Abstract
The developmental course of daily functioning prior to first psychosis-onset remains poorly understood. This study explored age-related periods of change in social and role functioning. The longitudinal study included youth (aged 12–23, mean follow-up years = 1.19) at clinical high risk (CHR) for psychosis (converters [CHR-C], n = 83; nonconverters [CHR-NC], n = 275) and a healthy control group (n =164). Mixed-model analyses were performed to determine age-related differences in social and role functioning. We limited our analyses to functioning before psychosis conversion; thus, data of CHR-C participants gathered after psychosis onset were excluded. In controls, social and role functioning improved over time. From at least age 12, functioning in CHR was poorer than in controls, and this lag persisted over time. Between ages 15 and 18, social functioning in CHR-C stagnated and diverged from that of CHR-NC, who continued to improve ( p = .001). Subsequently, CHR-C lagged behind in improvement between ages 21 and 23, further distinguishing them from CHR-NC ( p < .001). A similar period of stagnation was apparent for role functioning, but to a lesser extent ( p = .007). The results remained consistent when we accounted for the time to conversion. Our findings suggest that CHR-C start lagging behind CHR-NC in social and role functioning in adolescence, followed by a period of further stagnation in adulthood.
Disability in people with schizophrenia is evident in multiple areas of functioning, such as the quality of social contact and the fulfillment of roles at school or work (Green, Llerena, & Kern, 2015; Harvey et al., 2012). Although the importance of impaired social and role functioning as fundamental components of schizophrenia have been increasingly acknowledged (Burns & Patrick, 2007), little is known about their exact developmental course.
Studies of intellectual ability in schizophrenia indicate a slower increase in functioning in individuals who later develop the illness relative to the more rapid growth of healthy individuals (Reichenberg et al., 2010), indicating that cognitive abilities of future schizophrenia patients do increase over time, but with a pace slower than that of those who will never develop the illness. This finding is in congruence with the neurodevelopmental model, in which it is assumed that premorbid deficits in schizophrenia result from early neurodevelopmental disturbances, making it difficult to keep up with the “normal” functional growth that occurs later in life (Murray, O’Callaghan, Castle, & Lewis, 1992; Schmidt-Kastner, van Os, Esquivel, Steinbusch, & Rutten, 2012; Weinberger, 1987). The decade between early adolescence and young adulthood is specifically critical in this regard, as it has been suggested to represent a period during which growing societal/environmental challenges may negatively impact the final stages of brain maturation (Selemon & Zecevic, 2015; Weinberger, 1987).
In individuals who later develop schizophrenia, increasing deviations relative to the healthy population, prior to illness onset, have also been observed at the level of social and academic/vocational functioning (referred to as role functioning; Cole, Apud, Weinberger, & Dickinson, 2012; Horton, Tarbox, Olino, & Haas, 2015; Shapiro et al., 2009). Extensive work in this field stems from the 1950s, when Dr. Philips created the empirically derived Premorbid Adjustment Scale to retrospectively assess premorbid social and role functioning levels in patients diagnosed with schizophrenia (Phillips, 1953). Studies using the scale have since pointed to considerable heterogeneity, but overall it tends to show growing levels of premorbid social impairment in the phases closer to the illness (e.g., Allen, Frantom, Strauss, & van Kammen, 2005; Hafner, Nowotny, Loffler, an der Heiden, & Maurer, 1995; Monte, Goulding, & Compton, 2008; Strauss et al., 2012). Poorer premorbid functioning scores were shown to be associated with worse symptom course and overall severity of illness (Garmezy & Rodnick, 1959).
However, these previous studies generally used retrospective designs, introducing methodological limitations, such as recall biases. Although one recent population-based study showed a very similar pattern to the famous retrospective ABC studies (Hafner, Loffler, Maurer, Hambrecht, & an der Heiden, 1999), with increasing impairment compared to the general population in individuals tested closer to first hospitalization, this study included only one data point per individual (Velthorst et al., 2015). Collectively, these studies seem to point toward growing functional impairment over time, but longitudinal prospective studies are needed to confirm cross-sectional observations.
In addition, relatively little is known about the functional trajectories in the years following identification of risk of psychosis. Studying social and role functioning in this clinical high risk (CHR) phase is important, as it may provide important clues about the early developmental precursors of schizophrenia (Lin, Wood, & Yung, 2013). In most CHR studies carried out thus far, functional impairment is typically viewed as a single construct (e.g., as assessed with a single global assessment of functioning summary score; see Cotter et al., 2014), but distinct developmental trajectories may characterize different functional domains (Harvey et al., 2012). Previous studies from our group showed that, while impairments in both role and social functioning are persistent in individuals at CHR for psychosis (Addington, Cornblatt, et al., 2011), only deficits in the latter domain differentiate between those who eventually do and do not convert to psychosis (Cannon et al., 2008; Cornblatt et al., 2012), potentially suggestive of a differential developmental trajectory. Clarification of this matter is particularly important from the perspective of treatment of these different components of functional impairment.
In the present study, we explored if there are important periods during which impairments in the two key components of community functioning (social functioning and role functioning) start to become more pronounced in those who convert to psychosis. We compared the developmental trajectories of the two domains among CHR individuals who later converted to psychosis (CHR-C), CHR individuals who did not convert over a 2.5-year follow-up period (CHR-NC), and a healthy comparison group. More specifically, we examined whether the increased impairments over time in social and role functioning previously observed in CHR-C could be attributed to (age-related periods of) stagnation, delayed growth, or decline in functioning.
Methods
Subjects
We used data from 358 CHR and 164 healthy individuals (311 males, 211 females; baseline age: M = 17.37, SD = 3.0) who were participants in the North American Prodrome Longitudinal Study Phase 1 (NAPLS-1; Cannon et al., 2008) and/or the ongoing Center for Assessment and Prevention of Prodromal States (CAPPS) study at the University of California Los Angeles (UCLA) Semel Institute, and who had their baseline assessment in the decade between early adolescence and early adulthood (ages 12–23). Table 1 provides a detailed description of the baseline demographic and clinical characteristics broken down by group status. Forty-two percent of our sample (n = 217) was recruited as part of the CAPPS study. Of those, 41 (36 CHR, 5 controls) also participated in NAPLS-1. The other 176 CAPPS participants were recruited after the establishment of the NAPLS-1 cohort. CAPPS and NAPLS-1 used identical inclusion criteria and study designs; all participants (CHR and controls) were seen at 6-month intervals and prospectively followed for up to 2.5 years. At the UCLA Semel Institute, the same interviewers performed all clinical interviews and symptom ratings for both CAPPS and NAPLS-1.
Table 1.
Baseline demographic and clinical characteristics of the sample
| Variable | CHR | Controls (n = 164) | Statistics* | |
|---|---|---|---|---|
|
| ||||
| Converters (n = 83) | Nonconverters (n = 275) | |||
| Gender, no male (%) | 59 (69.9) | 175 (63.3) | 77 (47.0) | 1, 2 > 3 |
| Age at baseline, mean (SD) | 17.98 (2.51) | 16.86 (2.90) | 17.91 (3.25) | 1, 3 > 2 |
| White,a n (%) | 55 (68.75) | 192 (73.28) | 96 (61.54) | 1, 2 > 3 |
| IQ at baseline, mean (SD) | 102.72 (19.65) | 106.72 (15.99) | 111.51 (13.46) | 1, 2 < 3 |
| Years of education, mean (SD) | 10.86 (2.02) | 9.85 (2.68) | 10.95 (2.94) | 1, 3 > 2 |
| Lower educational level father,b n (%) | 17 (35.42) | 54 (29.83) | 19 (14.62) | 1, 2 > 3 |
| GF | ||||
| Social, mean (SD) | 5.49 (1.43) | 6.21 (1.50) | 8.61 (0.99) | 1 < 2 < 3 |
| Role, mean (SD) | 5.45 (1.74) | 6.12 (1.88) | 8.62 (0.97) | 1 < 2 < 3 |
| SIPS | ||||
| Positive symptoms | 14.00 (4.58) | 10.77 (3.92) | 0.89 (1.58) | 1 > 2 > 3 |
| Negative symptoms | 15.31 (6.87) | 11.70 (6.54) | 0.97 (1.62) | 1 > 2 > 3 |
| Disorganized symptoms | 8.25 (4.57) | 5.51 (3.50) | 0.39 (0.77) | 1 > 2 > 3 |
| General symptoms | 9.38 (4.46) | 7.57 (4.05) | 0.61 (1.05) | 1 > 2 > 3 |
Note: CHR, Clinical high risk; GF, global functioning; SIPS, Structured Interview for Prodromal Syndromes. Scores represent SIPS and GF at baseline.
Data for n = 24 missing.
Number of individuals without education higher than high school. Data for n = 163 missing.
p = .05.
CHR participants were help-seeking individuals who met criteria for one of three CHR categories, as assessed with the Structured Interview for Prodromal Syndromes (SIPS; McGlashan, Walsh, & Woods, 2010) by experienced MA/ PhD level clinicians: attenuated psychotic symptoms; transient, recent-onset psychotic symptoms; or a substantial drop in functioning in conjunction with schizotypal personality disorder or a first-degree relative with psychosis. Participants were excluded for current or past diagnosis of an Axis I psychotic disorder, including affective psychoses, as determined by the Structured Clinical Interview for the DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1997). Approximately 96% of the CHR participants met initial eligibility based on attenuated positive symptoms, and 4% met initial eligibility based on brief intermittent psychotic symptoms. While only 2 (<1%) patients were ascertained as prodromal exclusively in the genetic risk and deterioration category, 12% had a comorbid attenuated positive symptoms–genetic risk and deterioration prodromal diagnosis.
The typically developing control sample, matched on age to the CHR sample, did not meet CHR criteria or DSM-IV criteria for any major psychiatric disorder. Additional exclusion criteria for all participants were as follows: neurological disorder, drug/alcohol abuse or dependence within the past 6 months, insufficient English fluency, and/or IQ below 70 (see (Addington et al., 2007, for more details regarding inclusion/ exclusion criteria and diagnostic reliability procedures). The institutional review boards of the participating sites approved study protocols and informed consent documents.
Measures
Symptomatology
The SIPS and the Scale for Assessment of Prodromal Syndromes (McGlashan et al., 2010) were used to assess CHR criteria, severity of attenuated positive symptoms and negative symptoms, and to define conversion to psychosis (Cannon et al., 2008). Symptom domain scores were determined by the sum of symptom severity scores within each domain (positive, negative, disorganized, and general symptoms). Full details regarding SIPS criteria, reliability, and consensus procedures are described elsewhere (Addington et al., 2007; Meyer et al., 2005). The SCID (First et al., 1997) was used to establish current and past DSM IV Axis 1 diagnoses. Estimated full-scale IQ scores were derived from the Vocabulary and Block Design Tests of the Wechsler Intelligence Scale for Children—Third Edition (Wechsler, 1991) for individuals younger than 16 years and from the Wechsler Adult Intelligence Scale—Revised (Wechsler, 1981) for individuals 16 years or older (Carrion et al., 2013).
Social and role functioning
Social and role functioning were assessed using the GF: Social Scale and the GF: Role Scale (Cornblatt et al., 2012), two scales specifically designed to detect change in functioning in at-risk adolescents and young adults who are not as severely impaired as more chronic patients. The scales were initially validated as part of collaboration between the Zucker Hillside and UCLA sites, but later used as the common social/role measures across NAPLS-1 sites (Cornblatt et al., 2012). The GF: Social Scale assesses peer relationships, peer conflict, age appropriate intimate relationships, and involvement with family members. The GF: Role Scale assesses performance and amount of support needed in one’s specific roles (i.e., school/work). Scores of both scales range from 1 to 10 (with higher scores indicating better functioning). In the present study, we made use of the “current” functioning scores, that is, functioning levels in the month preceding assessment. Ratings for each scale were based on best estimates derived from all available information, an approach that has been shown to yield high interrater reliability scores (Cannon et al., 2008; Cornblatt et al., 2007).
Follow-up procedure
All 6-month interval follow-up interviews included SIPS ratings and the two global functioning scales. Conversion to psychosis was defined as the presence of a psychotic-level positive symptom (a score of 6 with a minimum duration of 1 week). For the majority of the cases, a follow-up SCID interview was conducted at the time of conversion to additionally determine DSM-IV diagnosis of psychotic disorder.
Statistical analyses
Because we were specifically interested in the period between early adolescence and early adulthood, and the number of data points per age group (specifically for converters) was substantially lower after this period, we restricted our analyses to participants aged 12–23 years. We included only those participants who had data on social and role functioning, and IQ scores available. We limited our analyses to data collected on functioning prior to first onset of psychosis only; thus, data from CHR-C youth gathered after first psychosis onset was excluded. Interdependence between the two functional domains (i.e., shared variance between deficits in role and social functioning) was examined with partial correlations, controlling for age, gender, and IQ. We used regression analyses to examine the association between functional impairment, age at baseline, and time-to-conversion. Differences in baseline demographic characteristics, baseline symptoms (positive, negative, disorganized, or general), IQ, and social and role functioning between the control group, CHR-NC, and CHR-C individuals were examined with regression analyses for continuous variables, and chi-square tests for dichotomous variables.
Functioning data from the 358 CHR and 164 healthy individuals included in our study (159 with 1 assessment, 363 with >1 assessment, range = 1–5) yielded 1,275 observations (including first assessment) to construct developmental trajectories. There were a significantly higher number of dropouts after first assessment among healthy individuals (43.3%; n = 71) versus in the CHR cohort (24.6%; n = 88). Some follow-up assessments were not included in the analyses as the participant aged-out of our age window: 9 participants had turned 24 by the time of the second assessment, another 4 by the time of the third, 2 by the time of the fourth, and 3 more by the time of the fifth assessment. CHR individuals with only one assessment were comparable to those with >1 on all clinical and functional measures and most demographic characteristics, but were marginally older (17.6 vs. 17.0; t = 1.98, p = .049). Healthy individuals with only one assessment did not differ from those with >1 on functional measures and demographic characteristics, but had significantly less positive ( p < .001), general ( p = .023), and disorganization symptoms ( p = .013).
Developmental trajectories of social and role functioning were analyzed separately with multilevel linear modeling analyses (Statacorp 14; StataCorp, 2014), taking multiple observations per individual into account. We explored the different functioning developmental trajectories per status group by entering age of assessment as an independent variable, and functioning score as the dependent variable in the model (controlling for IQ and gender). Both the main effect of group (CHR-NC, CHR-C, and controls) and the Group×Age interaction were effects of interest: a group main effect only would be suggestive of a static group difference, whereas a Group×Age interaction would be suggestive of stagnation, faster growth, or decline in functioning in one group compared to other groups.
We subsequently explored whether there were specific periods in the functional development of future converters that distinguished them from nonconverters by subdividing all social and role functioning assessments into age bands that capture different developmental epochs: early adolescence (ages 12–15 years), middle to late adolescence (15–18 years), young adulthood (18–21 years), and adulthood (21–23 years). We performed multilevel linear modeling analyses for each age band to identify time periods when levels of functioning in the CHR-C group started to diverge from the CHR-NC. We controlled for gender and IQ in these analyses as well.
To explore whether the baseline severity of symptoms attenuated our findings, all analyses were repeated controlling for positive, negative, general, and disorganization symptoms.
Results
Of the 358 CHR participants, 83 converted (23.2%) to a psychotic disorder within the follow-up period. Diagnostic outcome was available for 70 converters (84.3%). Of those, 36 (51.4%) received a diagnosis in the schizophrenia spectrum (schizophrenia, n = 18; schizophreniform, n = 11; schizoaffective, n = 7), 9 (12.9%) were diagnosed with a mood disorder with psychotic features, 19 (27.1%) with a psychosis not otherwise specified, 4 (5.7%) with a brief psychotic episode, and 2 (2.9%) with a delusional disorder.
Among those who converted, the mean time from baseline assessment to conversion to psychosis was 0.88 years (~10 months; SD = 0.81). Average time of follow-up assessment for the remainder of the sample was 1.19 years (SD = 0.97). Time to conversion was not significantly associated with social and role functioning at any time point ( p values ranged between p = .20 and p = .96 for social functioning, and between p = .08 and p = .79 for role functioning) or with age at baseline (B = –0.05, SE = 0.04, p = .18). Table 1 presents baseline demographic and clinical data for the 358 CHR participants and the 164 healthy comparison subjects. CHR individuals overall had lower IQ, and were significantly more often White and male relative to the comparison group. CHR-NC individuals had fewer years of education and were younger than CHR-C and the control group.
GF: Social functioning trajectories
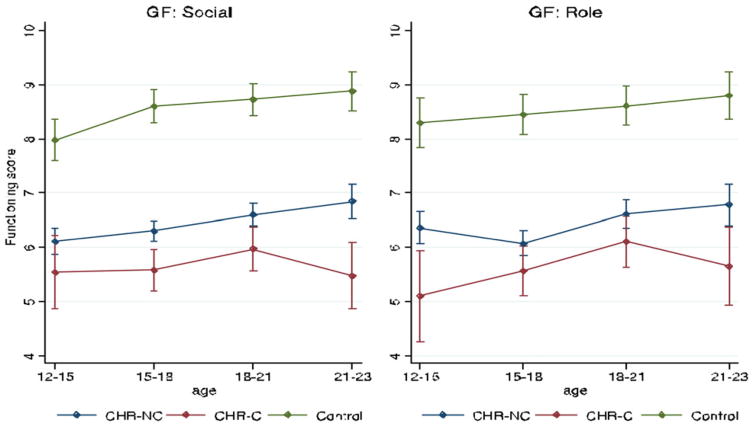
The square of correlations between role and social functioning was .34, indicating a moderate amount of common variance. We examined the natural course of social functioning by examining the social functioning data over time in controls. Social functioning in this group was dynamic, gradually improving with age (B = 0.08, SE = 0.02, p ≤ .001). Both the CHR-C and CHR-NC groups had lower levels of social functioning over time relative to the control group (main effect: B = –2.84, SE = 0.17, p < .001; B = –2.04; SE = 0.12, p < .001). There was a trend toward a Group×Age interaction when comparing functional trajectories across all groups (B = –0.10, SE = 0.06, p = .073), suggesting that functional trajectories may differ between groups. Examining this further, we observed that in contrast to CHR-NC individuals who (in congruence with the healthy control sample) significantly improved in their social functioning skills with age (B = 0.10, SE = 0.02; p < .001), the CHR-C group did not show such an increase (B = –0.0002, SE = 0.06, p = .997; see Figure 1). These findings held when the baseline severity of positive, negative, and disorganized symptoms were accounted for.
Figure 1.
(Color online) Developmental trajectories of social and role functioning. The Y-axis in the figure represents the GF: Social Scale and the GF: Role Scale scores, which range from 1 to 10, with higher scores meaning better functioning. The X-axis represents mean scores at the assessments conducted with individuals at ages 12–15, 15–18, 18–21, and 21–23.
Analyses within the CHR group suggest that there may be two potential periods during which converters started to lag behind in social functioning compared to nonconverters (see Table 2). Between ages 15 and 18, the growth seen in premorbid social functioning of nonconverters was not seen in the converting group (B = –0.84, SE = 0.25, p = .001; Cohen d = 0.56). Subsequently, a similar period occurred between ages 21 and 23, reflected by a relatively increasing deficit between converters and from nonconverters (B = –1.53, SE = 0.38; p < .001; Cohen d = 1.07). Controlling for prodromal symptoms did not attenuate the results.
Table 2.
Difference in social and role functioning between CHR converters and nonconverters per age band
| GF | B | SE | Z | p | CI 95% |
|---|---|---|---|---|---|
| Social | |||||
| 12–15 | −0.55 | 0.47 | −1.18 | .240 | [−1.47, 0.37] |
| 15–18 | −0.83 | 0.25 | −3.33 | .001 | [−1.32, −0.34] |
| 18–21 | −0.68 | 0.28 | −2.41 | .02 | [−1.23, −0.13] |
| 21–23 | −1.53 | 0.38 | −4.01 | <.0001 | [−2.27, −0.78] |
| Role | |||||
| 12–15 | −1.60 | 0.55 | −2.91 | .004 | [−2.69, −0.52] |
| 15–18 | −0.43 | 0.32 | −1.36 | .173 | [−1.05, 0.189] |
| 18–21 | −0.64 | 0.32 | −1.98 | .048 | [−1.26, −0.007] |
| 21–23 | −0.98 | 0.37 | −2.68 | .007 | [−0.07, −0.26] |
Note: CHR, Clinical high risk; GF, global functioning. All analyses are controlled for IQ and gender. Number of data points: 12–15 years: CHR converters (CHR-C), n = 25; CHR nonconverters (CHR-NC), n = 228; 15–18 years: CHR-C, n = 76; CHR-NC, n = 377; 18–21 years: CHR-C, n = 86; CHR-NC, n = 251; 21–23 years: CHR-C, n = 27; CHR-NC, n = 125.
GF: Role functioning trajectories
Similarly, in the domain of role functioning, controls marginally improved with age (B = 0.04, SE = 0.02, p = .04). Although an overall group difference was apparent (with the CHR-C and CHR-NC groups having lower role functioning scores compared to the control group; main effect: B = –2.75, SE = 0.20, p < .001; B = –2.05; SE = 0.14, p < .001), here there was no indication of a Group × Age interaction. Adjusting the analyses for prodromal symptom severity at baseline did not alter the results.
However, examining the developmental trajectories per group separately, we found the CHR-C group to show greater impairment in role functioning compared to CHR-NC between ages 12 and 15 (B = –1.60; SE = 0.55; p = .004; Co-hen d = 0.81). In addition, we detected a similar difference for role functioning between CHR-C and CHR-NC as observed for the social trajectories (see Figure 1) between the ages of 21 and 23 (B = –0.94, SE = 0.37, p = .01; Cohen d = 0.83). This latter effect decreased to trend level when negative symptoms or disorganization symptoms were accounted for (B = 0.74; SE = 0.45, p = .08, and B = –0.78, SE = 0.45, p = .059, respectively).
Converters and nonconverters between ages 21 and 23 were otherwise largely comparable to those with data at earlier ages. Apart from a higher IQ score in nonconverters age 21–23 compared to the nonconverters in the younger group (IQ = 106.0 vs. IQ = 113.4; p = .02), the CHR-C and CHR-NC groups with data between ages 21 and 23 did not significantly differ from the those with data at earlier ages in terms of positive, negative, general, or disorganization symptoms, nor in terms of ethnicity or gender.
Discussion
In a large sample of youth at high clinical risk for psychosis, we evaluated whether there are periods of change in the development of two key components of community functioning: social and role functioning. Taken together, our results indicate that both social and role functioning are more impaired in CHR individuals compared to controls from as early as age 12, and that differences between converters and nonconverters become increasingly apparent by age.
In congruence with the literature (Shapiro et al., 2009), functional levels of controls gradually increased with age. For social functioning, there were periods of early and late dynamic changes that distinguished CHR-C from CHR-NC, and results remained consistent when time to conversion was accounted for. During the first period, between ages 15 and 18, social functioning of converters stagnated and diverged from that of nonconverters, who, like controls, continued to improve. Next, the growth seen in nonconverters between ages 21 and 23 was not apparent for converters, further distinguishing them from nonconverters. Similar changes were observed for role functioning, with functioning levels that were lower in the CHR cohort, but initially increasing in a similar pace for both converters and nonconverters relative to the functional improvement observed in controls. However, a late change in role functioning became apparent for the converter group, as their role functioning scores seemed to stagnate between ages 21 and 23.
Similar patterns have been observed in a cross-sectional cohort (Velthorst et al., 2015) and in various retrospective studies to patients diagnosed with schizophrenia using the Premorbid Adjustment Scale (Allen et al., 2005; Monte et al., 2008; Shapiro et al., 2009; Strauss et al., 2012). The initial more pronounced difference in trajectories for social functioning between converters and nonconverters may be at least partly explained by findings of previous work from our research group, showing that social and role functional outcome may be driven by different cognitive functions (Carrion et al., 2013; Meyer et al., 2014). In this study, processing speed performance appeared to be particularly important for social outcome, while verbal memory was found more strongly related to successful academic and work achievement. Population-based studies point to an accelerated deficit in processing speed from the age of 13 in children who will develop adult schizophrenia, which could explain the stagnation in social functioning around that time (Meier et al., 2014; Reichenberg et al., 2010). In contrast, impairment in verbal memory has been found to be more stable (Stone et al., 2016), possibly accounting for the initially more subtle deficits in role functioning.
As argued previously, although accounting for baseline prodromal symptom severity did not alter our results, the subsequent period of stagnation in both social and role functioning in early adulthood may be caused by the interaction between prodromal symptoms and the increasingly complexity of social and work tasks that have to be taken on (Hafner et al., 1999; Velthorst et al., 2009). While in early adolescence support systems may still compensate and hide subtle functional deficits, early adulthood represents a phase during which people are no longer expected to heavily rely on others, potentially contributing to the increasing deficits during this time. It will be interesting for future studies to disentangle how stagnation in functional impairment may in turn directly contribute to increased risk of psychosis onset in the CHR samples; for example, it may be that functional impairment creates social distress, or reduces access to social support.
This study has several limitations. First, the minimum age of study participants was 12. We are therefore constrained to conclusions about functional trajectories, and symptom development only from this age onward. It would be interesting for future prospective population-based cohort studies to examine at which age first symptoms and functional impairments appear. Second, “a significant drop in functioning” was one of the necessary criteria for individuals included based on schizotypal personality disorder diagnosis. However, in our study only 0.8% of the CHR individuals were included solely based on the criterion “first-degree relative with psychosis or patient with schizotypal personality disorder and a 30% drop in global assessment of functioning score compared with that 1 year ago, sustained over the past month,” and omitting those subjects from the analyses did not significantly impact the results. Third, many, but not all, individuals who go on to develop psychosis experience subthreshold symptoms, and our conclusions are limited to those who do. In addition, some CHR subjects may convert to psychosis after the 2.5-year follow-up period (Nelson et al., 2013), and some converters may only have experienced brief full-blown psychotic complaints. Fourth, the potential misclassification of some subjects may have reduced differences observed between converters and nonconverters (Seidman et al., 2010). Because controls with DSM-IV psychiatric diagnosis were excluded, the control group may represent a higher functioning subgroup of the general population. However, the divergence in functioning we observed between CHR-C and CHR-NC would suggest that our findings are not simply a result of general distress, but are related to subsequent development of psychosis. Fifth, the sample size of converters in the 12–15 and 21–23 age bands was relatively small, and findings with regard to these age epochs should be interpreted with caution. Sixth, the lack of an association between time to conversion and functioning scores may have been partly accounted for by limited variability in the time to conversion, with the majority converting within the first year. Seventh, data on ongoing (pharmacological) interventions was not taken into account.
Despite these limitations, our study is largely consistent with results of previous studies, which suggest that early stagnation in social functioning may be a unique risk marker for psychosis (e.g., Cannon et al., 2008; Strauss et al., 2012; Velthorst et al., 2010). Our findings also provide new evidence supporting the neurodevelopmental model of psychosis, pointing to a developmental lag in social functioning between the ages of 15 and 18, followed byanother period of stagnation in earlyadulthood. Results we found related to age rather than to greater levels of functional impairment in those closer to overt illness. Role functioning trajectories of converters and nonconverters were initially more comparable, although also for role functioning, a period of relatively increasing impairment in early adulthood became apparent for the converting group.
Moreover, our findings further highlight the importance of examining social and role functioning separately, and moving away from “global” measures to more age-specific measures (Cornblatt et al., 2007). Treatment attempts to improve social functioning in psychotic disorders have been only marginally successful (Almerie et al., 2015), which may be partly due to the global measures used, as well as our limited knowledge of when deviations develop. Our results indicate that impaired social and role functioning in CHR individuals are apparent already in early adolescence, but that functioning levels of converters further diverge from nonconverters in early adulthood. We found middle adolescence to be a first period of change in the development of social functioning, as during this time trajectories of those with and without a psychotic disorder start to diverge. Worldwide, noninvasive cognitive (behavioral) interventions for young individuals at risk for psychosis are emerging (Addington, Epstein, et al., 2011; Landa et al., 2016; Okuzawa et al., 2014; van der Gaag et al., 2012, 2013) with few also focusing on the improvement of social functioning, or the preservation of social engagement (for a review, see Thompson et al., 2015). Certain cognitive interventions (e.g., see French & Morrison, 2004) may be promising in impeding the deteriorating functional course of adolescents at risk for psychosis.
It will be important for future studies to disentangle what neural and genetic processes contribute to this diversion and how these processes vary across different functions. The significance of these future studies has been highlighted by Hodgekins et al. (2015) who found that a large proportion of individuals with significant social disability do not improve over the first 12 months of service provision (66%).
Conclusion
In conclusion, our findings provide new insight into the developmental course of functioning in youth at CHR for psychosis, and inform the field about potentially important intervention windows. We hope that a better understanding of the origins and developmental course of functional impairment will ultimately help us decrease its debilitating consequences and increase our knowledge of the pathways to psychotic disorders.
Acknowledgments
This study was supported by grants from the National Institute of Mental Health of the National Institutes of Health (U01 MH081928 to L.J.S., U01 MH066134 to J.A., R01 MH60720 to K.S.C., R01 MH065079 to T.D.C., R01 MH061523 to B.A.C., U01 MH066069 and K23 MH001905 to D.O.P., RO1MH062066 to E.F.W., U01 MH066160 to S.W.W., and K05MH01654 to T.H.M.), the Commonwealth of Massachusetts (SCDMH82101008006 to L.J.S), and the Netherlands Organization for Scientific Research (VENI 916-15-005 to E.V.). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no conflicts of interest in relation to the subject of this study.
References
- Addington J, Cadenhead KS, Cannon TD, Cornblatt B, McGlashan TH, Perkins DO … North American Prodrome Longitudinal Study. North American prodrome longitudinal study: A collaborative multisite approach to prodromal schizophrenia research. Schizophrenia Bulletin. 2007;33:665–672. doi: 10.1093/schbul/sbl075. sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Cornblatt BA, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, … Heinssen R. At clinical high risk for psychosis: Outcome for nonconverters. American Journal of Psychiatry. 2011;168:800–805. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophrenia Research. 2011;125:54–61. doi: 10.1016/j.schres.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Allen DN, Frantom LV, Strauss GP, van Kammen DP. Differential patterns of premorbid academic and social deterioration in patients with schizophrenia. Schizophrenia Research. 2005;75:389–397. doi: 10.1016/j.schres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Almerie MQ, Okba Al Marhi M, Jawoosh M, Alsabbagh M, Matar HE, Maayan N, Bergman H. Social skills programmes for schizophrenia. Cochrane Database of Systematic Reviews. 2015;6:CD009006. doi: 10.1002/14651858.CD009006.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns T, Patrick D. Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatrica Scandinavica. 2007;116:403–418. doi: 10.1111/j.1600-0447.2007.01108.x. ACP1108. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, … Heinssen R. Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Archives of General Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion RE, McLaughlin D, Goldberg TE, Auther AM, Olsen RH, Olvet DM, … Cornblatt BA. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2013;70:1133–1142. doi: 10.1001/jamapsychiatry.2013.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole VT, Apud JA, Weinberger DR, Dickinson D. Using latent class growth analysis to form trajectories of premorbid adjustment in schizophrenia. Journal of Abnormal Psychology. 2012;121:388–395. doi: 10.1037/a0026922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, Cannon TD. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophrenia Bulletin. 2007;33:688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Carrion RE, Addington J, Seidman L, Walker EF, Cannon TD, … Lencz T. Risk factors for psychosis: Impaired social and role functioning. Schizophrenia Bulletin. 2012;38:1247–1257. doi: 10.1093/schbul/sbr136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter J, Drake RJ, Bucci S, Firth J, Edge D, Yung AR. What drives poor functioning in the at-risk mental state? A systematic review. Schizophrenia Research. 2014;159:267–277. doi: 10.1016/j.schres.2014.09.012. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV axis I disorders (SCID)—Clinician version. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- French P, Morrison AP. Early detection and cognitive therapy for people at high risk of developing psychosis: A treatment approach. Chichester: Wiley; 2004. [Google Scholar]
- Garmezy N, Rodnick EH. Premorbid adjustment and performance in schizophrenia: Implications for interpreting heterogeneity in schizophrenia. Journal of Nervous and Mental Disease. 1959;129:450–466. doi: 10.1097/00005053-195911000-00006. [DOI] [PubMed] [Google Scholar]
- Green MF, Llerena K, Kern RS. The “right stuff” revisited: What have we learned about the determinants of daily functioning in schizophrenia? Schizophrenia Bulletin. 2015;41:781–785. doi: 10.1093/schbul/sbv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner H, Loffler W, Maurer K, Hambrecht M, an der Heiden W. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatrica Scandinavica. 1999;100:105–118. doi: 10.1111/j.1600-0447.1999.tb10831.x. [DOI] [PubMed] [Google Scholar]
- Hafner H, Nowotny B, Loffler W, an der Heiden W, Maurer K. When and how does schizophrenia produce social deficits? European Archives of Psychiatry and Clinical Neuroscience. 1995;246:17–28. doi: 10.1007/BF02191811. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Sabbag S, Prestia D, Durand D, Twamley EW, Patterson TL. Functional milestones and clinician ratings of everyday functioning in people with schizophrenia: Overlap between milestones and specificity of ratings. Journal of Psychiatric Research. 2012;46:1546–1552. doi: 10.1016/j.jpsychires.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgekins J, Birchwood M, Christopher R, Marshall M, Coker S, Everard L, … Fowler D. Investigating trajectories of social recovery in individuals with first-episode psychosis: A latent class growth analysis. British Journal of Psychiatry. 2015;207:536–543. doi: 10.1192/bjp.bp.114.153486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton LE, Tarbox SI, Olino TM, Haas GL. Trajectories of premorbid childhood and adolescent functioning in schizophrenia-spectrum psychoses: A first-episode study. Psychiatry Research. 2015;227:339–346. doi: 10.1016/j.psychres.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa Y, Mueser KT, Wyka KE, Shreck E, Jespersen R, Jacobs MA, … Walkup JT. Development of a group and family-based cognitive behavioural therapy program for youth at risk for psychosis. Early Intervention in Psychiatry. 2016;10:511–521. doi: 10.1111/eip.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Wood SJ, Yung AR. Measuring psychosocial outcome is good. Current Opinion in Psychiatry. 2013;26:138–143. doi: 10.1097/YCO.0b013e32835d82aa. [DOI] [PubMed] [Google Scholar]
- McGlashan T, Walsh BC, Woods SW. The psychosis risk syndrome: Handbook for diagnosis and follow-up. New York: Oxford University Press; 2010. [Google Scholar]
- Meier MH, Caspi A, Reichenberg A, Keefe RS, Fisher HL, Harrington H, … Moffitt TE. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: Evidence from a population-representative longitudinal study. American Journal of Psychiatry. 2014;171:91–101. doi: 10.1176/appi.ajp.2013.12111438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EC, Carrion RE, Cornblatt BA, Addington J, Cadenhead KS, Cannon TD … NAPLS Group. The relationship of neurocognition and negative symptoms to social and role functioning over time in individuals at clinical high risk in the first phase of the North American prodrome longitudinal study. Schizophrenia Bulletin. 2014;40:1452–1461. doi: 10.1093/schbul/sbt235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer SE, Bearden CE, Lux SR, Gordon JL, Johnson JK, O’Brien MP, … Cannon TD. The psychosis prodrome in adolescent patients viewed through the lens of DSM-IV. Journal of Child and Adolescent Psychopharmacology. 2005;15:434–451. doi: 10.1089/cap.2005.15.434. [DOI] [PubMed] [Google Scholar]
- Monte RC, Goulding SM, Compton MT. Premorbid functioning of patients with first-episode nonaffective psychosis: A comparison of deterioration in academic and social performance, and clinical correlates of premorbid adjustment scale scores. Schizophrenia Research. 2008;104:206–213. doi: 10.1016/j.schres.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, O’Callaghan E, Castle DJ, Lewis SW. A neurodevelopmental approach to the classification of schizophrenia. Schizophrenia Bulletin. 1992;18:319–332. doi: 10.1093/schbul/18.2.319. [DOI] [PubMed] [Google Scholar]
- Nelson B, Yuen HP, Wood SJ, Lin A, Spiliotacopoulos D, Bruxner A, … Yung AR. Long-term follow-up of a group at ultra high risk (“prodromal”) for psychosis: The PACE 400 Study. JAMA Psychiatry. 2013;70:793–802. doi: 10.1001/jamapsychiatry.2013.1270. [DOI] [PubMed] [Google Scholar]
- Okuzawa N, Kline E, Fuertes J, Negi S, Reeves G, Himelhoch S, Schiffman J. Psychotherapy for adolescents and young adults at high risk for psychosis: A systematic review. Early Intervention in Psychiatry. 2014;8:307–322. doi: 10.1111/eip.12129. [DOI] [PubMed] [Google Scholar]
- Phillips L. Case history data and prognosis in schizophrenia. Journal of Nervous and Mental Disease. 1953;117:515–525. doi: 10.1097/00005053-195306000-00004. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, … Moffitt TE. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: A 30-year study. American Journal of Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, van Os J, Esquivel G, Steinbusch HW, Rutten BP. An environmental analysis of genes associated with schizophrenia: Hypoxia and vascular factors as interacting elements in the neurodevelopmental model. Molecular Psychiatry. 2012;17:1194–1205. doi: 10.1038/mp.2011.183. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD … North American Prodrome Longitudinal Study (NAPLS) Group. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: Relationship to family history and conversion to psychosis. Archives of General Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Zecevic N. Schizophrenia: A tale of two critical periods for prefrontal cortical development. Translational Psychiatry. 2015;5:e623. doi: 10.1038/tp.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro DI, Marenco S, Spoor EH, Egan MF, Weinberger DR, Gold JM. The premorbid adjustment scale as a measure of developmental compromise in patients with schizophrenia and their healthy siblings. Schizophrenia Research. 2009;112:136–142. doi: 10.1016/j.schres.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata statistical software: Release 14 [Computer software] College Station, TX: Author; 2014. [Google Scholar]
- Stone WS, Mesholam-Gately RI, Giuliano AJ, Woodberry KA, Addington J, Bearden CE, … Seidman LJ. Healthy adolescent performance on the MATRICS consensus cognitive battery (MCCB): Developmental data from two samples of volunteers. Schizophrenia Research. 2016 doi: 10.1016/j.schres.2016.02.003. Advance online publication. S0920-9964(16)30059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Miski P, Buchanan RW, Kirkpatrick B, Carpenter WT., Jr Differential patterns of premorbid social and academic deterioration in deficit and nondeficit schizophrenia. Schizophrenia Research. 2012;135:134–138. doi: 10.1016/j.schres.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E, Millman ZB, Okuzawa N, Mittal V, DeVylder J, Skadberg T, … Schiffman J. Evidence-based early interventions for individuals at clinical high risk for psychosis: A review of treatment components. Journal of Nervous and Mental Disease. 2015;203:342–351. doi: 10.1097/NMD.0000000000000287. [DOI] [PubMed] [Google Scholar]
- van der Gaag M, Nieman DH, Rietdijk J, Dragt S, Ising HK, Klaassen RM, … Linszen DH. Cognitive behavioral therapy for subjects at ultrahigh risk for developing psychosis: A randomized controlled clinical trial. Schizophrenia Bulletin. 2012;38:1180–1188. doi: 10.1093/schbul/sbs105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gaag M, Smit F, Bechdolf A, French P, Linszen DH, Yung AR, … Cuijpers P. Preventing a first episode of psychosis: Meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophrenia Research. 2013;149:56–62. doi: 10.1016/j.schres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Velthorst E, Nieman DH, Becker HE, van de Fliert R, Dingemans PM, Klaassen R, … Linszen DH. Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophrenia Research. 2009;109:60–65. doi: 10.1016/j.schres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Velthorst E, Nieman DH, Linszen D, Becker H, de Haan L, Dingemans PM, … Ruhrmann S. Disability in people clinically at high risk of psychosis. British Journal of Psychiatry. 2010;197:278–284. doi: 10.1192/bjp.bp.109.075036. [DOI] [PubMed] [Google Scholar]
- Velthorst E, Reichenberg A, Kapara O, Goldberg S, Fromer M, Fruchter E, … Weiser M. Developmental trajectories of impaired community functioning in schizophrenia. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.2253. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale—Revised (WAIS-R) San Antonio, TX: Psychological Corp; 1981. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: Psychological Corp; 1991. [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of General Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]