial Treatment Choice
| Odds Ratio |
95% CI | Wald | p value | |
|---|---|---|---|---|
| Factor 1 (counseling/detox) (df=6) | ||||
| Age (continuous) | 0.99 | 0.95–1.00 | 2.77 | 0.096 |
| Gender (male=1) | 1.87 | 1.19–2.93 | 7.47 | 0.006 |
| Insurance (y/n) | 0.50 | 0.29–0.87 | 6.11 | 0.013 |
| Insurance coverage for specific treatment (y/n) | 1.48 | 0.88–2.49 | 2.18 | 0.140 |
| Severe OUD (y/n) | 0.66 | 0.43–1.02 | 3.53 | 0.060 |
| Locally available (y/n) | 1.94 | 1.14–3.29 | 6.04 | 0.014 |
| Factor 2 (MAT) (df=5) | ||||
| Insurance coverage for specific treatment(y/n) | 2.80 | 1.28–6.10 | 7.63 | 0.006 |
| Severe OUD (y/n) | 2.39 | 1.11–5.12 | 5.00 | 0.026 |
| Locally available (y/n) | 2.39 | 1.06–5.39 | 4.41 | 0.036 |
| Heroin use in past 30 days (y/n) | 3.53 | 1.30–9.60 | 6.08 | 0.014 |
| Price affects access to treatment (continuous) | 0.99 | 0.98–1.00 | 2.57 | 0.110 |
| Physician Visit (df=3) | ||||
| Gender (male=1) | 0.67 | 0.40–1.13 | 2.24 | 0.135 |
| Insurance (y/n) | 2.28 | 1.07–4.88 | 4.51 | 0.034 |
| Locally available (y/n) | 3.27 | 1.68–6.37 | 12.15 | <0.001 |
Logistic Regression was used to determine which treatment a participant would try first. gender, age, income, insurance (binary), OUD severity, whether the treatment is available in the participant’s area, and the degree to which price affects accessibility. Variables that did not show marginal correlation (p≤.20) within the model were discarded and logistic regression was run a second time. Significant predictors are in bold (p<.05).
4. Discussion
This study evaluated treatment preferences of out-of-treatment individuals engaging in non-medical prescription opioid use, and quantified how practical barriers such as perceived treatment accessibility may impact tendency to seek out various treatment options. One-on-one counseling, physician visits, and 12-step groups were the top rated treatments in terms of familiarity and preference (i.e., the participant would be interested in using these options) (Table 2; Supplemental Table3), suggesting respondents were most likely to endorse options that were familiar to them (e.g., seeing a physician). On the other hand, oral/XR naltrexone had the lowest rating for both familiarity and preference. These results have clear public health implications and suggest that efforts to increase knowledge and familiarity of efficacious treatment options may increase willingness to utilize these treatments among persons seeking to initiate treatment for OUD.
A principal component analysis (PCA) of participant “perceived effectiveness” for each treatment option revealed treatment options clustered into two primary factors consisting of predominately counseling/detox (Factor 1) and MAT (Factor 2) (Table 2). Notably, many Factor 1 options are often coupled with opioid-MAT and, conversely, many Factor 2 options include elements in Factor 1 such as counseling (Lobmaier et al., 2010). Nevertheless, these data suggest that participants viewed these options as two discrete categories, and that willingness to initiate treatment with modalities within either factor was associated with different participant characteristics. Interestingly, respondents in this study had contrasting views of 12-step groups, evidenced by the fact that they were rated as both a top-3 option for “first treatment attempt” as well as in the top-3 treatments that were “not effective/would not use” (Table 2). Demographic and insurance related factors were not associated with attitudes toward 12-step groups.
The literature suggests that socioeconomic factors such as lack of insurance or use of Medicaid are associated with increased likelihood that an individual will develop OUD (Becker et al., 2008). In the current study, participants without insurance were more likely than those with insurance to endorse price as negatively affecting access to treatment. Participants without insurance were also less likely to endorse availability of residential facilities, sober living environments, and physicians in their local area. The current study also found an association between income and the amount an individual was willing to pay for one month of residential treatment or MAT. This relationship was not present among respondents who knew their insurance covered MAT, suggesting that individuals with insurance expect, to some degree, that insurance will alleviate the financial burden of medications that treat OUD. Taken together, these results suggest that individuals who do not have insurance may be discouraged from attempting many efficacious treatment options, including MAT. This is consistent with a previous study that reported perceived barriers for heroin-dependent individuals to enter MAT, including logistics, waiting lists, and lack of money/insurance (Peterson et al., 2010). The proportion of individuals in the current study that did not have insurance (22.7%; Table 1) was nearly double the rate of uninsured U.S. citizens between ages 18–64 (Cohen and Martinez, 2016); these outcomes are especially alarming in the context of efforts to decrease funding to Medicaid and/or decrease the number of individuals that have access to insurance through healthcare exchanges.
Geographic setting was also associated with differential perceived access to treatment, with individuals from rural/suburban areas endorsing less MAT availability relative to those from urban settings (Figure 1). Other studies have reported major geographic disparities in the number of physicians treating OUD in rural versus urban areas (Sigmon, 2014; Jones et al., 2015), potentially related to the fact that clinics specializing in opioid maintenance agonist therapy are often sparse in rural areas and may not offer a full range of treatment options compared to urban areas (Sigmon, 2014; Edmond et al., 2015). Finally, OUD severity was hypothesized to play a role in treatment preference since those with more severe OUD are likely to know others that have tried various treatment options and might have developed stronger preferences for certain treatments (specifically MATs) (Rounsaville and Kleber, 1985). Interestingly, respondents at both ends of the spectrum (no-OUD and severe OUD) endorsed higher rates of preference for buprenorphine and oral/XR naltrexone compared to those with mild/moderate OUD (Figure 1). Participants with mild/moderate OUD were more likely to prefer physician visits without the expressed purpose of MAT, relative to the other two groups. It is possible that individuals with mild/moderate OUD may be less interested in MAT because they don’t want to use a medication that interferes with the euphoric effects of opioids, or that they do not view their illness as severe enough to warrant undergoing MAT; more research on this interesting outcome is warranted as it may inform the point during a person’s OUD trajectory that he or she may decide to initiate different types of treatment.
Although academic researchers and public health officials have advocated that OUD be treated with MAT (Volkow et al., 2014; Blum et al., 2016), the current study suggests individuals engaging in non-medical prescription opioid use are interested in a range of treatment options that often include non-MAT care. Yet each form of treatment has inherent practical barriers; for instance, less than 10% of participants in the current study knew whether their insurance covered residential or inpatient detoxifications, despite the fact they were among the highest rated treatments for perceived effectiveness (Table 2; Supplemental Table4). Study results also suggest that primary care physicians may frequently be the point of first contact for opioid users who are initiating treatment. While office-based physicians might act as the most logical branching point in determining treatment course and level of care (i.e., whether patients engage in a path geared toward medical detoxification, ongoing MAT, or some combination) a recent survey of general internists reported that they did not feel prepared to screen for SUDs or provide brief interventions (Wakeman et al., 2016). Given that many individuals with OUD are interested in seeking treatment from primary care physicians, efforts should be made to train physicians to diagnose and treat OUD patients. This may be accomplished through enhanced autonomy treatment approaches that balance traditional physician “paternalism” and “independent choice” (Quill and Brody, 1996), or motivational interviewing interventions to encourage OUD patients to engage in long-term, meaningful recovery (Miller and Rollnick, 2012). Previous studies have also demonstrated that integrating primary health care with substance use disorder treatment can improve treatment outcomes (Weisner et al., 2001), providing further support for this approach.
This study has some limitations. The degree to which AMT workers are representative of the U.S. population is not known, though participant demographics in this study were similar to other large samples (Stein et al., 2015b) with the exception that our population was skewed toward middle class Caucasians. Also, this report does not include information on prior treatment attempts, a subject that is worth examining in future studies. In addition, surveys were done anonymously without clinical verification of OUD and instead relied upon a DSM-5 checklist to provide an assessment of “possible OUD severity”. Using this method, 25% of participants did not meet criteria for OUD yet used opioids at a rate similar to those meeting criteria for mild/moderate OUD, suggesting the non-OUD group may have been diagnosed with mild/moderate OUD following a formal diagnostic interview. Nevertheless, these data are representative of treatment preference based on personal assessment of OUD severity, which is valuable information for clinicians. Lastly, as these individuals were not treatment seeking, they might not be as aware of their insurance coverage for various treatment options.
5. Conclusion
This study is the first to examine the perceptions of various treatment options from an out-of-treatment population endorsing non-medical use of prescription opioids. Amongst these potentially treatment-seeking participants, the most familiar and preferred treatment options were counseling, 12-step groups, and physician visits, suggesting that this population is interested in psychological services, community support, and physician advice. Pharmacotherapies such as buprenorphine and oral/XR naltrexone were preferred by individuals with severe OUD. Factors that may affect access to treatment, such as insurance status and perceived local availability, did predict what treatment options respondents said they would be most willing to try first. Understanding the reasons that persons who are out-of-treatment but misusing opioids may choose to access different treatment modalities is crucial in developing targeted public health initiatives to reach individuals in need of treatment, and to adequately prepare the healthcare system to receive and manage these initial contacts in order to expand the availability of treatment options for OUD patients and combat the opioid epidemic.
Supplementary Material
Highlights.
Several factors shape individual preference for treatment of opioid use disorder
Access to treatment is predictive of initial treatment attempt in opioid users
Opioid users are more likely to utilize medical care if they have insurance
Opioid users in rural areas report less availability of methadone and naltrexone
Interest in pharmacotherapies is associated with severe opioid use disorder
Acknowledgments
Role of Funding Source
The work described in this manuscript was funded by the National Institute on Drug Abuse: NIDA R21 DA035327 (Dunn) and K23 DA029603.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Conflict of Interest
DAT has received medication supplies from Indivior (formerly Reckitt Benckiser Pharmaceuticals) for an investigator initiated study, is site PI for a clinical trial sponsored by Alkermes, and provided consulting services for AstraZeneca and Theravance. There are no conflicts to report for ASH or KED.
Contributions
All authors contributed to the research design and manuscript preparation. ASH performed all data analysis. All authors have approved the final article.
References
- Bailey GL, Herman DS, Stein MD. Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. J. Subst. Abuse Treat. 2013;45:302–305. doi: 10.1016/j.jsat.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker WC, Fiellin DA, Merrill JO, Schulman B, Finkelstein R, Olsen Y, Busch SH. Opioid use disorder in the United States: insurance status and treatment access. Drug Alcohol Depend. 2008;94:207–213. doi: 10.1016/j.drugalcdep.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Blum K, Gold M, Clark HW, Dushaj K, Badgaiyan RD. Should the United States government repeal restrictions on buprenorphine/naloxone treatment? Subst. Use Misuse. 2016;51:1674–9. doi: 10.1080/10826084.2016.1200097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opioid poisoning in the United States: 1999–2014. Am. J. Public Health. 2017;107:421–426. doi: 10.2105/AJPH.2016.303591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. 2015 National Survey on Drug use and Drug Health: Detailed Tables. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2016. [Google Scholar]
- Cohen RA, Martinez ME. Health Insurance Coverage, Early Release of Estimates from the National Health Interview Survey, 2011. National Center for Health Statistics 2016 [Google Scholar]
- Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N. Engl. J. Med. 2016;374:154–163. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL. Trends in opioid analgesic abuse and mortality in the United States. N. Engl. J. Med. 2015;2015:241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- Edmond MB, Aletraris L, Roman PM. Rural substance use treatment centers in the United States: An assessment of treatment quality by location. Am. J. Drug Alcohol Abuse. 2015;41:449–457. doi: 10.3109/00952990.2015.1059842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM. The paradox of decreasing nonmedical opioid analgesic use and increasing abuse or dependence—an assessment of demographic and substance use trends, United States, 2003–2014. Addict. Behav. 2017;65:229–235. doi: 10.1016/j.addbeh.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. J. Inform. 2015;105:e55–63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: A double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- Ling W, Charuvastra C, Collins JF, Batki S, Brown LS, Kintaudi P, Wesson DR, Mcnicholas L, Tusel DJ, Malkerneker U. Buprenorphine maintenance treatment of opiate dependence: A multicenter, randomized clinical trial. Addiction. 1998;93:475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- Lobmaier P, Gossop M, Waal H, Bramness J. The pharmacological treatment of opioid addiction—A clinical perspective. Eur. J. Clin. Pharmacol. 2010;66:537–545. doi: 10.1007/s00228-010-0793-6. [DOI] [PubMed] [Google Scholar]
- Mason W, Suri S. Conducting behavioral research on Amazon’s Mechanical Turk. Behav. Res. Methods. 2012;44:1–23. doi: 10.3758/s13428-011-0124-6. [DOI] [PubMed] [Google Scholar]
- Merlo LJ, Campbell MD, Skipper GE, Shea CL, DuPont RL. Outcomes for physicians with opioid dependence treated without agonist pharmacotherapy in physician health programs. J. Subst. Abuse Treat. 2016;64:47–54. doi: 10.1016/j.jsat.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. Guilford press; 2012. [Google Scholar]
- National Center for Health Statistics, Centers for Disease Control, Preventi. Health, United States, 2013, with Special Feature on Prescription Drugs. Government Printing Office; 2015. [PubMed] [Google Scholar]
- Paolacci G, Chandler J, Ipeirotis PG. Running experiments on amazon mechanical turk. Judg. Decis. Making. 2010;5:411–419. [Google Scholar]
- Peterson JA, Schwartz RP, Mitchell SG, Reisinger HS, Kelly SM, O’Grady KE, Brown BS, Agar MH. Why don’t out-of-treatment individuals enter methadone treatment programmes? Int. J. Drug Policy. 2010;21:36–42. doi: 10.1016/j.drugpo.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polydorou S, Ross S, Coleman P, Duncan L, Roxas N, Thomas A, Mendoza S, Hansen H. Integrating buprenorphine into an opioid treatment program: Tailoring care for patients with opioid use disorders. Psychiatr. Serv. 2016 doi: 10.1176/appi.ps.201500501. appi. ps. 201500501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quill TE, Brody H. Physician recommendations and patient autonomy: Finding a balance between physician power and patient choice. Ann. Intern. Med. 1996;125:763–769. doi: 10.7326/0003-4819-125-9-199611010-00010. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Kleber HD. Untreated opiate addicts: How do they differ from those seeking treatment? Arch. Gen. Psychiatry. 1985;42:1072–1077. doi: 10.1001/archpsyc.1985.01790340050008. [DOI] [PubMed] [Google Scholar]
- Schuman-Olivier Z, Greene MC, Bergman BG, Kelly JF. Is residential treatment effective for opioid use disorders? A longitudinal comparison of treatment outcomes among opioid dependent, opioid misusing, and non-opioid using emerging adults with substance use disorder. Drug Alcohol Depend. 2014;144:178–185. doi: 10.1016/j.drugalcdep.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Gryczynski J, O’Grady KE, Sharfstein JM, Warren G, Olsen Y, Mitchell SG, Jaffe JH. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995–2009. Am. J. Public Health. 2013;103:917–922. doi: 10.2105/AJPH.2012.301049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sees KL, Delucchi KL, Masson C, Rosen A, Clark HW, Robillard H, Banys P, Hall SM. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: A randomized controlled trial. JAMA. 2000;283:1303–1310. doi: 10.1001/jama.283.10.1303. [DOI] [PubMed] [Google Scholar]
- Sigmon SC. Access to treatment for opioid dependence in rural America: Challenges and future directions. JAMA Psychiatry. 2014;71:359–360. doi: 10.1001/jamapsychiatry.2013.4450. [DOI] [PubMed] [Google Scholar]
- Stein BD, Pacula RL, Gordon AJ, Burns RM, Leslie DL, Sorbero MJ, Bauhoff S, Mandell TW, Dick AW. Where is buprenorphine dispensed to treat opioid use disorders? The role of private offices, opioid treatment programs, and substance abuse treatment facilities in urban and rural counties. Milbank Q. 2015a;93:561–583. doi: 10.1111/1468-0009.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Anderson BJ, Bailey GL. Preferences for aftercare among persons seeking short-term opioid detoxification. J. Subst. Abuse Treat. 2015b;59:99–103. doi: 10.1016/j.jsat.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M, Bisaga A, Pavlicova M, Choi CJ, Mishlen K, Carpenter KM, Levin FR, Dakwar E, Mariani JJ, Nunes EV. Long-acting injectable naltrexone induction: A randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am. J. Psychiatry, appi. ajp. 2017;2016:16050548. doi: 10.1176/appi.ajp.2016.16050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins DA, Johnson PS, Smith MT, Strain EC, Edwards RR, Johnson MW. Temporal preference in individuals reporting chronic pain: Discounting of delayed pain-related and monetary outcomes. Pain. 2016;157:1724–1732. doi: 10.1097/j.pain.0000000000000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N. Engl. J. Med. 2014;370:2063–2066. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: Results from a survey of general internists. Subst. Abuse. 2016;37:635–41. doi: 10.1080/08897077.2016.1187240. [DOI] [PubMed] [Google Scholar]
- Weisner C, Mertens J, Parthasarathy S, Moore C, Lu Y. Integrating primary medical care with addiction treatment: A randomized controlled trial. JAMA. 2001;286:1715–1723. doi: 10.1001/jama.286.14.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Arch. Gen. Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR, Lintzeris N, Lea T. “Should I stay or should I go?” Coming off methadone and buprenorphine treatment. Int. J. Drug Policy. 2011;22:77–81. doi: 10.1016/j.drugpo.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Wu L, Zhu H, Swartz MS. Treatment utilization among persons with opioid use disorder in the United States. Drug Alcohol Depend. 2016;169:117–127. doi: 10.1016/j.drugalcdep.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra F, Veltman DJ, Booij J, van den Brink W, Franken IH. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug Alcohol Depend. 2009;99:183–192. doi: 10.1016/j.drugalcdep.2008.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.