ABSTRACT
Infective endocarditis is life-threatening; identification of the underlying etiology informs optimized individual patient management. Changing epidemiology, advances in blood culture techniques, and new diagnostics guide the application of laboratory testing for diagnosis of endocarditis. Blood cultures remain the standard test for microbial diagnosis, with directed serological testing (i.e., Q fever serology, Bartonella serology) in culture-negative cases. Histopathology and molecular diagnostics (e.g., 16S rRNA gene PCR/sequencing, Tropheryma whipplei PCR) may be applied to resected valves to aid in diagnosis. Herein, we summarize recent knowledge in this area and propose a microbiologic and pathological algorithm for endocarditis diagnosis.
KEYWORDS: clinical microbiology, endocarditis
INTRODUCTION
Despite recent advances in diagnostic and therapeutic strategies, the mortality of infective endocarditis remains high, with more than one-third of patients affected dying within a year following diagnosis (1, 2). Identification of the specific underlying microbial etiology is essential for optimal patient management; delays in microbial diagnosis may contribute to late initiation of effective antimicrobial therapy, influencing morbidity and mortality. The modified Duke criteria provide a basic scheme for diagnosis and definition of endocarditis and rely on detection of infecting microorganisms in addition to echocardiographic and clinical findings (1, 3). The finding of two (or more) blood cultures positive for a typical microorganism consistent with infective endocarditis is a major criterion for infective endocarditis as is positive Q fever serology (anti-phase I IgG titer of ≥1:800). Echocardiographic findings are also considered but are beyond the scope of the manuscript.
The epidemiology of endocarditis, which has shifted in recent years, should guide diagnostic testing. Today, staphylococci and streptococci combined cause ∼80% of cases. Staphylococcus aureus remains the dominant pathogen, associated with ∼25% to ∼30% of cases, while coagulase-negative staphylococci account for ∼11% of cases (4, 5). Streptococci, primarily viridans group streptococci, cause ∼30% of cases, with Streptococcus gallolyticus (a Streptococcus bovis group member) being involved in ∼20% to ∼50% of streptococcal cases (4, 5). Enterococci, especially Enterococcus faecalis, account for ∼10% of cases (4, 5). Gram-negative bacilli account for ∼5% of cases and include the HACEK group organisms (Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, and Kingella species) and, less commonly, non-HACEK Gram-negative bacilli, such as the Enterobacteriaceae and nonfermenting Gram-negative bacilli. Fungi are rare endocarditis causes, with Candida species being the most common. A number of uncultivable or challenging to cultivate organisms cause endocarditis, the most common of which are Coxiella burnetii, Bartonella species, and Tropheryma whipplei.
Endocarditis most often involves the aortic or mitral valves, with tricuspid valve involvement accounting for fewer than 10% of cases often in association with injection drug use (4, 6, 7). Endocarditis associated with prosthetic valves or cardiovascular implantable electronic devices accounts for approximately one-third of cases and is most commonly caused by staphylococci (4, 7). Coagulase-negative staphylococci are more frequent causes of prosthetic versus native valve endocarditis, while viridans group streptococci more commonly cause native than prosthetic valve endocarditis. Although the majority of endocarditis cases are community acquired, health care-associated endocarditis is increasing and now accounts for approximately one-third of endocarditis cases in North America (4). S. aureus, coagulase-negative Staphylococcus species, and enterococci are most frequently detected in health care-associated cases. Organisms acquired in health care settings are notable for being increasingly resistant to antibacterial agents; methicillin-resistant S. aureus, for example, is more frequently associated with health care-acquired than community-acquired endocarditis, and most cases of endocarditis caused by non-HACEK Gram-negative bacilli are health care associated (8).
ROLE OF BLOOD CULTURES IN DIAGNOSIS OF INFECTIVE ENDOCARDITIS
Endocarditis is an endovascular infection associated with the persistent presence of infecting microorganisms in blood. For this reason, blood cultures are the standard test to determine the microbiologic etiology of infective endocarditis. Routine blood cultures incubated on modern automated, continuous-monitoring blood culture systems allow recovery of almost all easily cultivable agents of endocarditis without additional specialized testing, such as prolonged incubation or terminal subculture. Recommendations regarding the number and timing of blood cultures differ by guideline set. The American Heart Association and the European Society of Cardiology recommend at least three sets of blood cultures collected from different venipuncture sites, with at least 1 h between the first and last draw (1, 6). The British Society for Antimicrobial Chemotherapy (BSAC) recommends collection of two sets of blood cultures within 1 h of each other in patients with suspected endocarditis and acute sepsis and three sets of blood cultures spaced ≥6 h apart in cases of suspected subacute or chronic endocarditis (9). Conventionally, three sets of blood cultures, with each set including one aerobic and one anaerobic bottle, are collected. Alternatively, two sets may be collected, with two aerobic and one anaerobic bottle per set (i.e., a total of six blood culture bottles) (10). Yield of blood cultures is directly related to volume of blood cultured, with properly filled blood culture bottles (i.e., 10 ml of blood per Bactec or BacT/Alert bottle) being essential. Most, if not all, blood cultures from patients with endocarditis caused by microorganisms able to grow in blood culture systems should be positive, provided that blood cultures are appropriately collected and drawn prior to the administration of antimicrobial therapy; a single positive blood culture does not typically represent an endocarditis pathogen. Although the concept of spacing blood culture draws to detect continuous bacteremia is promulgated in the above-referenced guidelines, separation of blood culture draws over time is not the norm for routine blood culture draws. We are not aware of evidence supporting the value of spaced blood culture draws for etiologic diagnosis of endocarditis; for these reasons, we do not recommend routinely spacing blood culture draws in cases of suspected endocarditis.
Standard blood culture incubation times of 5 days are adequate for recovery of almost all cultivable causes of endocarditis, including Candida species. The HACEK organisms were classically considered challenging to detect in blood cultures due to their fastidious nature; accordantly, in the past, prolonged incubation times were advised. With current blood culture systems, extended incubation (and terminal blind subculture) is unnecessary for recovery of these organisms, as they are easily grown and detected within the standard 5-day incubation period (11, 12). Current blood culture systems also contain sufficient supplements to support growth of Abiotrophia and Granulicatella species (nutritionally variant streptococci). Brucella species are infrequent causes of endocarditis in the United States, and detection in routine blood cultures is typically achieved within the standard 5-day incubation period (13); notably, serologic testing may be helpful if exposures are suggestive of Brucella endocarditis. Cutibacterium (formerly Propionibacterium) acnes deserves special consideration, however, as some strains of this species may require prolonged blood culture incubation (e.g., 14 days) (14). The Clinical and Laboratory Standards Institute (CLSI) guidelines recommend terminal subculture to chocolate agar if blood cultures are negative at 5 days and an endocarditis diagnosis is under consideration (15). However, current evidence fails to support the usefulness of blind subculture, and this practice is not recommended in the BSAC guidelines (9, 16). Fungal endocarditis is most commonly caused by Candida species, which should grow in routine blood cultures. Noncandidal fungal causes of endocarditis (e.g., Histoplasma capsulatum, Aspergillus species) are rare, require specialized testing (e.g., antigen detection, specialized fungal blood cultures), and should only be considered in patients with specific risks for these types of endocarditis (e.g., malignancy, injection drug use, prolonged health care exposure, presence of a prosthetic heart valve) after more common etiologies have been excluded.
DIAGNOSIS OF CULTURE-NEGATIVE ENDOCARDITIS
Blood cultures are negative in 2% to 40% of cases of endocarditis, with some studies reporting blood culture-negative rates up to 71% (4, 5, 17–19). The causes of so-called “culture-negative endocarditis” fall into two categories: negative blood cultures due to concomitant or antecedent antibacterial therapy or the presence of an organism that does not grow in routine blood cultures, with the first being more common. Even if considered necessary, antibacterial agents should not be started in patients with suspected endocarditis until after blood cultures have been collected. For cases in which antibiotics have been administered prior to blood culture collection, consideration may be given to stopping antibiotics if possible, with recollection of blood cultures after an antibiotic-free period. While 7 to 10 days off antimicrobial therapy has been recommended, the ideal length of time needed off therapy is unknown and may vary depending on the infecting organism, antibiotics used, and duration of therapy administered (9). Nevertheless, many patients with bacterial endocarditis receive antibiotics without blood cultures having been appropriately collected, obfuscating subsequent microbiologic diagnosis of endocarditis.
In patients who have not received antibiotics, the most common etiologies of culture-negative endocarditis are C. burnetii and Bartonella species, with the former accounting for 28% to 37% and the latter accounting for 12% to 28% of cases (7, 20). T. whipplei causes up to 6% of cases of culture-negative endocarditis (7, 20, 21). C. acnes, a rare cause of endocarditis, may cause culture-negative endocarditis due to the requirement for prolonged blood culture incubation for growth of some strains (14); in addition, some strains may not grow in blood cultures. Mycoplasmal endocarditis, while rare, is primarily caused by Mycoplasma hominis and is usually diagnosed using molecular methods. Traditionally, Mycoplasma pneumoniae has been considered an important cause of culture-negative endocarditis, but reported cases have relied primarily on serologic testing, rendering these historical diagnoses questionable. The incidence of extremely rare causes of endocarditis, such as those caused by Legionella species, Chlamydia/Chlamydophila species, and Mycoplasma species other than M. hominis, is unclear and requires further study, especially in light of evolving diagnostics.
A number of microbiologic tools have been developed to facilitate identification of an infectious agent in patients with suspected endocarditis and negative blood cultures. These technologies should be incorporated into a multimodal strategy to optimize detection of the etiological agent in culture-negative endocarditis.
Serologic testing.
For organisms that do not grow in routine bacterial cultures (e.g., C. burnetii) or are especially fastidious (e.g., Bartonella species), serologic evaluation may aid in diagnosis. In one study, when evaluated in conjunction with blood cultures, systematic serologic testing established an etiological diagnosis in an additional 8% (34/425) of patients (5). In a separate investigation focused on culture-negative endocarditis, serology provided a diagnosis in 77% (268/348) of cases (20). Organisms for which serologic tests have been shown to aid in the diagnosis of endocarditis include C. burnetii and Bartonella species (and, in areas where Brucella endocarditis occurs, Brucella species). Generally, these pathogens cause subacute endocarditis resulting in elevated IgG titers. Serology for C. burnetii is the best established serologic test for the diagnosis of endocarditis and is included as a major criterion in the modified Duke criteria (1, 3). In chronic Q fever with endocarditis, anti-phase I IgG C. burnetii titers of ≥1:800 are diagnostic. Aside from that for C. burnetii, specific serological criteria for the diagnosis of endocarditis have not been incorporated into the modified Duke criteria, although Bartonella endocarditis is often diagnosed by serologic testing. Dependence on antibody detection for etiological diagnosis of endocarditis may be complicated by serologic cross-reactivity; most notably, Chlamydia/Chlamydophila serologic assays demonstrate high level cross-reactivity with Bartonella species, possibly leading to erroneous diagnoses of chlamydial endocarditis (22). Low-level cross-reactivity has also been demonstrated between Bartonella and Coxiella, although in cases of endocarditis, antibody titers against the “true” agent are typically more elevated than those against the cross-reacting organism. Seroreactivity resulting from prior exposure to organisms unrelated to the episode of endocarditis under evaluation may confound interpretation. Serologic testing for extremely rare causes of endocarditis (e.g., Legionella species, Chlamydia/Chlamydophila species) is not recommended due to challenges with falsely positive results.
EVALUATION OF EXCISED CARDIAC VALVULAR TISSUE
Histopathology.
Surgical intervention is performed in 25% to 53% of cases of endocarditis (2, 23). If a microbial diagnosis has not been established at the time of surgery (e.g., by positive blood cultures or positive C. burnetii serology), excised valvular tissue should be submitted for histopathological and microbiological evaluation. If a microbial diagnosis has already been established, additional microbiological testing is typically unnecessary, although histopathological evaluation is often still performed.
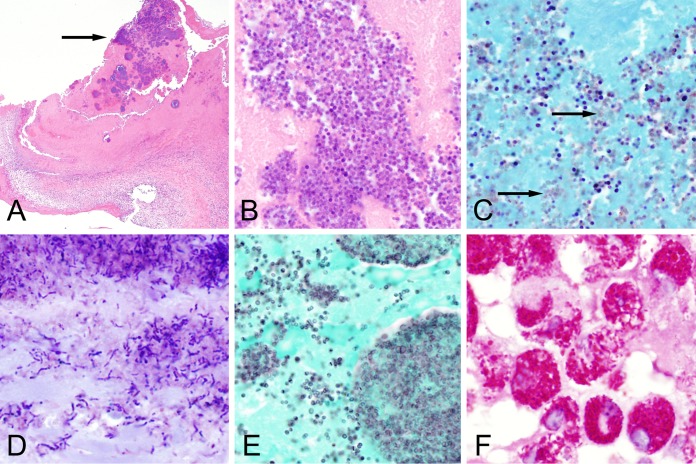
On gross examination, vegetations may be soft, friable, or firm and vary in size based on the infecting organism; discrete vegetations are not, however, always present. Representative sections of the valvular material should be processed for histopathology. On histologic examination of excised valve tissue, patterns and degrees of inflammation will vary depending on the infecting organism (Fig. 1). Endocarditis caused by highly virulent organisms, such as S. aureus, is often associated with acute inflammation characterized by extensive neutrophilic infiltration as well as large colonies of microorganisms with associated areas of tissue destruction. In cases of subacute endocarditis caused by less virulent organisms, such as viridans group streptococci, in addition to focal colonies and neutrophilic inflammation, evidence of healing, including fibrin deposition and mononuclear inflammatory cells, may be present. In cases of endocarditis caused by Bartonella species, C. burnetii, or T. whipplei, valves primarily show chronic inflammation and may be grossly normal in appearance. Mononuclear, rather than neutrophilic, infiltration predominates and macrophages are most frequently observed (24). Abundant foamy macrophages are the primary finding in T. whipplei endocarditis. Macrophages may also be found in C. burnetii endocarditis, although the infiltration is typically less pronounced than with T. whipplei infection and the inflammatory response may be mistaken for degenerative changes. Histopathologically, Bartonella endocarditis typically shows marked fibrosis with minimal vegetation formation, in addition to macrophage and lymphocytic infiltration.
FIG 1.
Histopathological findings of endocarditis. (A) Section of mitral valve from a case of streptococcal endocarditis showing focal basophilic bacterial colonies (arrow) at low magnification (×40 total magnification, hematoxylin and eosin [H&E]). (B) Higher magnification of the case shown in (A) demonstrating clearly defined cocci (×1,000 total magnification). (C) Gram stain of streptococcal endocarditis demonstrating Gram-positive cocci mixed with occasional Gram-negative staining organisms (arrows; ×1,000 total magnification, Twort's Gram stain). It is common to see inconsistent staining patterns in Gram-stained tissue sections. (D) Gram stain of Cutibacterium acnes endocarditis from a bioprosthetic aortic valve demonstrating Gram-positive bacilli (×1,000 total magnification, Twort's Gram stain). (E) Grocott-Gomori methenamine silver (GMS) stain of streptococcal endocarditis highlighting the external contours of the cocci. GMS stain often provides increased sensitivity over tissue Gram stain for the detection of bacteria in valvular tissue. (F) Endocarditis caused by Tropheryma whipplei. Note the large foamy macrophages with periodic acid-Schiff [PAS]-positive staining (×1,000 total magnification, PAS stain with diastase).
Bacteria are often apparent as basophilic or eosinophilic colonies on hematoxylin and eosin (H&E)-stained tissue (Fig. 1A). Not all bacteria are readily detectable in H&E-stained tissue, however, and it is common practice to evaluate a panel of stains when endocarditis is suspected, including Gram and Grocott-Gomori methenamine silver (GMS). The tissue Gram stain (e.g., Twort's, Brown and Brenn, Brown and Hopps) is commonly used for identifying and characterizing bacteria in cardiac valves (Fig. 1B and D) but may fail to highlight some organisms, particularly in the setting of prior antibiotic administration. The GMS stain, while classically used for the identification of fungi, may offer increased sensitivity for identification of bacteria in valve tissue (Fig. 1C). Additional stains that are useful in some settings include Warthin-Starry, Ziehl-Neelson, periodic acid-Schiff (PAS), and organism-specific immunohistochemical stains. Like GMS, PAS will highlight most bacteria and may offer increased sensitivity over tissue Gram stain. PAS with diastase is also the stain of choice for visualizing T. whipplei within foamy macrophages in cases of Whipple's endocarditis (Fig. 1D). Although Warthin-Starry stains may be used to identify weakly staining bacteria, such as Bartonella species, staining is nonspecific and, in our experience, heavy background precipitate often renders this stain difficult to interpret. The Ziehl-Neelson stain may be used for the detection of acid-fast bacteria, such as mycobacteria, but these are rare causes of endocarditis. Immunohistochemical techniques using organism-specific antibodies increase the sensitivity of histologic detection for difficult-to-identify organisms in tissue, but these methods may only be available in specialized reference laboratories and commercial antibodies are not available for many organisms. Caution should be exercised when interpreting staining properties of organisms in valves removed from patients on antimicrobial therapy, as bacterial morphologies and stain properties may be altered. Additionally, the presence of organisms in tissue does not necessarily indicate active endocarditis, as clearance of organisms is delayed compared to sterilization of the vegetation (17).
Importantly, histopathological analysis may facilitate the diagnosis of noninfectious causes of endocarditis, such as neoplastic and autoimmune causes, which account for up to 3% of cases of culture-negative endocarditis (7). Detection of autoantibodies in serum, such as rheumatoid factor, antinuclear antibodies, and anti-DNA antibodies, should be pursued in patients for whom no infectious etiology is apparent.
Culture.
Microorganism identification by culture of cardiac vegetations is considered a pathological criterion, meeting the definition of definite endocarditis by the modified Duke criteria; accordingly, excised valves are often submitted to the microbiology laboratory for culture and Gram stain (1). Current recommendations for the diagnosis of endocarditis also recommend culture of valvular tissue, with culture results being used to direct the duration of postoperative antimicrobial therapy (1, 6, 9). Gram stain of tissue processed in the microbiology laboratory may be more sensitive than histopathological Gram stain of tissue sections, 81% versus 67% in one study; however, in 10% of cases, the histopathology Gram stain detected organisms while the microbiology Gram stain was negative (17). Unfortunately, several studies have shown that culture of valve tissue suffers from low sensitivity and specificity, with positive cultures in only 6% to 26% of endocarditis cases (17, 18, 25). A microorganism different from that identified by blood culture or valve PCR was detected in 36% (10/28) of positive valve cultures in one study, suggesting a high rate of false positivity of valve culture (25). Likewise, cultures of valves from patients without evidence of endocarditis were falsely positive in 28% (293/1,030) of cases, a finding attributed to contamination during processing (25). Because of the low specificity of valve cultures, routine culture of valvular tissue removed for reasons other than possible endocarditis is not recommended. In cases of blood culture-positive endocarditis, results of valve cultures may cause unnecessary confusion if valve cultures generate discrepant (i.e., falsely positive) results. In cases of blood culture-negative endocarditis, valve tissue culture still suffers from low sensitivity and specificity, although growth of an organism does allow for antimicrobial susceptibility testing. When available tissue is insufficient for all tests of interest, in our opinion, culture should not be prioritized over more sensitive assays, such as molecular testing.
Molecular techniques.
Molecular methods are increasingly utilized to aid in the diagnosis of culture-negative endocarditis and have been applied to both blood and excised valve tissue. Molecular methods used in endocarditis diagnosis include organism-specific PCR and broad-range bacterial PCR followed by sequencing. Currently, these techniques are not widely available in clinical microbiology laboratories, but laboratory-developed tests (LDTs) performed in specialized reference laboratories and large clinical laboratories are available. LDTs using organism-specific primers have been developed for C. burnetii, Bartonella species, T. whipplei, C. acnes, and M. hominis, among others. Due to the relative abundance of bacterial DNA in valve tissue versus blood, testing of cardiac valve tissue with organism-specific PCR assays is more sensitive than testing blood or serum. For example, in one study, sensitivity of a Bartonella PCR assay on valve tissue was 92% (48/52) compared to 33% (20/60) and 36% (25/70) sensitivity in blood and serum, respectively (26). Nevertheless, a PCR result on blood may be helpful when positive.
Broad-range bacterial PCR, with amplification primers targeting the bacterial 16S rRNA gene, is a molecular method for detecting bacteria in general. Following amplification, bacterial identification is determined by sequencing amplified DNA followed by comparison of the sequence to established databases. Although broad-range bacterial PCR has been applied to blood sources, sensitivity is superior when performed on excised valve tissue, with an organism detected in 66% (150/227) of endocarditis cases in one study compared to just 14% (35/257) of cases when performed on EDTA blood (7). Broad-range bacterial PCR performed on valve tissue has a reported sensitivity of 33% to 90%, while sensitivity of valve culture in the same studies was 8% to 33% (7, 27–31). Differing patient populations and assay designs likely account for variations in sensitivities between studies. In cases of blood culture-negative endocarditis, an organism was identified by broad-range bacterial PCR of valve tissue in 60% to 100% of cases across five studies (28–30, 32, 33). Broad-range bacterial PCR assays have demonstrated high specificity (77% to 100%), with detection of contaminating organisms being rare (27, 29). While sensitivity may not be ideal in patients with infective endocarditis who undergo surgical excision of the affected valve and for whom no etiologic agent has yet been identified, we recommend testing valvular tissue by broad-range bacterial PCR when histopathologic examination of excised tissue shows acute inflammation.
Broad-range fungal PCR is technically possible but has low yield for endocarditis diagnosis due to the rarity of fungi as causes of endocarditis (7, 31). Specificity may also be an issue; in one study, 13% (15/117) of valves tested were positive by broad-range fungal PCR, with 53% (8/15) of positives determined to be contaminants (31).
When considering molecular testing of cardiac valves, it should be borne in mind that organism-specific PCR assays often demonstrate superior sensitivity compared to broad-range PCR. In cases where both broad-range bacterial PCR and organism-specific PCR were performed for the diagnosis of endocarditis, 62% (76/123) of specimens were positive by targeted PCR only, while only 2 specimens were positive by broad-range PCR only (34). For the diagnosis of Bartonella endocarditis, Bartonella-specific PCR applied to cardiac valve tissue was positive in 92% (48/52) of cases while broad-range PCR of valve tissue identified Bartonella in only 60% (21/35) of cases (26). Therefore, for diagnosis of culture-negative endocarditis, broad-range bacterial PCR should not be performed in lieu of organism-specific PCR.
Caution should be exercised in the interpretation of nucleic acid amplification test results from removed valves after completion of antibiotic therapy. Long-term persistence of bacterial DNA has been reported in patients who have completed a full course of antibiotic therapy, in some cases several years after diagnosis of endocarditis. In one study, PCR was more likely to be positive in valves with histologic evidence of endocarditis, although PCR was positive in 23% (7/30) of patients with a history of endocarditis but no histological findings consistent with active endocarditis, suggesting that bacterial DNA may persist even after resolution of tissue lesions (35). Conversely, results can be falsely negative due to the presence of PCR inhibitors, the presence of microbial nucleic acid below the limit of detection of the assay being used, or sampling error since microorganisms are often not homogenously distributed in resected valves.
A PROPOSED MICROBIOLOGIC AND PATHOLOGICAL DIAGNOSTIC ALGORITHM FOR ENDOCARDITIS
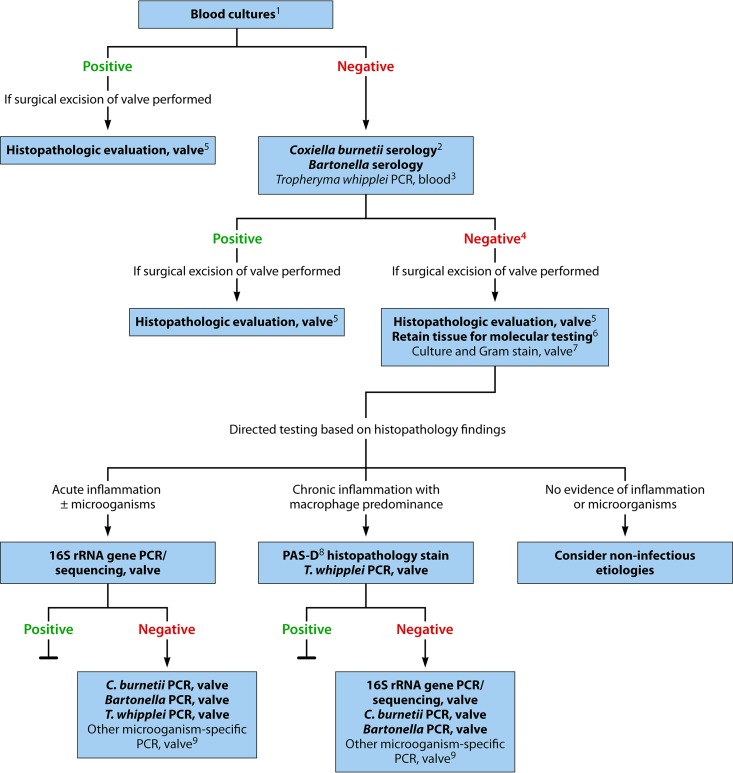
A multimodal testing strategy for the diagnosis of culture-negative endocarditis, employing culture, serology, histopathology, and molecular analysis, is essential for optimal sensitivity and specificity in identifying an infectious etiology in order to assist clinicians in selecting ideal antimicrobial therapy. A proposed testing strategy is shown in Fig. 2. Notably, this algorithm should be applied in the context of clinical evaluation of the patient and other findings (e.g., echocardiography) supportive of a diagnosis of endocarditis. Positive blood cultures are the standard means of microbial diagnosis of infective endocarditis; blood cultures should be collected prior to initiation of antibiotic therapy. In cases of culture-positive endocarditis (i.e., two positive blood cultures), no additional microbiology testing is necessarily needed, although histopathologic evaluation of valvular tissue, if excised, is typically performed to confirm the diagnosis of endocarditis. In cases of culture-negative endocarditis, C. burnetii and Bartonella serology should be performed and consideration given to performing T. whipplei PCR on blood. If the patient undergoes valvular resection, histopathology and staining of the resected valve is recommended. If a microbiological diagnosis has not been established, molecular testing of fresh excised valve tissue (or formalin-fixed, paraffin-embedded valve tissue if fresh tissue is unavailable) should be guided by histopathologic evaluation and includes broad-range bacterial PCR as well as specific PCR assays for T. whipplei, C. burnetii, and Bartonella species. Ideally, a representative sample of valvular tissue should be specifically collected in the operating room for molecular testing. With the advent and availability of molecular testing, routine culture of valve tissue appears to be less valuable than molecular testing, and molecular testing should be prioritized if sufficient tissue for all testing is unavailable. If no evidence of acute inflammation or other histological features of endocarditis is detected upon expert review of the valve tissue, and no organisms are detected by special stains, noninfectious etiologies should be considered.
FIG 2.
Diagnostic testing for identification of the microbiological etiology of infective endocarditis. This algorithm is intended for use in patients with clinical and/or echocardiographic findings suggestive of infective endocarditis based on the modified Duke criteria (3). Strong recommendations appear in boldface, with other diagnostic testing considerations shown in standard typeface. 1, Details on blood culture collection are provided in the text. 2, C. burnetii anti-phase I IgG antibody titer of ≥1:800 is considered positive. 3, The sensitivity of T. whipplei PCR from blood in endocarditis is unknown; a negative result should not be used to rule out T. whipplei endocarditis. 4, If surgery is not performed, consider testing for noninfectious etiologies. 5, Histologic evaluation is used to evaluate for infectious and noninfectious etiologies and for correlation with microbiology test results. 6, Ideally, a representative sample of valvular tissue should be specifically collected for molecular testing in a sterile fashion in the operating room. 7, If sufficient valvular tissue is available after sampling for histopathological and molecular (microorganism-specific and broad-range) testing, consider culture and microbiology Gram stain. Due to the low sensitivity and specificity of culture, molecular testing should be prioritized over culture. 8, PAS-D, periodic acid-Schiff with diastase. Macrophages infected with T. whipplei will stain PAS positive following diastase digestion. 9, Examples include Mycoplasma hominis and Cutibacterium (formerly Propionibacterium) acnes PCR. By permission of the Mayo Foundation for Medical Education and Research. All rights reserved.
UNMET NEEDS AND FUTURE RESEARCH QUESTIONS
In recent years, the epidemiology of endocarditis has changed and, at the same time, improved diagnostics have become available. However, there remain several unmet diagnostic needs. Improved strategies to promote collection of blood cultures before administration of antibiotics in patients with potential endocarditis are welcomed, as this will likely reduce rates of culture-negative endocarditis. Future studies are needed to define whether or not a third blood culture set is required when routine collection includes only two bottle sets, as given the continuous bacteremia characteristic of endocarditis, two sets may be adequate if properly performed (i.e., aerobic and anaerobic bottle, adequate blood volume). The use of valve cultures in directing duration of postoperative antimicrobial therapy should be reevaluated given the low specificity of valvular culture. Diagnostic options in blood culture-negative, serology-negative endocarditis where valvular tissue is unavailable remain inadequate. The relatively high and persistent microbial burden in blood of patients with endocarditis provides a potentially ideal setting for direct-from-blood molecular panels specifically tailored for the detection of cultivable as well as challenging-to-cultivate etiologic agents of endocarditis, in addition to relevant antimicrobial resistance genes (e.g., mecA). Such panels may also be applied to excised valvular tissue. Metagenomic shotgun sequencing approaches, while not routinely used at this time for infectious diseases diagnosis, are promising and may be applied to resected cardiac valves or even blood, plasma, or serum, providing the possibility of detecting not just bacteria but also fungi as well as markers of antimicrobial resistance. Improved serologic diagnostics for T. whipplei are welcomed, although serologic responses in asymptomatically colonized individuals may limit this approach. The establishment of formal diagnostic criteria for interpretation of Bartonella serology results in the context of endocarditis, and inclusion of these criteria in infective endocarditis guidelines, should be considered. The development of endocarditis serologic panels may ultimately help streamline test ordering. Finally, we note that expert histological examination of excised valvular tissue is of great diagnostic value; the development, standardization, and prospective evaluation of histologic criteria for the diagnosis of endocarditis are welcomed as is training of infectious diseases pathologists in this specific field.
ACKNOWLEDGMENTS
We thank James M. Steckelberg, Larry M. Baddour, Elitza S. Theel, L. Barth Reller, and Ferric Fang for their thoughtful comments.
R.P. reports grants from BioFire, Check-Points, Curetis, 3M, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, Allergan, and The Medicines Company. R.P. is a consultant to Curetis, Roche, Qvella, and Diaxonhit. In addition, R.P. has a patent on a Bordetella pertussis/B. parapertussis PCR assay with royalties paid by TIB, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an antibiofilm substance issued. R.P. serves on an Actelion data monitoring board. R.P. receives travel reimbursement and an editor's stipend from ASM and IDSA and honoraria from the USMLE, Up-to-Date, and the Infectious Diseases Board Review Course.
REFERENCES
- 1.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 2.Thuny F, Grisoli D, Collart F, Habib G, Raoult D. 2012. Management of infective endocarditis: challenges and perspectives. Lancet 379:965–975. doi: 10.1016/S0140-6736(11)60755-1. [DOI] [PubMed] [Google Scholar]
- 3.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 4.Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falco V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort study. Arch Intern Med 169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raoult D, Casalta JP, Richet H, Khan M, Bernit E, Rovery C, Branger S, Gouriet F, Imbert G, Bothello E, Collart F, Habib G. 2005. Contribution of systematic serological testing in diagnosis of infective endocarditis. J Clin Microbiol 43:5238–5242. doi: 10.1128/JCM.43.10.5238-5242.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL, Erol C, Nihoyannopoulos P, Aboyans V, Agewall S, Athanassopoulos G, Aytekin S, Benzer W, Bueno H, Broekhuizen L, Carerj S, Cosyns B, De Backer J, De Bonis M, Dimopoulos K, Donal E, Drexel H, Flachskampf FA, Hall R, Halvorsen S, Hoen B, Kirchhof P, Lainscak M, Leite-Moreira AF, Lip GY, Mestres CA, Piepoli MF, Punjabi PP, Rapezzi C, Rosenhek R, Siebens K, Tamargo J, Walker DM. 2015. 2015 ESC guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36:3075–3128. [DOI] [PubMed] [Google Scholar]
- 7.Fournier PE, Thuny F, Richet H, Lepidi H, Casalta JP, Arzouni JP, Maurin M, Celard M, Mainardi JL, Caus T, Collart F, Habib G, Raoult D. 2010. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis 51:131–140. doi: 10.1086/653675. [DOI] [PubMed] [Google Scholar]
- 8.Morpeth S, Murdoch D, Cabell CH, Karchmer AW, Pappas P, Levine D, Nacinovich F, Tattevin P, Fernandez-Hidalgo N, Dickerman S, Bouza E, del Rio A, Lejko-Zupanc T, de Oliveira Ramos A, Iarussi D, Klein J, Chirouze C, Bedimo R, Corey GR, Fowler VG Jr. 2007. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med 147:829–835. doi: 10.7326/0003-4819-147-12-200712180-00002. [DOI] [PubMed] [Google Scholar]
- 9.Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, Sandoe JA, Spry MJ, Watkin RW, Working Party of the British Society for Antimicrobial Chemotherapy. 2012. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 67:269–289. doi: 10.1093/jac/dkr450. [DOI] [PubMed] [Google Scholar]
- 10.Patel R, Vetter EA, Harmsen WS, Schleck CD, Fadel HJ, Cockerill FR III. 2011. Optimized pathogen detection with 30- compared to 20-milliliter blood culture draws. J Clin Microbiol 49:4047–4051. doi: 10.1128/JCM.01314-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petti CA, Bhally HS, Weinstein MP, Joho K, Wakefield T, Reller LB, Carroll KC. 2006. Utility of extended blood culture incubation for isolation of Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella organisms: a retrospective multicenter evaluation. J Clin Microbiol 44:257–259. doi: 10.1128/JCM.44.1.257-259.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers ST, Murdoch D, Morris A, Holland D, Pappas P, Almela M, Fernandez-Hidalgo N, Almirante B, Bouza E, Forno D, del Rio A, Hannan MM, Harkness J, Kanafani ZA, Lalani T, Lang S, Raymond N, Read K, Vinogradova T, Woods CW, Wray D, Corey GR, Chu VH. 2013. HACEK infective endocarditis: characteristics and outcomes from a large, multi-national cohort. PLoS One 8:e63181. doi: 10.1371/journal.pone.0063181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagi M, Nesher L, Yagupsky P. 2017. The Bactec FX blood culture system detects Brucella melitensis bacteremia in adult patients within the routine 1-week incubation period. J Clin Microbiol 55:942–946. doi: 10.1128/JCM.02320-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohail MR, Gray AL, Baddour LM, Tleyjeh IM, Virk A. 2009. Infective endocarditis due to Propionibacterium species. Clin Microbiol Infect 15:387–394. doi: 10.1111/j.1469-0691.2009.02703.x. [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2007. Principles and procedures for blood cultures; approved standard—1st ed CLSI document M47-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.Baron EJ, Scott JD, Tompkins LS. 2005. Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganisms from standard automated blood cultures. Clin Infect Dis 41:1677–1680. doi: 10.1086/497595. [DOI] [PubMed] [Google Scholar]
- 17.Morris AJ, Drinkovic D, Pottumarthy S, Strickett MG, MacCulloch D, Lambie N, Kerr AR. 2003. Gram stain, culture, and histopathological examination findings for heart valves removed because of infective endocarditis. Clin Infect Dis 36:697–704. doi: 10.1086/367842. [DOI] [PubMed] [Google Scholar]
- 18.Lamas CC, Fournier PE, Zappa M, Brandao TJ, Januario-da-Silva CA, Correia MG, Barbosa GI, Golebiovski WF, Weksler C, Lepidi H, Raoult D. 2016. Diagnosis of blood culture-negative endocarditis and clinical comparison between blood culture-negative and blood culture-positive cases. Infection 44:459–466. doi: 10.1007/s15010-015-0863-x. [DOI] [PubMed] [Google Scholar]
- 19.Topan A, Carstina D, Slavcovici A, Rancea R, Capalneanu R, Lupse M. 2015. Assesment of the Duke criteria for the diagnosis of infective endocarditis after twenty-years. An analysis of 241 cases. Clujul Med 88:321–326. doi: 10.15386/cjmed-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houpikian P, Raoult D. 2005. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore) 84:162–173. doi: 10.1097/01.md.0000165658.82869.17. [DOI] [PubMed] [Google Scholar]
- 21.Geissdorfer W, Moos V, Moter A, Loddenkemper C, Jansen A, Tandler R, Morguet AJ, Fenollar F, Raoult D, Bogdan C, Schneider T. 2012. High frequency of Tropheryma whipplei in culture-negative endocarditis. J Clin Microbiol 50:216–222. doi: 10.1128/JCM.05531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurin M, Eb F, Etienne J, Raoult D. 1997. Serological cross-reactions between Bartonella and Chlamydia species: implications for diagnosis. J Clin Microbiol 35:2283–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreillon P, Que YA. 2004. Infective endocarditis. Lancet 363:139–149. doi: 10.1016/S0140-6736(03)15266-X. [DOI] [PubMed] [Google Scholar]
- 24.Lepidi H, Houpikian P, Liang Z, Raoult D. 2003. Cardiac valves in patients with Q fever endocarditis: microbiological, molecular, and histologic studies. J Infect Dis 187:1097–1106. doi: 10.1086/368219. [DOI] [PubMed] [Google Scholar]
- 25.Munoz P, Bouza E, Marin M, Alcala L, Rodriguez Creixems M, Valerio M, Pinto A. 2008. Heart valves should not be routinely cultured. J Clin Microbiol 46:2897–2901. doi: 10.1128/JCM.02173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edouard S, Nabet C, Lepidi H, Fournier PE, Raoult D. 2015. Bartonella, a common cause of endocarditis: a report on 106 cases and review. J Clin Microbiol 53:824–829. doi: 10.1128/JCM.02827-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maneg D, Sponsel J, Muller I, Lohr B, Penders J, Madlener K, Hunfeld KP. 2016. Advantages and limitations of direct PCR amplification of bacterial 16S-rDNA from resected heart tissue or swabs followed by direct sequencing for diagnosing infective endocarditis: a retrospective analysis in the routine clinical setting. BioMed Res Int 2016:7923874. doi: 10.1155/2016/7923874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boussier R, Rogez S, Francois B, Denes E, Ploy MC, Garnier F. 2013. Two-step bacterial broad-range polymerase chain reaction analysis of heart valve tissue improves bacteriological diagnosis of infective endocarditis. Diagn Microbiol Infect Dis 75:240–244. doi: 10.1016/j.diagmicrobio.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Breitkopf C, Hammel D, Scheld HH, Peters G, Becker K. 2005. Impact of a molecular approach to improve the microbiological diagnosis of infective heart valve endocarditis. Circulation 111:1415–1421. doi: 10.1161/01.CIR.0000158481.07569.8D. [DOI] [PubMed] [Google Scholar]
- 30.Marsch G, Orszag P, Mashaqi B, Kuehn C, Haverich A. 2015. Antibiotic therapy following polymerase chain reaction diagnosis of infective endocarditis: a single centre experience. Interact Cardiovasc and Thorac Surg 20:589–593. doi: 10.1093/icvts/ivv006. [DOI] [PubMed] [Google Scholar]
- 31.Shrestha NK, Ledtke CS, Wang H, Fraser TG, Rehm SJ, Hussain ST, Pettersson GB, Blackstone EH, Gordon SM. 2015. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 99:33–37. doi: 10.1016/j.athoracsur.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 32.Miyazato A, Ohkusu K, Tabata M, Uwabe K, Kawamura T, Tachi Y, Ezaki T, Niinami H, Mitsutake K. 2012. Comparative molecular and microbiological diagnosis of 19 infective endocarditis cases in which causative microbes were identified by PCR-based DNA sequencing from the excised heart valves. J Infect Chemother 18:318–323. doi: 10.1007/s10156-011-0332-0. [DOI] [PubMed] [Google Scholar]
- 33.Harris KA, Yam T, Jalili S, Williams OM, Alshafi K, Gouliouris T, Munthali P, NiRiain U, Hartley JC. 2014. Service evaluation to establish the sensitivity, specificity and additional value of broad-range 16S rDNA PCR for the diagnosis of infective endocarditis from resected endocardial material in patients from eight UK and Ireland hospitals. Eur J Clin Microbiol Infect Dis 33:2061–2066. doi: 10.1007/s10096-014-2145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morel AS, Dubourg G, Prudent E, Edouard S, Gouriet F, Casalta JP, Fenollar F, Fournier PE, Drancourt M, Raoult D. 2015. Complementarity between targeted real-time specific PCR and conventional broad-range 16S rDNA PCR in the syndrome-driven diagnosis of infectious diseases. Eur J Clin Microbiol Infect Dis 34:561–570. doi: 10.1007/s10096-014-2263-z. [DOI] [PubMed] [Google Scholar]
- 35.Rovery C, Greub G, Lepidi H, Casalta JP, Habib G, Collart F, Raoult D. 2005. PCR detection of bacteria on cardiac valves of patients with treated bacterial endocarditis. J Clin Microbiol 43:163–167. doi: 10.1128/JCM.43.1.163-167.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]