Abstract
Background
Opioid use disorder is common in prison populations, and prison release is a high-risk time for relapse and overdose. Initiation of extended release injectable naltrexone (XR-NTX)) prior to prison release might decrease relapse among opioid-dependent persons.
Objective
This pilot study examined the feasibility and acceptability of XR-NTX injection prior to prison release among adult inmates with opioid use disorder, followed by six months of community XR-NTX treatment. It sought to determine effects on treatment retention and abstinence compared to post-release XR-NTX initiation.
Methods
Recruitment for the study took place at the RIDOC’s Adult Correctional Institute (ACI). Volunteers with a history of opioid dependence and a release date scheduled within 1–2 months were self-referred in response to recruitment fliers. Consented volunteers were randomized to XR-NTX treatment prior to release followed by 5 monthly treatments in the community (pre-release) or six XR-NTX treatments in the community (post-release).
Results
Of 26 volunteers consented, 15 were randomized (9 pre-release, 6 post-release). The pre-release group generally had better treatment retention: 100% received the first NTX injection (vs. 67% post-release), 78% received more than one injection (vs. 17%) and 22% received all 6 injections (vs. 0%). The pre-release group also had greater abstinence, with a higher proportion of self-reported opioid free days in the first month after release (83% vs. 46%, fewer positive urine drug tests in the 6 months after release (22% vs. 33%), and more days of opioid receptor blockade during the first two weeks after release, a high risk time for overdose death.
Conclusions
Initiation of XR-NTX injection prior to release from prison might be an effective approach to reduce relapse to opioids, but these findings require confirmation in a larger trial.
Introduction
Opioid use disorder is a chronic, relapsing disease that is pervasive among inmates reentering the community after incarceration (Polcin & Greenfield, 2003). Many opioid-addicted persons end up becoming incarcerated, and while they may detoxify in this setting the potential for relapse after prison release is very high. Loss of tolerance as a result of medication-free opioid addiction treatment approaches in correctional populations put opioid-addicted inmates at high risk of overdose on community reentry, with prisoners having a 12-fold increased risk of overdose during the first two weeks after re-entry compared to age-matched controls (Binswanger et al., 2007). Nonetheless, most correctional systems detoxify prisoners and do not provide evidence-based medical addiction treatment that could reduce overdose (Friedmann et al., 2012; Nunn et al., 2009; Volkow, Frieden, Hyde, & Cha, 2014).
Extended-release naltrexone (XR-NTX) has been shown to be an effective treatment (Lee et al., 2016). Naltrexone is non-addictive and has been more accepted in criminal justice settings (Finigan, Perkins, Zold-Kilbourn, Parks, & Stringer, 2011; Springer, Brown, Di Paola, & Altice, 2015). Because naltrexone is a full blocker of opiate effects, initiating treatment with extended-release naltrexone (XR-NTX) shortly before release might reduce opioid overdose during the critical first few weeks after release. A recent study of criminal-justice involved persons with opioid addiction found no overdoses during the treatment period with XR-NTX (Lee et al., 2016). This pilot study examined whether a strategy of starting XR-NTX treatment before release from prison increased abstinence and antagonist medication coverage during the high risk initial weeks after release to a greater extent than a strategy of referral for XR-NTX treatment in the community after release.
Methods
Study Design
This study was conducted as a supplement to the “Treatment Enhancement Study of Opioid Addiction Using Depot Naltrexone – Rhode Island Site” (1R01DA24549; Friedmann PI). The purpose of this pilot randomized controlled trial was to compare the feasibility and effectiveness of pre-release XR-NTX versus post-release XR-NTX treatment for prevention of opioid relapse among opioid dependent adults leaving prison in Rhode Island. Opioid-dependent volunteers not interested in opioid agonist treatment were randomized to one of two treatment conditions:
Pre-release, where the participant received one XR-NTX injection 1–2 weeks prior to release from prison and then up to five monthly injections in the community, or
Post-release, where the participant received up to six XR-NTX injections beginning immediately after prison release.
Study Setting
Recruitment took place at the Rhode Island Department of Corrections’ Adult Corrections Institute (ACI) from 2012 to 2014. Designated consultation rooms at the ACI were used by research staff to meet privately with potential participants. Informed consent and baseline measures were collected from inmate volunteers by research staff, while prison doctors were responsible for determining medical eligibility and initiating treatment. Study appointments after release from prison took place at research offices and patient areas of Rhode Island Hospital.
Participants
Potential participants were self-referred in response to recruitment fliers posted at the ACI. All potential participants were incarcerated but expected to be released within 1–2 months. Brief screens were conducted by research staff with all potential study volunteers to determine study eligibility. To be eligible potential participants had to be currently incarcerated with a known release date, not interested in agonist treatment, opioid-free with negative urine toxicology for all opioids prior to randomization, in good health, able to understand and sign the consent form and older than 18. They also had to meet DSM-IV criteria for current and/or prior opioid dependence, speak and understand English and not planning to become pregnant (females). Exclusion criteria included other drug or alcohol dependence requiring a higher level of care, untreated psychiatric disorder or medical condition that might make participation hazardous or current chronic pain diagnosis for which opioids are prescribed.
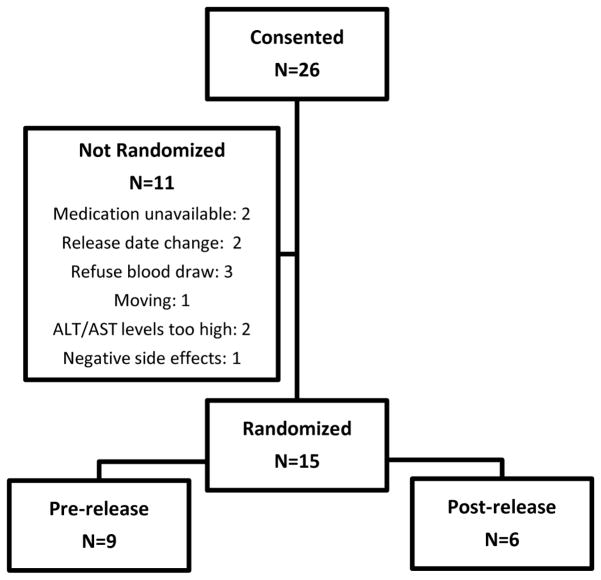
We obtained informed consent from 26 prisoners, of these 15 were randomized to the study. Participants were randomized in a non-blinded fashion, 9 to pre-release and 6 to post-release (Figure 1). The Miriam Hospital Institutional Review Board (IRB) and the U.S. Department of Health and Human Services Office for Human Research Protections (OHRP) approved the conduct of this study. The project was also reviewed and approved by the Rhode Island Department of Corrections Medical Research Advisory Group. The project was registered at clinicaltrials.gov prior to enrollment (NCT01563718).
Figure 1.
Consort diagram
Monitoring and Assessments
XR-NTX was administered at study visits once every four weeks for up to 24 weeks. At these visits the following assessments given at baseline were repeated: Time Line Follow Back (TLFB) self-reporting of drug use, Beck Depression Inventory (BDI), Non-Study Medication and Other Services (NSMOS), Quality of Life (QOL) and Crime and Legal Activities (CLA). Participants also provided urine samples for drug testing every two weeks up to week 25. Follow up visits were completed at 27, 52 and 78 weeks, at these visits additional baseline assessments were also repeated: Risk Assessment Battery (RAB) and Addiction Severity Index (ASI). All participants were encouraged to engage in addiction treatment within the prison and in the community after release. During baseline evaluation those not already so engaged were given referrals to appropriate programs. All participants met with a physician or nurse under study physician supervision for their monthly injection and received Medication Management counseling during these visits.
Statistical Methods
Equivalence between pre- and post-release treatment groups for baseline demographic, HIV risk behavior and drug use history variables was assessed using Fisher’s exact tests and t-tests. Proportions of self-reported opioid abstinent days during the first month after release from prison were compared by fitting a logistic GEE model adjusting for repeated measures. Mean days confirmed abstinent were compared by t-test and medians were compared using Wilcoxon ranked sum test. Opioid relapse status, as defined as 10 self-reported days of opioid use in a four week period and/or two consecutive positive or missing urine tests, was determined and the week of the start of relapse (if applicable) was calculated for each participant. Relapse and other study outcomes were compiled and summarized. Because of the small sample size of this pilot study, results are primarily meant to be descriptive. All analyses were performed using SAS v.9.4.
Results
The baseline characteristics were generally balanced between those randomized to pre- and post-release NTX treatment (Table 1) although the sample size was small. Males comprised 93% of the sample; 83% of participants were white and all participants reported having used heroin at some point in their lives. Age, education and HIV risk scores were similar for both groups.
Table 1.
Participant baseline characteristics
| Post-release (N=6) | Pre-release (N=9) | p-value* | |
|---|---|---|---|
|
|
|||
| White race (%) | 100 | 71.4 | 0.47 |
| Male (%) | 100 | 88.9 | 1.00 |
| Any lifetime use (%): | |||
| heroin | 100 | 100 | 1.00 |
| other opioid | 75.0 | 87.5 | 1.00 |
| cocaine | 75.0 | 100 | 0.33 |
| Injection drug | 25.0 | 62.5 | 0.55 |
| Age (mean) | 33.6 | 38.9 | 0.32 |
| Years of education (mean) | 11.0 | 11.6 | 0.61 |
| Employed (%) | 33.3 | 14.1 | 0.56 |
| Drug risk score (mean) | 1.0 | 1.9 | 0.66 |
| Sex risk score (mean) | 3.2 | 3.9 | 0.65 |
Fisher’s exact test for categorical, t-test for continuous variables
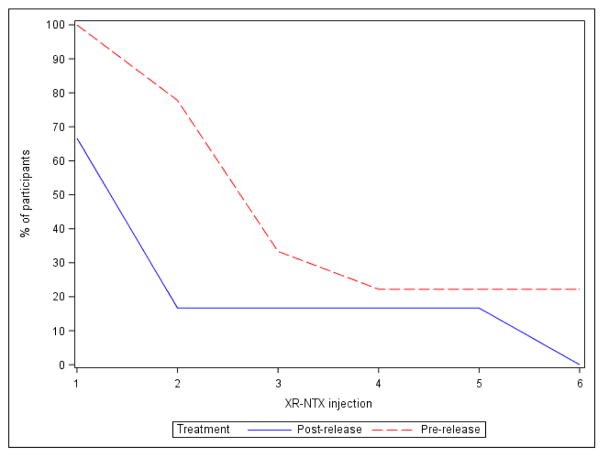
The pre-release XR-NTX group demonstrated a trend towards a greater percentage of days of confirmed abstinence in the first 4 weeks after release (odds ratio 5.6; 95% CI 0.8 to 37.9; P=.08) (Table 2). The pre-release group also reported a higher mean and median number of opioid free days when compared to the post-release group (Table 2). There was also some evidence of greater treatment retention and delayed time to relapse in the pre-release group, with increased median time to relapse for the pre-release group (9 weeks vs. 5 weeks, Table 3). Two of the nine pre-release NTX participants received all six study drug injections compared to none of the post-release participants, and these treatment completers were the only participants not meeting our definition of relapse.
Table 2.
Opioid self-reported abstinence during first 4 weeks post-release, by initiation time of XR-NTX
| Post-release | Pre-release | OR (95% CI) | p-value† | |
|---|---|---|---|---|
|
|
||||
| % Days confirmed abstinent | ||||
| 46% | 83% | 5.6 (0.8, 37.9) | 0.08 | |
| Days confirmed abstinent* | ||||
| Mean (Stand. Dev.) | 13 (16) | 23 (8) | 0.09 | |
| Median | 11 | 28 | 0.19 | |
confirmed by self report, missing days counted as not confirmed abstinent
GEE mixed effects model for proportion, ttest for mean, Wilcoxon rank sum test for medians
Table 3.
Participant outcomes, active treatment phase
| Post-release (N=6) | Pre-release (N=9) | |
|---|---|---|
| Number of NTX injections received: | ||
| Mean (std) | 1.3 (1.9) | 2.8 (1.9) |
| Median | 1 | 2 |
| Time to relapse (weeks)†: | ||
| Mean (std) | 7.5 (6) | 7.3 (3) |
| Median | 5 | 9 |
| Adverse events*: | ||
| Total # events reported | 3 | 13 |
| # participants reporting 1+ event | 2 | 4 |
| Total study drug related events | 2 | 6 |
| # participants reporting drug-related events | 1 | 2 |
| Total serious adverse events (SAE) | 0 | 2 |
| # participants reporting SAE | 0 | 1 |
| Overdoses | 0 | 0 |
| Deaths | 0 | 0 |
2 post-release and 1 pre-release had no AE data
of those who relapsed, 2 pre-release participants did not relapse during active study phase
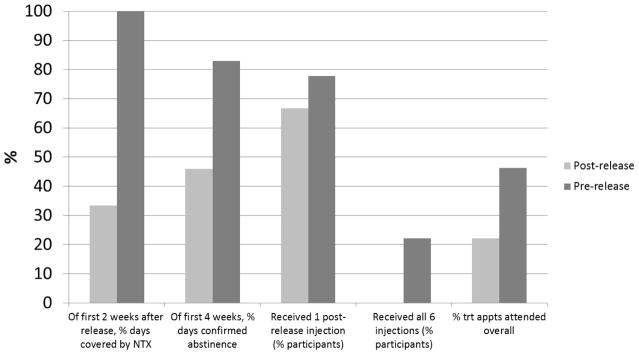
The pre-release group saw better treatment coverage, with 100% receiving first NTX injection (vs. 67% post-release), 78% receiving more than one injection (vs. 17%) and 22% receiving all 6 injections (versus none post-release). The pre-release group also showed a greater percentage of days covered by XR-NTX during the first two weeks after prison release (100% vs. 33%), days confirmed abstinent in first 28 days (83% vs. 46%), and treatment appointments attended (46% vs. 22%, Figure 3).
Figure 3.
Active study phase, participant outcomes, by XR-NTX initiation time
Adverse event data were collected for 12 of 15 participants. During the active treatment phase, 6 participants reported a total of 16 adverse events, including dry mouth, kidney stone pain, fatigue, lump at injection site, anxiety, blurred vision, jitters, abdominal pain, vomiting, nausea (2), insomnia (2), edema/gout, damaged eye socket, and broken/dislocated jaw. Of the 6 participants, 2 reported 1 event, 2 reported 2 events, 1 reported 3 events and 1 reported 7 events. Only 8 events among 3 participants were classified as drug related or possibly drug-related events (all not serious): dry mouth, fatigue, lump at injection site, nausea (2), insomnia (2) and edema/gout. Only one participant reported serious adverse events (not study-related): damaged eye socket and broken/dislocated jaw. The pre-release group reported a higher number of events during the study (Table 3).
Although all participants were encouraged to seek community treatment after prison release, only five did so – four in the pre-release group and one in the post-release group. In the pre-release group, two participants entered outpatient treatment and two entered residential treatment. The first subject reported one day of outpatient treatment every 2 weeks starting in week 21; he completed all 6 injections and did not relapse. The second participant started outpatient treatment at week 17 after a positive urine test; he had 2 injections, then relapsed. The third subject reported a 31 day residential treatment admission from baseline to first follow up; he completed all 6 injections and did not relapse. The fourth participant reported having been in a residential program at baseline; he received 2 injections, started missing urine tests at week 9 and was classified as relapsed. In the post-release group, only one participant reported receiving community treatment after release. He reported residential detoxification for 24 days prior to week 5 as well as outpatient treatment; he completed 5 injections but, after missing urine tests starting in week 17, was classified as having relapsed.
Discussion
This small, preliminary study found trends toward almost twice as many confirmed days of opioid abstinence and longer time-to-relapse the initial 4 weeks after release in the pre-release group compared to post-release. Participants who received NTX before release also tended to receive more NTX injections and have greater treatment retention. Perhaps most importantly, compared to the post-release group, the pre-release NTX group had three times more days of NTX medication coverage during the period right after release, a period associated with extremely high risk for relapse and overdose death (Binswanger et al., 2007).
These findings are consistent with other work from community-based criminal justice programs and clinical trials regarding the effectiveness of XR-NTX in increasing opioid abstinence (Finigan et al., 2011). A preliminary study of pre-release XR-NTX in jail populations, whose detention periods are much briefer and unpredictable than prisoners, similarly suggested a doubling of abstinence in week 4 after release (Lee et al., 2015); a larger, more definitive jail study is ongoing (McDonald et al., 2016). The one published single-arm feasibility study (N=27) of pre-release prisoners with a history of opioid use disorders is also consistent with these findings: it similarly found that among the 37% who received all 6 injections none tested positive for opioids (Gordon et al., 2015).
This study had several limitations, especially its small size. It was a pilot study, and thus underpowered to demonstrate definitely that pre-release XR-NTX is more effective than post-release administration. Study entry was voluntary, and so it cannot comment on the potential effectiveness of mandated treatment. However, most treatment with XR-NTX will likely remain voluntary because of ethical and legal concerns about its compulsory use (Bonnie, 2006; Wolfe et al., 2011).
XR-NTX has a growing role in the management of criminal-justice-involved persons with opioid use disorder. This preliminary study along with accumulating research and experience suggest that XR-NTX administration prior to community release from prison might reduce the risk of opioid relapse in the high risk period immediately after release, although larger studies are needed to demonstrate this inference. Pre-release XR-NTX might be especially effective in the majority of prisons in the U.S. that routinely withdraw opioid-dependent patients from all opioids and do not permit the use of opioid agonist treatments (Rich et al., 2015). XR-NTX will be more acceptable to the administrators and staff in these prisons because it does not require secure storage, there is no potential concern about diversion and abuse, and XR-NTX’s monthly administration minimizes medical staff dispensing burden (Gordon et al., 2015). Medical addiction treatment is the most effective option for the chronic disorder of opioid addiction. Future studies should also examine effects of pre-release XR-NTX on early overdose after community reentry, which effective medication coverage in the first 4 weeks after release will likely reduce as well.
Figure 2.
Participant retention, active study phase
Highlights.
Pilot randomized trial of XR-NTX injection prior to prison release (N=9) versus post-release (N=6) among adult inmates with opioid use disorder, followed by six months of community XR-NTX treatment.
Compared with subjects who received XR-NTX after release, those who received it pre-release trended towards more days of confirmed opioid abstinence during the first month in the community, better treatment retention, and more days of opioid receptor blockade during the first two weeks after release, a high risk time for overdose death.
Initiation of XR-NTX injection prior to release from prison might be an effective approach to reduce relapse to opioids, but these preliminary findings require more definitive study.
Acknowledgments
Supported by the National Institute on Drug Abuse (NIDA) R01DA024549-01S1. Trial medication was provided in kind from an investigator-initiated grant from Alkermes. Dr. Friedmann reports receiving fees for serving on an advisory board and travel support from Indivior, an honorarium for leading a roundtable discussion from Orexo, and travel and training from Braeburn.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, Koepsell TD. Release from prison--a high risk of death for former inmates. N Engl J Med. 2007;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnie RJ. Judicially mandated naltrexone use by criminal offenders: a legal analysis. J Subst Abuse Treat. 2006;31(2):121–127. doi: 10.1016/j.jsat.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Finigan MW, Perkins T, Zold-Kilbourn P, Parks J, Stringer M. Preliminary evaluation of extended-release naltrexone in Michigan and Missouri drug courts. J Subst Abuse Treat. 2011;41(3):288–293. doi: 10.1016/j.jsat.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Friedmann PD, Hoskinson R, Gordon M, Schwartz R, Kinlock T, Knight K, … Frisman LK. Medication-assisted treatment in criminal justice agencies affiliated with the criminal justice-drug abuse treatment studies (CJ-DATS): availability, barriers, and intentions. Subst Abus. 2012;33(1):9–18. doi: 10.1080/08897077.2011.611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MS, Kinlock TW, Vocci FJ, Fitzgerald TT, Memisoglu A, Silverman B. A Phase 4, Pilot, Open-Label Study of VIVITROL(R) (Extended-Release Naltrexone XR-NTX) for Prisoners. J Subst Abuse Treat. 2015;59:52–58. doi: 10.1016/j.jsat.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA, Jr, … O’Brien CP. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders. N Engl J Med. 2016;374(13):1232–1242. doi: 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, McDonald R, Grossman E, McNeely J, Laska E, Rotrosen J, Gourevitch MN. Opioid treatment at release from jail using extended-release naltrexone: a pilot proof-of-concept randomized effectiveness trial. Addiction. 2015;110(6):1008–1014. doi: 10.1111/add.12894. [DOI] [PubMed] [Google Scholar]
- McDonald RD, Tofighi B, Laska E, Goldfeld K, Bonilla W, Flannery M, … Lee JD. Extended-release naltrexone opioid treatment at jail reentry (XOR) Contemp Clin Trials. 2016;49:57–64. doi: 10.1016/j.cct.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn A, Zaller N, Dickman S, Trimbur C, Nijhawan A, Rich JD. Methadone and buprenorphine prescribing and referral practices in US prison systems: results from a nationwide survey. Drug Alcohol Depend. 2009;105(1–2):83–88. doi: 10.1016/j.drugalcdep.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcin DL, Greenfield TK. Factors associated with probation officers’ use of criminal justice coercion to mandate alcohol treatment. Am J Drug Alcohol Abuse. 2003;29(3):647–670. doi: 10.1081/ada-120023463. [DOI] [PubMed] [Google Scholar]
- Rich JD, McKenzie M, Larney S, Wong JB, Tran L, Clarke J, Zaller N. Methadone continuation versus forced withdrawal on incarceration in a combined US prison and jail: a randomised, open-label trial. Lancet. 2015;386(9991):350–359. doi: 10.1016/S0140-6736(14)62338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Brown SE, Di Paola A, Altice FL. Correlates of retention on extended-release naltrexone among persons living with HIV infection transitioning to the community from the criminal justice system. Drug Alcohol Depend. 2015;157:158–165. doi: 10.1016/j.drugalcdep.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–2066. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- Wolfe D, Carrieri MP, Dasgupta N, Wodak A, Newman R, Bruce RD. Concerns about injectable naltrexone for opioid dependence. Lancet. 2011;377(9776):1468–1470. doi: 10.1016/S0140-6736(10)62056-9. [DOI] [PubMed] [Google Scholar]