ABSTRACT
Diarrhea is responsible for the death of approximately 900,000 children per year worldwide. In children, typical enteropathogenic Escherichia coli (EPEC) is a common cause of diarrhea and is associated with a higher hazard of death. Typical EPEC infection is rare in animals and poorly reproduced in experimental animal models. In contrast, atypical EPEC (aEPEC) infection is common in both children and animals, but its role in diarrhea is uncertain. Mortality in kittens is often attributed to diarrhea, and we previously identified enteroadherent EPEC in the intestines of deceased kittens. The purpose of this study was to determine the prevalence and type of EPEC in kittens and whether infection was associated with diarrhea, diarrhea-related mortality, gastrointestinal pathology, or other risk factors. Kittens with and without diarrhea were obtained from two shelter facilities and determined to shed atypical EPEC at a culture-based prevalence of 18%. In contrast, quantitative PCR detected the presence of the gene for intimin (eae) in feces from 42% of kittens. aEPEC was isolated from kittens with and without diarrhea. However, kittens with diarrhea harbored significantly larger quantities of aEPEC than kittens without diarrhea. Kittens with aEPEC had a significantly greater severity of small intestinal and colonic lesions and were significantly more likely to have required subcutaneous fluid administration. These findings identify aEPEC to be prevalent in kittens and a significant primary or contributing cause of intestinal inflammation, diarrhea, dehydration, and associated mortality in kittens.
KEYWORDS: Escherichia coli enterocyte attaching and effacing (eae) gene, EPEC, animal models, histopathology, cat
INTRODUCTION
Diarrhea is responsible for the death of an estimated 900,000 children per year worldwide, with the majority of cases of mortality occurring in developing countries (1–3). Enteropathogenic Escherichia coli (EPEC) is responsible for over 81 million cases of diarrhea per year, of which 17 million are diagnosed in children (4). A recent Global Enteric Multicenter Study (GEMS) determined that the diarrheal death of children can be largely attributed to a mere few infectious agents (2). In particular, diarrhea caused by typical EPEC (tEPEC) is associated with a 2.6-fold higher hazard of death, the largest reported in the study (2). EPEC strains are separated into tEPEC and atypical EPEC (aEPEC) on the basis of the presence of the EAF plasmid, which contains the gene encoding the bundle-forming pilus (bfp). Typical EPEC is an established diarrheal pathogen in humans, whereas the role of aEPEC as a primary cause of diarrhea is not completely solved (5–7). A major issue is that aEPEC isolates can also be detected in healthy individuals. aEPEC is more prevalent than tEPEC in developed countries, where it is associated with prolonged diarrhea in some studies (8, 9) but is unassociated with diarrhea in others (10–12).
It is estimated that 180 million kittens are born in the United States each year, and inestimable numbers of these kittens are abandoned, orphaned, or relinquished to be fostered by thousands of U.S. animal shelters (13–15). While the exact statistics are unknown, approximately 15% of kittens fostered by these shelters will die or be euthanized because of severe illness before they reach 8 weeks of age (Jim Babbitt, chief veterinarian, San Diego Humane Society and Society for the Prevention of Cruelty to Animals [SPCA], and Mondy Lamb, marketing director, Wake County, NC, SPCA, personal communications). Infectious diseases are prevalent in this population, and as many as 50% of kittens have diarrhea at the time of death or evidence of enteritis postmortem (13, 16–18). Few studies have attempted to identify EPEC in the gastrointestinal tract or feces of cats (19–24). These studies either evaluated very few cats or were performed on healthy animals only. The studies largely focused on animals as a reservoir for EPEC and not on an association of EPEC with disease (19, 21, 23, 24). Two previous studies focusing on adult cats identified a 1% prevalence of EPEC in cats with diarrhea and a slightly higher (3 to 6%) prevalence in healthy cats (20, 22).
In susceptible species, EPEC bacteria cause diarrhea and severe dehydration by attaching to the microvillus brush border of intestinal epithelial cells by means of the adhesin intimin, which is encoded by the E. coli enterocyte attaching and effacing (eae) gene (25). During the course of investigating gastrointestinal lesions associated with death in kittens, we discovered a significant association between colonization of the intestinal epithelium by eae-positive E. coli strains and death or euthanasia due to severe illness (18). Given the established role of tEPEC infection as a leading cause of diarrhea and diarrhea-related mortality in children, we hypothesized a similar potential of EPEC infection in kittens. Consequently, the purpose of the present study was to determine the prevalence and type of EPEC infection in kittens ≤12 weeks of age and to establish any association between EPEC infection and diarrhea, diarrhea-related mortality, specific intestinal tract pathology, or factors promoting susceptibility to clinical disease. Our rationale was that identification of EPEC as an important cause of diarrhea and related mortality in kittens could provide a unique opportunity for the development of diagnostic, treatment, or prevention strategies having comparable benefits in both kittens and children with EPEC infection.
RESULTS
Naturally occurring atypical EPEC colonization is prevalent in kittens.
Feces from 61 live kittens from 2 different shelter facilities in North Carolina were evaluated for the presence of EPEC (see Table S1 in the supplemental material). Escherichia coli was cultured from the feces of all but one kitten. Among the 60 kittens from which E. coli was isolated, cultures positive for EPEC (eae-positive and stx1- and stx2-negative strains) were obtained for 11 kittens, resulting in an overall prevalence of 18%. There was no significant difference in prevalence of EPEC between kittens with diarrhea (6/27; 22%) and kittens without diarrhea (5/33; 15%). All EPEC isolates were identified to be atypical on the basis of the absence of the gene coding for the bundle-forming pilus (bfp) (26).
The gene for intimin (eae) could also be directly amplified from the fecal DNA of 22 kittens by means of quantitative PCR (qPCR), suggesting a prevalence of aEPEC exposure or a colonization rate as high as 36%. There was no significant difference in the prevalence of eae in feces from kittens with diarrhea (13/28; 46%) and kittens without diarrhea (9/33; 27%) (P = 0.199, χ2 test). On the basis of a standard curve for the correlation of the eae cycle threshold (CT) number to the number of CFU of aEPEC in feces (Fig. 1A), kittens with diarrhea were estimated to shed a median of 4.9 × 105 CFU of aEPEC (interquartile range [IQR] = 2.5 × 104 to 4.7 × 106 CFU) per 100 mg feces, whereas kittens without diarrhea were estimated to shed a median of 2.8 × 104 CFU of aEPEC (IQR = 3.8 × 103 to 1.5 × 105 CFU) per 100 mg feces (Fig. 1B). The results of quantitative PCR for amplification of the 16S rRNA gene from feces were not different between the kittens. The stx1, stx2, or bfp gene failed to be amplified from fecal DNA from each kitten.
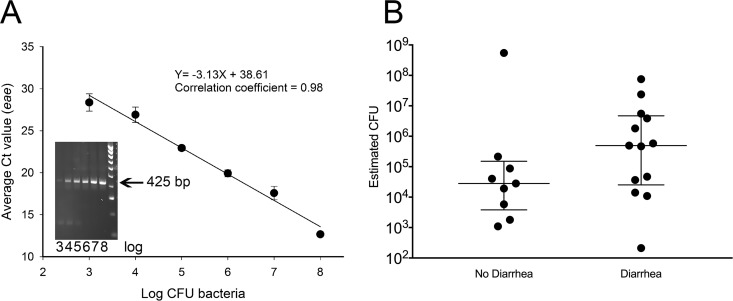
FIG 1.
Estimation of aEPEC quantity determined by quantitative PCR (qPCR) amplification of the E. coli enterocyte attaching and effacing (eae) gene from fecal DNA from 61 live kittens with and without diarrhea. (A) Standard curve obtained by inoculating serial dilutions of known numbers of CFU of aEPEC into 100-mg aliquots of cat feces, followed by DNA extraction and qPCR amplification of eae. The graph represents the results of 3 independent experiments each performed in triplicate at each dilution. The linear equation relates y = cycle threshold (CT) value to x = log10 number of CFU of bacteria. The insert shows an electrophoresis gel of the eae qPCR product from aEPEC log dilutions 3 to 8 (103 to 108) and a molecular weight ladder demonstrating the size of the PCR product (425 bp). (B) Estimated number of aEPEC CFU per 100 mg of feces determined from the standard curve and the cycle threshold (CT) values for qPCR amplification of eae from the fecal DNA of live kittens with and without diarrhea. The lines represent the medians and interquartile ranges of the data.
Diarrhea-related mortality is associated with a larger quantity of aEPEC.
Recent epidemiological studies in children have identified that EPEC infection and, in particular, tEPEC infection are associated with a higher hazard of diarrhea-related death (2, 4). To determine if there is any association between aEPEC infection and diarrhea-related mortality in kittens, we first identified the pathotypes of E. coli that could be cultured from the feces of apparently healthy kittens and compared them to the pathotypes that could be cultured from the feces of kittens that died or were euthanized due to severe diarrhea. Fifty-four deceased kittens from two different shelter facilities had feces cultured for the presence of diarrheagenic E. coli, including EPEC (Table S2). All apparently healthy kittens came from a single shelter and were euthanized after short-term housing because they were feral or otherwise unadoptable. In contrast, kittens with diarrhea were predominantly from a different shelter in which foster care was provided until the time of their death or euthanasia due to severe diarrhea. Kittens with diarrhea had a significantly lower body weight than apparently healthy kittens. Forty-four percent (15/34) of kittens with diarrhea died in foster care prior to their return to the shelter, which resulted in a significantly longer time period lapse between death and autopsy in the diarrhea group than in the group of apparently healthy kittens.
A diarrheagenic pathotype of E. coli was cultured from the feces of 9/19 (47%) apparently healthy kittens and 10/29 (34%) kittens that died or were euthanized due to diarrhea (Table 1). The most common pathotypes of E. coli identified were aEPEC and cnf1-positive E. coli. The presence of cnf1-positive E. coli was not significantly associated with diarrhea, and cnf1-positive E. coli was cultured from a greater number of kittens without diarrhea (5/19; 26%) than kittens with diarrhea (5/29; 17%) (Table 1). Atypical EPEC was cultured from the feces of 6/29 (21%) kittens with diarrhea-associated mortality and 3/19 (16%) apparently healthy kittens. In contrast, eae could be directly amplified from the fecal DNA of 18/34 (53%) kittens with diarrhea-associated mortality and 8/20 (40%) apparently healthy kittens (P = 0.524, χ2 test). There was no significant difference in the culture or qPCR-based prevalence of aEPEC infection between apparently healthy kittens and kittens that died or were euthanized due to diarrhea. However, all of the E. coli isolates from cultures of feces from kittens with aEPEC that died or were euthanized due to diarrhea were identified to be aEPEC. Moreover, kittens that died or were euthanized due to diarrhea had a greater estimated quantity of aEPEC in their feces than apparently healthy kittens as determined by amplification of eae (Fig. 2). On the basis of the quantity of eae determined by qPCR, kittens with diarrhea-associated mortality and having concurrent eae amplification shed an estimated median of 1.8 × 106 CFU of aEPEC (IQR = 2.3 × 105 to 5.3 × 107 CFU) per 100 mg feces, whereas kittens without diarrhea shed an estimated median of 1.4 × 104 CFU of aEPEC (IQR = 7.7 × 103 to 3.1 × 106 CFU) per 100 mg feces. The results of quantitative PCR for the amplification of the 16S rRNA gene from feces were not different between the two groups of kittens. The stx1, stx2, or bfp gene failed to be amplified from fecal DNA from each kitten.
TABLE 1.
Results of multiplex PCR for detection of a virulence gene(s) in E. coli cultured from feces of apparently healthy kittens and kittens that died or were euthanized due to diarrhea
| Culture findingc | Kittens with no diarrhea (n = 20) |
Kittens with diarrhea-associated mortality (n = 34) |
||||
|---|---|---|---|---|---|---|
| No. of kittens with indicated finding/total no. (%) | No. of isolates with indicated finding/total no. (%) | No. of hemolytic isolates/total no. (%) | No. of kittens with indicated finding/total no. (%) | No. of isolates with indicated finding/total no. (%) | No. of hemolytic isolates/total no. (%) | |
| Positive fecal bacterial culture | 19/20 (95) | 29/34 (85) | ||||
| Isolation of E. coli | 17/19 (89) | 49 | 12/49 (24) | 19/29 (68) | 54 | 9/54 (17) |
| Isolation of diarrheagenic E. coli | 9/19 (47) | 19/49 (39) | 12/19 (63) | 10/29 (34) | 30/54 (56) | 9/30 (30) |
| Enteropathogenic (eae positive, bfp and stx negative) | 3/19 (16) | 6 | 0/6 (0) | 6a/29 (21) | 18 | 1/18 (6) |
| CNF1 containing (e.g., necrotoxigenic) (cnf1 positive) | 5/19 (26) | 10 | 9/10 (90) | 5a,b/29 (17) | 14 | 8b/14 (57) |
| Enterotoxigenic (STa and STb positive) | 1/19 (5) | 3 | 3/3 (100) | 0/29 (0) | 0 | |
| Shiga toxin producing (eae negative, stx positive) | 0/19 (0) | 0 | 0/29 (0) | 0 | ||
| Enterohemorrhagic (eae and stx positive) | 0/19 (0) | 0 | 0/29 (0) | 0 | ||
| Enteroaggregative (pCVD432 positive) | 0/19 (0) | 0 | 1b/29 (3) | 1 | 1b/1 (100) | |
| Enteroinvasive (ipaH positive) | 0/19 (0) | 0 | 0/29 (0) | 0 | ||
| Isolation of nondiarrheagenic E. coli | 8/19 (42) | 30/49 (61) | 0/49 (0) | 9/29 (31) | 24/54 (44) | 0/54 (0) |
One kitten had E. coli with both cnf1 and eae.
One kitten had E. coli with both cnf1 and pCVD432.
cnf1, gene encoding cytotoxic necrotizing factor 1; STa and STb, heat-stable toxins a and b, respectively; eae, E. coli enterocyte attaching and effacing gene, stx, gene encoding Shiga-like toxin; pCVD432, enteroaggregative plasmid; ipaH, gene encoding invasion plasmid antigen H.
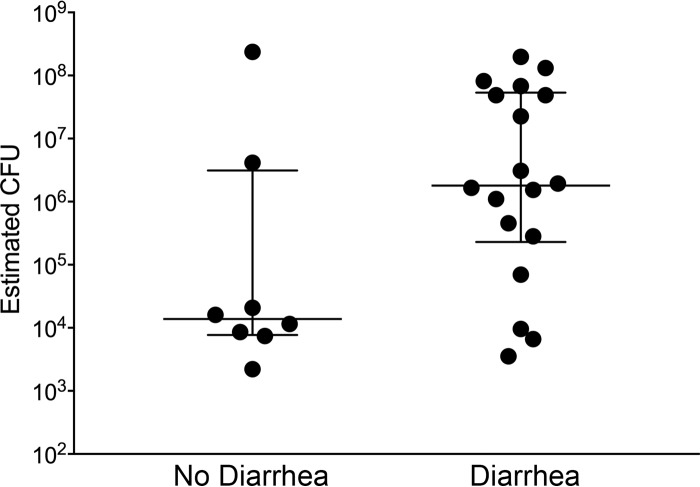
FIG 2.
Estimated number of aEPEC CFU per 100 mg feces from apparently healthy kittens euthanized by animal control and kittens that died or were euthanized because of severe diarrhea while in foster care. The lines represent the medians and the interquartile ranges of the data.
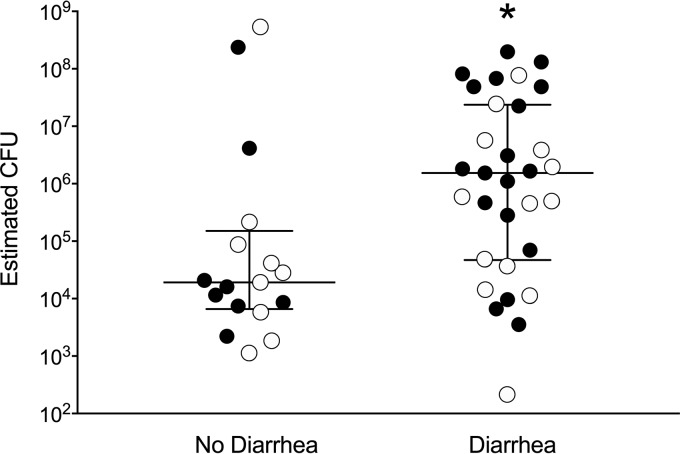
A closer examination of the data representing the burden of aEPEC colonization in kittens demonstrated a large increase in the median number of CFU of aEPEC in both live kittens with diarrhea (Fig. 1B) and deceased kittens with diarrhea (Fig. 2), although the difference was not significant (P = 0.142 and P = 0.101, respectively, by the Mann-Whitney rank sum test). The presence of outlier data points for both groups of healthy kittens and limitations of sample size within each subgroup prompted subsequent analysis of these data as a combined data set. When data for all live and deceased kittens were combined, a significantly greater burden of aEPEC was identified in kittens with diarrhea (Fig. 3) (P = 0.013, Mann-Whitney rank sum test).
FIG 3.
Estimated number of aEPEC CFU per 100 mg feces from all kittens with or without diarrhea. Lines represent the medians and the interquartile ranges of the data. Kittens with diarrhea had a significantly larger quantity of aEPEC, as determined by the eae qPCR and standard curve. White symbols, live kittens; black symbols, kittens that died or were euthanized. *, P = 0.013, Mann-Whitney rank sum test.
Kitten aEPEC isolates represent diverse serotypes.
A total of 24 kitten aEPEC isolates, including 6 aEPEC isolates from 3 kittens without diarrhea and 18 aEPEC isolates from 6 kittens with diarrhea-associated mortality, were serotyped. Eight different combinations of O serotypes and H types were observed, and more than one serotype of aEPEC was isolated from 3/9 (33%) kittens. As shown in Table 2, the most commonly identified O serotype was O153. Isolates with H21 were more closely associated with diarrhea-related mortality (P = 0.052, Fisher's exact test).
TABLE 2.
Distribution of serotypes among 24 aEPEC isolates from kittens with the different clinical outcomes examined in the study
| Serotype | No. (%) of isolates |
||
|---|---|---|---|
| Total (n = 24) | From kittens without diarrhea (n = 6) | From kittens with diarrhea-associated mortality (n = 18) | |
| O153:H7 | 8 (33) | 2 (33) | 6 (33) |
| O153:H21 | 6 (25) | 0 (0) | 6 (33) |
| O108:H21 | 3 (13) | 0 (0) | 3 (17) |
| O128:H2 | 3 (13) | 3 (50) | 0 (0) |
| O4:H+ | 1 (4) | 0 (0) | 1 (6) |
| O4:H5 | 1 (4) | 0 (0) | 1 (6) |
| O111:H8 | 1 (4) | 0 (0) | 1 (6) |
| O153:H− | 1 (4) | 1 (17) | 0 (0) |
Kitten aEPEC isolates are genotypically diverse.
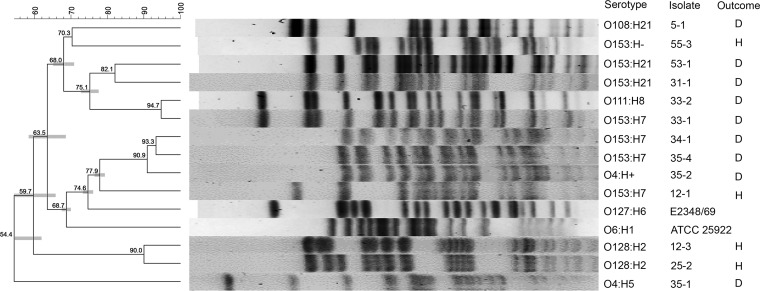
Pulsed-field gel electrophoresis (PFGE), performed on each isolate with a unique serotype recovered from each kitten, revealed a remarkable degree of genetic diversity (Fig. 4). No significant associations between the pulsotypes of aEPEC isolates from apparently healthy kittens and the pulsotypes of aEPEC isolates from kittens that died or were euthanized due to diarrhea were observed. A discordance between pulsotype and serotype was observed for several isolates.
FIG 4.
Pulsed-field gel electrophoresis (PFGE) of 13 aEPEC isolates obtained from 3 apparently healthy kittens euthanized by animal control and 6 kittens that died or were euthanized because of severe diarrhea while in foster care. The results for a typical EPEC strain (E2348/69) and a nonpathogenic E. coli strain (ATCC 25822) are included for comparison. The isolates were clustered using the unweighted pair group method with arithmetic means (UPGMA), and the scale on the left represents the percent similarity between each pulsotype. The serotype, isolate identifier, and clinical outcome (H, healthy; D, diarrhea) for each pulsotype are shown on the right.
Gross and microscopic lesions are common in the small intestine and colon of kittens with severe diarrhea.
On the basis of the photographic appearance of the colonic contents at autopsy, kittens that died or were euthanized due to diarrhea were confirmed to have significantly higher fecal scores than apparently healthy kittens (Table S3). Four kittens with diarrhea and one apparently healthy kitten did not have their feces scored due to a lack of stool. Gross lesions were observed at the time of autopsy in 32/34 (94%) kittens that died or were euthanized due to diarrhea and in only 7/20 (35%) apparently healthy kittens (Table S3). Gross and light microscopic lesions were significantly more common and severe in the small intestine and colon of kittens with diarrhea than apparently healthy kittens. Diarrhea was significantly associated with crypt dilation or distortion in the small intestine. Diarrhea was significantly associated with epithelial injury in the colon. Among all kittens undergoing autopsy, there was no association between India ink-marked gross lesions and any specific lesion identified on light microscopic examination of the corresponding tissue section (Table S4 and Fig. S1). No gross lesions were identified to be specifically associated with the presence of aEPEC (Table S3).
Kitten aEPEC infection is associated with a greater severity of inflammatory infiltrates in the small intestine and colon.
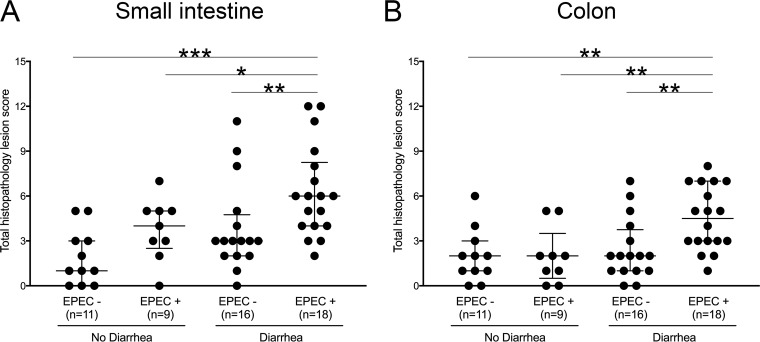
On the basis of the photographic appearance of the colon contents at the time of autopsy, kittens with evidence of aEPEC infection (i.e., kittens whose feces were eae positive and/or from whose feces aEPEC was cultured) did not have more severe diarrhea scores than kittens in which evidence of aEPEC infection was not identified (Table S3). However, kittens with diarrhea and positive culture or eae qPCR results for aEPEC had significantly higher small intestinal and colonic lesion scores than healthy kittens and kittens with diarrhea but no evidence of aEPEC, as determined by culture and qPCR (Fig. 5 and Table S3). The increase in small intestinal lesion scores in kittens with diarrhea and aEPEC was due to increased epithelial injury and increased amounts of inflammatory infiltrate in the lamina propria. The increase in colonic lesion scores in kittens with diarrhea and aEPEC was due to increased amounts of inflammatory infiltrate in the lamina propria (Fig. 6 and Table S3). Autolysis was observed in intestinal tissue sections from 33 of the 54 kittens (61%) and was significantly more common in apparently healthy kittens (Table S3).
FIG 5.
Small intestinal and colonic histopathology lesion scores based on results of fecal culture and/or qPCR for eae in apparently healthy kittens and those dying or euthanized due to severe diarrhea. Kittens with aEPEC that died with diarrhea had significantly higher small intestinal (A) and colonic (B) histopathology lesion scores than kittens with other causes of diarrhea and apparently healthy kittens with and without aEPEC. P values were determined by the Mann-Whitney rank sum test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 6.
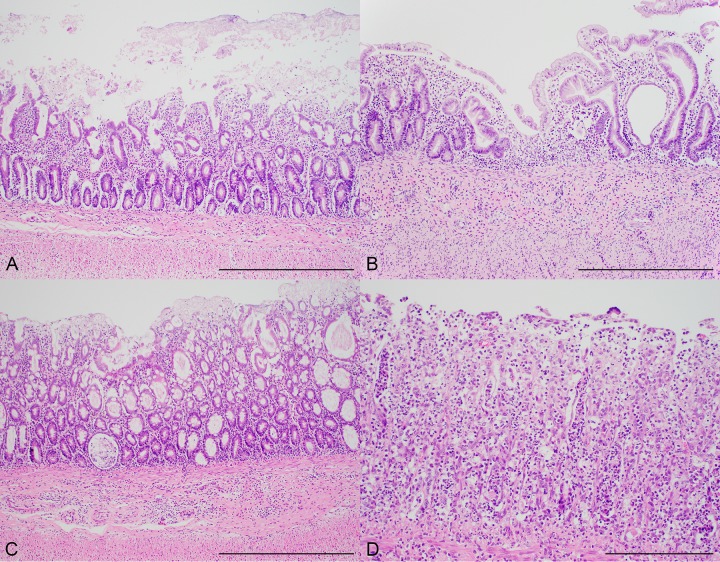
Representative photomicrographs of lesions significantly associated with the presence of aEPEC (determined by culture and/or eae qPCR) in kittens. (A) Photomicrograph of small intestine from a kitten with aEPEC demonstrating (i) epithelial cell loss and (ii) inflammatory infiltrate composed of lymphocytes and plasma cells in the lamina propria. (B) Increased lesion severity. Also noted in this panel are crypt dilation and villus blunting in the small intestine. (C) Inflammatory infiltrate in the lamina propria of the colon along with crypt dilation in a kitten with aEPEC. (D) Severe inflammation composed predominantly of neutrophils obscuring the crypts and architecture of the colon. Hematoxylin and eosin stain. Bars = 500 μm (A to C) and 200 μm (D).
Enteroadherent E. coli was demonstrated in the intestines of kittens that died or were euthanized due to diarrhea.
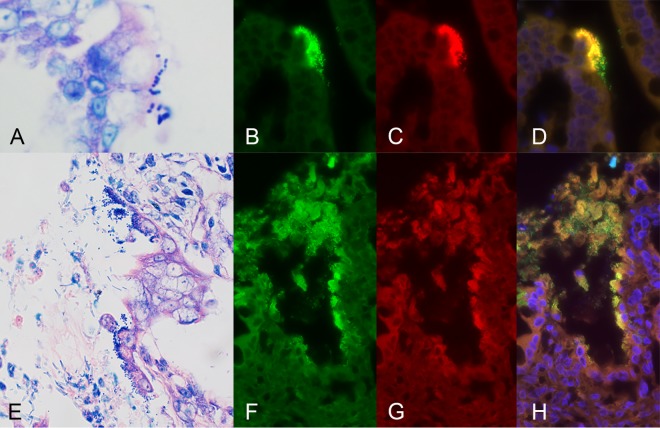
Light microscopic examination of Giemsa-stained sections of gastrointestinal tissue obtained from each kitten identified the presence of mucosa-associated bacteria and/or debris in 17/34 (50%) kittens that were euthanized or died due to severe diarrhea and 5/20 (25%) apparently healthy kittens. Using an E. coli-specific oligonucleotide probe to identify the presence of mucosa-associated E. coli by means of fluorescence in situ hybridization (FISH), enteroadherent E. coli was observed in the small intestine and colon, respectively, of two kittens that were euthanized due to diarrhea (Fig. 7). One kitten was both qPCR positive for eae and positive for aEPEC by culture, while the other kitten was only qPCR positive for eae. Other mucosa-associated bacteria that did not fluoresce with the E. coli-specific probe were identified in 6 kittens with diarrhea (spirochetes and rods) and 4 apparently healthy kittens (segmented filamentous bacteria and rods).
FIG 7.
Representative fluorescence microscopy images of focally adherent E. coli in the small intestine of one kitten (A to D) and the colon of another kitten (E to H) that were euthanized due to severe diarrhea. (A and E) Appearance of enteroadherent bacteria with Giemsa stain. Fluorescence in situ hybridization was performed using oligonucleotide probes specific for eubacterial 16S (probe Eub338; 3′ 6-FAM green) (B and F) and specific for E. coli and Shigella (5′ Cy3; red) (C and G). (D and H) Overlay of fluorescence signals. The nuclei in the specimens were counterstained with DAPI (4′,6-diamidino-2-phenylindole; blue).
There was no association between aEPEC infection and intestinal parasitism.
Gastrointestinal parasites were identified in 28/51 (55%) autopsied kittens. Three kittens that died or were euthanized due to diarrhea did not contain enough feces to perform parasite evaluations. Intestinal parasites were more often identified in the apparently healthy kittens than in kittens that were euthanized or died due to diarrhea (Table S5 and Fig. S2). This difference was attributed to the less frequent administration of preventative medications to euthanized healthy kittens, as documented in their medical records. The parasites identified in kittens that were culture or eae qPCR positive for aEPEC infection included Toxocara cati in 10/27 (37%) kittens and Isospora spp. in 8/27 (30%) kittens. There was not a statistically significant association between aEPEC infection and the presence of intestinal parasites.
Kittens with aEPEC were significantly more likely to require administration of parenteral fluids.
To determine if any individual, environmental, clinical, or therapeutic variables were associated with aEPEC infection, all objective data that could be obtained from the medical record of each kitten was examined. A summary of these variables and their association with the combined results of aEPEC culture and the eae qPCR for each group of kittens is shown in Table S6. Live kittens with aEPEC were significantly more likely to have a decreased appetite and to have required administration of parenteral fluids. When all live and deceased kittens were combined into groups on the basis of their aEPEC culture and eae qPCR results, factors significantly increasing the odds of aEPEC infection were not identified. However, kittens with aEPEC were significantly more likely to have required parenteral fluid administration (odds ratio, 3.36; 95% confidence interval, 1.34 to 8.41; P = 0.015). The specific medications given to kittens within each category are shown in Table S7. Antemortem testing of feces for the presence of feline panleukopenia virus antigen was performed for 68% (23/34) of the kittens with diarrhea-related mortality, and 4 kittens tested positive. There was no significant association between a diagnosis of panleukopenia virus infection and the presence of aEPEC in kittens that underwent testing for this virus.
DISCUSSION
This study identified that 40% of kittens from two different shelter environments in the United States sampled postmortem shed isolates of diarrheagenic Escherichia coli (DEC) in their feces. Most of the DEC isolates recovered from these kittens were identified to be positive for the virulence marker eae or cnf1. We chose to focus on further investigation of the eae-positive E. coli isolates based on the findings of our prior study, in which these bacteria were observed to colonize the intestinal epithelium of 18% of kittens that died and 0% of healthy kittens (18). Because we had no precedent observations regarding cnf1-positive E. coli and there was not a compelling association between diarrhea-related death and the presence of cnf1-positive E. coli in this study, we did not investigate this pathotype further. That said, given the high prevalence of cnf1-positive E. coli isolates observed in these kittens (21%), as well as in others (27, 28), and their established role in extraintestinal infections (29), further study of their contribution to diarrheal disease and especially nondiarrheal causes of death in kittens is warranted.
On the basis of the positive results of fecal culture, 18% of kittens in this study were identified to be colonized by aEPEC. This prevalence is higher than that reported by previous studies of EPEC infection in cats (19, 20, 22) and is likely due to our focus on kittens. In developing countries, the prevalence of EPEC infection in children with diarrhea is 8% and the hazard of diarrhea-related death in children with tEPEC is the greatest for infants under the age of 11 months (2, 30, 31). In developed countries, the prevalence of aEPEC in children ranges from 5 to 25% and is the highest in infants ages 0 to 12 months (10, 32–36). In this study, aEPEC was cultured from the feces of apparently healthy kittens, live kittens with diarrhea, and kittens that died or were euthanized due to severe clinical signs of diarrhea. This finding is similar to that from some reports describing the detection of aEPEC in children, where the prevalence of infection is similar in individuals with diarrhea and those without diarrhea (10, 37).
In this study, a nonsignificantly higher percentage of kittens with diarrhea than kittens without diarrhea was culture positive for aEPEC. Additionally, 100% of E. coli isolates cultured from the feces of aEPEC-positive kittens with diarrhea-associated mortality were identified to be aEPEC. Therefore, to investigate whether diarrhea could be related to the burden of aEPEC infection, we developed a qPCR assay for the quantification of eae in DNA extracted from the feces of kittens. As a surrogate for the detection of aEPEC, eae was amplified from the feces of 42% of kittens in the study, which is a percentage much larger than that identified by culture of feces for aEPEC. Similar to the fecal culture results, the percentage of kittens that were positive for eae did not differ significantly between apparently healthy kittens and kittens with diarrhea. However, on the basis of the qPCR for eae, there was a significantly larger quantity of aEPEC in the feces of kittens with diarrhea when both live and deceased kittens with diarrhea were considered as a single data set. These findings indirectly suggest that larger quantities of aEPEC are associated with diarrhea and diarrhea-related mortality in kittens. These results are similar to those of studies of children where the EPEC load, as measured by qPCR, was higher in individuals with diarrhea than in those with asymptomatic EPEC infection (38). It is tempting to propose the utilization of qPCR for eae as an alternative to the more laborious fecal culture techniques otherwise needed to diagnose EPEC infection. This approach has been applied to insightful epidemiological studies of EPEC infection in children (38, 39). Unfortunately, a high prevalence of eae positivity in kittens and children with and without diarrhea precludes the use of qPCR for the determination of disease causation. This is particularly true in kittens and children with clinical disease, in which a myriad of potential infectious agents may be responsible for diarrhea and positive results of qPCR cannot differentiate live versus dead bacteria. The potential for horizontal transfer of the eae gene between different bacteria makes the results of the eae qPCR incompletely specific for EPEC (40). Nonetheless, on the basis of our positive aEPEC culture results, it is likely that positive qPCR amplification of eae demonstrated kittens with a recent history of aEPEC infection.
On the basis of their serotypes and pulsotypes, the kitten aEPEC isolates in this study had a high level of genetic diversity, which is similar to the findings observed for human and environmental aEPEC isolates (41, 42). Most of the identified serotypes have been previously associated with aEPEC. There is little information available for the most common serotype (O153) identified in kittens in this study, other than an association with EPEC (6, 42–44). However, a study of environmental isolates of EPEC from French coastal regions identified serotype O153:H2 to be among the serotypes with the highest number of virulence genes (42). While serotype O128 was observed only in kittens without diarrhea, it has been associated with aEPEC or enterohemorrhagic E. coli (EHEC) from humans with diarrheal disease (43, 45). A single kitten with diarrhea in this study was colonized by a serotype O111 aEPEC isolate. Strains of serotype O111 have been identified to be aEPEC, tEPEC, enteroaggregative E. coli, and non-O157 EHEC strains (46–50). This is of interest due to the ability of these aEPEC strains to arise from the loss of stx genes by EHEC or to acquire stx genes by infection with phages whose genomes encode stx (51–54). Accordingly, it is possible that kittens harbor serotypes of aEPEC that have lost and can reacquire virulence factors from EHEC. The significance of our finding that H type 21 was identified only in kittens with diarrhea is unclear. The H type of E. coli refers to the flagellin component of the flagella (55). The presence of flagellin is important for adherence and stimulation of cytokines by aEPEC in in vitro studies (56–58), and mutations in the fliC gene of O113:H21 Shiga-toxin producing E. coli lead to a decreased association with the colonic epithelium in experimentally infected mice (59). However, in that study, it is unclear if the impaired epithelial association in the mutated H21 was specific to that H type (H21).
Some studies have described the pathological lesions associated with EPEC infection in children (60–63). The main histopathological findings in children with EPEC are villus atrophy, crypt hyperplasia, enteroadherent bacteria, and mucosal inflammatory infiltrate in the small intestine (60–63). In this study, we identified a significant association between aEPEC and lesions in both the small intestine and colon of kittens that died or were euthanized due to diarrhea. Lesions were defined by the presence of epithelial injury in the small intestine and an inflammatory infiltrate in the small intestine and colon. The median inflammation score in the small intestine was higher than that in the colon, suggesting that the pathological effects of aEPEC in kittens may be more pronounced in the small intestine, similar to EPEC infection in children (60–62). Both epithelial injury and inflammatory cell infiltrate could contribute directly to the pathogenesis of aEPEC diarrhea by mechanisms related to increased paracellular permeability, intestinal malabsorption, and increased epithelial secretion. Cell culture-based studies of tEPEC pathogenesis support the presence of effector proteins that can alter the permeability of the tight junction barrier, electrolyte transport, and the apical plasma membrane microvillus architecture and function and promote the secretion of proinflammatory cytokines (64–71). The presence of the same effector proteins in aEPEC supports the likelihood of comparable pathological effects on the intestinal epithelium. While the observed histopathological lesions were significantly more severe in kittens with aEPEC infection, they lack specificity because they were also observed in kittens with diarrhea that did not have aEPEC.
On the basis of the results of FISH utilizing a DNA probe specific for E. coli, colonization of both the small intestine and colon was evidenced by palisades of E. coli isolates attached to either the villus or the crypt epithelium. We observed a lower prevalence of FISH-positive results for EPEC in this study (2/34; 6%) than in our prior study (9/50; 18%), in which kittens with all causes of mortality were examined (18). The reason for this difference is unclear but may be due to differences in the medications (such as antibiotics) administered to kittens with diarrhea or a higher prevalence of autolysis of tissues from kittens in the present study. Nonetheless, the number of FISH-positive kittens is likely a gross underestimate on the basis of the size of the samples examined (5 μm) compared to the length of the entire gastrointestinal tract (2.1 m) (72).
A significant gap in knowledge exists regarding what host or bacterial factors are responsible for why some aEPEC-infected children remain asymptomatic while others develop severe diarrhea that can result in death. Examination of the demographic, environmental, clinical, and treatment history of individual kittens from both live and deceased populations failed to disclose any factors distinguishing aEPEC-infected kittens with diarrhea from aEPEC-infected kittens without diarrhea. We hypothesized that weaning would be associated with increased susceptibility to aEPEC diarrhea due to an association with stress, altered intestinal function, and increased susceptibility to infection and disease in multiple species (73, 74). In addition, maternal milk has been shown to protect against EPEC infection in rabbits and infants (75–77). In the present study, however, nearly all of the kittens were weaned, which likely precluded our ability to meaningfully assess the impact of weaning on susceptibility to aEPEC diarrhea.
Given the myriad medications administered to kittens in this study for the treatment of many ostensible causes for diarrhea, it is remarkable that kittens diagnosed with aEPEC infection were significantly more likely than other kittens to have required parenteral fluid administration. This finding implies that kittens with aEPEC infection were likely to have dehydration of a severity greater than that in kittens with other causes of diarrhea. The greater severity of dehydration parallels the association of aEPEC with intestinal histopathological lesions of increased severity and suggests greater fecal water loss in kittens with aEPEC diarrhea.
Several limitations to this study are worth mentioning. First, the study was undertaken with kittens under naturally occurring conditions from two different shelter facilities with different population sizes, processing procedures, and foster care policies. While adding considerable extraneous variability to the study, we did not find any statistically significant differences in aEPEC infection in kittens on the basis of shelter identity. Second, in both live and deceased kitten populations, apparently healthy kittens spent a significantly shorter time in foster care and received fewer medications and preventatives than kittens with diarrhea. In healthy kittens, fecal samples were mostly obtained within 24 h of shelter intake, prior to administration of preventatives, and then kittens were transferred to foster care (live kittens) or euthanized due to their feral nature, overpopulation, or health concerns arising due to the death of their littermates (deceased kittens). In contrast, kittens with diarrhea were returned dead or alive to the shelter by their foster caregiver for clinical examination by the shelter veterinarian. Despite these limitations, we observed no significant association between the presence of aEPEC infection and the number of days in foster care or the duration of time between death and autopsy. Finally, with the exception of the parasitological and histopathological examinations, we did not conduct an exhaustive diagnostic investigation for other infectious culprits of diarrhea in kittens with or without aEPEC. Accordingly, the results of our study do not identify aEPEC to be a definitive cause of diarrhea or diarrheal death in the kittens reported on here.
Overall, this study demonstrates a strong association between aEPEC infection and the presence of histopathological lesions of epithelial injury and inflammatory infiltrate in the intestinal tract and an increased need for parenteral fluid administration in shelter kittens. An increased burden of aEPEC infection, on the basis of the result of qPCR for eae, was significantly associated with diarrhea and related mortality. These findings indicate that aEPEC is likely an important contributor to intestinal disease in kittens. In many ways, the prevalence, population demographics, circumstances, and clinical observations for kittens with aEPEC infection mirror those for children with aEPEC infection. Accordingly, studies of the infection in kittens may offer unique insights into host and bacterial factors responsible for differences in disease susceptibility and lead to novel treatment approaches to ameliorate the negative impact of aEPEC infection in both kittens and children.
MATERIALS AND METHODS
Kitten case selection.
Kittens that were ≤12 weeks of age, ≤1 kg of body weight, unrelated, and housed separately were prospectively identified at two independent shelter facilities over a period of 2 years. During phase I of the study, fecal samples were collected from live kittens with and without clinical signs of diarrhea. During phase II of the study, kittens that died or were euthanized due to severe clinical signs of diarrhea were selected. A cohort of apparently healthy kittens that were euthanized for non-health-related reasons was selected as a control group. No kittens were euthanized for the purpose of the study. For all kittens, medical records were obtained when available. The study was approved by the North Carolina State University Institutional Animal Care and Use Committee.
Fecal sample collection and culture isolation of E. coli.
Fecal samples were collected by shelter personnel (phase I) or were collected from each kitten at the time of autopsy by study investigators (phase II). Feces (live kittens) and rectal contents (kittens that died) were additionally swabbed by shelter personnel for the preservation of E. coli. Samples and swabs in Cary-Blair transport medium (Becton, Dickinson and Company, Franklin Lakes, NJ) were transported to the laboratory on ice packs within 24 h of collection. Culture swabs were streaked onto MacConkey agar for isolation of bacteria and incubated at 37°C overnight for detection of enteric, Gram-negative bacteria. For each sample, 12 morphologically distinct, lactose-positive bacterial colonies were subcultured onto blood agar plates. Subcultures identified to be indole positive, pyrrolidonyl arylamidase test negative, and oxidase negative by dry slides (Becton, Dickinson and Company, Franklin Lakes, NJ) were determined to be E. coli. Isolates of E. coli were frozen in Luria-Bertani broth (LB)–glycerol at −80°C.
Identification of EPEC isolates cultured from live kittens.
Isolates of E. coli were heated at 100°C for 30 min for DNA extraction. Conventional PCR was performed to identify the presence of the intimin gene (eae). If the isolate was positive for eae, it was additionally tested for the presence of genes encoding bundle-forming pilus (bfp) and Shiga toxins 1 and 2 (stx1, stx2). All conventional PCR analyses were performed using published primer sequences and reaction conditions and AmpliTaq Gold DNA polymerase (Thermo Fisher Scientific, Waltham, MA) (25, 26, 78–80). All EPEC isolates were further confirmed to be E. coli by use of a matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) analyzer (Vitek MS; bioMérieux, Marcy l'Etoile, France) as previously described for Gram-negative bacteria (81).
Multiplex PCR for E. coli virulence gene detection.
Individual isolates of E. coli obtained during phase II of the study were shipped overnight on LB agar for testing by means of multiplex PCR (E. coli Reference Center, Pennsylvania State University, University Park, PA) for the presence of virulence genes encoding intimin (eae), Shiga toxins 1 and 2 (stx1, stx2), heat-stable and heat-labile toxins (STa, STb, LT), invasion plasmid antigen H (ipaH), enteroaggregative plasmid pCVD432 (82), and cytotoxic necrotizing factors 1 and 2 (cnf1, cnf2) using published protocols (83). Conventional PCR for identification of bfp was performed as previously described (26).
Fecal DNA extraction.
DNA was extracted from 100-mg fecal samples using a commercial kit (Zymo Research, Irvine, CA) as previously described and stored at −80°C (84).
Quantitative PCR.
Quantitative PCR was used to amplify bacterial 16S and E. coli 16S rRNA genes from extracts of fecal DNA. The primers used for bacterial 16S rRNA were previously published (85). The primers used for E. coli 16S rRNA were forward primer 5′-CATGCCGCGTGTATGAAGAA-3′ and reverse primer 5′-CGGGTAACGTCAATGAGCAAA-3′. The primers were used at a concentration of 0.5 μM in a 20-μl reaction volume with a commercially available qPCR master mix (PerfeCTa SYBR supermix for iQ; Quantabio, Beverly, MA). Amplifications were performed with an initial denaturation at 95°C for 2 min, followed by 30 cycles of denaturation at 95°C for 15 s, annealing at 60.7°C for 45 s, and extension at 68°C for 1 min.
A quantitative PCR assay for eae was optimized and validated for quantifying EPEC in feces. An isolate of EPEC from a kitten with diarrhea was inoculated in LB, incubated overnight at 37°C, and serially diluted 10-fold in sterile phosphate-buffered saline (101 to 108). The dilutions were simultaneously plated for counting of the number of CFU and spiked into 100-mg aliquots of feline feces prior to DNA extraction. The primers used for amplification of eae were previously published (79, 86). Amplifications were performed with an initial denaturation at 95°C for 2 min, followed by 30 cycles of denaturation at 95°C for 15 s, annealing at 50°C for 45 s, and extension at 68°C for 1 min.
Serotyping.
Serotyping of the O antigen was performed using antisera directed to all identified O antigens (O1 to O187) (87). H typing was performed by PCR amplification of the fliC (flagellar) gene, followed by analysis of HhaI restriction fragment length polymorphism (E. coli Reference Center, Pennsylvania State University, University Park, PA) as previously described (55).
Pulsed-field gel electrophoresis.
Pulsed-field gel electrophoresis was performed as described for PulseNet analysis of E. coli, Shigella, and Salmonella (88). Overnight cultures of aEPEC were equilibrated in cell suspension buffer solution to an absorbence of 1.08 to 1.1 at a 610-nm wavelength and mixed with 1% SeaKem gold agarose (Thermo Fisher Scientific, Waltham, MA) for plug formation. The plugs were sectioned to an equal size with a 2-mm width and then individually digested with the restriction enzyme XbaI (New England BioLabs, Ipswich, MA). Plugs were embedded into an agarose gel, and restriction fragments were separated by electrophoresis at 6 V/cm for 19 h. Standardization across gels was confirmed by including Salmonella enterica serotype Braenderup H9812 digested with XbaI in each gel. A nonpathogenic E. coli strain (ATTC 25922; American Type Culture Collection, Manassas, VA) and tEPEC strain E2348/69 were also included for PFGE pattern comparison. Gels were stained with a commercially available DNA stain (GelRed; Biotium, Fremont, CA) following the manufacturer's protocol and imaged with a UV imager (Bio-Rad, Hercules, CA). The band patterns produced by PFGE were evaluated with BioNumerics software (Applied Maths Inc., Austin, TX).
Autopsy and sample collection.
Deceased kittens were kept at 4°C for less than 24 h and then transported on ice from each collaborating shelter to the laboratory for autopsy. The gastrointestinal tract from the distal esophagus to the distal colon was removed and opened longitudinally to expose the entire lumen. The gastrointestinal tract and colonic contents of each kitten were photographed. Fecal samples were obtained from the colon for culture isolation of E. coli and DNA extraction. The gastrointestinal tract was then immersed in phosphate-buffered saline and shaken to dislodge the contents. Any grossly abnormal section(s) of the gastrointestinal tract was described and selectively marked for later inclusion during tissue sampling. The entire gastrointestinal tract was then submerged in 10% neutral buffered formalin for a minimum of 24 to 48 h. The gastrointestinal contents and remaining feces were stored at 4°C until they were utilized for parasitological examination.
Fecal scoring.
Fecal scores were assigned by 3 individuals blind to the kitten's health status, utilizing photographs of the colonic content that were taken at the time of autopsy. The fecal score was calculated on the basis of a published scale ranging from 1 (very hard and dry) to 7 (watery with no texture) (89). A median fecal score was calculated from the score of each individual. If the colon was empty, perineal soiling was considered representative of severe diarrhea and was given a score of 7. No score was assigned if the colon was empty and no perineal soiling was present.
Histopathology.
Full-thickness samples of the stomach (fundus and antrum), proximal duodenum, midjejunum, ileum, and colon (proximal and distal) were obtained from the formalin-fixed gastrointestinal tract of each kitten. Any grossly abnormal tissue that was identified at autopsy was also included and marked with India ink. The sampled tissues were paraffin embedded, sectioned at a thickness of 5 μm, and stained with hematoxylin and eosin or Giemsa. Microscopic examination of each tissue was performed by an American College of Veterinary Pathologists-boarded pathologist (V.E.W.) that was blind to the identity of each kitten. Hematoxylin- and eosin-stained sections from each gastrointestinal region were examined and scored on the basis of a simplified histopathological model for determining gastrointestinal inflammation (90). Each lesion was scored as absent (score, 0), mild (score, 1), moderate (score, 2), or severe (score, 3). In addition to scoring the inflammation, sections were evaluated for lesions consistent with or indicative of a specific gastrointestinal disease or etiology. The severity of autolysis was recorded using the following guidelines: lifting of epithelial cells from the villi (mild), sloughed epithelial cells (moderate), and hypereosinophilia with a loss of tissue architecture (severe). Giemsa-stained sections were specifically evaluated for the presence of bacteria in close association with the brush border of the intestinal epithelium.
FISH.
Formalin-fixed and paraffin-embedded 5-μm sections of gastrointestinal tissue from each kitten were mounted on poly-l-lysine-coated slides, and fluorescence in situ hybridization (FISH) was performed as previously described (18, 91–93). The probes used for hybridizations included a universal eubacterial probe, Eub338 (5′-GCTGCCTCCCGTAGGAGT-6-FAM-3′, where 6-FAM is 6-carboxyfluorescein) (92); a probe specific for E. coli and Shigella (5′-Cy3-GCAAAGGTATTAACTTTACTCCC-3′) (92); and a negative-control non-Eub probe (5′-Cy3-CGACGGAGGGCQTCCTCA-3′). The probes were reconstituted with sterile water and diluted with hybridization buffer to final working concentrations of 5 ng/μl. Formalin-fixed and paraffin-embedded intestinal tissue from a puppy diagnosed with enteroadherent E. coli was included in each hybridization experiment as a positive control.
Parasitological examination.
Gastrointestinal contents were grossly examined for the presence of helminths using a dissecting microscope. Collected helminths were fixed and processed as previously described (94). Three parasitological techniques were utilized for analysis of each fecal sample. For direct fecal microscopic examination, a scant amount of feces (micrograms) was mixed in 0.9% saline on a microscope slide and covered with a 22- by 22-mm square coverslip. The sample was systematically examined at ×100 magnification with a compound microscope for the detection of helminth ova and larvae and again at ×400 magnification for the detection of protozoan cysts and trophozoites. Centrifugation of fecal material was used for detection of coccidian oocysts and nematode and cestode ova as previously detailed (95). Fecal material (≤1 g) was mixed in sodium nitrate flotation solution at a specific gravity of 1.20, strained, and centrifuged for 5 min. After centrifugation, additional flotation solution was added to the tube mixture until a meniscus formed. A 22- by 22-mm square coverslip was added to the meniscus and allowed to stand for 10 min. The coverslip was transferred to a microscope slide, and the sample was systematically examined for helminth ova at ×100 magnification. Fecal sedimentation was performed for detection of trematode eggs and nematode larvae. Fecal material (≤1 g) was mixed in 0.9% saline, strained, and centrifuged for 5 min. After centrifugation, sediment from the bottom of the centrifuge tube was transferred with a Pasteur pipette to a microscope slide. The sample was covered with a 22- by 40-mm coverslip and systematically examined for helminth ova and nematode larvae at ×100 magnification. The transfer and examination of sediment from the bottom of the centrifuge tube were repeated until all the sediment was examined.
Medical record review.
If available, individual medical records were reviewed. Recorded parameters included age, sex, weaning status, presence and nature of antemortem clinical signs, the medications and vaccinations administered, results of testing for fecal panleukopenia virus antigen, whether the kitten was euthanized or died, the length of time in foster care, the source (shelter), and body weight.
Statistical analysis.
Discrete data were analyzed for significant differences in observations between groups (number of kittens) by the χ2 test and determination of the odds ratio or Fisher's exact test. Continuous data were analyzed for significant differences in mean values between groups using Student's t test and median values between groups using the Mann-Whitney rank sum test. Continuous data were graphed as vertical point plots. The linear correlation between variables was determined using the linear regression line of plotted data as well as Pearson product moment correlation tests. Statistical analyses were performed using commercial software (SigmaPlot, version 12; Systat Software, Inc., San Jose, CA), and significance was assigned a P value of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
The research reported in this publication was supported by grants from the Winn Feline Foundation (grant numbers W14-1616 and W11-012). V.E.W. was supported by a Ruth L. Kirschstein National Research Service Award through the National Institutes of Health (NIH) under grant number T32OD011130 and as part of North Carolina State University's Comparative Medicine and Translational Research Training Program.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
We thank the Wake County Animal Center and Wake County Society for the Prevention of Cruelty to Animals for their invaluable assistance in acquiring cases and samples from the kittens. We also thank Allison Baker, Ashley Williams, and Stephen Stauffer for their technical support.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00403-17.
REFERENCES
- 1.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE. 2012. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 3.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. 2010. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 4.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Dopfer D, Fazil A, Fischer-Walker CL, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ. 2015. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 12:e1001921. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochoa TJ, Contreras CA. 2011. Enteropathogenic Escherichia coli infection in children. Curr Opin Infect Dis 24:478–483. doi: 10.1097/QCO.0b013e32834a8b8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandes RT, Elias WP, Vieira MA, Gomes TA. 2009. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol Lett 297:137–149. doi: 10.1111/j.1574-6968.2009.01664.x. [DOI] [PubMed] [Google Scholar]
- 7.Hu J, Torres AG. 2015. Enteropathogenic Escherichia coli: foe or innocent bystander? Clin Microbiol Infect 21:729–734. doi: 10.1016/j.cmi.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afset JE, Bevanger L, Romundstad P, Bergh K. 2004. Association of atypical enteropathogenic Escherichia coli (EPEC) with prolonged diarrhoea. J Med Microbiol 53:1137–1144. doi: 10.1099/jmm.0.45719-0. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen RN, Taylor LS, Tauschek M, Robins-Browne RM. 2006. Atypical enteropathogenic Escherichia coli infection and prolonged diarrhea in children. Emerg Infect Dis 12:597–603. doi: 10.3201/eid1204.051112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vernacchio L, Vezina RM, Mitchell AA, Lesko SM, Plaut AG, Acheson DW. 2006. Diarrhea in American infants and young children in the community setting: incidence, clinical presentation and microbiology. Pediatr Infect Dis J 25:2–7. doi: 10.1097/01.inf.0000195623.57945.87. [DOI] [PubMed] [Google Scholar]
- 11.Contreras CA, Ochoa TJ, Ruiz J, Lacher DW, Durand D, DebRoy C, Lanata CF, Cleary TG. 2012. Genetic diversity of locus of enterocyte effacement genes of enteropathogenic Escherichia coli isolated from Peruvian children. J Med Microbiol 61:1114–1120. doi: 10.1099/jmm.0.045443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochoa TJ, Ecker L, Barletta F, Mispireta ML, Gil AI, Contreras C, Molina M, Amemiya I, Verastegui H, Hall ER, Cleary TG, Lanata CF. 2009. Age-related susceptibility to infection with diarrheagenic Escherichia coli among infants from periurban areas in Lima, Peru. Clin Infect Dis 49:1694–1702. doi: 10.1086/648069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cave TA, Thompson H, Reid SW, Hodgson DR, Addie DD. 2002. Kitten mortality in the United Kingdom: a retrospective analysis of 274 histopathological examinations (1986 to 2000). Vet Rec 151:497–501. doi: 10.1136/vr.151.17.497. [DOI] [PubMed] [Google Scholar]
- 14.New JC Jr, Kelch WJ, Hutchison JM, Salman MD, King M, Scarlett JM, Kass PH. 2004. Birth and death rate estimates of cats and dogs in U.S. households and related factors. J Appl Anim Welf Sci 7:229–241. doi: 10.1207/s15327604jaws0704_1. [DOI] [PubMed] [Google Scholar]
- 15.Kitts-Morgan SE. 2015. Companion animals symposium: sustainable ecosystems: domestic cats and their effect on wildlife populations. J Anim Sci 93:848–859. doi: 10.2527/jas.2014-8557. [DOI] [PubMed] [Google Scholar]
- 16.Nutter FB, Levine JF, Stoskopf MK. 2004. Reproductive capacity of free-roaming domestic cats and kitten survival rate. J Am Vet Med Assoc 225:1399–1402. doi: 10.2460/javma.2004.225.1399. [DOI] [PubMed] [Google Scholar]
- 17.Sparkes AH, Rogers K, Henley WE, Gunn-Moore DA, May JM, Gruffydd-Jones TJ, Bessant C. 2006. A questionnaire-based study of gestation, parturition and neonatal mortality in pedigree breeding cats in the UK. J Feline Med Surg 8:145–157. doi: 10.1016/j.jfms.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh A, Stauffer SH, Borst L, Suyemoto M, Moisan P, Zurek L, Gookin JL. 2013. Mortality in kittens is associated with a shift in ileum mucosa-associated enterococci from Enterococcus hirae to biofilm-forming Enterococcus faecalis and adherent Escherichia coli. J Clin Microbiol 51:3567. doi: 10.1128/JCM.00481-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krause G, Zimmermann S, Beutin L. 2005. Investigation of domestic animals and pets as a reservoir for intimin- (eae) gene positive Escherichia coli types. Vet Microbiol 106:87–95. doi: 10.1016/j.vetmic.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Morato EP, Leomil L, Beutin L, Krause G, Moura RA, Pestana de Castro AF. 2009. Domestic cats constitute a natural reservoir of human enteropathogenic Escherichia coli types. Zoonoses Public Health 56:229–237. doi: 10.1111/j.1863-2378.2008.01190.x. [DOI] [PubMed] [Google Scholar]
- 21.Goffaux F, China B, Janssen L, Mainil J. 2000. Genotypic characterization of enteropathogenic Escherichia coli (EPEC) isolated in Belgium from dogs and cats. Res Microbiol 151:865–871. doi: 10.1016/S0923-2508(00)01153-0. [DOI] [PubMed] [Google Scholar]
- 22.Puno-Sarmiento J, Medeiros L, Chiconi C, Martins F, Pelayo J, Rocha S, Blanco J, Blanco M, Zanutto M, Kobayashi R, Nakazato G. 2013. Detection of diarrheagenic Escherichia coli strains isolated from dogs and cats in Brazil. Vet Microbiol 166:676–680. doi: 10.1016/j.vetmic.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Pospischil A, Mainil JG, Baljer G, Moon HW. 1987. Attaching and effacing bacteria in the intestines of calves and cats with diarrhea. Vet Pathol 24:330–334. doi: 10.1177/030098588702400407. [DOI] [PubMed] [Google Scholar]
- 24.Mian KA. 1959. Isolation of enteropathogenic Escherichia coli from household pets: relation to infantile diarrhea. JAMA 171:1957–1961. doi: 10.1001/jama.1959.03010320047011. [DOI] [Google Scholar]
- 25.Jerse AE, Yu J, Tall BD, Kaper JB. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A 87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunzburg ST, Tornieporth NG, Riley LW. 1995. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J Clin Microbiol 33:1375–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanco J, Blanco M, Wong I, Blanco JE. 1993. Haemolytic Escherichia coli strains isolated from stools of healthy cats produce cytotoxic necrotizing factor type 1 (CNF1). Vet Microbiol 38:157–165. doi: 10.1016/0378-1135(93)90082-I. [DOI] [PubMed] [Google Scholar]
- 28.Beutin L. 1999. Escherichia coli as a pathogen in dogs and cats. Vet Res 30:285–298. [PubMed] [Google Scholar]
- 29.Sura R, Van Kruiningen HJ, DebRoy C, Hinckley LS, Greenberg KJ, Gordon Z, French RA. 2007. Extraintestinal pathogenic Escherichia coli-induced acute necrotizing pneumonia in cats. Zoonoses Public Health 54:307–313. doi: 10.1111/j.1863-2378.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 30.Ochoa TJ, Mercado EH, Durand D, Rivera FP, Mosquito S, Contreras C, Riveros M, Lluque A, Barletta F, Prada A, Ruiz J. 2011. Frequency and pathotypes of diarrheagenic Escherichia coli in Peruvian children with and without diarrhea. Rev Peru Med Exp Salud Publica 28:13–20. doi: 10.1590/S1726-46342011000100003 (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 31.Dias RCB, dos Santos BC, dos Santos LF, Vieira MA, Yamatogi RS, Mondelli AL, Sadatsune T, Sforcin JM, Gomes T, Hernandes RT. 2016. Diarrheagenic Escherichia coli pathotypes investigation revealed atypical enteropathogenic E. coli as putative emerging diarrheal agents in children living in Botucatu, São Paulo State, Brazil. APMIS 124:299–308. doi: 10.1111/apm.12501. [DOI] [PubMed] [Google Scholar]
- 32.Paciorek J. 2002. Virulence properties of Escherichia coli faecal strains isolated in Poland from healthy children and strains belonging to serogroups O18, O26, O44, O86, O126 and O127 isolated from children with diarrhoea. J Med Microbiol 51:548–556. doi: 10.1099/0022-1317-51-7-548. [DOI] [PubMed] [Google Scholar]
- 33.Beutin L, Marches O, Bettelheim KA, Gleier K, Zimmermann S, Schmidt H, Oswald E. 2003. HEp-2 cell adherence, actin aggregation, and intimin types of attaching and effacing Escherichia coli strains isolated from healthy infants in Germany and Australia. Infect Immun 71:3995–4002. doi: 10.1128/IAI.71.7.3995-4002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Araujo JM, Tabarelli GF, Aranda KR, Fabbricotti SH, Fagundes-Neto U, Mendes CM, Scaletsky IC. 2007. Typical enteroaggregative and atypical enteropathogenic types of Escherichia coli are the most prevalent diarrhea-associated pathotypes among Brazilian children. J Clin Microbiol 45:3396–3399. doi: 10.1128/JCM.00084-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santona S, Diaz N, Fiori PL, Francisco M, Sidat M, Cappuccinelli P, Rappelli P. 2013. Genotypic and phenotypic features of enteropathogenic Escherichia coli isolated in industrialized and developing countries. J Infect Dev Ctries 7:214–219. doi: 10.3855/jidc.3054. [DOI] [PubMed] [Google Scholar]
- 36.Sakkejha H, Byrne L, Lawson AJ, Jenkins C. 2013. An update on the microbiology and epidemiology of enteropathogenic Escherichia coli in England 2010-2012. J Med Microbiol 62:1531–1534. doi: 10.1099/jmm.0.062380-0. [DOI] [PubMed] [Google Scholar]
- 37.Knutton S, Shaw R, Phillips AD, Smith HR, Willshaw GA, Watson P, Price E. 2001. Phenotypic and genetic analysis of diarrhea-associated Escherichia coli isolated from children in the United Kingdom. J Pediatr Gastroenterol Nutr 33:32–40. doi: 10.1097/00005176-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Barletta F, Ochoa TJ, Mercado E, Ruiz J, Ecker L, Lopez G, Mispireta M, Gil AI, Lanata CF, Cleary TG. 2011. Quantitative real-time polymerase chain reaction for enteropathogenic Escherichia coli: a tool for investigation of asymptomatic versus symptomatic infections. Clin Infect Dis 53:1223–1229. doi: 10.1093/cid/cir730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, Becker SM, Blackwelder WC, Breiman RF, Faruque AS, Fields B, Gratz J, Haque R, Hossain A, Hossain MJ, Jarju S, Qamar F, Iqbal NT, Kwambana B, Mandomando I, McMurry TL, Ochieng C, Ochieng JB, Ochieng M, Onyango C, Panchalingam S, Kalam A, Aziz F, Qureshi S, Ramamurthy T, Roberts JH, Saha D, Sow SO, Stroup SE, Sur D, Tamboura B, Taniuchi M, Tennant SM, Toema D, Wu Y, Zaidi A, Nataro JP, Kotloff KL, Levine MM, Houpt ER. 2016. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng W, Li Y, Vallance BA, Finlay BB. 2001. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect Immun 69:6323–6335. doi: 10.1128/IAI.69.10.6323-6335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingle DJ, Tauschek M, Edwards DJ, Hocking DM, Pickard DJ, Azzopardi KI, Amarasena T, Bennett-Wood V, Pearson JS, Tamboura B, Antonio M, Ochieng JB, Oundo J, Mandomando I, Qureshi S, Ramamurthy T, Hossain A, Kotloff KL, Nataro JP, Dougan G, Levine MM, Robins-Browne RM, Holt KE. 2016. Evolution of atypical enteropathogenic E. coli by repeated acquisition of LEE pathogenicity island variants. Nat Microbiol 1:15010. doi: 10.1038/nmicrobiol.2015.10. [DOI] [PubMed] [Google Scholar]
- 42.Baliere C, Rince A, Delannoy S, Fach P, Gourmelon M. 2016. Molecular profiling of Shiga toxin-producing Escherichia coli and enteropathogenic E. coli strains isolated from French coastal environments. Appl Environ Microbiol 82:3913–3927. doi: 10.1128/AEM.00271-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tennant SM, Tauschek M, Azzopardi K, Bigham A, Bennett-Wood V, Hartland EL, Qi W, Whittam TS, Robins-Browne RM. 2009. Characterisation of atypical enteropathogenic E. coli strains of clinical origin. BMC Microbiol 9:117. doi: 10.1186/1471-2180-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tilak GP, Mudaliar JL. 2012. Role of enteropathogenic Escherichia coli in paediatric diarrhoeas in South India. Mater Sociomed 24:178–181. doi: 10.5455/msm.2012.24.178-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whittam TS, Wolfe ML, Wachsmuth IK, Orskov F, Orskov I, Wilson RA. 1993. Clonal relationships among Escherichia coli strains that cause hemorrhagic colitis and infantile diarrhea. Infect Immun 61:1619–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen S, Sanderson MW, Lee C, Cernicchiaro N, Renter DG, Lanzas C. 2016. Basic reproduction number and transmission dynamics of common serogroups of enterohemorrhagic Escherichia coli. Appl Environ Microbiol 82:5612–5620. doi: 10.1128/AEM.00815-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Stanford K, McAllister TA, Johnson RP, Chen J, Hou H, Zhang G, Niu YD. 2016. Biofilm formation, virulence gene profiles, and antimicrobial resistance of nine serogroups of non-O157 Shiga toxin-producing Escherichia coli. Foodborne Pathog Dis 13:316–324. doi: 10.1089/fpd.2015.2099. [DOI] [PubMed] [Google Scholar]
- 48.Brooks JT, Sowers EG, Wells JG, Greene KD, Griffin PM, Hoekstra RM, Strockbine NA. 2005. Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983-2002. J Infect Dis 192:1422–1429. doi: 10.1086/466536. [DOI] [PubMed] [Google Scholar]
- 49.Botelho BA, Bando SY, Trabulsi LR, Moreira-Filho CA. 2003. Identification of EPEC and non-EPEC serotypes in the EPEC O serogroups by PCR-RFLP analysis of the fliC gene. J Microbiol Methods 54:87–93. doi: 10.1016/S0167-7012(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 50.Scotland SM, Willshaw GA, Smith HR, Gross RJ, Rowe B. 1989. Adhesion to cells in culture and plasmid profiles of enteropathogenic Escherichia coli isolated from outbreaks and sporadic cases of infant diarrhoea. J Infect 19:237–249. doi: 10.1016/S0163-4453(89)90729-9. [DOI] [PubMed] [Google Scholar]
- 51.Hazen TH, Sahl JW, Fraser CM, Donnenberg MS, Scheutz F, Rasko DA. 2013. Refining the pathovar paradigm via phylogenomics of the attaching and effacing Escherichia coli. Proc Natl Acad Sci U S A 110:12810–12815. doi: 10.1073/pnas.1306836110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Z, Li X, Liu B, Beutin L, Xu J, Ren Y, Feng L, Lan R, Reeves PR, Wang L. 2010. Derivation of Escherichia coli O157:H7 from its O55:H7 precursor. PLoS One 5:e8700. doi: 10.1371/journal.pone.0008700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wick LM, Qi W, Lacher DW, Whittam TS. 2005. Evolution of genomic content in the stepwise emergence of Escherichia coli O157:H7. J Bacteriol 187:1783–1791. doi: 10.1128/JB.187.5.1783-1791.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bielaszewska M, Prager R, Kock R, Mellmann A, Zhang W, Tschape H, Tarr PI, Karch H. 2007. Shiga toxin gene loss and transfer in vitro and in vivo during enterohemorrhagic Escherichia coli O26 infection in humans. Appl Environ Microbiol 73:3144–3150. doi: 10.1128/AEM.02937-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Machado J, Grimont F, Grimont PA. 2000. Identification of Escherichia coli flagellar types by restriction of the amplified fliC gene. Res Microbiol 151:535–546. doi: 10.1016/S0923-2508(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 56.Sampaio SC, Gomes TA, Pichon C, du Merle L, Guadagnini S, Abe CM, Sampaio JL, Le Bouguenec C. 2009. The flagella of an atypical enteropathogenic Escherichia coli strain are required for efficient interaction with and stimulation of interleukin-8 production by enterocytes in vitro. Infect Immun 77:4406–4413. doi: 10.1128/IAI.00177-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sampaio SC, Luiz WB, Vieira MA, Ferreira RC, Garcia BG, Sinigaglia-Coimbra R, Sampaio JL, Ferreira LC, Gomes TA. 2016. Flagellar cap protein FliD mediates adherence of atypical enteropathogenic Escherichia coli to enterocyte microvilli. Infect Immun 84:1112–1122. doi: 10.1128/IAI.01001-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moraes CT, Polatto JM, Rossato SS, Izquierdo M, Munhoz DD, Martins FH, Pimenta DC, Farfan MJ, Elias WP, Barbosa AS, Piazza RM. 2015. Flagellin and GroEL mediates in vitro binding of an atypical enteropathogenic Escherichia coli to cellular fibronectin. BMC Microbiol 15:278. doi: 10.1186/s12866-015-0612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rogers TJ, Paton JC, Wang H, Talbot UM, Paton AW. 2006. Reduced virulence of an fliC mutant of Shiga-toxigenic Escherichia coli O113:H21. Infect Immun 74:1962–1966. doi: 10.1128/IAI.74.3.1962-1966.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill SM, Phillips AD, Walker-Smith JA. 1991. Enteropathogenic Escherichia coli and life threatening chronic diarrhoea. Gut 32:154–158. doi: 10.1136/gut.32.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rothbaum R, McAdams AJ, Giannella R, Partin JC. 1982. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology 83:441–454. [PubMed] [Google Scholar]
- 62.Ulshen MH, Rollo JL. 1980. Pathogenesis of Escherichia coli gastroenteritis in man—another mechanism. N Engl J Med 302:99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- 63.Fagundes-Neto U, Kallas MR, Patricio FR. 1997. Morphometric study of the small bowel mucosa in infants with diarrhea due to enteropathogenic Escherichia coli strains. Hepatogastroenterology 44:1051–1056. [PubMed] [Google Scholar]
- 64.Tomson FL, Viswanathan VK, Kanack KJ, Kanteti RP, Straub KV, Menet M, Kaper JB, Hecht G. 2005. Enteropathogenic Escherichia coli EspG disrupts microtubules and in conjunction with Orf3 enhances perturbation of the tight junction barrier. Mol Microbiol 56:447–464. doi: 10.1111/j.1365-2958.2005.04571.x. [DOI] [PubMed] [Google Scholar]
- 65.Shaw RK, Smollett K, Cleary J, Garmendia J, Straatman-Iwanowska A, Frankel G, Knutton S. 2005. Enteropathogenic Escherichia coli type III effectors EspG and EspG2 disrupt the microtubule network of intestinal epithelial cells. Infect Immun 73:4385–4390. doi: 10.1128/IAI.73.7.4385-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thanabalasuriar A, Koutsouris A, Weflen A, Mimee M, Hecht G, Gruenheid S. 2010. The bacterial virulence factor NleA is required for the disruption of intestinal tight junctions by enteropathogenic Escherichia coli. Cell Microbiol 12:31–41. doi: 10.1111/j.1462-5822.2009.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511–520. doi: 10.1016/S0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 68.Dean P, Kenny B. 2004. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol Microbiol 54:665–675. doi: 10.1111/j.1365-2958.2004.04308.x. [DOI] [PubMed] [Google Scholar]
- 69.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. 2007. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117:428–437. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McNamara BP, Koutsouris A, O'Connell CB, Nougayrede JP, Donnenberg MS, Hecht G. 2001. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest 107:621–629. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raymond B, Crepin VF, Collins JW, Frankel G. 2011. The WxxxE effector EspT triggers expression of immune mediators in an Erk/JNK and NF-kappaB-dependent manner. Cell Microbiol 13:1881–1893. doi: 10.1111/j.1462-5822.2011.01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith RM. 1890. The physiology of the domestic animals: a text-book for veterinary and medical students and practitioners. F. A. Davis, Philadelphia, PA. [Google Scholar]
- 73.Moeser AJ, Klok CV, Ryan KA, Wooten JG, Little D, Cook VL, Blikslager AT. 2007. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol 292:G173–G181. [DOI] [PubMed] [Google Scholar]
- 74.Rakoff-Nahoum S, Kong Y, Kleinstein SH, Subramanian S, Ahern PP, Gordon JI, Medzhitov R. 2015. Analysis of gene-environment interactions in postnatal development of the mammalian intestine. Proc Natl Acad Sci U S A 112:1929–1936. doi: 10.1073/pnas.1424886112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gallois M, Gidenne T, Tasca C, Caubet C, Coudert C, Milon A, Boullier S. 2007. Maternal milk contains antimicrobial factors that protect young rabbits from enteropathogenic Escherichia coli infection. Clin Vaccine Immunol 14:585–592. doi: 10.1128/CVI.00468-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rogier EW, Frantz AL, Bruno ME, Wedlund L, Cohen DA, Stromberg AJ, Kaetzel CS. 2014. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci U S A 111:3074–3079. doi: 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blake PA, Ramos S, MacDonald KL, Rassi V, Gomes TA, Ivey C, Bean NH, Trabulsi LR. 1993. Pathogen-specific risk factors and protective factors for acute diarrheal disease in urban Brazilian infants. J Infect Dis 167:627–632. doi: 10.1093/infdis/167.3.627. [DOI] [PubMed] [Google Scholar]
- 78.Kobayashi H, Shimada J, Nakazawa M, Morozumi T, Pohjanvirta T, Pelkonen S, Yamamoto K. 2001. Prevalence and characteristics of Shiga toxin-producing Escherichia coli from healthy cattle in Japan. Appl Environ Microbiol 67:484–489. doi: 10.1128/AEM.67.1.484-489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franck SM, Bosworth BT, Moon HW. 1998. Multiplex PCR for enterotoxigenic, attaching and effacing, and Shiga toxin-producing Escherichia coli strains from calves. J Clin Microbiol 36:1795–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paton AW, Paton JC, Manning PA. 1993. Polymerase chain reaction amplification, cloning and sequencing of variant Escherichia coli Shiga-like toxin type II operons. Microb Pathog 15:77–82. doi: 10.1006/mpat.1993.1058. [DOI] [PubMed] [Google Scholar]
- 81.Stephan R, Cernela N, Ziegler D, Pfluger V, Tonolla M, Ravasi D, Fredriksson-Ahomaa M, Hachler H. 2011. Rapid species specific identification and subtyping of Yersinia enterocolitica by MALDI-TOF mass spectrometry. J Microbiol Methods 87:150–153. doi: 10.1016/j.mimet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt H, Knop C, Franke S, Aleksic S, Heesemann J, Karch H. 1995. Development of PCR for screening of enteroaggregative Escherichia coli. J Clin Microbiol 33:701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DebRoy C, Maddox CW. 2001. Identification of virulence attributes of gastrointestinal Escherichia coli isolates of veterinary significance. Anim Health Res Rev 1:129–140. [PubMed] [Google Scholar]
- 84.Stauffer SH, Birkenheuer AJ, Levy MG, Marr H, Gookin JL. 2008. Evaluation of four DNA extraction methods for the detection of Tritrichomonas foetus in feline stool specimens by polymerase chain reaction. J Vet Diagn Invest 20:639–641. doi: 10.1177/104063870802000518. [DOI] [PubMed] [Google Scholar]
- 85.Ritchie LE, Steiner JM, Suchodolski JS. 2008. Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis. FEMS Microbiol Ecol 66:590–598. doi: 10.1111/j.1574-6941.2008.00609.x. [DOI] [PubMed] [Google Scholar]
- 86.Yu J, Kaper JB. 1992. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol 6:411–417. doi: 10.1111/j.1365-2958.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 87.Orskov I, Orskov F, Jann B, Jann K. 1977. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev 41:667–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis 3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- 89.Bybee SN, Scorza AV, Lappin MR. 2011. Effect of the probiotic Enterococcus faecium SF68 on presence of diarrhea in cats and dogs housed in an animal shelter. J Vet Intern Med 25:856–860. doi: 10.1111/j.1939-1676.2011.0738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jergens AE, Evans RB, Ackermann M, Hostetter J, Willard M, Mansell J, Bilzer T, Wilcock B, Washabau R, Hall EJ, Minami T, Wang C, Day MJ. 2014. Design of a simplified histopathologic model for gastrointestinal inflammation in dogs. Vet Pathol 51:946–950. doi: 10.1177/0300985813511123. [DOI] [PubMed] [Google Scholar]
- 91.Nicklas JL, Moisan P, Stone MR, Gookin JL. 2010. In situ molecular diagnosis and histopathological characterization of enteroadherent Enterococcus hirae infection in pre-weaning-age kittens. J Clin Microbiol 48:2814–2820. doi: 10.1128/JCM.00916-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Janeczko S, Atwater D, Bogel E, Greiter-Wilke A, Gerold A, Baumgart M, Bender H, McDonough PL, McDonough SP, Goldstein RE, Simpson KW. 2008. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet Microbiol 128:178–193. doi: 10.1016/j.vetmic.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 93.Simpson KW, Dogan B, Rishniw M, Goldstein RE, Klaessig S, McDonough PL, German AJ, Yates RM, Russell DG, Johnson SE, Berg DE, Harel J, Bruant G, McDonough SP, Schukken YH. 2006. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect Immun 74:4778–4792. doi: 10.1128/IAI.00067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pritchard MH, Kruse GO. 1982. The collection and preservation of animal parasites. Technical bulletin no. 1. The Harold W. Manter Laboratory, University of Nebraska Press, Lincoln, NE. [Google Scholar]
- 95.Dryden MW, Payne PA, Ridley R, Smith V. 2005. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet Ther 6:15–28. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.