Abstract
Objective
To determine whether applying an assistance force to the pelvis and legs during treadmill training can improve walking function in children with cerebral palsy (CP).
Design
Twenty-three children with CP were randomly assigned to the robotic or treadmill only group. For participants who were assigned to the robotic group, a controlled force was applied to the pelvis and legs during treadmill walking. For participants who were assigned to the treadmill only group, manual assistance was provided as needed. Each participant trained 3 times/week for 6 weeks. Outcome measures included walking speed, 6-minute walking distance, and clinical assessment of motor function, which were evaluated pre, post training, and 8 weeks after the end of training, and were compared between two groups.
Results
Significant increases in walking speed and 6-minute walking distance were observed after robotic training (p = 0.03), but no significant change was observed after treadmill training only. A greater increase in 6-minute walking distance was observed after robotic training than that after treadmill only training (p = 0.01).
Conclusions
Applying a controlled force to the pelvis and legs, for facilitating weight-shift and leg swing, respectively, during treadmill training may improve walking speed and endurance in children with CP.
Keywords: Locomotion, treadmill training, cerebral palsy, pelvis weight shifting
Introduction
Treadmill training has been used as a promising technique for improving locomotor function in children with cerebral palsy (CP)1–3. While previous studies suggest that it is feasible to improve walking speed and endurance for some children with CP through treadmill training, many studies had a small sample size and/or did not have a control group4, 5. Recent literature reviews suggested that there is still insufficient evidence to determine the effectiveness of current treadmill training paradigm for improving walking function in children with CP3, 6. In addition, treadmill training requires greater involvement from physical therapist and it can be a labor intensive work for physical therapists.
Recently, robotic gait training systems have been developed for improving walking function in children with CP7, 8. While these robotic systems are effective in reducing the labor intensity of therapists during treadmill training, the functional gains are relatively small for some children with CP after training. For instance, a recent randomized controlled study indicated that robotic treadmill training induced no significant change in walking speed in children with CP9, and it was not more effective than individual exercises with a physical therapist. In contrast, results from other studies indicated that robotic treadmill training induced significant improvements in walking speed10, and gross motor function in children with CP7, but these studies did not have a control group, which may preclude a firm conclusion about the efficacy of robotic treadmill training in children with CP. Thus, there is still insufficient evidence for determining the effect of robotic treadmill training on walking function in children with CP, suggesting a need for improving current robotic treadmill training paradigm. Possible reasons why robotic treadmill training may not be optimally effective for improving walking function in children with CP include the lack of training of lateral weight shift and/or lack of variability in leg and pelvis kinematics. Specifically, lateral movement of the pelvis is constrained during robotic treadmill training due to the limitations of the current robotic system7, which may significantly affect gait dynamics11. In addition, a fixed trajectory control strategy (i.e., repeated movements of the limbs via fixed kinematic trajectories) used in the current pediatric robotic treadmill training system may abolish variation in the kinematics and the sensorimotor pathways, a fundamental feature of the neural control of repetitive movements such as stepping12. Thus, a fixed trajectory movement of the limbs may produce habituation to sensory input, resulting in reduced sensory responses associated with locomotor training13. As a consequence, the training effect could be suboptimal.
Weight shifting is one of key components for a natural gait pattern14, but many children with CP have an impaired weight shift capacity15, which is related to their impaired walking function. For instance, children with CP were less efficient at weight shift, demonstrated as a shorter range of motion of the center of pressure and slower velocity of the center of pressure displacement during visually guided weight shifting than children with normal development15. The impairment of weight shift of children with CP may be related to the weakness of the hip abductors/adductors16, which are suggested to be a crucial muscle groups for maintaining lateral balance during walking17. It is suggested that weight shifting training may be an effective strategy for improving walking function in children with CP. However, while the importance of weight shifting training has been acknowledged, it remains unclear whether the integration of weight shift training in the locomotor training will improve the efficacy of treadmill training. In addition, results from animal studies indicated that locomotor training was more effective while allowing some level of variability than that with a fixed trajectory control13. However, it remains unclear whether allowing for some level of variability in limb kinematics during locomotor training will improve the efficacy of robotic treadmill training.
The goal of this study was to determine whether the integration of weight shifting training into treadmill training and allowing for variability in limb kinematics would be effective for improving walking function in children with CP. We hypothesized that applying assistance at the pelvis for the purpose of facilitating weight shifting and allowing for variability in limb kinematics during treadmill training would improve the efficacy of treadmill training in children with CP.
Methods
Participants
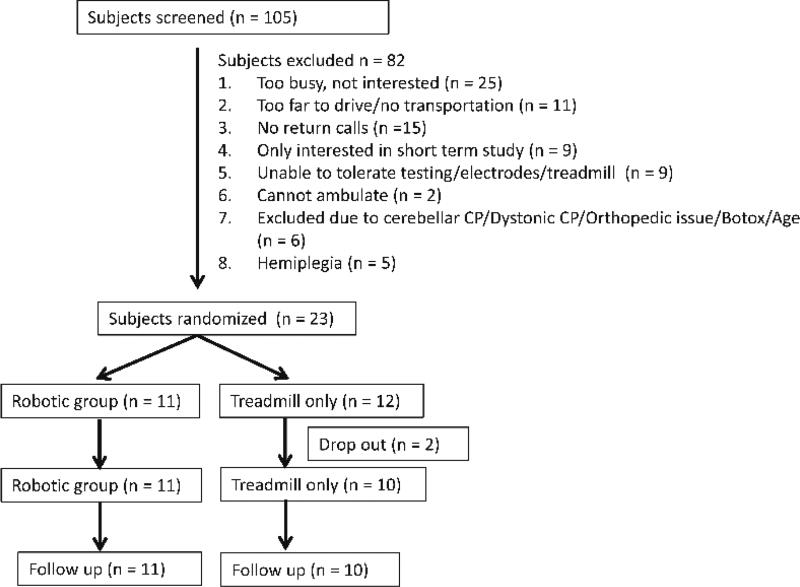
Participants were recruited through the pediatric outpatients of the Rehabilitation Institute of Chicago from 2012–2015. Specifically, 105 participants were contacted, 82 participants were excluded, and 23 children with CP were recruited (14 boys and 9 girls, average age was 10.9 ± 3.2 years old, Gross Motor Function Classification System (GMFCS) levels were I to IV), see Figure 1. Each subject was randomly assigned to either a robotic training group (n = 11) or treadmill only training group (n = 12), see Table 1. Randomization was performed by a research physical therapist using opaque envelope.
Figure 1.
Flowchart of participants’ enrollment and randomization.
Table 1.
Characteristics of participants for baseline comparisons.
| Characteristics | Robot- assisted |
PT-assisted | p |
|---|---|---|---|
| Age (y) | 11.3 ± 3.8 | 10.5 ± 2.6 | 0.57 |
| Gender (M/F) | 6/5 | 8/4 | |
| Race (white/other) | |||
| African American | 1 | 4 | |
| Asian | 1 | 0 | |
| Hispanic | 4 | 3 | |
| White | 5 | 5 | |
| Extremity distribution | |||
| Diplegia | 4 | 4 | |
| Quadriplegia | 6 | 8 | |
| Triplegia | 1 | 0 | |
| GMFCS | |||
| I | 1 | 2 | |
| II | 6 | 3 | |
| III | 3 | 5 | |
| IV | 1 | 2 | |
| Assistive Device | |||
| RW/RRW | 4 | 7 | |
| None | 7 | 5 | |
| Ankle braces | |||
| None | 1 | 0 | |
| BAFO/SMO | 9/1 | 12/0 | |
| GMFM | |||
| Total score | 62.4 ± 6.7 | 61.0 ± 10.4 | 0.46 |
| Dimension D | 25.7 ± 9.2 | 21.9 ± 11.2 | 0.38 |
| Dimension E | 35.3 ± 19.4 | 28.4 ± 21.4 | 0.43 |
| Self-selected gait speed (m/s) | 0.70 ± 0.20 | 0.69 ± 0.29 | 0.40 |
| Fast walking gait speed (m/s) | 1.13 ± 0.33 | 1.06 ± 0.52 | 0.66 |
| 6-minute walking distance (m) | 314.0 ± 73.7 | 341.7 ± 212.9 | 0.69 |
| MAS | 0.62 ± 0.46 | 0.62 ± 0.39 | 0.95 |
Abbreviations: PT, physical therapist; GMFCS, Gross Motor Function Classification System; GMFM, Gross Motor Function Measure; RW, rolling walker, RRW, reverse rolling walker; BAFO, Bilateral Ankle Foot Orthosis; SMO, Supra-Malleolar Orthosis; MAS, Modified Ashworth Scale.
Inclusion criteria
1) a diagnosis of bilateral spastic CP attributed to complications of prematurity, intracranial hemorrhage, and periventricular leukmalacia according to the definition of Bax18; 2) age ranged from 4 to 16 years old; 3) GMFCS levels ranged from I to IV; 4) able to signal pain, fear or discomfort reliably; 5) passive range of motion within functional limits (ankle dorsiflexion = neutral; knee flexion = 0–120°; hip flexion = 0–90°; and hip extension = 0–10°); 6) if scoliosis is present, Cobb angle < 20°; 7) no Botulinum toxin treatment within past 3 months; 8) no orthopedic surgery or neurosurgery within the past 6 months.
Exclusion criteria
Children with severe lower extremity contractures, fractures, osseous instabilities (joint dislocation), osteoporosis, severe disproportional bone growth, unhealed skin lesions in the lower extremities, thromboembolic diseases, cardiovascular instability, and aggressive or self-harming behaviors.
All participants required medical clearance for participation, i.e., the primary physician of each subject was contacted to obtain a permission to participate in this study. All procedures were approved by the institutional review board of the medical school of Northwestern University. Written informed consent was obtained from all participants and their parents.
Apparatus
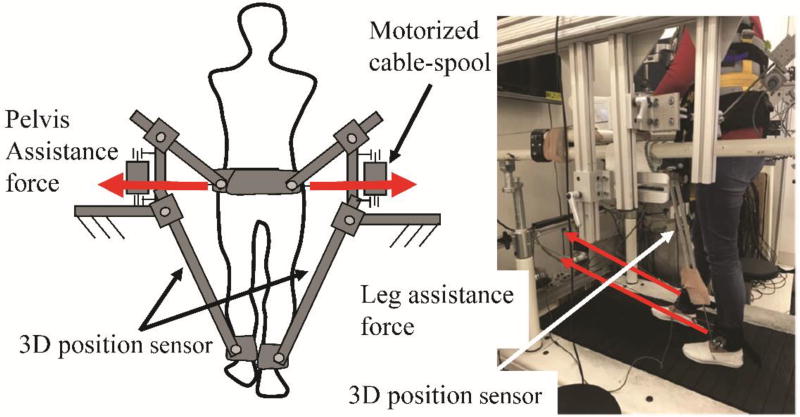
A custom designed 3D cable-driven robotic gait training system, 3DCaLT, was used to apply controlled forces to the pelvis and legs during treadmill walking19. Specifically, the 3D cable-driven robotic gait training system consists of four motors (AKM 33H, Kollmorgen) and cable spools with two of them located at the front of the treadmill and two of them located at the side of the treadmill. The two motors located at the front of the treadmill were used to provide controlled swing assistance to legs through two motorized cables and leg straps, and the two motors located at the side of treadmill were used to provide assistance force to the pelvis through two motorized cables and a waist belt, see Figure 2. Two sets of custom designed 3D position sensors were attached to the legs above the ankle and were used to record ankle position during treadmill walking20. The recorded ankle position signals were used to trigger pelvis and leg loading. A custom LabVIEW (NI, Austin, TX) program was used to control the coordinated movement of 4 motors. The cable-driven robotic gait training system is highly backdrivable21, i.e., the cable driven system can be moved by the patient with smallest possible resistance opposed by the robot. Thus, the cable system allows the patients greater flexibility in controlling their gait pattern.
Figure 2.
Illustration of the 3D cable-driven robotic gait training system. Two motors and cable-spools were attached to a fixed frame located at the side of treadmill and were used to provide pelvis assistance force in the mediolateral direction, and two motors and cable-spools were attached to a fixed frame located at the front of the treadmill and were used to provide leg assistance force in the anterior-posterior direction. A PC was used to control the coordinated movement of four motors.
Protocol
Treadmill training was performed 3 times/week for 6 weeks with the training time for each visit set at 30–40 minutes, as tolerated, excluding setup time. Treadmill speed was set at the subject’s maximum comfortable walking speed and gradually increased during the course of training. Body weight support was provided to 3 participants (1 in the robotic group) to prohibit knee buckling or toe drag during treadmill training. The level of body weight support and treadmill training speed were determined based on the tolerance of participants by a licensed physical therapist. For participants who were assigned to the robotic training group, a controlled assistance load was applied to the pelvis and legs. The assistance force applied to legs started from toe-off to mid-swing to facilitate leg swing, with peak force at ~4–5% of body weight (ranging from 13 to 30 N). The assistance force applied to the pelvis in the mediolateral direction started from heel strike to mid-stance of the ipsilateral leg to facilitate weight shifting, with peak force at ~9% of body weight (ranging from 30 to 70 N). The peak force was adjusted based on tolerance of each subject. One physical therapist was monitoring the robotic training but provided no manual assistance. For participants from the treadmill only training group, manual assistance was provided to legs for facilitating leg swing by a physical therapist if necessary. Verbal encouragement was provided to subjects from both groups.
Outcome measures
Primary outcome measures were overground walking speed and endurance, which were assessed pre, post 6 weeks of training, and at 8 weeks after the end of training. Gait speed was tested using an instrumented walkway22, GaitRite (CIR Systems Inc. Sparta, NJ). Participants were instructed to walk on the mat at their self-selected and fast walking speeds for 3 trials for each condition. Endurance was assessed using the 6-minute walk distance, which has been validated by a previous study23.
Secondary outcome measures included clinical measures of motor function (the dimensions D (standing) and E (walking, running, jumping) of the Gross Motor Function Measure (GMFM-66))24, muscle tone of hip flexors and extensors, which was assessed using the Modified Ashworth Scale25, and the Pediatric Outcomes Data Collection Instrument (PODCI)26. Spatial-temporal gait parameters were also calculated using the data collection software and averaged across three trials and two legs. Assessing physical therapists were not blinded for group assignment.
Data analysis
Baseline characteristics and training parameters were compared between two groups using t tests, and Wilcoxon rank sum tests, as appropriate, and data normality was checked using Shapiro-Wilk test. All parametric measures were analyzed using repeated measures (pre, post, and follow up tests) ANOVAs for within group comparison. If a significant difference was detected, Tukey-Kramer post-hoc tests were conducted to determine which conditions were different from each other. Modified Ashworth Scale (MAS) scores were analyzed using Friedman tests with post-hoc Wilcoxon tests. Changes in primary outcomes were calculated by subtracting the baseline value from the value obtained at post and follow up tests, and analyzed using repeated-measures ANOVA with main factor of group (robotic vs. treadmill only), and repeated for time (post training and follow up tests). Data were analyzed using Matlab_R2016 (The MathWorks, Natick, Massachusetts). Statistical significance for all tests was set at p < 0.05. Effect sizes were calculated and expressed as Cohen d. Effect sizes of 0.20–0.49 were considered small, 0.50–0.79 were considered moderate, and ≥ 0.80 were considered large.
Results
Eleven participants from the robotic training group and 10 participants from the treadmill only group completed all the training and evaluation sessions. Two participants dropped out, one of them was due to poor attendance, and another one was due to difficulty in transportation. The dropout rate was 8.7%. Thus, data from subjects who completed all the training and evaluation sessions were analyzed27. Treadmill training speed, distance, and time were gradually increased for both groups (p < 0.01) during the course of training with no significant difference between two groups. In addition, the training intensity had no significant difference between two groups, see Table 2. At baseline, there were no significant differences between two groups in age, walking speed, 6-minute walk distance, and GMFM scores, see Table 1.
Table 2.
Training paradigms, including treadmill speed, time, and training intensity at session 1, session 9, and session 18 for robotic treadmill training or treadmill only group.
| Robotic training | Treadmill only | p | |
|---|---|---|---|
| Section 1 | |||
| Speed (m/s) | 0.53 ± 0.15 | 0.49 ± 0.13 | 0.67 |
| Distance (km) | 0.99 ± 0.35 | 0.99 ± 0.31 | 0.83 |
| Time (min) | 30.4 ± 3.7 | 33.1 ± 3.7 | 0.09 |
| Intensity | 11.7 ± 1.7 | 12.2 ± 1.4 | 0.62 |
| Section 9 | |||
| Speed (m/s) | 0.60 ± 0.15 | 0.60 ± 0.18 | 0.92 |
| Distance (km) | 1.41 ± 0.33 | 1.41 ± 0.49 | 0.92 |
| Time (min) | 38.5 ± 2.3 | 37.2 ± 4.5 | 0.49 |
| Intensity | 12.0 ± 1.5 | 11.5 ± 0.8 | 0.33 |
| Section 18 | |||
| Speed (m/s) | 0.66 ± 0.19 | 0.68 ± 0.17 | 0.68 |
| Distance (km) | 1.58 ± 0.46 | 1.64 ± 0.42 | 0.62 |
| Time (min) | 39.5 ± 2.0 | 39.8 ± 0.6 | 0.57 |
| Intensity | 12.7 ± 1.6 | 11.8 ± 1.0 | 0.19 |
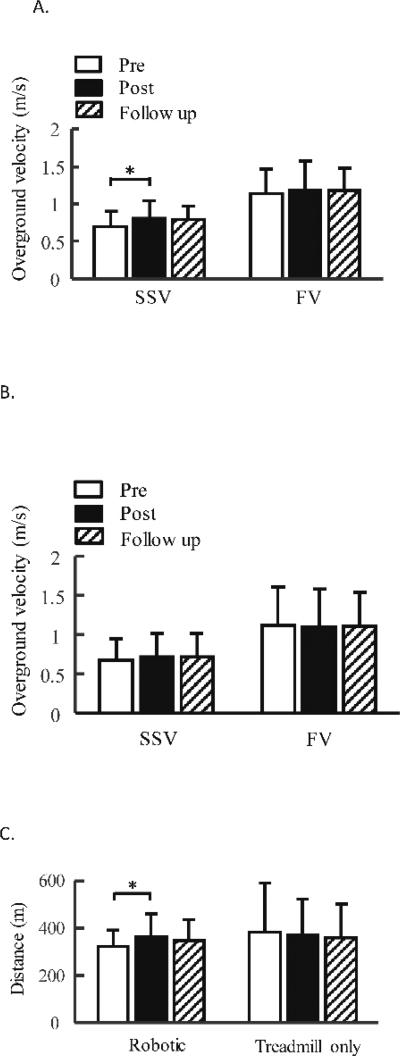
Walking function of children with CP improved after 3D robotic treadmill training. Specifically, self-selected walking speed significantly increased after robotic training (p = 0.03), see Figure 3. Post-hoc test indicated a significant difference between pre vs. post training tests (15.4% increase, p = 0.04, effect size d = 0.46), although there were no significant differences between pre vs. follow up tests (p = 0.08). Fast walking speed had no significant change after robotic training (p = 0.74, effect size d = 0.11). Six-minute walking distance significantly increased after robotic training (p = 0.048), Figure 3. Post-hoc test indicated a significant difference between pre vs. post training tests (12.8% increase, p = 0.04, effect size d = 0.48), although there were no significant differences between pre vs. follow up tests (p = 0.28). The GMFM scores had no significant changes after robotic training, see Table 3.
Figure 3.
Average of self-selected, fast walking velocities pre, post 6 weeks of robotic treadmill training, A, or treadmill only training, B, and 8 weeks after the end of treadmill training, i.e., follow up. Three trials were tested and averaged across each test sessions and averaged across participants for each group. C. average of 6-minute walking distance pre, post 6 weeks of robotic treadmill training or treadmill only training, and 8 weeks after the end of training. Error bars indicate standard deviation of each gait parameters (n = 10 for the robotic treadmill training group, data from one subject was excluded for 6-minute walking distance test because this subject was sick immediately before post test, which significantly impacted his endurance performance based on subject’s self-report). Error bars indicate standard deviation of each gait parameters. SSV, self-selected velocity; FV, fast velocity. * indicates significant difference, p < 0.05.
Table 3.
Gross Motor Function Measure scores, Modified Ashworth Scale, and the Pediatric Outcomes Data Collection Instrument after robotic training and treadmill only training.
| Robotic training | Treadmill only training | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | FU | p | Pre | Post | FU | p | |
| GMFM | ||||||||
| Total score | 64.0±8.3 | 64.7±9.2 | 64.7±9.4 | 0.57 | 62.6±10.7 | 64.5±11.1 | 63.8±10.5 | 0.08 |
| Dimension D | 25.7±9.2 | 26.2±10.9 | 25.4±11.3 | 0.84 | 23.3±11.5 | 27.4±10.0 | 27.6±9.8 | 0.01* |
| Dimension E | 35.3±19.4 | 36.5±20.0 | 37.6±20.5 | 0.06 | 32.2±21.5 | 33.6±22.7 | 33.4±22.9 | 0.34 |
| MAS | 0.62±0.46 | 0.67±0.60 | 0.41±0.38 | 0.18 | 0.65±0.36 | 0.48±0.47 | 0.58±0.44 | 0.19 |
| PODCI (self) | 10.0±14.6 | −1.0±24.8 | 16.0±17.45 | 0.52 | 23.0±23.6 | 19.5±12.1 | 24.0±16.0 | 0.73 |
| PODCI (parent) | 12.9±16.2 | 19.4±12.9 | 17.2±16.0 | 0.17 | 17.2±17.6 | 18.2±19.4 | 21.4±21.2 | 0.34 |
Abbreviations: FU, Follow Up; MAS, Modified Ashworth Scale; PODCI, Pediatric Outcomes Data Collection Instrument; GMFM, Gross Motor Function Measure.
indicates significant difference.
Walking speed and endurance had no significant change after treadmill only training (p > 0.05, effect sizes d = 0.14, d = −0.05, d = −0.07, for self-selected walking speed, fast walking speed, and endurance, respectively), see Figure 3. In addition, treadmill only training induced no significant change in the dimension E of GMFM (p = 0.34), but induced significant increase in the dimension D of GMFM (p = 0.01). Post-hoc tests indicated significant difference between pre vs. post training tests (p = 0.03), and pre vs. follow up test (p = 0.02), see Table 3.
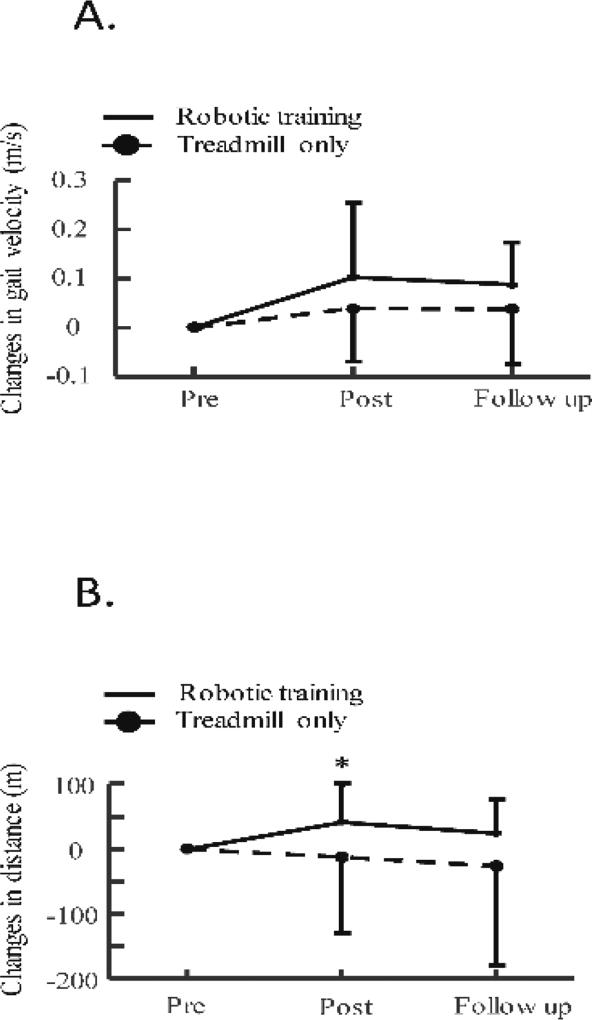
A greater gain in 6-minute walking distance was obtained for the participants from the robotic group than that from the treadmill only group (p = 0.01), but gains in self-selected and fast walking speeds had no significant difference between the two groups (p = 0.12 and p = 0.29 for self-selected and fast walking speeds, respectively), see Figure 4, probably due to small functional gains and large variability for subjects from the treadmill only group. Specifically, changes in 6-minute walking distance were 42.2 ± 57.4 m (post training) and 25.1 ± 52.0 m (follow up) after robotic training, and were −3.8 ± 35.9 m (post training) and −8.2 ± 46.8 m (follow up) after treadmill only training. Changes in self-selected walking speed were 0.10 ± 0.15 m/s (post training) and 0.09 ± 0.09 m/s (follow up) after robotic training, and were 0.04 ± 0.11 m/s (post training) and 0.04 ± 0.11 m/s (follow up) after treadmill only training. Changes in fast walking speed were 0.04 ± 0.22 m/s (post training) and 0.04 ± 0.18 m/s (follow up) after robotic training, and were −0.03 ± 0.16 m/s (post training) and −0.01 ± 0.15 m/s (follow up) after treadmill only training. In addition, step frequency significantly increased after robotic training (p = 0.03), but had no significant change after treadmill only training. Step length had no significant changes after both the robotic and treadmill only training, Table 4. MAS, and PODCI (including both self-report and parent report) had no significant changes after both the robotic and treadmill only training, Table 3.
Figure 4.
Changes in walking speeds, A, and walking distance, B, immediately after robotic/treadmill only training, and 8 weeks after the end of training.
Table 4.
Spatial-temporal gait parameters pre, post robotic treadmill training or treadmill only training.
| Robotic training | Treadmill only training | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | FU | p | Pre | Post | FU | p | |
| SSV | 0.70 ± 0.20 | 0.80 ± 0.24 | 0.79 ± 0.19 | 0.03* | 0.68 ± 0.26 | 0.72 ± 0.28 | 0.72 ± 0.23 | 0.42 |
| Step length (m) | 0.44 ± 0.05 | 0.46 ± 0.05 | 0.46 ± 0.04 | 0.16 | 0.42 ± 0.10 | 0.44 ± 0.09 | 0.43 ± 0.10 | 0.56 |
| Step frequency (steps/min) | 95.6 ± 24.0 | 104.2 ± 29.0 | 102.8 ± 24.6 | 0.03* | 98.0 ± 29.1 | 96.4 ± 28.6 | 98.0 ± 27.2 | 0.77 |
| Stance time (%) | 63.7± 4.9 | 63.6 ± 3.6 | 63.4± 2.3 | 0.95 | 64.4 ± 5.2 | 65.9 ± 6.6 | 65.4 ± 7.0 | 0.53 |
| Single leg support time | 37.0± 6.6 | 36.4 ± 3.6 | 36.6 ± 2.3 | 0.90 | 35.5 ± 5.2 | 34.2 ± 6.6 | 34.5 ± 7.0 | 0.52 |
| FV | 1.13 ± 0.33 | 1.18 ± 0.40 | 1.17 ± 0.31 | 0.74 | 1.13 ± 0.49 | 1.10 ± 0.47 | 1.12 ± 0.43 | 0.85 |
| Step length (m) | 0.52 ± 0.06 | 0.52 ± 0.10 | 0.53 ± 0.07 | 0.59 | 0.51 ± 0.11 | 0.52 ± 0.10 | 0.52 ± 0.10 | 0.88 |
| Step frequency (steps/min) | 132.4 ± 35.7 | 133.8 ± 39.1 | 133.1 ± 31.2 | 0.98 | 129.3 ± 40.6 | 123.9 ± 41.8 | 127.7 ± 37.8 | 0.38 |
| Stance time (%) | 59.8± 2.2 | 59.5 ± 4.4 | 59.9± 3.2 | 0.91 | 59.8 ± 4.8 | 61.4 ± 6.6 | 60.8 ± 6.0 | 0.24 |
| Single leg support time | 40.1± 2.3 | 40.5 ± 4.6 | 40.2 ± 3.2 | 0.87 | 40.3 ± 4.8 | 38.6 ± 6.6 | 39.2 ± 6.0 | 0.23 |
Abbreviations: FU, Follow Up; SSV, Self-selected velocity; FV, fast velocity.
indicates significant difference.
Discussion
We observed significant improvements in self-selected walking speed and endurance after robotic training, but not after treadmill only training. Further, we observed greater increase in 6-minute walking distance for the participants from the robotic training group than that from the treadmill training only group, although observed no significant difference in changes in overground walking speeds between the two groups. Thus, treadmill training when combined with pelvic assistance to facilitate weight shifting seems more effective than conventional treadmill training in improving endurance in children with CP.
Applying a controlled assistance force to the pelvis during treadmill training may increase the efficacy of locomotor training in children with CP. Many children with CP have an impaired lateral weight shifting capacity15, 28, a key component for an efficient gait pattern because an insufficient weight shift to the ipsilateral leg may prevent participants from taking a longer step with the contralateral leg. One possible reason for impaired weight shifting may be due to weakness of hip adductors/abductors16, a key muscle group for maintaining lateral balance during walking17. In this study, the controlled lateral pelvis assistance load applied during the stance phase may facilitate weight shifting to the ipsilateral leg, which may help to release the load on the contralateral leg, resulting in enhanced load afferents to the spinal locomotor center, which may facilitate an efficient transition from stance to swing of the contralateral leg. Alternatively, applying external pelvis assistance might induce additional challenges in lateral balance control for children with CP, which may enhance muscle activation of hip abductors/adductors to maintain lateral balance during walking. Repeated exposure to the pelvic assistance force during treadmill training may improve motor control of hip abductors/adductors, resulting in an improvement in lateral balance of children with CP after robotic training. The improvement in lateral balance of the standing leg may allow participants to take a longer step with the contralateral leg. These could be the potential reasons why we observed significant improvements in walking function in children with CP after robotic training.
In contrast, results from this study indicated that treadmill only training did not induce significant improvements in walking function of children with CP, which is consistent with several previous systematic reviews1, 3, although another systematic review suggested that treadmill training may be effective in improving walking speed, but not endurance. For instance, the average gain in self-selected walking speed obtained after treadmill only training was 0.04 ± 0.11 m/s, which is comparable to gains obtained from a previous randomized controlled study using treadmill training9, but is 40% of the gain obtained after robotic treadmill training, i.e., 0.10 ± 0.15 m/s (> minimal clinical important difference, MCID)29. In addition, the average gain in 6 minute walking distance decreased −3.8 ± 35.9 m (although this change was not significant) after treadmill only training, which is comparable to gains obtained from a previous randomized controlled study (i.e., −28.7 ± 88.2 m)6, although 10 minute walking distance test was used, and only children with GMFCS level at III and IV were recruited in this study, and was smaller than that obtained after robotic treadmill training (i.e., 42.2 ± 57.4 m > MCID). A possible reason for the less effectiveness of treadmill only training may be due to the challenge that was applied to these participants during treadmill training was not strong enough to induce improvement. For instance, balance was less challenged in the treadmill only training group, particularly when participants were allowed to hold onto the frontal bar. The dimension D of the GMFM significantly increased after treadmill only training, which is consistent with previous studies2, and may be due to task-specific practice.
In addition, the functional gains only partially retained at follow up test, see Figure 4, suggesting that some children with CP might lose some of the progress during the 8 weeks follow up period. A possible reason may be due to these participants had less physical activities after the end of treadmill training because participants were requested to refrain from initiating a new physical therapy during the follow up period. Further studies are needed to determine how to effectively retain the functional gains, such as provide maintenance therapy, after the intervention.
Allowing for variability in limb kinematics during treadmill training may facilitate transfer of motor skills from treadmill training to overground walking in children with CP. Variability in leg kinematics has been suggested to be a key component for motor learning during locomotor training13. Results from animal studies indicated that artificially reducing variability in leg kinematics during locomotor training decreased the training effect13. In this study, the robotic system allowed variability in leg and pelvis kinematics while a controlled assistance force was applied to the pelvis and legs because the cable-driven robotic system used in this study is highly backdrivable21. This may be one possible reason for improved overground gait speed and endurance after robotic treadmill training. In contrast, results from a randomized controlled study indicated that robotic treadmill training induced no significant changes in walking speeds in children with CP9. A possible reason for the less effectiveness may be due to the limitations of the system, such as position control strategy, which may reduce leg kinematic variability, and lack of the pelvis lateral movement. However, results from another study using the same robotic system induced promising increases in walking function, but this study had no control group7.
Results from the current study may have some clinical applications. For instance, the results suggest that applying lateral pelvis assistance to facilitate weight shifting during treadmill training is an effective strategy for improving walking function in children with CP. Thus, to facilitate improvements in walking function, clinical physical therapists may focus greater attention on lateral weight shifting training when treating children with CP.
This study has several limitations. The sample size was small, which warrants further studies involving a larger cohort. In addition, the functional level of children with CP at baseline may also impact improvements in walking function after training. For instance, we observed significant correlation between changes in 6-minute walking distance and scores of GMFCS for subjects from the robotic training group (p = 0.02) with a greater increase was obtained from subjects who had smaller GMFCS score (i.e., higher function), although we observed no significant correlation between changes in 6-minute walking distance and scores of GMFCS for subjects from treadmill only training group (p = 0.10). In addition, we observed no significant correlation between changes in walking speed and scores of GMFCS for subjects from the robotic training group (p = 0.46 and p = 0.21 for self-selected and fast walking speeds, respectively), and for subjects from treadmill only training group (p = 0.57 and p = 0.94 for self-selected and fast walking speeds, respectively). The range of GMFCS scores was also large. Further studies with a more narrowed GMFCS score range are warranted. The age of subjects may also impact improvements in walking function after training. However, we observed no significant correlation between changes in 6-minute walking distance and self-selected walking speed, and age of subjects across two groups (p = 0.78 and p = 0.40 for walking distance and speed, respectively). All participants in this study could ambulate with/without assistive devices. We do not know whether this type of paradigm will be beneficial for these participants with lower functional levels, and participants who cannot ambulate with assistive device. In addition, while participants were randomly assigned into two different groups, physical therapists who conducted intervention and outcome assessments were not blinded, which might potentially bias the results. Five (3 from the robotic group and 2 from the treadmill only group) out of 23 participants had orthopedic surgery or neurosurgery, which may also impact their gait performance. However, these surgeries were conducted at 1 to 4 years before they participated in this study. Thus, we do not believe that these surgeries systemically impacted the results. We did not systematically evaluate the impact of the robotic system on the variability in leg kinematics of children with CP in this study, a previous study in humans with spinal cord injury indicated that cable-driven robotic system had no significant impact on the variability in leg kinematics21.
Conclusions
Applying a controlled lateral pelvic assistance force, which may facilitate weight shifting and/or apply an additional challenge to lateral balance control during locomotor training, may increase the efficacy of robotic treadmill training in children with CP. Results from this study may be used to develop intervention paradigms for improving walking function in children with CP.
Acknowledgments
We thank Dr. Feng Wei, for his assistance during data collection. We thank Ms. Jill Landry for her comments and suggestions for this manuscript. This study was supported by NIDRR/RERC, H133E100007.
Abbreviations
- CP
cerebral palsy
- GMFCS
Gross Motor Function Classification System
- GMFM
Gross Motor Function Measure
- 3D
3 Dimensional
- PODCI
Pediatric Outcomes Data Collection Instrument
- ANOVA
Analysis of variance
Footnotes
Suppliers:
GaitRite, CIR Systems Inc. 12 Cork Hill Road, Bldg #2, Franklin, NJ, 07416
Woodway, WOODWAY USA, Inc. W229 N591 Foster Ct., Waukesha, WI, 53186
References
- 1.Damiano DL, DeJong SL. A systematic review of the effectiveness of treadmill training and body weight support in pediatric rehabilitation. J Neurol Phys Ther. 2009;33:27–44. doi: 10.1097/NPT.0b013e31819800e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emara HA, El-Gohary TM, Al-Johany AA. Effect of body-weight suspension training versus treadmill training on gross motor abilities of children with spastic diplegic cerebral palsy. Eur J Phys Rehabil Med. 2016;52:356–363. [PubMed] [Google Scholar]
- 3.Valentin-Gudiol M, Bagur-Calafat C, Girabent-Farres M, Hadders-Algra M, Mattern-Baxter K, Angulo-Barroso R. Treadmill interventions with partial body weight support in children under six years of age at risk of neuromotor delay: a report of a Cochrane systematic review and meta-analysis. Eur J Phys Rehabil Med. 2013;49:67–91. [PubMed] [Google Scholar]
- 4.Schindl MR, Forstner C, Kern H, Hesse S. Treadmill training with partial body weight support in nonambulatory patients with cerebral palsy. Arch Phys Med Rehabil. 2000;81:301–306. doi: 10.1016/s0003-9993(00)90075-3. [DOI] [PubMed] [Google Scholar]
- 5.Dodd KJ, Foley S. Partial body-weight-supported treadmill training can improve walking in children with cerebral palsy: a clinical controlled trial. Dev Med Child Neurol. 2007;49:101–105. doi: 10.1111/j.1469-8749.2007.00101.x. [DOI] [PubMed] [Google Scholar]
- 6.Willoughby KL, Dodd KJ, Shields N, Foley S. Efficacy of partial body weight-supported treadmill training compared with overground walking practice for children with cerebral palsy: a randomized controlled trial. Arch Phys Med Rehabil. 2010;91:333–339. doi: 10.1016/j.apmr.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Meyer-Heim A, Borggraefe I, Ammann-Reiffer C, et al. Feasibility of robotic-assisted locomotor training in children with central gait impairment. Dev Med Child Neurol. 2007;49:900–906. doi: 10.1111/j.1469-8749.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 8.Smania N, Bonetti P, Gandolfi M, et al. Improved gait after repetitive locomotor training in children with cerebral palsy. Am J Phys Med Rehabil. 2011;90:137–149. doi: 10.1097/PHM.0b013e318201741e. [DOI] [PubMed] [Google Scholar]
- 9.Druzbicki M, Rusek W, Snela S, et al. Functional effects of robotic-assisted locomotor treadmill thearapy in children with cerebral palsy. J Rehabil Med. 2013;45:358–363. doi: 10.2340/16501977-1114. [DOI] [PubMed] [Google Scholar]
- 10.Meyer-Heim A, Ammann-Reiffer C, Schmartz A, et al. Improvement of walking abilities after robotic-assisted locomotion training in children with cerebral palsy. Arch Dis Child. 2009;94:615–620. doi: 10.1136/adc.2008.145458. [DOI] [PubMed] [Google Scholar]
- 11.Veneman JF, Menger J, van Asseldonk EH, van der Helm FC, van der Kooij H. Fixating the pelvis in the horizontal plane affects gait characteristics. Gait Posture. 2008;28:157–163. doi: 10.1016/j.gaitpost.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Hausdorff JM. Gait variability: methods, modeling and meaning. J Neuroeng Rehabil. 2005;2:19. doi: 10.1186/1743-0003-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai LL, Fong AJ, Otoshi CK, et al. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J Neurosci. 2006;26:10564–10568. doi: 10.1523/JNEUROSCI.2266-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inman V, Ralston HJ, Todd F. Human Walking. Baltimore: Williams and Wilkins; 1981. [Google Scholar]
- 15.Ballaz L, Robert M, Parent A, Prince F, Lemay M. Impaired visually guided weight-shifting ability in children with cerebral palsy. Res Dev Disabil. 2014;35:1970–1977. doi: 10.1016/j.ridd.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Dallmeijer AJ, Rameckers EA, Houdijk H, de Groot S, Scholtes VA, Becher JG. Isometric muscle strength and mobility capacity in children with cerebral palsy. Disabil Rehabil. 2015:1–8. doi: 10.3109/09638288.2015.1095950. [DOI] [PubMed] [Google Scholar]
- 17.Winter DA, MacKinnon CD, Ruder GK, Wieman C. An integrated EMG/biomechanical model of upper body balance and posture during human gait. Prog Brain Res. 1993;97:359–367. doi: 10.1016/s0079-6123(08)62295-5. [DOI] [PubMed] [Google Scholar]
- 18.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–576. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 19.Wu M, Kim J, Arora P, Gaebler-Spira DJ, Zhang Y. Kinematic and EMG Responses to Pelvis and Leg Assistance Force during Treadmill Walking in Children with Cerebral Palsy. Neural Plast. 2016;2016:5020348. doi: 10.1155/2016/5020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yen SC, Schmit BD, Landry JM, Roth H, Wu M. Locomotor adaptation to resistance during treadmill training transfers to overground walking in human SCI. Exp Brain Res. 2012;216:473–482. doi: 10.1007/s00221-011-2950-2. [DOI] [PubMed] [Google Scholar]
- 21.Wu M, Hornby TG, Landry JM, Roth H, Schmit BD. A cable-driven locomotor training system for restoration of gait in human SCI. Gait Posture. 2011;33:256–260. doi: 10.1016/j.gaitpost.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Wondra VC, Pitetti KH, Beets MW. Gait parameters in children with motor disabilities using an electronic walkway system: assessment of reliability. Pediatr Phys Ther. 2007;19:326–331. doi: 10.1097/PEP.0b013e3181577d6d. [DOI] [PubMed] [Google Scholar]
- 23.Maher CA, Williams MT, Olds TS. The six-minute walk test for children with cerebral palsy. Int J Rehabil Res. 2008;31:185–188. doi: 10.1097/MRR.0b013e32830150f9. [DOI] [PubMed] [Google Scholar]
- 24.Russell DJ, Avery LM, Rosenbaum PL, Raina PS, Walter SD, Palisano RJ. Improved scaling of the gross motor function measure for children with cerebral palsy: evidence of reliability and validity. Phys Ther. 2000;80:873–885. [PubMed] [Google Scholar]
- 25.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy ML, Silberstein CE, Atkins EA, Harryman SE, Sponseller PD, Hadley-Miller NA. Comparing reliability and validity of pediatric instruments for measuring health and well-being of children with spastic cerebral palsy. Dev Med Child Neurol. 2002;44:468–476. doi: 10.1017/s0012162201002377. [DOI] [PubMed] [Google Scholar]
- 27.Dziura JD, Post LA, Zhao Q, Fu Z, Peduzzi P. Strategies for dealing with missing data in clinical trials: from design to analysis. Yale J Biol Med. 2013;86:343–358. [PMC free article] [PubMed] [Google Scholar]
- 28.Liao HF, Jeng SF, Lai JS, Cheng CK, Hu MH. The relation between standing balance and walking function in children with spastic diplegic cerebral palsy. Dev Med Child Neurol. 1997;39:106–112. doi: 10.1111/j.1469-8749.1997.tb07392.x. [DOI] [PubMed] [Google Scholar]
- 29.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]