Abstract
Large observational databases linking kidney function and other routine patient health data are increasingly being used to study acute kidney injury (AKI). Routine health care data show an apparent rise in the incidence of population AKI and an increase in acute dialysis. Studies also report an excess in mortality and adverse renal outcomes after AKI, although with variation depending on AKI severity, baseline, definition of renal recovery, and the time point during follow-up. However, differences in data capture, AKI awareness, monitoring, recognition, and clinical practice make comparisons between health care settings and periods difficult. In this review, we describe the growing role of large databases in determining the incidence and prognosis of AKI and evaluating initiatives to improve the quality of care in AKI. Using examples, we illustrate this use of routinely collected health data and discuss the strengths, limitations, and implications for researchers and clinicians.
Key Words: Acute kidney injury, Incidence, Prognosis, Big-data, Quality improvement
Clinical Summary.
-
•
Large database studies show that AKI pervades health care systems, with poor outcomes and an increasing use of renal replacement therapy.
-
•
Risk stratification based on acute kidney injury (AKI) severity, baseline, and recovery to baseline and follow-up time may be helpful to improve post-AKI care, but such an approach would need to be evaluated.
-
•
Temporal and regional comparisons of AKI incidence and prognosis may be biased by changes in AKI awareness, monitoring, recognition, recording, and clinical practice.
-
•
Researchers evaluating quality initiatives using observational data should be careful to capture all who may be affected by policy changes, to evaluate both intended and unintended consequences, and to recognize the limitations of potential confounding.
Introduction—Why Use Large Databases to Study Acute Kidney Injury?
…if you are going to teach people to make observations, you should show that something wonderful can come from them…
–Richard Feynman, nobel laureate1
For over a century, epidemiologists have exploited routinely collected health data to observe the causes and natural history of human diseases; 150 years ago, William Farr pioneered the collection of vital statistics and leveraged them for insight into the cholera epidemic in England. The same approach now forms the basis of the International Classification of Diseases (ICD) used by health systems worldwide.2 In Sweden, for example, data contained within each of 90 national registries can be linked by unique personal identity numbers for use in medical research. Swedish data have been instrumental for developing innovative medical devices, monitoring policy, and improving care quality.3 Well-conducted large observational studies using routinely collected health data are crucial in health research in the current era of big data and technologic innovation.4 Large observational studies are more capable than trials of determining the true burden of a disease in the “real world.” This makes them valuable resources for evaluating disease incidence, prevalence, prognosis, and responses to policy changes. With the increasing financial pressure to design trials efficiently, routine electronic records also show promise for rapid case finding and inexpensive follow-up.5 However, observational evidence from routinely collected data also comes with caveats. There may be unaccounted confounding and results can mislead if not interpreted carefully.6 In this review, we describe recent large database studies that have furthered understanding of acute kidney injury (AKI) and discuss the advantages and drawbacks of using routine health data in observational research. We focus on 3 themes that are topical in AKI: the apparent increasing incidence of AKI, the long-term prognosis of AKI, and the role of large databases in AKI care quality.
AKI—The Growing Awareness of a Global Burden
AKI is common (8-16% of hospital admissions7), serious (fourfold increased hospital mortality8), and many aspects of its natural history remain uncertain.9, 10 In the United Kingdom, >1% of health service expenditure is attributed to AKI.11 Over 20 years, studies of AKI have moved from detailed descriptions of small but well-characterized cohorts12 to large-scale analyses involving thousands of people classified using ICD hospital episode codes.13, 14 Also in the last decade, the former term “acute renal failure” has been replaced with standardized AKI criteria incorporating small changes in creatinine and urine output, such as the “Acute Kidney Injury Network” (AKIN)15 and “Kidney Disease: Improving Global Outcomes” (KDIGO) criteria.16 In many health systems, kidney function data are increasingly available either in integrated electronic health records or through data linkage. In high-income countries, these developments have led to recognition that even small changes in creatinine carry an adverse prognosis8 and that some poor outcomes after AKI are preventable.17 The International Society of Nephrology 0by25 initiative has an ambitious aim to prevent all avoidable death from AKI worldwide by 2025.18 It carried out a global study over 10 weeks in 2014 to capture a snapshot of the burden of KDIGO-based AKI across 289 centers and 72 countries.19 Seven-day mortality in this select snapshot was 10-12% in both high- and low-income countries. Key etiologic risk factors varied between countries but included dehydration, shock, infection, sepsis, cardiac disease, and nephrotoxic medications. The study showcased the potential for global data collection to inform international strategy.
The Population Incidence of AKI
Numerous studies have exploited ICD coding of hospital episodes to quantify the incidence of AKI across a range of clinical settings (Table 1). They consistently report a rising incidence of AKI over time, whether requiring or not requiring renal replacement therapy (RRT).45 If real, this represents a major global public health concern, but it is hard to interpret these findings amid changes in AKI definitions, awareness of AKI, and clinical practice.45
Table 1.
Summary of Studies that Have Described Temporal Trends in the Incidence of AKI
| Author | Time Period | AKI Definition | Country | Data Source | Clinical Setting | Reported Change in Population AKI Incidence |
|---|---|---|---|---|---|---|
| Xue et al (2006)20 | 1992-2001 | ICD-9 AKI | United States | Medicare | All hospitalizations | Increase from 15 to 36 cases per 1000 hospitalizations |
| Waikar et al (2006)21 | 1988-2002 | AKI-D codes ICD-9 AKI |
United States | US sample | All hospitalizations | Increase in incidence of AKI-D from 40 to 270 pmpyr Increase in incidence of AKI from 61 to 288 pmpyr |
| Bagshaw et al (2007)22 | 1996-2005 | Creatinine and urine criteria | Australia and New Zealand | National Intensive Care Database | Intensive care admissions | Increase in incidence of AKI (4.8% vs 5.6%) |
| Swaminathan et al (2007)23 | 1988-2003 | AKI-D codes ICD-9 AKI |
United States | US sample | Cardiopulmonary bypass | Increase in age-sex-morbidity adjusted incidence of AKI-D from 0.33% to 0.35% Increase in age-sex-morbidity adjusted incidence of AKI from 1.1% to 4.1% |
| Hsu et al (2007)24 | 1996-2003 | AKI-D codes Creatinine change criteria |
United States | Kaiser Permanente | All hospitalizations | Increase in incidence of AKI-D from 195 to 295 pmpyr Increase in incidence of AKI from 3227 to 5224 pmpyr |
| Thakar et al (2007)25 | 1993-2002 | AKI-D Creatinine change criteria |
United States | Cleveland Clinic Foundation | Cardiac surgery | Increase in incidence of AKI-D from 1.5% to 2.0% Increase in incidence of AKI from 5.1% to 6.6% |
| Liu et al (2010)26 | 2003-2007 | AKI-D codes ICD-10 AKI |
Canada | National Discharge Abstract Database | All hospital obstetric deliveries | No change in incidence of AKI-D (40 per million deliveries) Increase in incidence of AKI from 160 to 230 per million deliveries |
| Callaghan et al (2012)27 | 1998-2009 | ICD-9 AKI | United States | US sample | All hospital obstetric deliveries | Increase in incidence of AKI from 229 to 452 per million deliveries |
| Amin et al (2012)28 | 2000-2008 | AKIN creatinine change criteria | United States | Cerner Corporation Health Facts database | Acute myocardial infarction | Decrease in incidence of AKI from 26.6% to 19.7% |
| Siddiqui et al (2012)29 | 1995-2009 | AKI-D codes | Canada | Ontario Provincial Database | All major elective surgery | Increase in incidence from 0.2% to 0.6% |
| Lenihan et al (2013)30 | 1999-2008 | AKI-D codes ICD-9 AKI |
United States | US sample | Cardiac surgery | Increase in incidence of AKI-D from 0.45% to 1.28% Increase in incidence of AKI from 4.5% to 12.8% |
| Hsu et al (2013)31 | 2000-2009 | AKI-D codes | United States | US sample | All hospitalizations | Increase from 222 to 533 pmpyr |
| Khera et al (2013)32 | 2002-2010 | AKI-D codes ICD-9 AKI |
United States | US sample | Cardiac catheterization | Decrease in age-sex-morbidity adjusted incidence of AKI-D from 0.6% to 0.4% Increase in age-sex-morbidity adjusted incidence of AKI from 5.6% to 14.2% |
| Mehrabadi et al (2014)33 | 2003-2010 | ICD-10 AKI | Canada | National Discharge Abstract Database | All hospital obstetric deliveries | Increase in incidence of AKI from 166 to 268 per million deliveries |
| Sakhuja et al (2015)34 | 2000-2009 | AKI-D codes | United States | US sample | Severe sepsis | Increase in incidence of AKI-D from 5.2% to 6.6% |
| Kolhe et al (2015)35 | 1998-2013 | AKI-D codes | United Kingdom | NHS England | All hospitalizations | Increase from 15.9 to 208.7 pmpyr |
| Nadkarni et al (2015)36 | 2002-2011 | AKI-D codes | United States | US sample | Stroke | Increase in incidence of AKI-D from 0.09% to 0.18% |
| Nadkarni et al (2015)37 | 2002-2010 | AKI-D codes | United States | US sample | Adults with HIV | Increase in incidence of AKI-D from 0.7% to 1.35% |
| Nadkarni et al (2016)38 | 2006-2012 | AKI-D codes | United States | US sample | Decompensated cirrhosis | Increase in incidence of AKI-D from 1.5% to 2.23% |
| Nadkarni et al (2016)39 | 2004-2012 | AKI-D codes | United States | US sample | Adults with hepatitis C | Increase in incidence of AKI-D from 0.86% to 1.28% |
| Nadkarni 201640 | 2002-2012 | ICD-9 AKI | United States | US sample | Orthopedic surgery | Increase in the incidence of AKI from 0.5% to 1.8% |
| Hsu 201641 | 2007-2009 | AKI-D codes | United States | US | All hospitalizations | Increase in incidence of AKI-D by 11% per year |
| Kolhe et al (2016)42 | 1998-2013 | ICD-10 AKI | United Kingdom | NHS England | All hospitalizations | Increase from 317 to 3995 pmpyr |
| Brown et al (2016)43 | 2001-2011 | AKI-D codes ICD-9 AKI |
United States | US sample | Cardiac catheterization | Increase in incidence of AKI-D from 16 to 30 pmp Increase in incidence of AKI from 155 to 416 pmp |
| Carlson et al (2016)44 | 2000-2012 | AKI-D codes | Denmark | National registry | All hospitalizations | Increase in incidence of AKI-D from 143 to 366 pmpyr |
| Sawhney 2017 (this article) | 2001-2014 | KDIGO AKI criteria ICD-10 AKI |
United Kingdom | Regional population cohort | Whole population | Increase in incidence of KDIGO AKI from 11,269 to 12,923 pmpyr Increase in incidence of ICD-10 AKI from 663 to 2647 pmpyr |
Abbreviations: AKI-D, dialysis-requiring AKI; ICD, International Classification of Diseases.
AKI Rates Using ICD Coding
Over a combined period spanning 25 years, rates of hospital episode coding of non–dialysis-requiring AKI have increased in the United States,20, 21, 32, 40 Canada,26, 33 and United Kingdom.42 Studies from general hospital settings also report a concurrent decline in AKI mortality.20, 21, 42 However, wide between-study variation in AKI rates also exist (Table 1). An important limitation is that increased coding of AKI may reflect changes in coding practice more generally, changing diagnostic criteria (eg, RIFLE, AKIN, KDIGO) or increased awareness of AKI rather than a true change in disease incidence. Increased recognition of milder AKI may also explain the rising incidence in the context of falling mortality. However, it seems unlikely that an up to 10-fold increase in AKI incidence is attributable purely to changes in ICD coding.21
Similar increased rates of dialysis-requiring AKI (AKI-D) have also been reported over the last 25 years across America and Europe in settings including general hospital admissions,21, 24, 31, 35, 41, 44 elective surgery,29 cardiac surgery,23, 25, 30 coronary interventions,43 infectious diseases,37, 39 liver disease,38 obstetrics,26, 27, 33 sepsis,34 and stroke.36 Many of these studies also showed a declining mortality over time but not consistently.35 The rise in acute RRT across countries and health care settings is striking.45 The reasons are unclear but could include increased availability of RRT (eg, for the frail), increased recording of RRT use, financial incentives (eg, rising incidence of billing for RRT in the United States), or changing trends in medical practice (eg, a lower threshold for starting RRT). Taxation-based health care systems, such as in Denmark and the United Kingdom, have also seen increased AKI-related RRT, suggesting that financial incentives are unlikely to be the sole explanation.35, 44
AKI Rates Using Creatinine Change Criteria
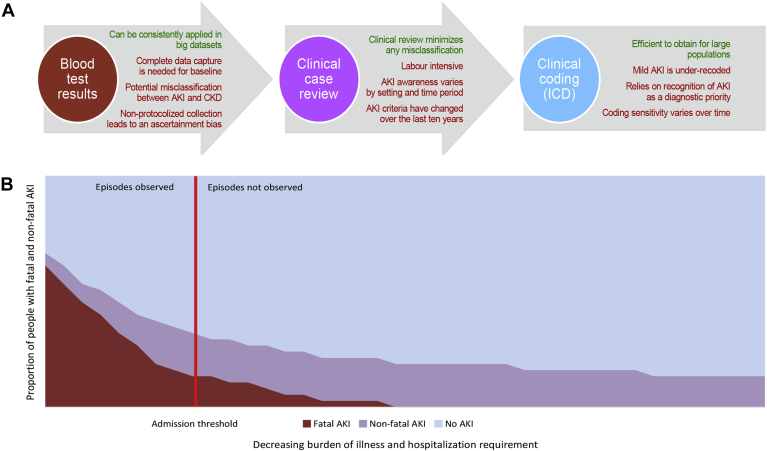
The changing rates of code-classified AKI have been extensively reported, but there is a paucity of literature reporting trends in the incidence of AKI using creatinine change criteria.22, 24, 25, 28 Only 1 study has used KDIGO/AKIN–based criteria28 and only 1 has incorporated urine output (in an intensive care setting).22 Major strengths of creatinine change criteria are that they can be applied retrospectively in big data sets, capture a larger subset of AKI than that which is clinically recognized and coded,46, 47, 48 and enable a more consistent case ascertainment over time than is possible using ICD coding. In comparison, coding of AKI is dependent on clinical recognition and diagnosis, which in turn is dependent on a blood result suggesting AKI (Fig 1A). For this reason, KDIGO-based AKI criteria provide a much higher estimated incidence (10-fold) of AKI than code-classified AKI.48, 49
Figure 1.
Methodologic challenges in AKI epidemiology. (A) Approaches to studying AKI using observational data and their advantages and disadvantages. (B) Bias that may arise because of convenience sampling of those admitted to hospital. In this scenario, of 1000 people in the population, 250 people had AKI (25% population incidence) including 93 who died (37% fatality). If only people above the threshold are observable, 113 people have observed AKI (11% estimated population incidence) including 80 observed deaths (71% fatality). If the admission threshold changes (eg, with a new policy), this would affect both the incidence and fatality of hospital AKI. Abbreviations: AKI, acute kidney injury; ICD, International Classification of Diseases.
Nevertheless, creatinine change AKI incidence studies also have limitations, primarily relating to definitions of AKI and “baseline creatinine” (Fig 1A). Over the last decade, consensus AKI criteria have been updated 3 times.15, 16, 50 As each new study adopts the latest criteria, it is difficult to compare different studies, even if the definition within each study is applied consistently. Moreover, a consistently applied KDIGO-based AKI definition can only provide a representative estimate of population incidence if capture of the whole at-risk population is complete. For example, until recently, AKI that occurred and remained in the community (without admission) was under recognized.51 An increase in AKI observed in hospital may, therefore, reflect greater awareness and a lower threshold for hospital admission among people with AKI who were not previously admitted.51, 52 As this subgroup also have a substantially better prognosis,52 admission thresholds for community AKI confound many hospital AKI studies that lack complete capture of biochemistry from all clinical settings (Fig 1B). In addition, AKI can only be identified when previous (baseline) tests are available for comparison, but blood testing is clinically driven, either in acute or chronic disease management. This means that a baseline creatinine value often needs to be estimated from those previous blood tests that are available, introducing an inherent selection bias.53 If previous tests are infrequent and distant, then bidirectional misclassification between progressive CKD and AKI may result. Variation in the intensity of blood sampling can also introduce an ascertainment bias as more tests are generally done on sicker patients or those in a more monitored setting.52 Finally, if multiple tests are performed in quick succession, Lin and colleagues54 demonstrated through simulation that fluctuations in creatinine because of sampling variation can be sufficient to lead to a false-positive diagnosis of AKI.
Notwithstanding these limitations, 3 studies with creatinine-based definitions of AKI have reported a rising incidence of AKI in the US databases from Kaiser Permanente,24 Cleveland Clinic,25 and a national intensive care database covering Australia and New Zealand.22 These changes in AKI incidence were smaller (<2-fold) than in studies of code-classified AKI. In addition, 1 US study reporting AKI based on AKIN criteria after myocardial infarction actually reported fall in AKI incidence and mortality,28 which was attributed to improved clinician awareness, care and prevention of AKI, and may also relate to case selection.
AKI Rates Using ICD-10 Coding and KDIGO-Based Criteria Simultaneously
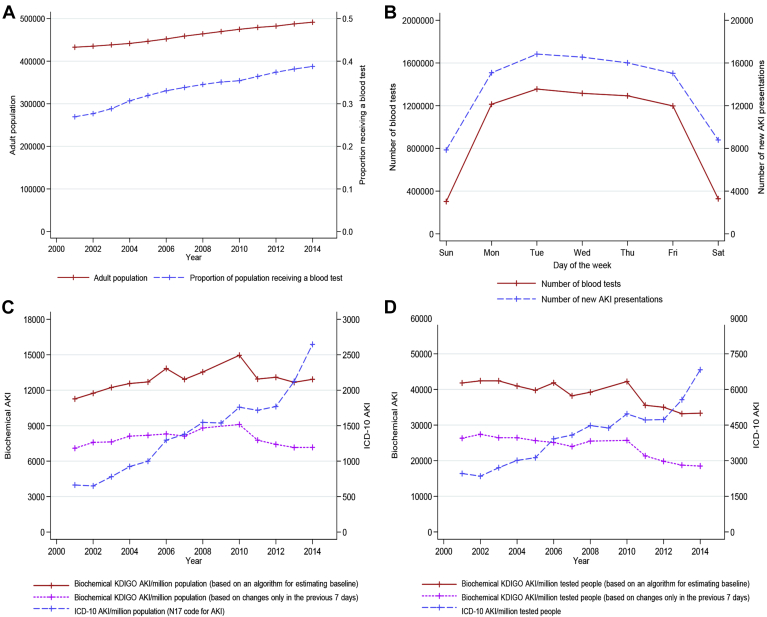
Although studies suggest that AKI and RRT use are increasing, the limitations of ICD coding accuracy and biochemistry testing intensity mean that true trends in population AKI remain hard to interpret. To illustrate the influence of these methodologic issues with AKI identification, in Figure 2A-D, we have described the incidence of AKI using all biochemistry in the Grampian (UK) population (n = 500,000). We have contrasted ICD-10 code-classified AKI (N17), KDIGO-based AKI (first, estimating baseline as described by Sawhney and colleagues7 and second, estimating baseline only using blood tests strictly taken in the past week), and the intensity of biochemistry sampling. Those already receiving long-term RRT were excluded as previously described.7 Although the Grampian population has grown slowly over 14 years, there has been a disproportionate increase in the intensity of biochemical testing; 39% of the population now receive at least 1 blood test/year (Fig 2A) compared with 27% in 2001. In addition, although KDIGO-AKI has increased slowly 2001-2014 (from 11,269 to 12,923 per million population/year, pmpyr), code-classified AKI has quadrupled over the same period (from 663 to 2647 pmpyr) (Fig 2C). If AKI were reported as a ratio of cases to the number of tested individuals at risk, the rate of KDIGO AKI could actually be considered to have fallen (from 41,816 to 33,337/million people receiving blood tests) (Fig 2D). Ascertainment bias because of testing intensity is also suggested by a crude comparison of the day of the week on which AKI episodes initially present vs number of tests performed on each day (Fig 2B).
Figure 2.
Study of the incidence of AKI in the Grampian population 2001-2014. (A) Growth of Grampian population (red solid) and increase in the proportion of people receiving a blood test (blue dash). (B) Association between testing intensity and the incidence of new AKI presentations by day of the week 2001-2014. (C) Rates of KDIGO-AKI using creatinine change criteria (red solid and pink dot) and ICD-10 code-classified AKI (blue dash). (D) AKI incidence represented as a proportion of the tested population at risk. Abbreviations: AKI, acute kidney injury; ICD-10, International Classification of Diseases, Tenth Revision; KDIGO-AKI, Kidney Disease: Improving Global Outcomes.
The Long-term Prognosis of AKI
An association between AKI and poor long-term outcomes is supported by evidence from systematic reviews of over 50 studies linking AKI with increased mortality, CKD, and long-term RRT.55, 56, 57 Accordingly, KDIGO AKI guidelines advocate follow-up of patients after hospital discharge.16 Biologic plausibility is also supported by basic science models58 and evidence from extended follow-up of kidney transplant donors revealing increased long-term RRT requirement.59 However, given the high incidence of hospital AKI and the fact that only a minority see a nephrologist after discharge,60 there is a need to help clinicians prioritize those with the greatest risk and the most modifiable risk of poor outcomes. Large observational studies can inform this clinical need because subgroup analyses are possible with adequate power. Several large population and hospital-based cohort studies from Canada, the US Veteran Affairs (VA) health system, and the UK National Health Service have evaluated outcomes of AKI within subgroups of baseline kidney function, AKI severity, renal recovery, and follow-up time.
AKI Outcomes Grouped by Baseline CKD and Follow-up Time
In 864,933 VA patients including 82,711 meeting AKIN criteria for AKI, Lafrance and colleagues61 reported AKI mortality in baseline subgroups over 2.4 years. AKI mortality hazard ratios (HRs) were greater among those with a higher baseline estimated glomerular filtration rate (eGFR) than a lower baseline eGFR: HRs 1.45, 1.35, 1.31, and 1.23 for eGFR ≥90, 60-89, 45-59, and 30-44 mL/min/1.73 m2, respectively. In 29,388 separate VA individuals undergoing cardiac surgery, Ishani and others grouped patients according to the severity of postoperative creatinine rise (≤0%, 1-24%, 25-49%, 50-99%, and ≥100%) and in time intervals at risk to determine if mortality, new CKD, and long-term RRT varied over follow-up time. This concept of a varying excess risk following an exposure is familiar in renal transplantation research,62 where an insult (eg, transplant procedure) may initially be detrimental before later yielding benefit, but the application in AKI was novel. The investigators found outcomes were poorer among those with greater postoperative creatinine increase, but even small rises in creatinine were associated with poor renal outcomes. Although the excess risk of AKI was greatest over the first year after hospitalization, it persisted up to 5 years.63 A limitation was that this study was limited to AKI in a cardiac setting, which may not be generalizable. In addition, both studies were conducted in cohorts of older US veterans with few women.
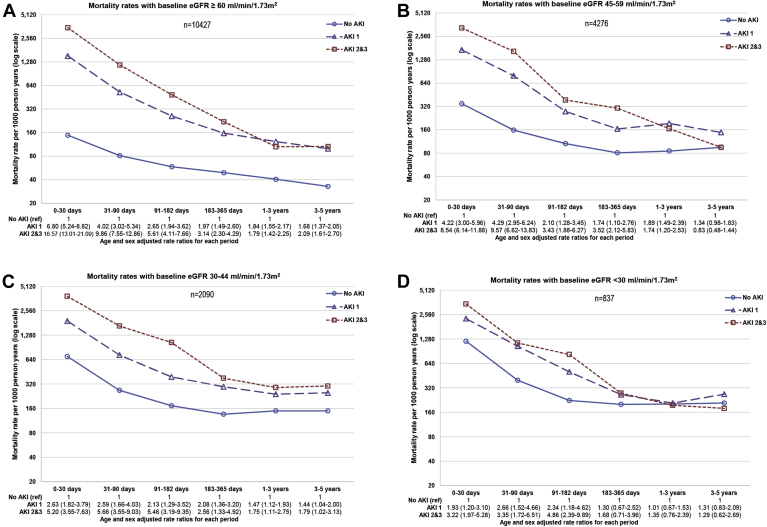
The concurrent interactions of baseline CKD and follow-up time on the relationship between AKI and death were recently tested together in a single general population study of 17,630 hospital admissions with and without AKI in the UK.7 The authors separated outcomes by baseline eGFR, AKI severity, and short (0-30 days), intermediate (31-364 days), and long-term (1-10 years) time periods for those at risk. Consistent with the 2 previous Veteran Affairs studies, over 10-year follow-up mortality was greatest among those with AKI and severe baseline CKD (eGFR <30 mL/min/1.73 m2), and the excess risk from AKI was greater among those with a higher baseline eGFR than with a lower baseline eGFR (who were already at elevated risk irrespective of AKI) (Fig 3A-D). Excess risk of AKI and AKI severity also diminished over time, such that for patients with baseline eGFR <30 mL/min/1.73 m2, even a severe AKI episode no longer conferred additional mortality risk among people who had already survived 1 year. This study also identified previous AKI episodes as an additional adverse prognostic factor in those with AKI (37% greater long-term mortality). However, the primary outcome was mortality and progression of CKD not reported.7
Figure 3.
Mortality rates and age- and sex-adjusted rate ratios by (A-D) baseline eGFR group and acute kidney injury (AKI; 1-3 denote severity stage). Abbreviations: AKI, acute kidney injury; ref, reference group; eGFR, estimated glomerular filtration rate. Note the log scale: each increment on the y axis represents a doubling of mortality rates.
Modified from Sawhney et al.,7http://dx.doi.org/10.1053/j.ajkd.2016.05.018 under the terms of the Creative Commons Attribution License (CC BY).
AKI Outcomes Grouped by Renal Recovery
Further large database subgroup analyses of AKI outcomes have focused on renal recovery to baseline as a stratifying risk factor. In a population study of hospitalizations in Alberta, Pannu and others64 compared people with AKI who subsequently recovered to within 25% of baseline and people with AKI who did not recover. Over a median 34 months, those who did not recover had increased mortality (adjusted HR 1.26, 95% confidence interval [CI] 1.10-1.43) and renal outcomes (HR 4.13, CI 3.38-5.04). A feature of this study was a detailed sensitivity analysis of different thresholds for defining renal recovery, with the worst outcomes among those with little or no recovery. A further US single-center study propensity matched 1610 people hospitalized with AKI who recovered to within 90% of baseline (vs hospitalization without AKI). Over a median 3.3 years, AKI patients with near-complete recovery still had increased mortality (HR 1.5, CI 1.21-1.85) and de novo CKD (HR 1.91, 1.75-2.09).65 However, this was a single-center study and findings may not be generalizable. The authors also did not account for changes in the HR over follow-up time.
Implications and Limitations
Collectively, these studies provide evidence of greater mortality and adverse renal outcomes after AKI, with baseline eGFR, previous episodes, renal recovery extent, and follow-up time as key factors on which to further risk stratify and ensure those whose risk of poor outcomes have increased are sufficiently monitored. However, the consequences of new models of care should be evaluated, and we note that a dedicated post-AKI clinic is currently under evaluation in Canada.66 The greater excess risk of AKI among those with normal baseline kidney function is perhaps counterintuitive, but those who have baseline CKD are already at high risk and may share many confounding risk factors (measurable and unmeasurable) that predispose toward AKI, CKD, and mortality.
For the clinician, the main implication is that any form of renal impairment, acute or chronic, is associated with poorer outcomes, but the excess of poor outcomes after AKI are observed mainly in the first year after hospitalization. Whereas early reassessment could be guided by the severity of the acute illness and extent of recovery, long-term monitoring could be guided by chronic factors, such as new or worsening chronic kidney disease.
These observational studies using creatinine change criteria also have important limitations. Causality cannot necessarily be inferred from these observational studies. Even high-quality cohort studies are subject to risk of bias and confounding, and causality is, therefore, contested.9, 10 On the other hand, many relevant randomized trial designs would be unethical. Moreover, bidirectional misclassification of AKI and CKD due to incomplete baseline data means that some renal outcomes attributed to AKI may actually represent rapidly progressing CKD. Variation in blood testing intensity across clinical settings and time will influence the extent of CKD misclassification and the extent of this bias. Similarly, as previously described, variation in data capture of people with AKI in the community will skew the impression of the overall burden and distribution of AKI. Those who meet KDIGO AKI criteria and are not promptly admitted to hospital have a substantially better short-term prognosis, which means that failure to account for this subgroup will adversely affect overall AKI prognosis and incidence estimates.52 Admission thresholds may also vary between health care settings and over time. Convenience sampling in AKI studies may lead to bias, with a focus on severe, high-mortality AKI, and under-ascertainment of milder AKI cases with a low mortality. As a result, the full population burden is underestimated, and the case fatality rate is overestimated (Fig 1B). Notably, similar sampling bias can also occur in studies limited to a critical care setting or restricted to code-classified AKI. Finally, studies of renal recovery should also be interpreted carefully due to the effect of critical illness on muscle mass and accordingly creatinine assays. A study of 700 National Health Service intensive care survivors reported discharge creatinine values below baseline for those without AKI. Adjusting for this confounding effect of major illness led to an estimated 135% increase in post-discharge CKD diagnoses.67
The Role of Large Databases and AKI in Care Quality
Following a UK enquiry into patient outcome and death (National Confidential Enquiry into Patient Outcome and Death) from AKI, there was international recognition of the need to improve care.17, 68 Given the poor outcomes after AKI across all clinical settings,8 interest has focused on interventions that may improve recognition and early management. Initiatives include AKI e-alerts and care bundles which are covered in a later article within this issue. The “Tackling Acute Kidney Injury” trial is notable because it will use data linkage to follow-up outcomes in 5 participating hospitals.69 However, other approaches to quality improvement use large databases to monitor policy changes and initiatives. This is illustrated by 2 quazi-experimental studies using interrupted time-series analysis.70, 71
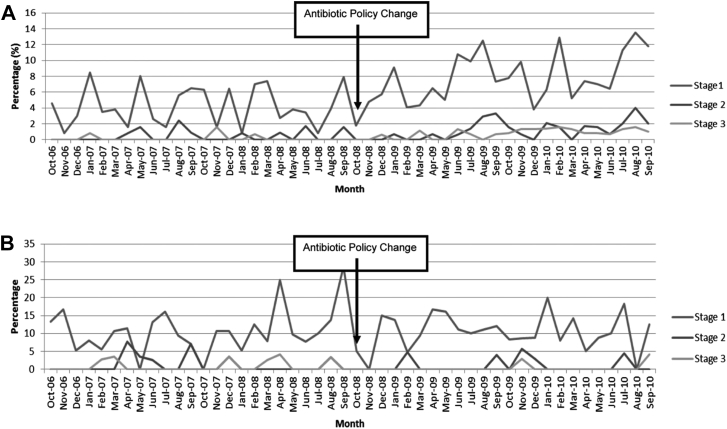
Bell and colleagues monitored the consequences of a regional policy change from co-amoxiclav to gentamicin prophylaxis for non-neck of femur (non-NOF) orthopedic procedures as part of a target to reduce Clostridium difficile in Scotland. They assessed the rates of C. difficile (an intended consequence) and of AKI (an unintended consequence). NOF procedures were a control group where policy did not change.
The gentamicin policy did not reduce C. difficile rates but caused an unintended 94% increase in KDIGO AKI in non-NOF procedures (Fig 4A) compared with no change in NOF procedures (Fig 4B).70 This increase in AKI subsequently reduced 63% when the policy was reversed.71 This demonstrates the power of routine data for monitoring intended and unintended consequences of health care changes and inform new policy.
Figure 4.
Percentage of people undergoing surgery who developed postoperative AKI stages 1, 2, and 3: (A) following a gentamicin policy change among people undergoing orthopedic surgery (excluding NOF). (B) People undergoing surgery of an NOF fracture (for whom the policy change did not involve gentamicin). Abbreviations: AKI, acute kidney injury; NOF, non-neck of femur.
Reproduced from Bell et al.,70http://dx.doi.org/10.1681/ASN.2014010035, with permission from the authors and publisher.
Conclusion
Large database studies show that AKI pervades health care systems worldwide, with poor outcomes and an increasing use of RRT. Given the high incidence of AKI hospital survivors, prognostic risk stratification on AKI severity, baseline, recovery to baseline, and follow-up time, may be helpful to improve post-AKI care, but such an approach would need careful evaluation. Temporal and regional comparisons of AKI incidence and prognosis may be biased by changes in AKI awareness, monitoring, recognition, and clinical practice. For this reason, researchers monitoring quality initiatives using observational data should be careful to capture all who may be affected by policy changes and ensure that both intended and unintended consequences are recognized.
Acknowledgments
S.S. is supported by a Clinical Research Training Fellowship from the Wellcome Trust (Ref 102729/Z/13/Z). We also acknowledge the support from The Farr Institute of Health Informatics Research. The Farr Institute is supported by a 10-funder consortium: Arthritis Research UK, the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, the Engineering and Physical Sciences Research Council, the Medical Research Council, the National Institute of Health Research, the National Institute for Social Care and Health Research (Welsh Assembly government), the Chief Scientist Office (Scottish government Health Directorates), and the Wellcome Trust (MRC grant nos: Scotland MR/K007017/1). The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Feynman R. What is science? Phys Teach. 1969;7(6):313–320. [Google Scholar]
- 2.Lilienfeld D. Celebration: William Farr (1807–1883)—an appreciation on the 200th anniversary of his birth. Int J Epidemiol. 2007;36(5):985–987. [Google Scholar]
- 3.Webster P.C. Sweden's health data goldmine. Can Med Assoc J. 2014;186(9):E310. doi: 10.1503/cmaj.109-4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagshaw S.M., Goldstein S.L., Ronco C., Kellum J.A. Acute kidney injury in the era of big data: the 15th consensus conference of the Acute Dialysis Quality Initiative (ADQI) Can J Kidney Health Dis. 2016;3(1):1–5. doi: 10.1186/s40697-016-0103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coorevits P., Sundgren M., Klein G.O. Electronic health records: new opportunities for clinical research. J Intern Med. 2013;274(6):547–560. doi: 10.1111/joim.12119. [DOI] [PubMed] [Google Scholar]
- 6.Sackett D.L., Rosenberg W.M.C., Gray J.A.M., Haynes R.B., Richardson W.S. Evidence based medicine: what it is and what it isn't. BMJ. 1996;312(7023):71. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawhney S., Marks A., Fluck N., Levin A., Prescott G.J., Black C. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: a large population-based cohort study. Am J Kidney Dis. 2017;69(1):18–28. doi: 10.1053/j.ajkd.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chertow G.M., Burdick E., Honour M., Bonventre J.V., Bates D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 9.Rifkin D.E., Coca S.G., Kalantar-Zadeh K. Does AKI truly lead to CKD? J Am Soc Nephrol. 2012;23(6):979–984. doi: 10.1681/ASN.2011121185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu C. Yes, AKI truly leads to CKD. J Am Soc Nephrol. 2012;23(6):967–969. doi: 10.1681/ASN.2012030222. [DOI] [PubMed] [Google Scholar]
- 11.Kerr M., Bedford M., Matthews B., O'Donoghue D. The economic impact of acute kidney injury in England. Nephrol Dial Transplant. 2014;29(7):1362–1368. doi: 10.1093/ndt/gfu016. [DOI] [PubMed] [Google Scholar]
- 12.Liano F., Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50(3):811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 13.Wald R., Quinn R.R., Luo J., University of Toronto Acute Kidney Injury Research, Group Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 14.James M.T., Hemmelgarn B.R., Wiebe N., Alberta Kidney Disease Network Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376(9758):2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 15.Mehta R.L., Kellum J.A., Shah S.V., Acute Kidney Injury Network Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Inter Suppl. 2012;2:1–138. [Google Scholar]
- 17.Stewart J., Findlay G., Smith N., Kelly K., Mason M. National Confidential Enquiry into Patient Outcome and Death; London: 2009. Adding Insult to Injury: a Review of the Care of Patients Who Died in Hospital with a Primary Diagnosis of Acute Kidney Injury. [Google Scholar]
- 18.Mehta R.L., Cerda J., Burdmann E.A. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385(9987):2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 19.Mehta R.L., Burdmann E.A., Cerdá J. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387(10032):2017–2025. doi: 10.1016/S0140-6736(16)30240-9. [DOI] [PubMed] [Google Scholar]
- 20.Xue J.L., Daniels F., Star R.A. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 21.Waikar S.S., Curhan G.C., Wald R., McCarthy E.P., Chertow G.M. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17(4):1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 22.Bagshaw S.M., George C., Bellomo R. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11:R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swaminathan M., Shaw A.D., Phillips-Bute B.G. Trends in acute renal failure associated with coronary artery bypass graft surgery in the United States. Crit Care Med. 2007;35(10):2286–2291. doi: 10.1097/01.ccm.0000282079.05994.57. [DOI] [PubMed] [Google Scholar]
- 24.Hsu C., McCulloch C., Fan D., Ordonez J., Chertow G., Go A. Community-based incidence of acute renal failure. Kidney Int. 2007;72(2):208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakar C.V., Worley S., Arrigain S., Yared J., Paganini E.P. Improved survival in acute kidney injury after cardiac surgery. Am J Kidney Dis. 2007;50(5):703–711. doi: 10.1053/j.ajkd.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Liu S., Joseph K.S., Bartholomew S. Temporal trends and regional variations in severe maternal morbidity in Canada, 2003 to 2007. J Obstetrics Gynaecol Can. 2010;32(9):847–855. doi: 10.1016/S1701-2163(16)34656-4. [DOI] [PubMed] [Google Scholar]
- 27.Callaghan W.M., Creanga A.A., Kuklina E.V. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstetrics Gynecol. 2012;120(5):1029–1036. doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 28.Amin A.P., Salisbury A.C., McCullough P.A. Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Arch Intern Med. 2012;172(3):246–253. doi: 10.1001/archinternmed.2011.1202. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui N.F., Coca S.G., Devereaux P.J. Secular trends in acute dialysis after elective major surgery—1995 to 2009. Can Med Assoc J. 2012;184(11):1237–1245. doi: 10.1503/cmaj.110895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenihan C.R., Montez-Rath M.E., Mora Mangano C.T., Chertow G.M., Winkelmayer W.C. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20–28. doi: 10.1016/j.athoracsur.2012.05.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu R.K., McCulloch C.E., Dudley R.A., Lo L.J., Hsu C. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37–42. doi: 10.1681/ASN.2012080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khera S., Kolte D., Aronow W.S. Trends in acute kidney injury and outcomes after early percutaneous coronary intervention in patients ≥75 years of age with acute myocardial infarction. Am J Cardiol. 2013;112(9):1279–1286. doi: 10.1016/j.amjcard.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Mehrabadi A., Liu S., Bartholomew S. Hypertensive disorders of pregnancy and the recent increase in obstetric acute renal failure in Canada: population based retrospective cohort study. BMJ. 2014;349:g4731. doi: 10.1136/bmj.g4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakhuja A., Kumar G., Gupta S., Mittal T., Taneja A., Nanchal R.S. Acute kidney injury requiring dialysis in severe sepsis. Am J Respir Crit Care Med. 2015;192(8):951–957. doi: 10.1164/rccm.201502-0329OC. [DOI] [PubMed] [Google Scholar]
- 35.Kolhe N.V., Muirhead A.W., Wilkes S.R., Fluck R.J., Taal M.W. National trends in acute kidney injury requiring dialysis in England between 1998 and 2013. Kidney Int. 2015;88(5):1161–1169. doi: 10.1038/ki.2015.234. [DOI] [PubMed] [Google Scholar]
- 36.Nadkarni G.N., Patel A.A., Konstantinidis I. Dialysis requiring acute kidney injury in acute cerebrovascular accident hospitalizations. Stroke. 2015;46(11):3226. doi: 10.1161/STROKEAHA.115.010985. [DOI] [PubMed] [Google Scholar]
- 37.Nadkarni G.N., Patel A.A., Yacoub R. The burden of dialysis-requiring acute kidney injury among hospitalized adults with HIV infection: a nationwide inpatient sample analysis. AIDS. 2015;29(9):1061–1066. doi: 10.1097/QAD.0000000000000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadkarni G.N., Simoes P.K., Patel A. National trends of acute kidney injury requiring dialysis in decompensated cirrhosis hospitalizations in the United States. Hepatol Int. 2016;10(3):525–531. doi: 10.1007/s12072-016-9706-9. [DOI] [PubMed] [Google Scholar]
- 39.Nadkarni G.N., Patel A., Simoes P.K. Dialysis-requiring acute kidney injury among hospitalized adults with documented hepatitis C virus infection: a nationwide inpatient sample analysis. J Viral Hepat. 2016;23(1):32–38. doi: 10.1111/jvh.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadkarni G.N., Patel A.A., Ahuja Y. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop. 2016;45(1):E12–E19. [PubMed] [Google Scholar]
- 41.Hsu R.K., McCulloch C.E., Heung M. Exploring potential reasons for the temporal trend in dialysis-requiring AKI in the United States. Clin J Am Soc Nephrol. 2016;11(1):14–20. doi: 10.2215/CJN.04520415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolhe N.V., Muirhead A.W., Wilkes S.R., Fluck R.J., Taal M.W. The epidemiology of hospitalised acute kidney injury not requiring dialysis in England from 1998 to 2013: retrospective analysis of hospital episode statistics. Int J Clin Pract. 2016;70(4):330–339. doi: 10.1111/ijcp.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown J.R., Rezaee M.E., Nichols E.L., Marshall E.J., Siew E.D., Matheny M.E. Incidence and in-hospital mortality of acute kidney injury (AKI) and dialysis-requiring AKI (AKI-D) after cardiac catheterization in the national inpatient sample. J Am Heart Assoc. 2016;5(3):e002739. doi: 10.1161/JAHA.115.002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlson N., Hommel K., Olesen J.B. Trends in one-year outcomes of dialysis-requiring acute kidney injury in Denmark 2005-2012: a population-based nationwide study. PLoS One. 2016;11(7):e0159944. doi: 10.1371/journal.pone.0159944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siew E.D., Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46–61. doi: 10.1038/ki.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomlinson L., Riding A., Payne R. The accuracy of diagnostic coding for acute kidney injury in England—a single centre study. BMC Nephrol. 2013;14(1):58. doi: 10.1186/1471-2369-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grams M.E., Waikar S.S., MacMahon B., Whelton S., Ballew S.H., Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682–689. doi: 10.2215/CJN.07650713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawhney S., Fluck N., Marks A. Acute kidney injury—how does automated detection perform? Nephrol Dial Transplant. 2015;30(11):1853–1861. doi: 10.1093/ndt/gfv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bedford M., Stevens P., Wheeler T., Farmer C. What is the real impact of acute kidney injury? BMC Nephrol. 2014;15(1):95. doi: 10.1186/1471-2369-15-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellomo R., Ronco C., Kellum J., Mehta R., Palevsky P., the ADQI Workgroup Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hobbs H., Bassett P., Wheeler T. Do acute elevations of serum creatinine in primary care engender an increased mortality risk? BMC Nephrol. 2014;15:206. doi: 10.1186/1471-2369-15-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawhney S., Fluck N., Fraser S.D. KDIGO-based acute kidney injury criteria operate differently in hospitals and the community—findings from a large population cohort. Nephrol Dial Transplant. 2016;31(6):922–929. doi: 10.1093/ndt/gfw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siew E.D., Ikizler T.A., Matheny M.E. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7(5):712–719. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin J., Fernandez H., Shashaty M.G.S. False-positive rate of AKI using consensus creatinine–based criteria. Clin J Am Soc Nephrol. 2015;10(10):1723–1731. doi: 10.2215/CJN.02430315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coca S.G., Yusuf B., Shlipak M.G., Garg A.X., Parikh C.R. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawhney S., Mitchell M., Marks A., Fluck N., Black C. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open. 2015;5(1):e006497. doi: 10.1136/bmjopen-2014-006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Venkatachalam M.A., Griffin K.A., Lan R., Geng H., Saikumar P., Bidani A.K. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Ren Physiol. 2010;298(5):F1078–F1094. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mjoen G., Hallan S., Hartmann A. Long-term risks for kidney donors. Kidney Int. 2014;86(1):162–167. doi: 10.1038/ki.2013.460. [DOI] [PubMed] [Google Scholar]
- 60.Siew E.D., Peterson J.F., Eden S.K. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23(2):305–312. doi: 10.1681/ASN.2011030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lafrance J.P., Miller D.R. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21(2):345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 63.Ishani A., Nelson D., Clothier B. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171(3):226–233. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 64.Pannu N., James M., Hemmelgarn B., Klarenbach S., for the Alberta Kidney Disease Network Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8(2):194–202. doi: 10.2215/CJN.06480612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bucaloiu I.D., Kirchner H.L., Norfolk E.R., Hartle J.E., II, Perkins R.M. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81(5):477–485. doi: 10.1038/ki.2011.405. [DOI] [PubMed] [Google Scholar]
- 66.Silver S.A., Goldstein S.L., Harel Z. Ambulatory care after acute kidney injury: an opportunity to improve patient outcomes. Can J Kidney Health Dis. 2015;2:36. doi: 10.1186/s40697-015-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prowle J.R., Kolic I., Purdell-Lewis J., Taylor R., Pearse R.M., Kirwan C.J. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol. 2014;9(6):1015–1023. doi: 10.2215/CJN.11141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacLeod A. NCEPOD report on acute kidney injury—must do better. Lancet. 2009;374(9699):1405–1406. doi: 10.1016/S0140-6736(09)61843-2. [DOI] [PubMed] [Google Scholar]
- 69.Selby N.M., Casula A., Lamming L., Mohammed M., Caskey F. Tackling AKI Investigators: design and rationale of ‘Tackling acute kidney injury’, a multicentre quality improvement study. Nephron. 2016;134(3):200–204. doi: 10.1159/000447675. [DOI] [PubMed] [Google Scholar]
- 70.Bell S., Davey P., Nathwani D. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol. 2014;25(11):2625–2632. doi: 10.1681/ASN.2014010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker H., Patton A., Bayne G. Reduction in post-operative acute kidney injury following a change in antibiotic prophylaxis policy for orthopaedic surgery: an observational study. J Antimicrob Chemother. 2016;71(9):2598–2605. doi: 10.1093/jac/dkw166. [DOI] [PubMed] [Google Scholar]