Abstract
Background
Previous studies identified B cell gene signatures and predominance of specific B cell subsets as a marker of operational tolerance following kidney transplantation. These findings suggested a role for B cells in the establishment or maintenance of tolerance. Here we analyzed B cell recovery in 4 subjects, 3 of whom achieved tolerance after combined kidney/bone marrow transplantation.
Methods
Peripheral B cell subsets were examined longitudinally by flow cytometry. Immunoglobulin heavy chain repertoire analysis was performed using next generation sequencing. Lastly, the patients’ serum reactivity to HLA was assessed by Luminex.
Results
B cell counts recovered approximately 1 year posttransplant except for 1 subject who experienced delayed reconstitution. This subject resumed immunosuppression for acute rejection at 10 months posttransplant and underwent preemptive retransplantation at 3 years for chronic rejection. B cell recovery was accompanied by a high frequency of CD20+CD24highCD38high transitional B cells and a diversified clonal repertoire. However, all 4 subjects showed prevalence of CD20+CD27+ memory B cells around 6 months posttransplant when B cell counts were still low and the clonal B cell repertoire very limited. The predominance of memory B cells was also associated with high levels of somatically mutated IGHV sequences and transient serum reactivity to HLA.
Conclusions
Our observations reveal the presence of memory B cells early posttransplant that likely escaped the preparative regimen at a time consistent with the establishment of tolerance. Further studies are warranted to characterize the functional properties of these persisting memory cells and evaluate their potential contribution to tolerance induction.
Introduction
Several independent studies have now reported distinctive B cell markers in operationally tolerant kidney transplant recipients1–8. Gene profiling experiments first revealed increased expression of specific immunoglobulin light chain variable region genes in tolerant subjects compared to controls4,7. Expression of these genes is now being evaluated for its capacity to identify subjects who may benefit from immunosuppression withdrawal. Phenotypic studies also examined the composition of peripheral B cell pools in these subjects. A consensus emerged around transitional B cells, a population of presumably immature cells, as it was shown that this B cell subset was increased in tolerant subjects in several independent studies2,4,5,8. Other B cell subsets were also identified as elevated in tolerant subjects, including memory B cells and granzyme B+ cells with a plasma cell phenotype1,3,6.
Aside from their usefulness as predictive biomarkers, B cells were also investigated in the hope that they contributed to the establishment of tolerance. Their function might then provide key elements to understanding the mechanisms of tolerance. Because of their increased numbers in operationally tolerant subjects and their recently discovered regulatory properties9, it was suggested that transitional B cells were directly involved in graft acceptance. On the other hand, these immature B cells were detected at a time when tolerance was already established ie, when transplant recipients were no longer being treated with immunosuppressive drugs2,4,5. For this reason, it is still uncertain whether transitional B cells are effectively related to the mechanisms that result in this state of tolerance. Ideally, the contribution of B cells to tolerance would be examined at the time when it is established. This specific time window is difficult to capture.
In the present study, we investigated a well-defined group of subjects who received combined kidney/bone marrow transplantation (CKBMT) at Massachusetts General Hospital from HLA haplo-matched donors as a strategy to induce tolerance to the organ graft (ITN036 trial)10,11. Three of the 5 subjects enrolled in this trial, sponsored by the Immune Tolerance Network, successfully accepted their transplants without the need for long-term immunosuppression10,11. The precise timing of the trial, conditioning regimen and immunosuppression withdrawal delineated the time when tolerance was induced in these subjects. We took this opportunity to examine B cells at this critical time and determine which subset may contribute to graft acceptance.
Materials and Methods
Subject characteristics
Five subjects were enrolled in this study. CKBMT was performed at Massachusetts General Hospital from HLA haplomatched donors. The detailed conditioning regimen (ITN036) and clinical outcomes have been reported separately10,11. All subjects received 4 doses of rituximab (375mg/m2/dose) on days -7, -2 pretransplant and days 5 and 12 posttransplant. For the sake of concordance, we used the same subject identifying code, namely subjects 6, 7, 8, 9 and 10, as that used in a prior publication from our group10. Subject 8 lost her graft 6 months posttransplant because of thrombotic microangiopathy, likely related to tacrolimus toxicity. This subject is not described further in the present report. For the 4 remaining subjects, immunosuppression (IS) was slowly tapered over several months and completely discontinued at 8 months. Graft function remained stable for 5–6 years for subjects 6, 7 and 9. As described in detail elsewhere10, subject 10 experienced acute T cell-mediated rejection approximately 2 months after immunosuppression (IS) was discontinued and following an episode of pyelonephritis treated with antibiotics. IS was resumed but graft function never fully recovered. He underwent preemptive retransplantation with standard immunosuppression 36 months after receiving his first transplant. The collection of all specimens used in this study was approved by the MGH internal review board.
Flow cytometry
CD19+ B cell counts were determined on whole blood after red blood cell lysis using mAbs specific for CD19 (BD Bioscience, San Jose, CA). Absolute numbers of B cells were calculated based on patient white blood count and the immunophenotypic data. Phenotyping analysis of B cells was performed by labeling blood lymphocytes with titrated volumes of anti-CD24 FITC (BD biosciences), anti-CD38 PE (Beckman Coulter, Fullerton, CA), anti-CD27APC/Cy7 (Biolegend, San Diego, CA). Cells were analyzed using a FACSVerse flow cytometer (BD Biosciences). The flow cytometer calibrated by methods described previously12.
Detection of reactivity to HLA molecules
The reactivity of subjects’ sera at different time points to HLA Class I and HLA Class II was assessed using beads coated with mixed HLA molecules (LABScreen Mixed, One Lambda, Los Angeles, CA). A non-corrected mean fluorescence intensity (MFI) of 1000 was arbitrarily used as a cutoff value. MFI values obtained for negative control beads ranged from 8 to 60. Serum samples showing positive reactivity to HLA Class I or HLA Class II were further tested using beads coated with single HLA molecules (LABScreen Single Antigen HLA Class I and Class II; One Lambda). Bound antibodies were detected with anti-IgG (One Lambda) PE-conjugated secondary antibody on a Luminex 200 apparatus (Luminex, Austin, TX).
Molecular Analysis of Rearranged Immunoglobulin Heavy Chain transcripts
Total RNA was extracted from subjects’ PBMC collected at different time points using a PureLink RNA Mini Kit (Invitrogen, Carlsbad, CA). Superscript III reverse transcriptase kit (Invitrogen, Carlsbad, CA) was used to generate cDNA. Variable regions of the Ig heavy chain were then amplified by PCR using 6 family-specific forward primers (VH1–VH6) and a consensus JH reverse primer as previously described13,14 with minor modifications. All 6 forward VH primers and the JH reverse primer used for each specimen (subject time point) included a unique barcode. The PCR conditions were as follows: 95°C for 5 minutes (95°C for30 seconds; 56°C for 30 seconds; 72°C for 30 seconds) × 35 cycles; 72°C for 10 minutes. PCR products were then purified after electrophoresis on agarose gel and used as template for next generation sequencing (NGS) of immunoglobulin heavy chain variable regions (IGHV). NGS was carried out by Beckman-Coulter Genomics services.
Analysis of IGHV sequences and Somatic Hypermutations
IGHV sequences were analyzed using the Immunoglobulin Analysis Tool (IgAT)15. Using this tool, the different framework (FR1~FR3) domains and complementarity determining regions (CDR1~CDR3) were identified for each sequence. All sequences including identical CDR3 amino acid segments were considered as originating from the same clone and grouped together to constitute partial IGHV repertoires. IGHV somatic hypermutations (SHM) were also identified using IgAT software. Somatic mutation rate was calculated to show the SHM status of the sequence pool generated from subject specimens at different time points. The diversity of sequences generated from specimens collected at different time points was calculated and presented as Shannon Diversity Index.
Results
B cell reconstitution following combined kidney bone marrow transplant
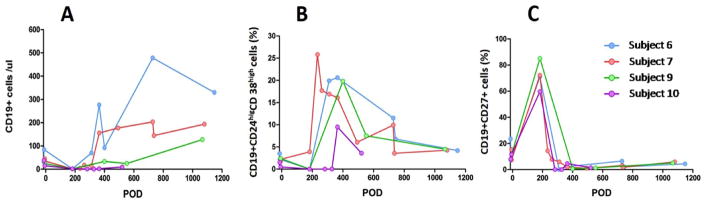
In addition to a preparative regimen including cyclophosphamide, subjects enrolled in the ITN036 trial received rituximab (375mg/m2/dose) on days -7 and -2 pretransplant and days 5 and 12 post transplant10. This combined treatment almost eliminated B cells from peripheral blood. As shown in Figure 1A, the kinetics of B cell reconstitution varied between individuals, starting early in subject 6 and comparatively later in subjects 7 and 9. B cell reconstitution was also delayed in subject 10, who received steroids and anti-thymocyte globulin 10 months posttransplant for acute T cell-mediated rejection. Peripheral blood B cell counts typically recovered to pretransplant levels (>30~40 cells/μl blood), by around 1 year posttransplant, except for subject 10 who experienced delayed reconstitution. This subject lost his graft to rejection at 36 months posttransplant while the other subjects achieved operational tolerance. Although barely detectable by flow cytometry, all subjects had peripheral blood B cells at day 182. We carried out a longitudinal phenotypic analysis of reconstituting B cells. As shown in Figure 1B, transitional B cells, identified as CD19+CD24highCD38high 16, were detectable at low frequency in subject 7 (~5%) and virtually non-existent in subjects 6, 9 and 10 at 6 months posttransplant. In contrast, we observed a prevalence of CD20+CD27+ memory B cells among reduced B cell pools in all subjects at this time-point (Figure 1C). These memory cells were likely B cells that had escaped rituximab treatment. Accordingly, the percentage of memory B cells sharply diminished as the percentage of transitional B cells increased as reconstitution followed its course (Figure 1B, C).
Figure 1. Phenotypic assessment of B cell reconstitution.
Peripheral CD19+ B cell absolute number (A), percentages of CD19+CD24highCD38hightransitional cells (B) and CD19+CD27+ memory B cells (C) were determined by flow cytometry on serial samples collected from the 4 subjects at various times after transplantation.
Longitudinal IGHV repertoire analysis in peripheral blood of CKBMT recipients, diversity and somatic hypermutation
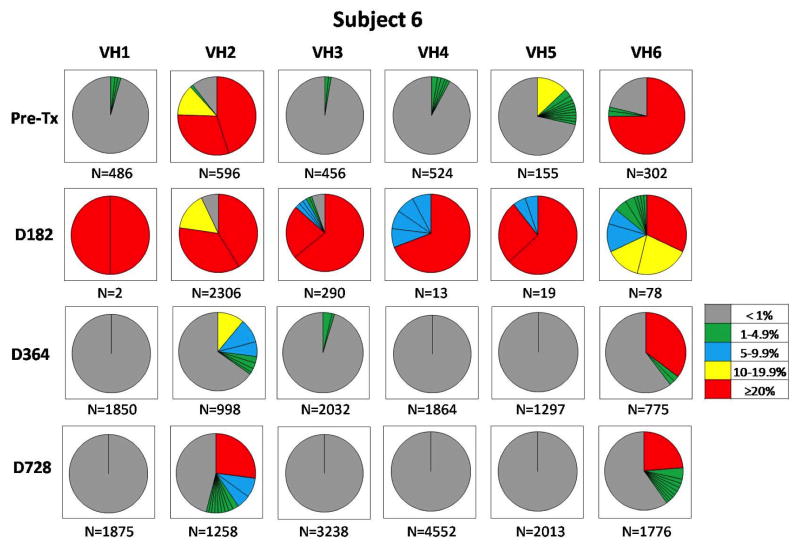
To evaluate B cell reconstitution at the clonal level, we conducted IGHV repertoire analyses at various time points for all subjects. We used a next generation sequencing strategy to generate between 2500–28 500 reads per samples. All sequences were analyzed and grouped by clonally rearranged complementarity determining region 3 (CDR3) sequences. Pie charts reported in Figure 2 for subject 6, shown as an example, and in Figures S1–S3, depict the frequency of clonotypic CDR3 sequences for each the 6 IGHV families (VH1~VH6) for each time point. The diversity of B cell repertoires was high in the pretransplant blood as well as at 1 and 2 years posttransplant. Although B cell counts were low at day 182, we successfully obtained enough sequences to carry out repertoire analysis in all subjects. As expected, B cell repertoires were markedly skewed at day 182, when B cell reconstitution was not detectable by flow cytometry. At this timepoint, a few prevalent clones dominated the B cell pools. Based on the phenotypic results, these clones were likely memory B cells that had escaped the cytolytic activity of rituximab and possibly expanded in a B cell lymphopenic environment.
Figure 2. Subject 6 longitudinal peripheral blood IGHV repertoire analysis.
Pie chart representation of the frequency of clonotypic CDR3 sequences for each the 6 IGHV families (VH1~VH6) in the peripheral blood of subject 6. Each pie chart section represents a distinct clonotypic CDR3. The size of each section corresponds to the frequency of the clonotypic sequences among all analyzed sequences. The frequency of each clonotypic sequence is also indicated by a color code (right box). The total number of sequences analyzed for each IGHV family is indicated below each pie. IGHV, immunoglobulin heavy chain variable gene.
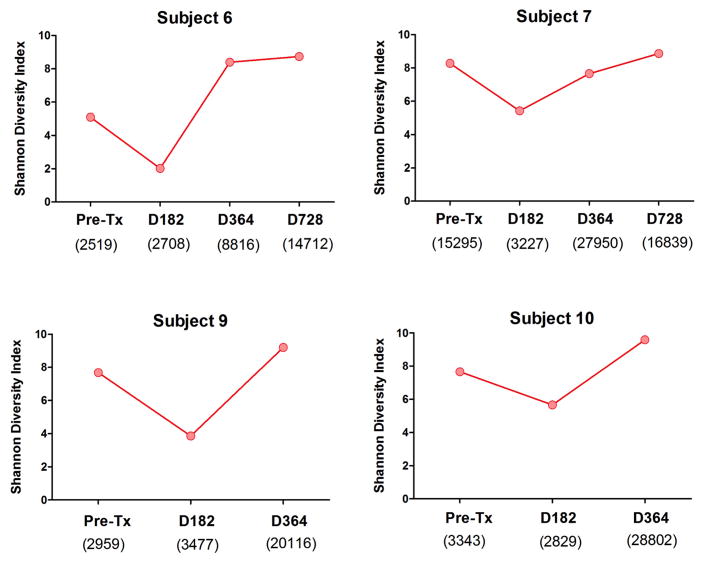
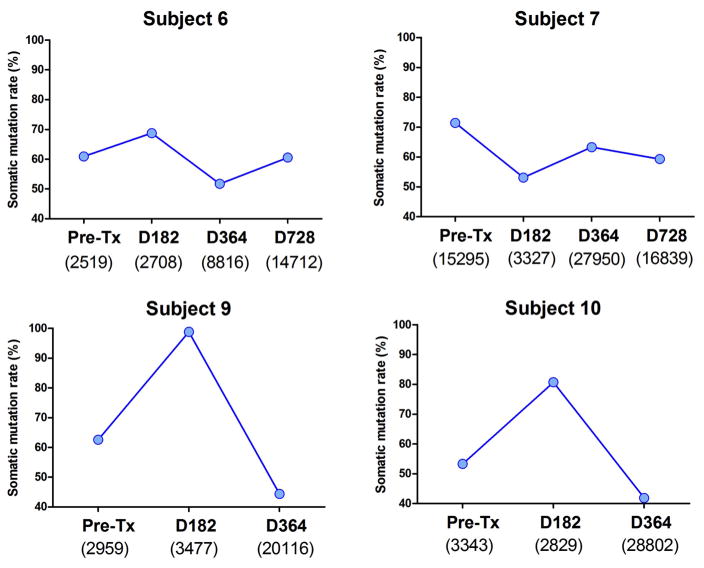
As a more reliable marker of IGHV sequence diversity, we calculated the Shannon Diversity Index for each subject time point. This index provides a direct measurement of the diversity in the composition of each repertoire. The Shannon index was sharply reduced for all subjects at day 182 posttransplant but recovered to pretransplant levels at 1 year posttransplant (Figure 3). We also calculated the somatic mutation rate of the sequences generated for all the time points. As shown in Figure 4, the somatic mutation rate was increased at day 182 for all but 1 subject (subject 7) in accordance with the predominance of memory B cells at this timepoint. Of note, subject 7 was the only subject with detectable transitional B cells at day 182.
Figure 3. Shannon Diversity Index of the IGHV sequence pools.
All sequences including identical CDR3 amino acid segments were considered originating from the same clone. Shannon Diversity Index was calculated to show diversity of sequences generated from 4 subjects’ specimens at various time points. Shannon Index was calculated using the formula: .
Figure 4. Somatic hypermutation rate of IGHV sequences.
Somatic mutations (SHM) within IGHV sequences were identified using IgAT software. SHM rates were calculated for all time points. The total number of sequences analyzed for each sample is indicated below the time point markers. Somatic mutation rate = (Number of mutated sequences/Number of total sequences) ×100%.
Serum reactivity to HLA molecules
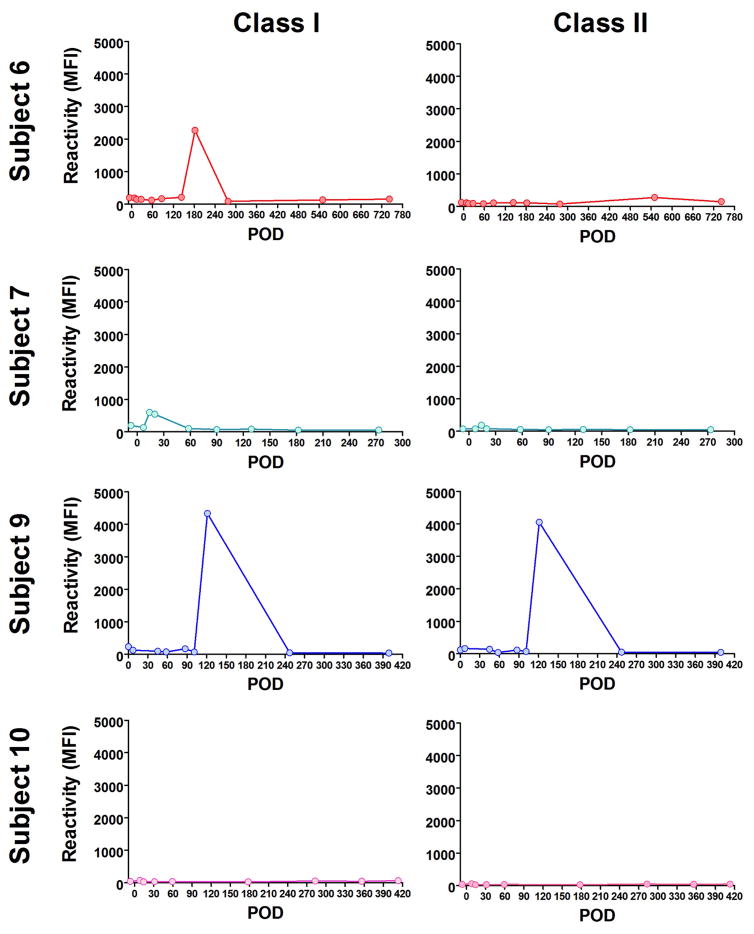
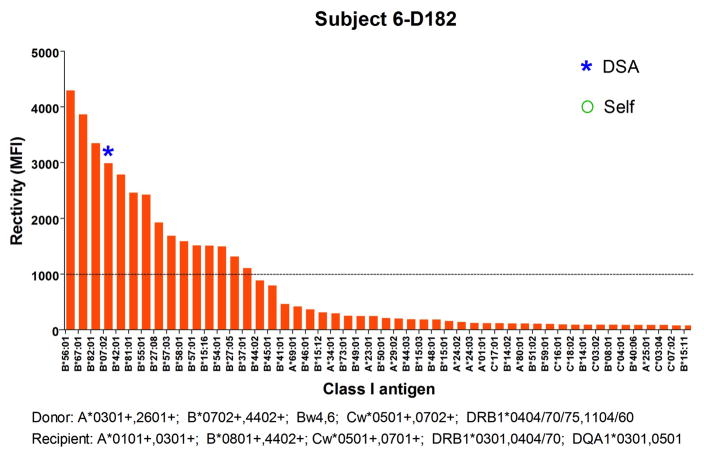
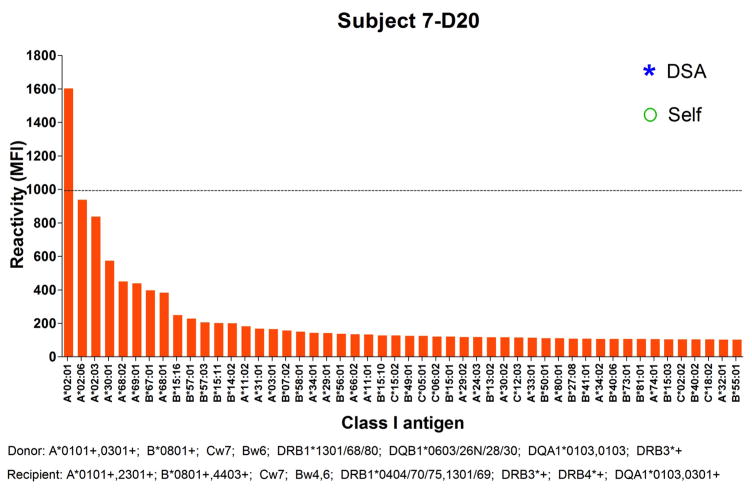
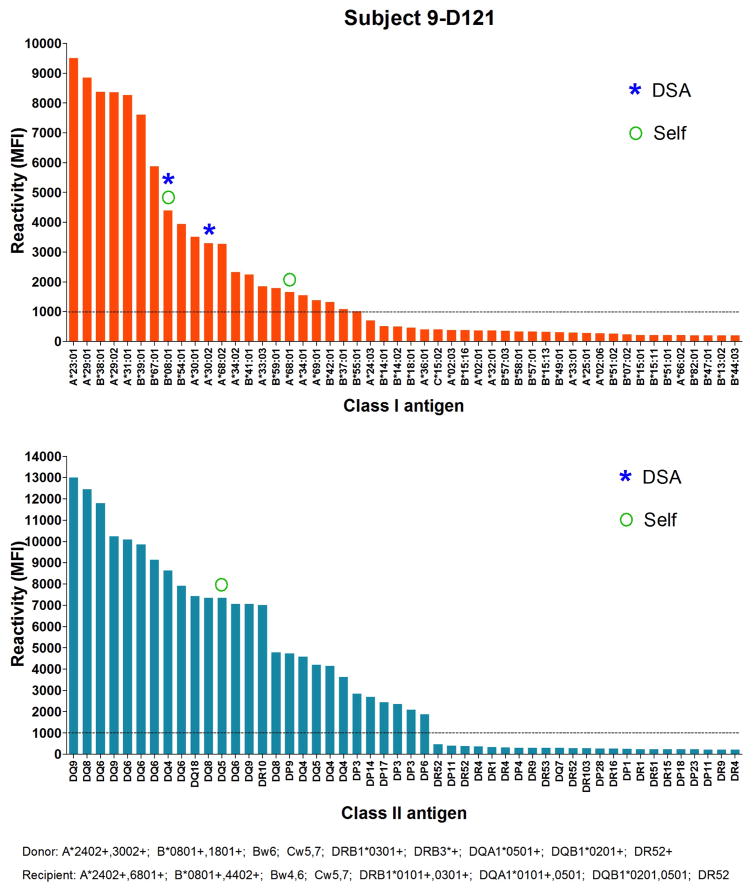
In addition to markers of B cell reconstitution, we assessed the subjects’ serum reactivity to HLA class I and class II at all time points by Luminex using beads coated with mixed HLA molecules. As shown in Figure 5, we detected a spike of reactivity within the first 8 months posttransplant for subjects 6, 7 and 9 before IS was discontinued. The serum of subject 6 reacted to HLA class I at day182; the serum of subject 7 weakly reacted to HLA Class I at day 20 and the serum of subject 9 reacted to both HLA class I and class II at day 121 posttransplant. To identify the recognized antigens, we subsequently assessed the positive serum samples using beads coated with single HLA molecules. As shown in Figure 6, subject 6 reacted to multiple class I antigens at day 182, including B*07:02 expressed on donor cells. The serum of subject 7 only showed weak reactivity to multiple HLA class I molecules at day 20 (Figure 7). However, as revealed in Figure 8, the serum of subject 9 strongly reacted to multiple HLA class I and class II, including several donor (A*30:01), and self-antigens (A*68:01, B*08:01, DQB1*0501).
Figure 5. Longitudinal assessment of serum reactivity to HLA molecules.
The reactivity of subject 6, 7, 9 and 10, serum specimens collected at various times to HLA Class I and HLA Class II was assessed using beads coated with mixed HLA molecules. Results are reported as mean fluorescence intensity (MFI).
Figure 6. Subject 6 serum reactivity to HLA class I.
The reactivity of subject 6 serum at Day 182 posttransplant to HLA class I antigens was tested by Luminex using single antigen beads. Each bar represents reactivity to 1 corresponding class I molecule labeled below. Only the 50 antigens towards which the serum reacted the most are depicted. Antigens are sorted based on reactivity. Donor and recipient HLA are listed below the x-axis.
Figure 7. Subject 7 serum reactivity to HLA class I.
The reactivity of subject 7 serum at Day 20 posttransplant to HLA class I antigens was tested by Luminex using single antigen beads. Each bar represents reactivity to 1 corresponding class I molecule labeled below. Only the 50 antigens towards which the serum reacted the most are depicted. Antigens are sorted based on reactivity. Donor and recipient HLA are listed below the x-axis.
Figure 8. Subject 9 serum reactivity to HLA class I and class II.
The reactivity of subject 9 serum at Day 121 posttransplant to HLA class I and class II antigens was tested by Luminex using single antigen beads. Each bar represents reactivity to 1 corresponding class I or class II molecule labeled below. Only the 50 antigens towards which the serum reacted the most are depicted. Antigens are sorted based on reactivity. Donor and recipient HLA are listed below the x-axis.
Discussion
In this study we examined B cell reconstitution in patients who achieved tolerance following CKBMT from HLA haploidentical donors. This clinical scenario provided us with a unique time window to investigate B cells during tolerance induction and identify specific subsets that may have contributed to the immune mechanisms underlying graft acceptance.
Previous studies have examined elements of immune recovery following HSCT combined with organ transplantation17,18. The present results depict the gradual reconstitution of B cell pools following CKBMT. Based on peripheral B cell counts, the reconstitution varied between individuals, starting early in subject 7 and comparatively later in subjects 6 and 9. B cell reconstitution was also delayed in subject 10 who received steroids and anti-thymocyte globulin 10 months posttransplant for acute T cell-mediated rejection. Phenotypic studies showed a high frequency of CD27+ memory B cells immediately after transplant at a time of low B cell counts. This population likely corresponds to memory cells that escaped rituximab treatment and even possibly expanded in the context of B cell lymphopenia19. We also examined transitional B cells in the patients’ blood as these cells are among the first to emerge during B cell reconstitution following HSCT (review in20). Transitional B cells were defined as CD19+CD24highCD38high cells as reported in multiple studies2,4,5,8,16,21. Transitional B cells surged in frequency and numbers later posttransplant and after immunosuppression withdrawal. Next generation sequencing analysis corroborated this data by revealing a skewed B cell repertoire at 6 months posttransplant for all 4 subjects examined. A higher frequency of mutated IGHV genes harbored somatic mutations at that time consistent with differentiated memory B cells. Notwithstanding individual variations, the B cell repertoire normalized around 1 year posttransplant, following the production of new B cells bearing an immature transitional phenotype. The normalization was accompanied by an increase in the Shannon diversity index and a decrease in the frequency of mutated IGHV genes.
Converging studies including ours have described elevated populations of transitional B cells in tolerant patients2,4,5,8,21. Despite this observation, it seems incongruous at first to consider these otherwise immature cells as key tolerogenic elements. Conventional wisdom would dictate that transitional cells observed in peripheral blood are unlikely to have had any function in vivo precisely because, as immature cells, they have never been stimulated22. Blair et al, showed that transitional cells have regulatory capabilities, especially through the production of IL-109. However, this function is acquired after sustained stimulation, resulting in the loss of their immature phenotype. Likewise, regulatory cells can originate from transitional B cells in vivo but by doing so they would lose their immature phenotype. It is therefore unlikely that transitional B cells detected in peripheral blood samples could have contributed to tolerance. Furthermore, our present investigation reveals a marked increase in CD24highCD38high transitional cells but only after patients had stopped immunosuppression and therefore after tolerance to the organ graft was established, with the exception of patient 7 who had 5% transitional B cells 6 months posttransplant. This distinction is crucial because it virtually excludes these transitional cells as possible immune elements contributing to tolerance induction in CKBMT patients.
In contrast, memory B cells were the predominant B cell subset at the time tolerance was evolving in CKBMT patients. Increased memory B cell pools have been associated with operational tolerance in previous studies1,4,6 even though this observation was mainly obscured by an initial focus on transitional B cells. Moreover, regulatory functions were also attributed to memory cells by several investigators, including the groups of Tedder and Mauri23,24. Our present data, together with the published literature, suggest that memory B cells could have contributed to graft acceptance following CKBMT. On the contrary, transitional B cells, virtually undetectable in peripheral blood as tolerance was evolving, seem less likely to have played a significant role. Of note, the situation observed in CKBMT recipients may differ from that of operationally tolerant kidney recipients who did not undergo B cell-depleting conditioning regimen. Nevertheless, a recent study by the group of Hernandez-Fuentes, reported that the initial identification of transitional B cells in operationally tolerant patients may have been due to immunosuppression withdrawal25.
Only very few B cells, expressing a memory phenotype, were detected in the peripheral blood of CKBMT patients 6 months after transplant. These low counts are consistent with the slow B cell reconstitution following hematopoietic cell transplantation. Memory B cells may have been spared by the non-myeloablative regimen, including several rounds of rituximab treatment. These cells are considered to have increased longevity compared to naïve B cells26. On the one hand, it is difficult to conceive that such a small number of cells could have contributed to any form of immune reaction, tolerogenic or not, nor does this retrospective study demonstrate such a contribution. On the other hand, we detected a transient increase in serum IgG reactivity to multiple HLA antigens within the first year posttransplant. The antigens towards which the serum reacted included HLA alleles expressed by the donor, the recipient, neither or both. This broad reactivity is characteristic of polyreactive antibodies, as we recently described14,27. Polyreactive antibodies cross-react to a wide range of HLA alleles that do not necessarily share a public epitope. While we cannot formally demonstrate that polyreactive antibodies contributed to the serum reactivity in ITN036 subjects, their possible contribution is suggested by the fact that recipient-specific HLA were among the antigens recognized. The origin of such spike of serum reactivity is uncertain. It is plausible that a cytokine flare associated with the engraftment syndrome may have caused a non-specific activation of memory B cells. The functional significance of these antibodies is also unclear. Consistent with the possibility that B cells producing such antibodies may be contributing to tolerance, Tedder and colleagues reported the polyreactive nature of IL-10-producing regulatory B cells28. Because of limited specimens collected from CKBMT patients, we could not assess whether circulating memory B cells detected 6 months posttransplant secreted IL-10 and corresponded to B10 cells. It is worth noting that patient 10, who experienced delayed B cell reconstitution, developed acute rejection at 10 months posttransplant and was eventually retransplanted approximately 2 years later for chronic rejection.
Our study is inherently limited by the small number of patients examined. Nevertheless, it sheds light on the composition of B cells detected in the peripheral blood at different stages of the immune reconstitution following CKBMT, which may have implications for their possible role in tolerance induction.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the Immune Tolerance Network, National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number UM1AI109565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors are indebted to Drs. Yong-Guang Yang and Chen Xiojuan for reviewing the manuscript.
Abbreviations
- CKBMT
combined kidney bone marrow transplant
- IS
immunosuppression
- DSA
donor-specific antibodies
- SHM
somatic hypermutation
- MFI
mean fluorescence intensity
- CDR3
complementarity-determining region 3
- IGHV
immunoglobulin heavy chain variable regions
- NGS
next generation sequencing
Footnotes
Authorship Page
EZ was the principal investigator who coordinated the research. BG, YG, CR, CM and FM performed the laboratory work for this study. FP performed immunophenotyping. WW, SLS, YF, BC, DHS, TK and MS recruited the subjects, provided clinical data and participated in the design of the study. BG and EZ wrote the manuscript.
Disclosure
This study was supported by the Immune Tolerance Network (NIH).
References
- 1.Chesneau M, Michel L, Dugast E, et al. Tolerant kidney transplant patients produce B cells with regulatory properties. J Am Soc Nephrol. 2015;26(10):2588–2598. doi: 10.1681/ASN.2014040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chesneau M, Pallier A, Braza F, et al. Unique B cell differentiation profile in tolerant kidney transplant patients. Am J Transplant. 2014;14(1):144–155. doi: 10.1111/ajt.12508. [DOI] [PubMed] [Google Scholar]
- 3.Durand J, Huchet V, Merieau E, et al. Regulatory B cells with a partial defect in CD40 signaling and overexpressing granzyme B transfer allograft tolerance in rodents. J Immunol. 2015;195(10):5035–5044. doi: 10.4049/jimmunol.1500429. [DOI] [PubMed] [Google Scholar]
- 4.Newell KA, Asare A, Kirk AD, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newell KA, Asare A, Sanz I, et al. Longitudinal studies of a B cell-derived signature of tolerance in renal transplant recipients. Am J Transplant. 2015;15(11):2908–2920. doi: 10.1111/ajt.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pallier A, Hillion S, Danger R, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78(5):503–513. doi: 10.1038/ki.2010.162. [DOI] [PubMed] [Google Scholar]
- 7.Sagoo P, Perucha E, Sawitzki B, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shabir S, Girdlestone J, Briggs D, et al. Transitional B lymphocytes are associated with protection from kidney allograft rejection: a prospective study. Am J Transplant. 2015;15(5):1384–1391. doi: 10.1111/ajt.13122. [DOI] [PubMed] [Google Scholar]
- 9.Blair PA, Norena LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14(7):1599–1611. doi: 10.1111/ajt.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai T, Sachs DH, Sykes M, Cosimi AB, Immune Tolerance N. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2013;368(19):1850–1852. doi: 10.1056/NEJMc1213779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagwell CB, Hill BL, Wood BL, et al. Human B-cell and progenitor stages as determined by probability state modeling of multidimensional cytometry data. Cytometry B Clin Cytom. 2015;88(4):214–226. doi: 10.1002/cyto.b.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferdman J, Porcheray F, Gao B, et al. Expansion and somatic hypermutation of B-cell clones in rejected human kidney grafts. Transplantation. 2014;98(7):766–772. doi: 10.1097/TP.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porcheray F, DeVito J, Helou Y, et al. Expansion of polyreactive B cells cross-reactive to HLA and self in the blood of a patient with kidney graft rejection. Am J Transplant. 2012;12(8):2088–2097. doi: 10.1111/j.1600-6143.2012.04053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogosch T, Kerzel S, Hoi KH, et al. Immunoglobulin analysis tool: a novel tool for the analysis of human and mouse heavy and light chain transcripts. Front Immunol. 2012;3:176. doi: 10.3389/fimmu.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuss AK, Avery DT, Cannons JL, et al. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J Immunol. 2006;176(3):1506–1516. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- 17.Leventhal JR, Elliott MJ, Yolcu ES, et al. Immune reconstitution/immunocompetence in recipients of kidney plus hematopoietic stem/facilitating cell transplants. Transplantation. 2015;99(2):288–298. doi: 10.1097/TP.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 18.Mathes DW, Chang J, Hwang B, et al. Simultaneous transplantation of hematopoietic stem cells and a vascularized composite allograft leads to tolerance. Transplantation. 2014;98(2):131–138. doi: 10.1097/TP.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Zelm MC, Szczepanski T, van der Burg M, van Dongen JJ. Replication history of B lymphocytes reveals homeostatic proliferation and extensive antigen-induced B cell expansion. J Exp Med. 2007;204(3):645–655. doi: 10.1084/jem.20060964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marie-Cardine A, Divay F, Dutot I, et al. Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation. Clin Immunol. 2008;127(1):14–25. doi: 10.1016/j.clim.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Porcheray F, Wong W, Saidman SL, et al. B-cell immunity in the context of T-cell tolerance after combined kidney and bone marrow transplantation in humans. Am J Transplant. 2009;9(9):2126–2135. doi: 10.1111/j.1600-6143.2009.02738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105(11):4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoder A, Sarvaria A, Alsuliman A, et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. 2014;124(13):2034–2045. doi: 10.1182/blood-2014-04-571125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebollo-Mesa I, Nova-Lamperti E, Mobillo P, et al. Biomarkers of tolerance in kidney transplantation: are we predicting tolerance or response to immunosuppressive treatment? Am J Transplant. 2016;16(12):3443–3457. doi: 10.1111/ajt.13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong AS, Sciammas R. Memory B cells in transplantation. Transplantation. 2015;99(1):21–28. doi: 10.1097/TP.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao B, Rong C, Porcheray F, et al. Evidence to support a contribution of polyreactive antibodies to HLA serum reactivity. Transplantation. 2016;100(1):217–226. doi: 10.1097/TP.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maseda D, Smith SH, Dilillo DJ, et al. Regulatory b10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol. 2012;188(3):1036–1048. doi: 10.4049/jimmunol.1102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.