Abstract
Lay Abstract
Children with Autism Spectrum Disorder (ASD) have numerous impairments in social interaction that can severely impede mental and physical development, learning, and behavioral functioning at home and in the community and also make treatment difficult. In addition, many individuals have difficulty performing movements of the body that involve both large and small actions. We explored the role of an overlooked dimension of social interaction, social motor synchrony, in ASD, and evaluated its relationship with body movement. In particular we examined the ability of children with and without ASD to perform body movements alone or with another individual. We found that children with ASD were less able to synchronize their body with an experimenter and children with ASD performed single-person motor movements that were slower and more variable in both spacing and timing. Children with ASD had trouble performing movements that were of a consistent size and tempo over the course of the interaction. Such lack of consistency in movement likely makes coordination with another person more difficult. This raises the possibility that these types of body movements could provide new insights into understanding the social problems in ASD.
Scientific Abstract
Impairments in social interaction and communicating with others are core features of autism spectrum disorder (ASD) but the specific processes underlying such social competence impairments are not well understood. An important key for increasing our understanding of ASD-specific social deficits may lie with the social motor synchronization that takes place when we implicitly coordinate our bodies with others. Here, we tested whether dynamical measures of synchronization differentiate children with ASD from controls and further explored the relationships between synchronization ability and motor control problems. We found (a) that children with ASD exhibited different and less stable patterns of social synchronization ability than controls; (b) children with ASD performed motor movements that were slower and more variable in both spacing and timing; and (c) some social synchronization that involved motor timing was related to motor ability but less rhythmic synchronization was not. These findings raise the possibility that objective dynamical measures of synchronization ability and motor skill could provide new insights into understanding the social deficits in ASD that could ultimately aid clinical diagnosis and prognosis.
Keywords: autism, social synchronization, dynamics, social interaction, motor skill
Impairments in social interaction and communication are core features of autism spectrum disorder (ASD). These impairments negatively affect typical social-cognitive development in multiple settings and are associated with poor clinical outcomes (American Psychiatric Association, 2013; Howlin, Goode, Hutton, & Rutter, 2004). One noteworthy characteristic of most social interactions is the coordination of bodies that occurs in turn-taking and jointly created actions. For example, neonates have been found to coordinate their body movements to human speech almost immediately after birth (Condon & Sander, 1974a, 1974b) and 4- and 9-month-olds coordinate their gaze and vocalizations based on when mothers start or stop talking (Feldstein et al., 1993; Jasnow & Feldstein, 1986). While problems with fundamental motor skills have been widely documented in ASD (Ghaziuddin & Butler, 1998; Pan et al., 2009; Fournier et al., 2010), much of the research relies on behavioral observation or self or parental reports of motor skill rather than a more objective analysis of the patterning of the dynamic movement structure itself. In addition, social motor synchronization remains a largely overlooked dimension of social interaction and communication in ASD research.
This is unfortunate because in the developmental literature, behavioral coordination and synchrony have been found to play important roles in social development. For example, Condon and Sander (1974a, 1974b) proposed that the interactional synchrony found in mother-infant interactions is important not only for language learning but also for the development of social relations and intersubjectivity whereby movement abnormalities may have numerous consequences for cognitive and emotional development (Feldman, 2007; Trevarthen & Delafield-Butt, 2013; Jaffe, Bebe, Feldstein, Crown, & Jasnow, 2001). Other research has shown that bodily coordination influences infant and toddler’s social perceptions. For example, toddlers prefer to play with or help adults who have mimicked their actions (Carpenter, Uebel, & Tomasello, 2013; Fawcett & Liszkowski, 2012) and 12-month old infants demonstrate a preference for social stimuli that moved synchronously with them, although 9-month old infants had not yet developed the social preference for synchronously moving objects (Tunçgenç, Cohen, & Fawcett, 2015). This type of behavioral coordination and synchronization is typically learned implicitly and deficits in such implicit learning could result in social problems. In fact, Klinger, Klinger, and Pohlig (2007) have found that individuals with ASD do indeed have impairments in implicit learning and argue that this impacts their ability to attend to and integrate information in the environment.
Some preliminary evidence suggests that children with ASD may have difficulty synchronizing their movements with another person. For example, Fitzpatrick et al. (2013) reported pilot data in which they found that school-age children with ASD had lower synchronization abilities than controls and Marsh et al. (2013) found that preschoolers with ASD were less able to exhibit spontaneous social rocking than controls. Similarly, Fitzpatrick et al. (2015) found that adolescents with ASD performed worse on synchronization tasks than controls.
Other research points to other types of motorically-based connections to others, such as those in imitative matching, as important in the early development of social competence. Imitation, for example, is evident within hours after birth (Meltzoff & Moore, 1977) and has been proposed to be a precursor to more complex social cognition such as joint attention and understanding agency that continues to develop during the first few years of life (Meltzoff, 1990; Meltzoff, 2009). Indeed some researchers have proposed that understanding early deficits in the ability to imitate others, along with the possible role of an atypically functioning mirror neuron system, are key to understanding the social deficits in ASD (Charman et al., 1997; Colombi et al., 2009; Gallese, 2006; Oberman & Ramachandran, 2007; Rizzolatti, & Fabbri-Destro, 2010; Rogers et al., 2003; Rogers & Pennington, 1991; Williams et al., 2001). Other research, however, suggests that some children with ASD do not have deficits in imitative movements and that the mirror neuron system of the social brain may not be damaged (Fan, et al., 2010; Hamilton et al., 2007; Gowen et al., 2008). The lack of consensus with regards to impairments in imitation may be due to methodological differences, including variability in task difficulty and participant characteristics. Alternatively, perhaps important clues to understanding the imitative movements of those with ASD lies more in understanding the nature of how the movements are performed rather than whether or not imitation occurs.
Other research points to the importance of early impairments in motor functioning, rather than impairments in imitation, for understanding social problems in ASD. Almost 80% of individuals diagnosed with ASD also have problems performing motor activities (Fournier et al., 2010; Ghaziuddin & Butler,1998; Pan et al., 2009) and research has documented that individuals with ASD can have difficulties in fine and gross motor coordination, control of posture and sway, bimanual arm coordination and performance of gestures and complex movement sequences (Henderson & Sugden, 1992; Ghaziuddin et al., 1994; Ghaziuddin & Butler, 1998; Isenhower et al., 2012; Jansiewicz et al., 2006; Minshew et al., 2004; Mostofsky et al., 2006). Importantly, there is evidence that these problems in motor functioning appear early in development during the first year of life. Flanagan, Landa, Bhat, and Bauman (2012), for example, found that infants at high risk for ASD demonstrated head lag during pull to sit at 6 months, and head lag was associated with ASD at 36 months. In addition, Esposito and colleagues report that lower levels of motor symmetry in sitting and lying differentiated infants with and without ASD (Esposito, Venuti, Meastro, & Juratori, 2009; Esposito & Venuti, 2009).
The development of language, communication, interaction with others and play all rely on using motor skills (Gernsbacher et al., 2008; Blaesi and Wilson, 2010; Clearfield, 2011) so motor difficulties can have potentially far-reaching consequences for individuals with ASD. For example, Bedford, Pickles, and Lord (2016) recently found that gross motor skill was a predictor of expressive and receptive language development in children with ASD. Dowd et al. (2010) have argued for the importance of understanding motor impairments in autism because motor impairments happen in parallel with social and behavioral deficits, may contribute to the social deficits and may share similar neural circuits. Similarly, Bhat et al. (2011) also suggests that motor problems may contribute to the social difficulties of those with ASD.
Our approach involves relying on the tools of behavioral dynamics, which provides researchers with methodologies and techniques that enable a fine-grained analysis of an individual’s movement as it unfolds in time. These techniques can be used to identify the degree of coordination between the movements of two people, as well as analyze the patterning of the movement variability (Richardson et al., 2007a; 2007b; 2008, 2014; Thiel et al., 2002). Such innovative techniques provide a new, more objective way to understand the complex, interactive and time-dependent emergent nature of social interactions and thus hold much promise for uncovering an ASD-specific movement signature underlying social interactions.
Given the importance of social synchrony for developing, initiating and maintaining social connections, understanding its possible role in the social problems evident in ASD seems well warranted. This research explored whether social motor synchronization is disrupted in ASD and evaluated the relationship between motor ability and social motor synchronization. In particular, we tested whether dynamical measures of social synchronization and motor ability differentiate children with ASD from controls and evaluated whether the degree of synchronization depended on whether the movements were performed at the same time as another person (synchrony condition), as an imitative sequence after a demonstration (imitation condition) or as a more free-flowing interactive two-person movement game (interpersonal hand-clapping). We also compared dynamical measures of motor ability of children with and without ASD and explored the relationship between synchronization ability and motor ability. Based on preliminary findings of Fitzpatrick et al. (2013), Isenhower et al. (2012), and Marsh et al. (2013), we predicted that children with ASD would demonstrate weaker synchronization ability and more variable and less coordinated motor ability. We also predicted that there would be an association between synchronization ability and motor ability (Bhat et al., 2011; Dowd et al., 2010).
Method
Participants
A total of 103 children, 50 with a diagnosis of ASD and 53 controls, participated. Five of the children in the ASD group were classified as non-spectrum and were eliminated from the analysis, resulting in a final sample of 98 children (mean age of ASD group 103.8 months, range 72–129; mean age of controls 99.70 months, range 75–131). The gender and age distribution of the participants is found in Table 1. The sample was chosen to reflect the prevalence of ASD in males and females.
Table 1.
Age and Gender Distribution of Participants
| Gender | Age (in years) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Male | Female | 6 | 7 | 8 | 9 | 10 | |
| ASD | 39 | 6 | 8 | 8 | 8 | 11 | 10 |
| Control | 40 | 13 | 13 | 11 | 10 | 10 | 9 |
The groups did not differ in chronological age (see Table 2) and the language and cognitive ability (as measured by the CELF-4 and DAS-II, see below) for both groups was in the normal range of 7–13 and 85–115, respectively. However, the language and cognitive scores of the ASD group were slightly lower than the control group (see Table 2). All parents of participants gave informed, written consent for their children to take part in the study and releases were also obtained for the video recordings. Participants received a $100 gift certificate for participating in the study. The project was approved by the Cincinnati Children’s Hospital Medical Center’s (CCHMC) Institutional Review Board and participants were recruited from CCHMC and local communities through print advertising, a recruitment brochure, email, social media and community events.
Table 2.
Participant Characteristics and Clinical Phenotyping
| ASD (n = 45) | Control (n = 53) | Group Difference | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Mean | SD | Mean | SD | t (96) | p | |
| CA (months) | 103.8 | 16.06 | 99.70 | 17.24 | 1.18 | .24 |
| CELF FD | 7.58 | 3.62 | 10.11 | 3.21 | −3.57 | < .001 |
| CELF FS | 8.31 | 4.35 | 11.64 | 2.92 | −4.40 | < .001 |
| DAS GCA | 95.82 | 15.18 | 108.06 | 14.03 | −4.12 | < .001 |
| DAS SNC | 97.50 | 16.22 | 109.08 | 14.24 | −3.74 | < .001 |
| ADOS | ||||||
| SA | 9.51 | 3.76 | ||||
| RRB | 3.07 | 1.68 | ||||
| Comparison Score | 7.12 | 1.66 | ||||
The participants with ASD had previously been diagnosed by a licensed clinical psychologist or medical doctor based on DSM-IV_TR criteria (APA, 2000) and diagnosis was confirmed using the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2, Lord et al., 2012). The ADOS-2 is a semi-structured, standardized assessment of communication, social interaction, and play for individuals referred because of possible autism. Five participants were administered Module 2 and 40 were administered Module 3. The mean ADOS scores for the ASD group are reported in Table 2.
Clinical Phenotyping
The Clinical Evaluation of Language Fundamentals-4 (CELF 4) and Differential Abilities Scales, 2nd Edition (DAS-II) were administered at the first visit. The CELF-4 (Semel, Wiig, & Secord, 2003) is a standardized language assessment appropriate for individuals 5 through 21 years of age. The concepts and following directions subscale (C & FD) was used to assess the child’s ability to follow directions and the formulated sentences subscale (FS) was used to assess the ability to communicate with sentences. The general conceptual ability score (GCA) and the special nonverbal composite score (SNC) from the DAS-II (Elliot, 2007) were used as measures of cognitive ability. The School Age form was used for 74 participants and the Early Years form for 24 participants (21 of whom were 6-years old and hence needed to be administered the Early Years form and 3 who failed to receive the minimum score on a subtest of the DAS-II School Age). Additional parental-report questionnaires were administered as part of a larger study but are not being reported here.
Social Motor Tasks
Children completed a battery of simple social motor imitation and synchrony tasks in order to examine the dynamic unfolding of social motor synchronization. The session was video recorded and small light weight wireless motion tracking sensors (Polhemus Liberty Latus, Polhemus Corporation, Colchester, VT) were also attached to the wrists of the child and experimenter using comfortable nylon sleeves in order to obtain high resolution recordings of the child and experimenter’s movements during the tasks. The motion data recording and reduction is explained in the Appendix.
Social Motor Coordination Battery
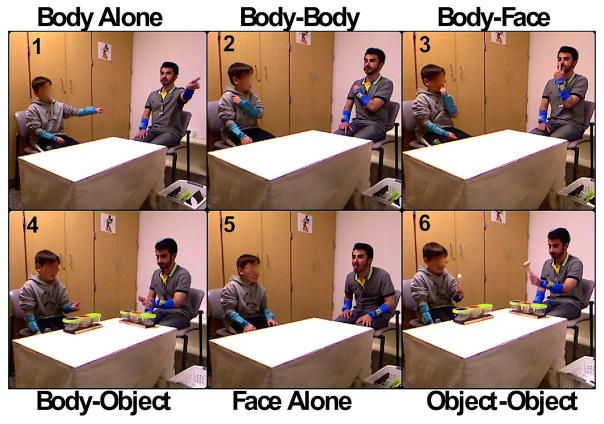
Five action sequences were used that involved the interpersonal coordinating of objects, bodies and faces to evaluate imitation and synchronization ability. These sequences were chosen to standardize the types of movements involved while varying the object of the coordination (physical objects, bodies or faces, see Figure 1). Half of the participants performed the sequences as an imitation task (the child watched the experimenter perform one of the sequences and then child then imitated the sequence) and half as a synchronization task (the child performed the actions at the same time as the experimenter). The manner in which the experimenter interacted with the participants was standardized across participants to minimize uncontrolled influences. There were six replications of each of the six movement sequences. The weighted coherence was used as the dependent variable to assess synchronization ability. Weighted coherence was calculated from the movement time series and is a measure of the coordination that occurred between the child and experimenter by estimating the correlation between their movements. A coherence of 1 reflects perfect correlation of the movements (absolute synchrony) and 0 reflects no correlation (no synchrony).
Figure 1.
Experimental set-up for the social motor coordination tasks. A body alone sequence (BA) involved the child tapping his/her finger in front of his/her body (in front of the right shoulder, in front of midline, in front of the left shoulder). A body-body sequence (BB) required the child to tap his/her own body in sequence (shoulder-head-shoulder) with his/her index finger. In the body-face sequence (BF), the child tapped his left ear, nose, right ear with his finger. In the body-object sequence (BO), the child tapped the cylinders in sequence with his/her index finger. In the face alone sequence (FA) the child stuck his tongue out in three locations (in front of the right shoulder, in front of midline, in front of the left shoulder). In the object-object sequence (OO), three cylinders were arranged on a table and a stick was used to tap each of the cylinders in sequence.
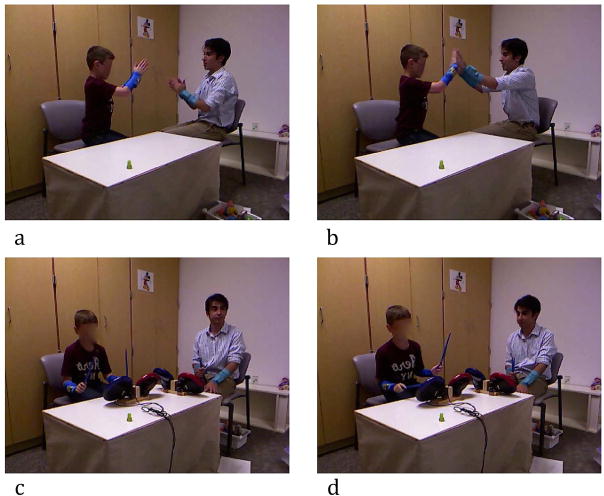
Interpersonal Hand-Clapping Task
An interpersonal hand-clapping task was also used as a second interpersonal motor coordination task. This task was somewhat less stereotyped, more dynamic, required more precise movement timing, and was more socially interactive. For the clapping task, the experimenter played a two-person pat-a-cake game with the child (see Figure 2a and 2b). As mentioned, this task was more social than the imitation/synchrony battery since the experimenter and child sat facing each other and made body contact with their hands. Two replications of the task were completed and weighted coherence was calculated from the time series movements of the child and experimenter as a measure of synchronization ability.
Figure 2.
The experimenter sang the pat-a-cake song and alternated individual claps with two-handed claps with the child in the hand-clapping task (panels a and b). In the in-phase drumming task (panel c), the child had one drumstick in each hand and moved them up and down so that the two sticks were moving together at the same tempo and both sticks contacted the drum together and were up in the air at the same time. In the anti-phase drumming condition (panel d), the participant drummed in an alternating pattern so that one stick was up in the air while the other stick contacted the drum.
Motor Control Tasks
Motor control ability was assessed with a drumming task. This task has been used in previous research to successfully differentiate children with and without ASD (Isenhower et al., 2012). Participants were handed one drumstick and completed a single-handed drumming trial with their dominant hand and in-phase drumming (see Figure 2c) and anti-phase drumming condition (see Figure 2d) with two sticks for the two-handed drumming tasks. The experimenter demonstrated each drumming task before the trial and the child was told to drum at a steady tempo until the experimenter said to stop. Drumming was recorded for 15s bouts. In the single-handed drumming task, period, period standard deviation (SD), amplitude and amplitude SD were calculated from the dominant hand wrist-movement time series as measures of movement patterning and variability. For the two-handed drumming tasks, weighted coherence was calculated from the child’s right and left wrist movement time-series to measure the degree of synchronization between the two limbs.
Procedure
Participants completed two sessions, no more than one-month apart. In the first session clinical pheonotyping was completed and lasted approximately three hours. The examiner who conducted the clinical assessment has extensive experience in the assessment of children with ASD and has obtained research reliability on ADOS-2. Screening for medical and psychopathology was conducted, participants completed the appropriate ADOS-2 module based on their language- and developmental level and the DAS-II and CELF-4 were administered. Only the ASD group completed the ADOS-2. Additional parental reports were completed as part of a larger study but are not being reported here.
In the second visit, the social motor coordination and motor coordination tasks were completed. (Additional social cognitive tasks were also completed as part of a larger study but are not being reported here.) The study was conducted in a 10 by 12 foot laboratory room at Cincinnati Children’s Hospital Medical Center (University of Cincinnati, Cincinnati, OH). Children came into the laboratory room and were asked to sit at a 2 foot wide × 4 foot long × 2 foot high table next to the seated experimenter (see Figure 1). Four Polhemus Latus receptors were attached to the underside of the tabletop, one in each corner, to create a 10 × 12 × 8 foot capture volume around the table. As soon as the child was seated, the four Polhemus wireless markers/sensors were placed in wristbands and slipped over the child’s and experimenter’s wrists (one marker on each wrist of the child and experimenter). The complete experimental protocol for the larger study was quite lengthy and in order to ensure fidelity, the order of presentation of the experimental conditions was identical for all participants. For the two tasks being reported in this study, the imitation or synchrony sequence (completed in order, object-object, body-object, body-body, body-alone, face-body) was completed first followed by the motor control tasks, in the following order: single-handed drumming, two-hand in-phase drumming and two-handed anti-phase drumming). Half of the participants in each diagnostic group were randomly assigned to complete the imitation sequence and half randomly assigned to complete the synchrony sequence.
Diagnostic group (ASD, control) and type of social motor coordination (imitation or synchrony) were between-subjects variables. Group differences in interpersonal hand-clapping and single-handed drumming were evaluated with independent samples t-tests. To evaluate the level of synchrony demonstrated in the social motor coordination battery, ANOVAs were performed with diagnostic group and type of social motor coordination as between-subjects variables, and type of action sequence (object-object, body-object, body-body, body-alone, body-face) as a within-subjects variable. To evaluate motor control ability, ANOVAs were performed with diagnostic group as a between-subjects variable and type of drumming (in-phase or anti-phase) as a within-subjects variable. Bivariate correlations were calculated to evaluate the relationship between the motor and social motor variables and motor and social motor variables and language and cognitive abilities. These correlations were calculated using the data from both the ASD and control groups.
Results
Group Differences in Synchronization Ability
Social motor coordination battery
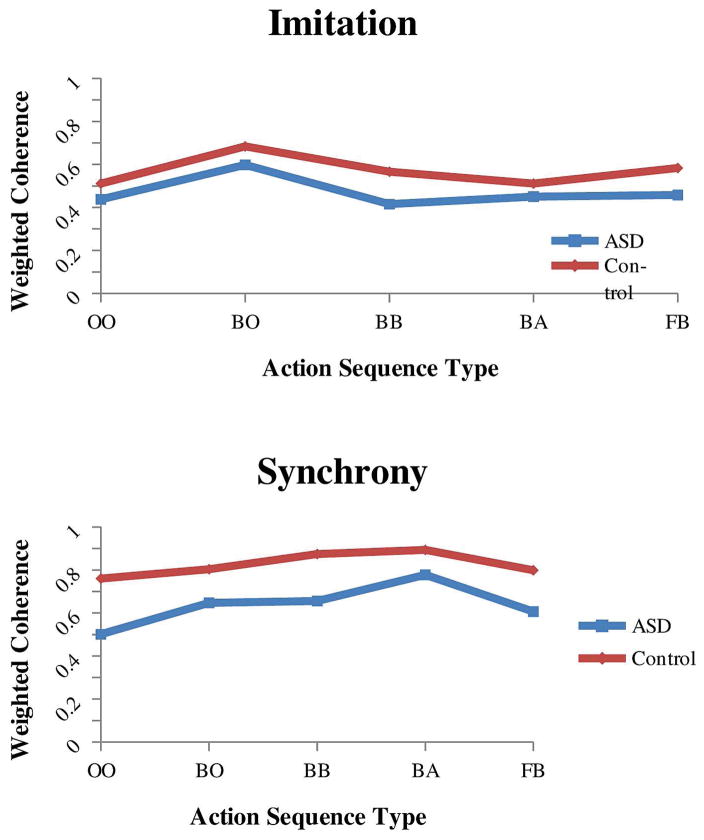
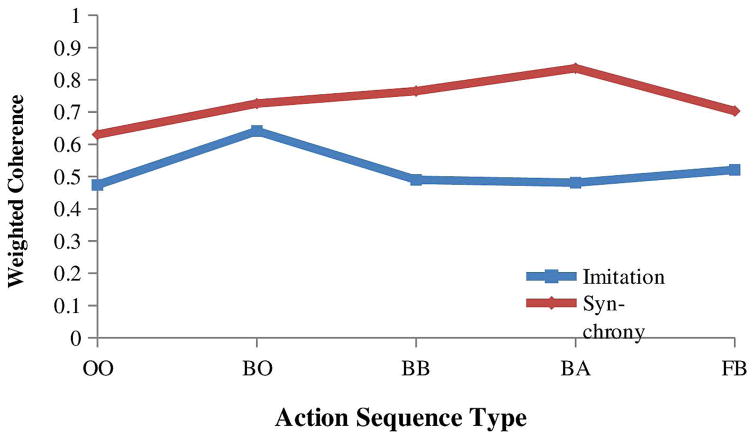
For the social motor coordination battery, a 2 (diagnostic group) × 2 (social motor coordination type, imitation or synchrony) × 5 (action sequence type) mixed ANOVA with weighted coherence as the dependent variable resulted in significant main effects of diagnostic group (F (1, 89) = 27.87, p < .001, η2 = .24), type of social motor coordination (F (1, 89) = 59.42, p<.001, η2 = .40) and action sequence type (F (4,356) = 9.34, p <.001, η2 =.10). The main effect of diagnostic group indicated that children with ASD had lower coherence scores (.56) than the control group (.70) for both social motor coordination types and for all action sequences (see Figure 3). In addition, the social motor coordination type and action sequence type 2-way interaction was significant (F (4,356) = 10.47, p <.001, η2 =.11). As seen in Figure 4, coherence scores were lower for the imitation task than the synchrony task for all action sequences. However, the body-body, body-alone and face-body sequences differentiated the imitation and synchrony tasks the best. No other two-way interactions were significant, nor was the 3-way interaction significant.
Figure 3.
Synchronization ability differences for the ASD and control group. The main effect of diagnosis condition revealed that the children with ASD had lower coherence scores than the control group in both the imitation and synchrony tasks and for all action sequence types.
Figure 4.
Synchronization ability differences for the social motor coordination battery. For both the ASD and control groups, the imitation task had overall lower coherence scores than the synchrony task. However, the body-body, body-alone, and face-body sequences better differentiated the imitation and synchrony tasks.
Interpersonal hand-clapping
A one-way ANOVA with diagnosis group as the independent variable revealed that the ASD group had significantly lower coherence scores (.84) than the control group (.93) for the interpersonal hand-clapping task (F (1, 88) = 16.30, p < .001, η2 = .17).
Group Differences in Motor Coordination
Single-handed drumming
To analyze differences in single-handed drumming performance, independent samples t-tests were conducted on the period, period standard deviation, amplitude and amplitude standard deviation. Results revealed that the ASD group had a higher movement period than the control group (.88 and .76, respectively, t (89) = 2.67, p = .009) but the movement amplitude of the ASD group was not significantly different than the control group. These results indicate that the ASD group drummed more slowly but covered the same spatial extent with their movements. The ASD group had significantly higher movement variability on measures of both period SD (.50 and .20, for ASD and control, respectively, t (89) = 4.44, p < .001) and amplitude SD (.78 and .60, for ASD and control, respectively, t (89) = 1.95, p = .05) indicating that in the ASD group drumming was more variable in both space and time.
Bimanual drumming
A 2 (diagnostic group) × 2 (type of drumming, in-phase and anti-phase) mixed ANOVA with weighted coherence as the dependent variable resulted in main effects of diagnosis group (F (1, 90) = 13.84, p < .001, η2 = .13) and type of drumming (F (1, 90) = 25.12, p < .001, η2 = .22). The ASD group had significantly lower coherence (.54) than the control group (.73) and both groups performed better on the in-phase drumming (coherence = .73 and .55 for in-phase and anti-phase, respectively). The interaction between diagnostic group and type of drumming was not significant.
Relationship Between Social Synchronization and Motor Measures
Bivariate correlations were calculated to examine the relationship between synchronization ability and motor measures. As seen in Table 3, interpersonal hand-clapping coherence had significant, negative correlations with single-handed drumming period, period SD, and amplitude SD and a significant positive correlation with in-phase drumming coherence. The correlations with amplitude and anti-phase drumming were not significant. The synchrony/imitation battery was not correlated with any of the motor measures for single-handed or two-handed drumming.
Table 3.
Correlations between Synchronization Ability and Motor Measures
| Interpersonal Hand-clapping | Imitation/Synchrony Battery | |||
|---|---|---|---|---|
|
|
|
|||
| r | p | r | p | |
| Single-handed Drumming | ||||
| Period | −.53** | <.001 | −.08 | .45 |
| Period SD | −.50** | <.001 | −.19 | .06 |
| Amplitude | −.02 | .83 | −.02 | .83 |
| Amplitude SD | −.27** | .008 | −.16 | .11 |
| Two-handed Drumming | ||||
| In-phase | .29** | .004 | .04 | .70 |
| Anti-phase Drum | .13 | .20 | .08 | .45 |
Note: all correlations calculated using the data from both the ASD and control groups
p < .05
p < .01
Correlations of Social Motor and Motor Measures with Language and Cognitive Ability
Although the language (as measured by the CELF) and cognitive (as measured by the DAS) ability of both groups was in the normal range, the scores of the ASD group were lower than the control group for both (see Table 2). As a result, we calculated bivariate correlations to evaluate the relationship between social motor synchronization ability and language and cognitive ability. Interpersonal hand-clapping and imitation/synchrony battery coherence were not correlated with language and cognitive ability, except for one significant correlation between interpersonal hand-clapping and CELF formulated sentences (see Table 4). We also examined the relationship between motor ability and language and cognitive ability. As seen in Table 5, there were no significant correlations between motor ability and language and cognitive ability.
Table 4.
Correlations between Social Motor Synchronization and Language and Cognitive Ability
| Interpersonal Hand-clapping | Imitation/Synchrony Battery | |||
|---|---|---|---|---|
|
|
|
|||
| r | p | r | p | |
| CELF FD | .20 | .06 | .16 | .15 |
| CELF FS | .29** | .007 | .19 | .07 |
| DAS GCA | .21 | .052 | .14 | .18 |
| DAS SNC | .13 | .24 | .15 | .16 |
Note: all correlations calculated using the data from both the ASD and control groups
p < .05
p < .01
Table 5.
Correlations between Motor Ability and Language and Cognitive Ability
| In-phase Drum | Anti-phase Drum | |||
|---|---|---|---|---|
|
|
|
|||
| r | p | r | p | |
| CELF FD | .08 | .47 | .14 | .19 |
| CELF FS | .20 | .06 | .14 | .19 |
| DAS GCA | .14 | .19 | .08 | .47 |
| DAS SNC | .13 | .22 | .06 | .54 |
Note: all correlations calculated using the data from both the ASD and control groups
p < .05
p < .01
Discussion
In summary, this research demonstrated (a) that children with ASD exhibited lower social synchronization ability than controls in all three types of social motor synchronization tasks (synchrony battery, imitation battery, and interpersonal hand-clapping); and (b) social synchronization was highest in interpersonal hand-clapping, moderate in the synchrony battery and lowest in the imitation battery for both groups. In addition, the children with ASD performed drumming movements that were slower and more variable in both spacing and timing than those of control children. Finally, social synchronization ability was correlated with motor skill and sentence formation for the interpersonal hand-clapping game but not for either the synchrony battery or the imitation battery.
As stated above, children with ASD had significantly poorer movement synchrony (as indexed by the dynamical measure coherence) during both an interpersonal hand-clapping task that involved more implicit and dynamic movement matching and a social motor coordination battery that involved more explicit, intentional coordination of a simple, well-specified movement sequence that involved actions on objects or the body. This disruption in synchronization ability was found both for the imitation and synchrony version of the tasks. This replicates previous research which has found individuals with ASD demonstrate problems in imitation (Charman et al., 1997; Colombi et al., 2009; Gallese, 2006; Oberman & Ramachandran, 2007; Rizzolatti, & Fabbri-Destro, 2010; Rogers et al., 2003; Rogers & Pennington, 1991; Williams et al., 2001) as well as other research which has found lower levels of synchronization ability in children and adolescents with ASD (Fitzpatrick et al., 2013; Fitzpatrick et al., 2015, 2016; Marsh et al., 2013). Our research makes two important contributions to this literature. First, we found that the manner in which movements are performed is different in children with ASD and such an analysis of the dynamics of the movement structure has not been investigated previously in imitation tasks. Children with ASD had trouble performing movements that were of a consistent size and tempo over the course of the interaction. Such lack of consistency in movement production likely makes coordination with another person more difficult. This suggests that how movements are performed may be an important component of the social information that can facilitate or hinder social connection. Second, previous research investigating synchronization has been conducted with small sample sizes and we found group differences with strong effect sizes using a much larger sample.
Synchronizing one’s body with another person does involve a number of cognitive and communicative abilities—the ability to follow directions as well as non-verbal and spatial reasoning. We would argue, however, that it is unlikely that the synchronization differences we found are due to cognitive and language differences between the groups. First, the language and cognitive ability measures fell within the average range for both groups. Second, synchronization ability was not correlated with language and cognitive ability, except for one correlation between interpersonal handclapping coherence and formulated sentences. The lack of correlation between most of the measures suggests that difficulties in following directions or non-verbal and spatial reasoning abilities are unlikely to be underlying factors responsible for the social synchronization differences we observed. The finding that synchronization was correlated with sentence formation is, however, potentially important because both synchronization ability and speech prosody require rhythmic timing and anticipation. These findings raise the intriguing possibility that the difficulty some children with ASD have in acquiring language (Eigsti, Schuh, Mencl, Schultz, & Paul, 2012) and their difficulties synchronizing with another person may be due to fundamental problems with rhythm production during social interactions. Additional research is needed to explore these relationships across a larger range of language, cognitive and synchronization abilities.
Importantly, the synchronization of the movements of the child and experimenter was lower for the imitation battery than synchrony battery for both groups. One essential difference between the imitation battery and the synchrony battery is that imitation requires performing the movements in terms of both spacing and timing from memory while the synchrony version requires sustained attention and monitoring of the other person’s movements while the task is being performed. Thus, it is unclear whether the increased memory load is responsible for this difference or if there is another feature of the imitation task that results in poorer performance. Future research should be designed to disentangle this issue. Either way, however, these findings suggest that how movements are performed may be important for understanding both the imitative and synchronization abilities of those with ASD.
It should also be noted that the difference in coherence scores between groups was greater for the synchrony battery than the imitation battery, suggesting behavioral synchrony tasks (having the child attempt to match the behavior or movements of the experimenter at the same time) may be better at differentiating children with ASD than imitation tasks. One possible reason for this is that synchronizing movements in time requires more social monitoring of the other person’s movements, which may make it an inherently more social task.
In addition to the differences in synchronization ability for the imitation and synchrony batteries noted above, we also found that coherence was somewhat higher for the interpersonal hand-clapping game than the imitation or synchrony batteries. This is somewhat surprising given that the interpersonal hand-clapping could be considered more social since it was completed with the child sitting face-to-face with the experimenter rather than side-to-side as in the imitation and synchrony batteries. One might also say the interpersonal hand-clapping game had more social ecological validity since it is a dynamic, fluid exchange between two people.
However, three important differences between the interpersonal hand-clapping game and the social motor batteries may account for this difference. First, the social motor battery was a novel task while the interpersonal hand-clapping game was a more familiar childhood game. It is widely known that individuals with ASD have a strong preference for familiarity and routines (Schaaf et al., 2011) so understanding the role of novelty in contributing to the social interaction difficulties is a factor that should be systematically explored in future research. Second, one could argue that since the interpersonal hand-clapping game is more familiar and consequently the coordination mechanism is easily assembled and the coordination can be executed with less on-going monitoring. In contrast, the social motor battery is a somewhat more discrete and novel series of movements and more attention to the on-going execution of the task may be needed. Finally, the child was performing the movements independently during the social motor batteries while the synchronization was jointly created by both the child and the experimenter in the interpersonal hand-clapping game. The degree to which the experimenter compensated for the child’s inability to synchronize could have resulted in the higher performance during the interpersonal hand-clapping game. Future research should be designed to explore this bi-directional influence. While more research is needed to understand the underlying mechanisms involved in these differences, the poorer performance of children with ASD on all three tasks suggests disruptions in all three of these factors—novelty, task initiation, and task execution.
Furthermore, children with ASD displayed a motor deficit both in terms of their ability to coordinate their arms during two-handed drumming (as indicated by lower coherence scores) and the ability to make consistent, smooth movements in both space and time during single-handed drumming (as indicated by higher variability). These findings are similar to work that found poor drumming performance in participants with ASD (Isenhower et al., 2012). It is important to note, however, that these motor measures rely on rhythmic timing of movements. While these findings are consistent with other research that indicates motor impairments across a range of motor behaviors are pervasive in ASD (Fournier, Hass, Naik, Lodha, & Cauraugh, 2010), additional research is needed to explore the relationship between motor coordination measures that focus on timing of movements and those that do not. Moreover, further research is needed to more closely evaluate the relationship between ASD deficits in rhythmic timing and in motor anticipation (Brisson et al, 2011) as well as how these more generally affect social interaction outcomes (Sebanz & Knoblich, 2009).
In addition, coherence was lower for anti-phase than in-phase drumming for both groups. This replicates other research which has found that anti-phase coordination is less stable than in-phase coordination (Turvey, Rosenblum, Schmidt, & Kugler, 1986) and can also be explained by the finding that interlimb coordination continues to develop throughout childhood (Fitzpatrick, Schmidt, & Lockman, 1996) and is not mature until the corpus callosum has fully developed after the age of 10 (Jeeves, Silver, & Milne, 1988; Wolff, Kotwica, & Obregon, 1998).
Interestingly, however, only interpersonal hand-clapping coherence was correlated with the motor measures. This suggests that the relationship between motor skill and social motor synchronization may not be straightforward. Both the hand-clapping game and the drumming were familiar, very rhythmic movements that are timed in space and time and also highlight movement initiation. The hand-clapping game also requires anticipating the partner’s movement, which was more difficult due to the highly variable movements of the child with ASD. This may account for the association between them and suggest there may be shared underlying mechanisms in both motor performance and social motor interactions. In contrast, the imitation and synchrony batteries were less rhythmic and hence depended less on perfect timing. Instead, these batteries may have relied more on sustained attention and continuous monitoring of the other person’s movements, which could have been heighted due to the novelty of the task. The lack of association with motor skills may suggest that this dimension of social motor coordination is unique to the social context. It is also possible, however, that measures of motor ability that do not require precise timing of movements may demonstrate a different pattern. Future research is needed to explore whether the relationship between motor coordination that does not rely on timing has similar relationships with two types of social motor synchronization. Clearly, more research is needed to understand whether an overarching neuromotor impairment is contributing to the social problems in ASD (Dowd et al., 2010; Gernsbacher et al., 2008) or whether there are underlying mechanisms that are unique to social motor synchronization. Alternatively, it is possible there may be some shared underlying mechanisms that contribute to both motor and social motor synchronization problems.
Our finding that motor ability was not correlated with language or cognitive ability is somewhat surprising given that other research suggests there may be a connection between the two. This difference could be due to the fact that much of the other research relies on behavioral observation of gross motor skill that typically does not involve movement timing like our measure did. Bedford et al. (2016), for example, used the gross motor subscale of the Mullen Scales of Early Learning and found it was a was a predictor of expressive and receptive language development in children with ASD. This suggests that motor ability that involves movement timing may be different in important ways from overall gross motor behavior. In addition, we used objective, time-dependent measures of motor ability that provide a more fine-grained analysis of movement than is possible with behavioral coding which can be subjective. Another possible reason for the different pattern of results could be due to the fact that Bedford et al.’s (2016) sample was infants and toddlers and ours was school-aged children. It is possible that the relationship between motor and language ability changes developmentally. Finally, our language measures involved concepts and following directions and formulated sentences while Bedford et al. (2016) measured receptive and expressive language. This may mean that the relationship between motor ability and language is different for different dimensions of language ability. These are important issues that need to be disentangled in future research.
Conclusion and Clinical Implications
Taken together, these findings suggest that children with ASD have difficulty synchronizing their movements with another person and highlight the need for further research to understand the contribution of synchronization ability to the social interaction problems evident in individuals with ASD. In particular, the roles of novelty, sustained attention/ongoing-monitoring of information, and movement initiation should be evaluated. Social interactions are complex—they are dynamic events that unfold over time, can be inherently unpredictable and thus require ongoing attention and monitoring and anticipation of the partner’s actions. In addition, each social interaction is a unique and novel situation that needs to be negotiated anew each time. This research makes an important contribution to the literature by demonstrating that individuals with ASD are less able to synchronize their movements with another person, which may be an important factor contributing to their problems initiating and maintaining social interactions. In addition, our findings implicate a number of dimensions of sociality that are important in social interactions that need to be more fully and systematically investigated in future research.
Furthermore, our research focuses on the importance of understanding how behavior unfolds during social interactions. This is noteworthy because the meaning behind our movements may depend not only on whether we perform certain actions, but whether they are performed at the appropriate time and in a manner that facilitates social connection. Such a focus may provide new insights for understanding underlying mechanisms and new pathways for designing interventions for ASD. Current interventions, such as social skills training, focus on teaching individuals what to do in a social interaction and have been only moderately successful (Rao, Beidel, & Murray, 2008). Our research raises the important possibility that perhaps by creating a learning environment that better facilitates implicit learning (Klinger et al., 2007) we can change how social interactions unfold over time through interventions that provide opportunities for improving social motor coordination. This could be a potential path for new interventions that could be successful for individuals across the autism spectrum because synchronization ability is non-verbal.
These findings also have potentially important clinical implications for early diagnosis and early intervention because movement synchronization can be easily and objectively measured at an early age. For example, neonates have been found to synchronize their movements to adult speech (Condon & Sander, 1974) and disruptions in such synchronization could be helpful in identifying children for early intervention services.
Quantification of synchronization ability thus reflects a potential neurophysiological biomarker. Social synchronization has been largely overlooked as a contributor to the social skills deficit in ASD and our research indicates that it is disrupted in ASD. In addition, the relationship between sentence formation and social synchronization suggests that disruptions in rhythmic timing and motor anticipation could be contributing to communication problems evident in ASD that manifest in difficulties acquiring language and synchronizing with another person. Further research that relies on objective dynamical measures of social synchronization and perceptual and motor processes is needed to determine whether enhancing synchronization ability has the potential to increase social competence in clinically relevant ways. If that turns out to be the case, synchronization intervention programs could provide an alternative intervention for improving social skills in children with ASD. Objective measures of social synchronization may also offer insights to aid clinical diagnosis and prognosis of ASD.
Acknowledgments
Research reported in this article was supported by the National Institute of Mental Health of the National Institutes of Health under award number R21MH094659. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Appendix Motion Data Recording and Reduction
All of the data extraction and analysis methods for the dynamical measures were completed using custom MATLAB (Mathworks, Inc., Natick, MA) applications and functions developed by the authors. These MATLAB applications and functions, as well as example time-series can be downloaded from www.xkiwilabs.com.
The x-plane (left-right), y-plane (forward-back) and z-plane (up-down) positional coordinates of the Polhemus Latus sensors placed on the wrists of the experimenter and child were recorded for each task. These sensors have been used extensively in previous research (e.g., see Schmidt & Richardson (2008) for a review of research employing such sensors in interpersonal coordination work) and use a passive magnetic field to track movements in 3-dimension space. The motion of the Polhemus sensors was recorded at 94 Hz on a Dell PC computer using a custom software application written by the authors using the Polhemus Latus C/C++ SDK Library.
To best determine the stability and patterning of the behavioral coordination that occurred between the child and experimenter, we first isolated the primary plane of motion for each task. Since the primary plane of motion for the drumming and pointing tasks was in the left-right plane, the x-plane movement time-series was used to assess the behavioral coordination that occurred for these two tasks. For the interpersonal hand-clapping task, the largest amplitude of movement was in the up-down, z-plane, with the intrapersonal clapping events occurring at a lower height than the interpersonal clap events. Accordingly, this plane of motion was employed to assess the behavioral coordination that occurred for this task.
For the tapping and pointing tasks, we then performed an analysis of interpersonal coordination (see below for details) using the primary plane of motion time-series of the experimenter’s right forearm (the experimenter always used his right hand/arm for all the tasks) and the primary plane of motion time-series of the forearm used by the child for analysis. Note that for the tapping and pointing tasks the child was free to use either left or right arm/hand. Although both arms/hands were employed by the experimenter and child for the hand clapping game, we only extracted the right forearm movements of the experimenter and child for analysis, as the coordination that occurred between the left forearm movements was completely redundant with the right forearm movements.
Prior to analysis all of the pre- and post- non-task relevant movement transient periods were cropped from the different time-series. These final motion time-series were then low-passed filtered using 10 Hz 4th order Butterworth filter to removed system measure noise. To determine the stability and patterning of the social motor coordination that occurred between the children and the experimenter for each task and condition the bidirectional weighted cross spectral coherence (Porges et al., 1980; Goldfield, Wolff, & Schmidt, 1999) was calculated. Commonly referred to as coherence, this measure evaluated the coordination that occurred between the child and experimenter by estimating the correlation between their movements across the frequency band from 0 to 2 Hz that captures time scales of the rhythms inherent in the movement time series. The weighted coherence is a weighted average measure of the correlation (actually an r2 value) of the two time series across this frequency range and ranges on a scale from 0 to 1. A coherence of 1 reflects perfect correlation of the movements (absolute synchrony) and 0 reflects no correlation (no synchrony).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Bedford R, Pickles A, Lord C. Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder. Autism Research. 2016;9:993– 1001. doi: 10.1002/aur.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Landa RJ, Galloway JC. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical Therapy. 2011;91:1116–1129. doi: 10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- Blaesi S, Wilson M. The mirror reflects both ways: Action influences perception of others. Brain and Cognition. 2010;72:306–309. doi: 10.1016/j.bandc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Brisson J, Warreyn P, Serres J, Foussier S, Adrien-Louis J. Motor anticipation failure in infants with autism: A retrospective analysis of feeding situations. Autism. 2012;16:420–429. doi: 10.1177/1362361311423385. [DOI] [PubMed] [Google Scholar]
- Carpenter M, Uebel J, Tomasello M. Being mimicked increases prosocial behavior in 18-month-old infants. Child Development. 2013;84:1511–1518. doi: 10.1111/cdev.12083. [DOI] [PubMed] [Google Scholar]
- Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. Infants with autism: An investigation of empathy, pretend play, joint attention, and imitation. Developmental Psychology. 1997;33(5):781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- Clearfield MW. Learning to walk changes infants’ social interactions. Infant Behavior and Development. 2011;34:15–25. doi: 10.1016/j.infbeh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Colombi C, Liebal K, Tomasello M, Young G, Warneken F, Rogers S. Examining correlates of cooperation in Autism: Imitation, joint attention, and understanding intentions. Autism. 2009;13(2):143–163. doi: 10.1177/1362361308098514. [DOI] [PubMed] [Google Scholar]
- Condon WS, Sander LW. Neonate movement is synchronized with adult speech: Interactional participation and language acquisition. Science. 1974a;183:99–101. doi: 10.1126/science.183.4120.99. [DOI] [PubMed] [Google Scholar]
- Condon WS, Sander LW. Synchrony demonstrated between movements of the neonate and adult speech. Child Development. 1974b;45:456–462. [PubMed] [Google Scholar]
- Dowd AM, Rinehart NJ, McGinley J. Motor function in children with autism: Why is this relevant to psychologists? Clinical Psychologist. 2010;14:90–96. [Google Scholar]
- Elliot CD. Differential Ability Scales II. New York: Pearson Education, Inc; 2007. [Google Scholar]
- Eigsti IM, Schuh J, Mencl E, Schultz RT, Paul R. The neural underpinnings of prosody in autism. Child Neuropsychology. 2012;18:600–17. doi: 10.1080/09297049.2011.639757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Venuti P, Meastro S, Juratori F. An exploration of symmetry in early autism spectrum disorders: Analysis of lying. Brain & Development. 2009;31:131–138. doi: 10.1016/j.braindev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Esposito G, Venuti P. Symmetry in infancy: Analysis of motor development in autism spectrum disorders. Symmetry. 2009;1:215–225. [Google Scholar]
- Fan Y, Decety J, Yang C, Liu J, Cheng Y. Unbroken mirror neurons in autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2010;51(9):981–988. doi: 10.1111/j.1469-7610.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- Fawcett C, Liszkowski U. Mimicry and play initiation in 18-month-old infants. Infant Behavior and Development. 2012;35:689–696. doi: 10.1016/j.infbeh.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent-infant synchrony: Biological foundations and developmental outcomes. Current Directions in Psychological Science. 2007;16(6):340–345. [Google Scholar]
- Feldstein S, Jaffe J, Beebe B, Crown CL, Jasnow M, Fox H, Gordon S. Coordinated interpersonal timing in adult-infant vocal interactions: A cross-site replication. Infant Behavior and Development. 1993;16:455–470. [Google Scholar]
- Fitzpatrick P, Diorio R, Richardson MJ, Schmidt RC. Dynamical methods for evaluating the time-dependent unfolding of social coordination in children with autism. Frontiers in Integrative Neuroscience. 2013;7(21):1–13. doi: 10.3389/fnint.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P, Frazier J, Mitchell T, Cochran D, Coleman C, Schmidt RC. Impairments of social motor synchrony evident in autism spectrum disorder. Frontiers in Psychology. 2016;7:1323. doi: 10.3389/fpsyg.2016.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P, Mitchell T, Frazier J, Cochran D, Coleman C, Schmidt RC. Exploring the behavioral and neural processes underlying social synchronization of individuals with and without social deficits. In: Weast-Knapp J, Malone M, Abney D, editors. Studies in perception and action XIII: Eighteenth international conference on perception and action. New York, NY: Psychology Press, Taylor & Francis Group; 2015. [Google Scholar]
- Fitzpatrick PA, Schmidt RC, Lockman JJ. Dynamical patterns in the development of clapping. Child Development. 1996;67:2691–2708. [Google Scholar]
- Flanagan JE, Landa R, Bhat A, Bauman M. Head lag in infants at risk for autism: A preliminary study. The American Journal of Occupational Therapy. 2012;66(5):577–585. doi: 10.5014/ajot.2012.004192. [DOI] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders. 2010;40(10):1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Gallese V. Intentional attunement: A neurophysiological perspective on social cognition and its disruption in autism. Brain Research Cognitive Brain Research. 2006;1079:15–24. doi: 10.1016/j.brainres.2006.01.054. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Sauer EA, Geye HM, Schweigert EK, Hill Goldsmith H. Infant and toddler oral- and manual-motor skills predict later speech fluency in autism. Journal of Child Psychology and Psychiatry. 2008;49:43–50. doi: 10.1111/j.1469-7610.2007.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaziuddin M, Butler E. Clumsiness in autism and Asperger syndrome: A further report. Journal of Intellectual Disability Research. 1998;42:43–48. doi: 10.1046/j.1365-2788.1998.00065.x. [DOI] [PubMed] [Google Scholar]
- Goldfield EC, Wolff PH, Schmidt RC. Dynamics of oral-respiratory coordination in full-term and preterm infants: I. Comparisons at 38–40 weeks postconceptional age. Developmental Science. 1999;2:363–373. [Google Scholar]
- Gowen E, Stanley J, Miall C. Movement interference in autism-spectrum disorder. Neuropsychologia. 2008;46:1060–1068. doi: 10.1016/j.neuropsychologia.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AF, Brindley RM, Frith U. Imitation and action understanding in autistic spectrum disorders: How valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia. 2007;45:1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. The Journal of Child Psychology and Psychiatry. 2004;45(2):212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Isenhower RW, Marsh KL, Richardson MJ, Helt M, Schmidt RC, Fein D. Rhythmic bimanual coordination is impaired in children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2012;6:25–31. [Google Scholar]
- Jaffe J, Beebe B, Feldstein S, Crown CL, Jasnow M. Rhythms of dialogue in infancy. Monographs of the Society for Research in Child Development, Series 264. 2001;66(2):1–132. [PubMed] [Google Scholar]
- Jasnow M, Feldstein S. Adult-like temporal characteristics of mother–infant vocal interactions. Child Development. 1986;57:754–761. [PubMed] [Google Scholar]
- Jeeves MA, Silver PH, Milne AB. Role of the corpus callosum in the development of a bimanual motor skill. Developmental Neuropsychology. 1988;4(4):305–323. [Google Scholar]
- Klinger LG, Klinger MR, Pohlig RL. Implicit learning impairments in autism spectrum disorders. In: Perez JM, Gonzalez PM, Comi ML, Nieto C, editors. New developments in autism: The future is today. Philidelphia, PA: Jessia Kinglsley Publishers; 2007. pp. 76–103. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule, second edition (ADOS-2) (Part I): Modules 1–4 [Manual] Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- Marsh KL, Isenhower RW, Richardson MJ, Helt M, Verbalis AD, Schmidt RC, Fein D. Autism and social disconnection in interpersonal rocking. Frontiers in Integrative Neuroscience. 2013;7(4):1–8. doi: 10.3389/fnint.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. Foundations for developing a concept of self: The role of imitation in relating self to other and the value of social mirroring, social modeling, and self practice in infancy. In: Cicchetti D, Beeghly M, editors. The self in transition: Infancy to childhood. Chicago: University of Chicago Press; 1990. pp. 139–164. [Google Scholar]
- Meltzoff AN. Roots of social cognition: The like-me framework. In: Cicchetti D, Gunnar MR, editors. Minnesota symposium on child psychology: Meeting the challenge of translational research in child psychology. Vol. 35. Hoboken, NJ: John Wiley & Sons; 2009. pp. 29–58. [Google Scholar]
- Oberman LM, Ramachandran VS. Social Neuroscience on Mirror Neurons and Communication. 2007. Evidence for deficits in mirror neuron functioning, multisensory integration, and sound-form symbolism in autism spectrum disorders. Invited submission to special issue of. [DOI] [PubMed] [Google Scholar]
- Pan CY, Tsai CL, Chu CH. Fundamental movement skills in children diagnosed with autism spectrum disorders and attention deficit hyperactivity disorder. Journal of Autism and Developmental Disorders. 2009;39:1694–1705. doi: 10.1007/s10803-009-0813-5. [DOI] [PubMed] [Google Scholar]
- Porges SW, Bohrer RE, Cheung MN, Drasgow F, McCabe PM, Keren G. New time-series statistics for detecting rhythmic co-occurrence in the frequency domain: The weighted coherence and its application to psychophysiological research. Psychological Bulletin. 1980;88:580–587. [PubMed] [Google Scholar]
- Rao PA, Beidel DC, Murray MJ. Social skills interventions for children with Asperger’s syndrome or high-functioning autism: A review and recommendations. Journal of Autism and Developmental Disorders. 2008;38:353–361. doi: 10.1007/s10803-007-0402-4. [DOI] [PubMed] [Google Scholar]
- Richardson MJ, Kay BA, Schmidt RC. Distinguishing the noise and attractor strength of coordinated limb movements using recurrence analysis. Biological Cybernetics. 2007a;96:59–78. doi: 10.1007/s00422-006-0104-6. [DOI] [PubMed] [Google Scholar]
- Richardson MJ, Lopresti-Goodman S, Mancini M, Kay BA, Schmidt RC. Comparing the attractor strength of intra- and interpersonal interlimb coordination using cross recurrence analysis. Neuroscience Letters. 2008;438:340–345. doi: 10.1016/j.neulet.2008.04.083. [DOI] [PubMed] [Google Scholar]
- Richardson MJ, Marsh KL, Isenhower RW, Goodman JR, Schmidt RC. Rocking together: Dynamics of intentional and unintentional interpersonal coordination. Human Movement Science. 2007b;26(6):867–891. doi: 10.1016/j.humov.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Richardson MJ, Dale R, Marsh KL. Complex dynamical systems in social and personality psychology: Theory, modeling and analysis. In: Reis HT, Judd CM, editors. Handbook of Research Methods in Social and Personality Psychology. 2. New York, NY: Cambridge University Press; 2014. [Google Scholar]
- Rizzolatti G, Fabbri-Destro M. Mirror neurons: From discovery to autism. Experimental Brain Research. 2010;200:223–37. doi: 10.1007/s00221-009-2002-3. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology and Psychiatry. 2003;44:763–781. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Rogers S, Pennington B. A theoretical approach to the deficits in infantile autism. Developmental Psychopathology. 1991;3:137–162. [Google Scholar]
- Sebanz N, Knoblich G. Predictions in joint action: What, when, and where. Topics in Cognitive Science. 2009;1:353–367. doi: 10.1111/j.1756-8765.2009.01024.x. [DOI] [PubMed] [Google Scholar]
- Schaaf RC, Toth-Cohen S, Johnson SL, Outten G, Benevides TW. The everyday routines of families of children with autism: Examining the impact of sensory processing difficulties on the family. Autism. 2011;15:373–389. doi: 10.1177/1362361310386505. [DOI] [PubMed] [Google Scholar]
- Schmidt RC, Richardson MJ. Dynamics of interpersonal coordination. In: Fuchs A, Jirsa V, editors. Coordination: Neural, behavioral and social dynamics. Heidelberg: Springer-Verlag; 2008. pp. 281–308. [Google Scholar]
- Semel EM, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals–4. San Antonio, TX: Psychological Corp/Harcourt; 2003. [Google Scholar]
- Thiel M, Romano MC, Kurths J, Meucci R, Allaria E, Arecchi FT. Influence of observational noise on the recurrence quantification analysis. Physica D. 2002;171:138–152. [Google Scholar]
- Trevarthen C, Delafield-Butt JT. Autism as a developmental disorder in intentional movement and affective engagement. Frontiers in Integrative Neuroscience. 2013;7:49. doi: 10.3389/fnint.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunçgenç B, Cohen E, Fawcett C. Rock with me: The role of movement synchrony in infants’ social and nonsocial choices. Child Development. 2015;86:976–984. doi: 10.1111/cdev.12354. [DOI] [PubMed] [Google Scholar]
- Turvey MT, Rosenblum LD, Schmidt RC, Kugler PN. Fluctuations and phase symmetry in coordinated rhythmic movement. Journal of Experimental Psychology: Human Perception and Performance. 1986;12:564–583. doi: 10.1037//0096-1523.12.4.564. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Reviews. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Wolff PH, Kotwica K, Obregon M. The development of interlimb coordination during bimanual finger tapping. International Journal of Neuroscience. 1998;93(1–2):7–28. doi: 10.3109/00207459808986408. [DOI] [PubMed] [Google Scholar]