Abstract
Background
The Stewart model for analyzing acid–base disturbances emphasizes serum albumin levels, which are ignored in the traditional Boston model. We compared data derived using the Stewart model to those using the Boston model in patients with nephrotic syndrome.
Methods
Twenty-nine patients with nephrotic syndrome and six patients without urinary protein or acid–base disturbances provided blood and urine samples for analysis that included routine biochemical and arterial blood gas tests, plasma renin activity, and aldosterone. The total concentration of non-volatile weak acids (ATOT), apparent strong ion difference (SIDa), effective strong ion difference (SIDe), and strong ion gap (SIG) were calculated according to the formulas of Agrafiotis in the Stewart model.
Results
According to the Boston model, 25 of 29 patients (90%) had alkalemia. Eighteen patients had respiratory alkalosis, 11 had metabolic alkalosis, and 4 had both conditions. Only three patients had hyperreninemic hyperaldosteronism. The Stewart model demonstrated respiratory alkalosis based on decreased PaCO2, metabolic alkalosis based on decreased ATOT, and metabolic acidosis based on decreased SIDa. We could diagnose metabolic alkalosis or acidosis with a normal anion gap after comparing delta ATOT [(14.09 − measured ATOT) or (11.77 − 2.64 × Alb (g/dL))] and delta SIDa [(42.7 − measured SIDa) or (42.7 − (Na + K − Cl)]). We could also identify metabolic acidosis with an increased anion gap using SIG > 7.0 (SIG = 0.9463 × corrected anion gap—8.1956).
Conclusions
Patients with nephrotic syndrome had primary respiratory alkalosis, decreased ATOT due to hypoalbuminemia (power to metabolic alkalosis), and decreased levels of SIDa (power to metabolic acidosis). We could detect metabolic acidosis with an increased anion gap by calculating SIG. The Stewart model in combination with the Boston model facilitates the analysis of complex acid–base disturbances in nephrotic syndrome.
Keywords: Acid–base disturbance, Metabolic acidosis, Nephrotic syndrome, Renin–angiotensin–aldosterone system, Respiratory alkalosis
Introduction
Several methods for analyzing acid–base disturbances have been proposed. One method is the traditional Boston model based on the CO2/HCO3 method [1, 2]. Another is the Copenhagen-Danish model based on base excess (BE) [3, 4]. A third method is the Stewart model based on the physicochemical method [5–7]. The Boston model makes it easy for beginners to understand and analyze acid–base disturbances. However, without the use of compensatory formulas, this model is limited in the setting of complex conditions such as respiratory abnormalities and two or more concurrent metabolic abnormalities. The BE model is useful only in metabolic acidosis. In contrast, the Stewart model is theoretically superior to the other models mentioned above in complex situations because it is based on physicochemical data. However, it is very difficult for physicians to understand the underlying concepts and make calculations using the relevant formulas in clinical practice. The Stewart model emphasizes the importance of albumin (Alb), which carries a negative charge in serum and influences acid–base balance; Alb is not taken into account by the traditional Boston model. There have been no reports about acid–base disturbances in nephrotic syndrome. In this study, we compared data derived using the Stewart model to data from the traditional Boston model in the context of acid–base disturbances in patients with nephrotic syndrome.
Materials and methods
This study was approved by the Ethics Committee of Aichi Medical University (14-164).
Patient characteristics (Table 1)
Table 1.
Characteristics and laboratory data of the study patients
| Patient | Age | Gender | Diagnosis | U-protein | TP | Albumin | BUN | Cr | eGFR | IgG | IgA | IgM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) | Renal biopsy | Association | g/day | g/dL | g/dL | mg/dL | mg/dL | ml/min | mg/dL | mg/dL | mg/dL | ||
| 1 | 47 | F | Not done | SLE | 2.1 | 4.5 | 1.3 | 9.4 | 0.41 | 126 | 447 | 597 | 75 |
| 2 | 37 | F | MCNS | 4.1 | 5.5 | 1.9 | 12.6 | 0.48 | 114 | 1677 | 264 | 125 | |
| 3 | 55 | M | Not done | Rectal cancer | 5.2 | 5.2 | 2.1 | 11.6 | 0.58 | 111 | 1020 | 190 | 86 |
| 4 | 61 | F | endo-GN | 9.6 | 5.0 | 1.8 | 12.4 | 0.59 | 78 | 818 | 336 | 115 | |
| 5 | 32 | F | endo-GN | 7.6 | 4.6 | 1.9 | 13.5 | 0.71 | 77 | 715 | 346 | 103 | |
| 6 | 70 | M | MN | 7.0 | 5.7 | 2.9 | 12.0 | 0.77 | 76 | 712 | 264 | 92 | |
| 7 | 71 | F | MCNS | 10.2 | 5.2 | 2.4 | 17.2 | 0.65 | 68 | 633 | 270 | 85 | |
| 8 | 61 | M | MCNS | 14.3 | 4.8 | 1.0 | 21.9 | 0.92 | 65 | 1011 | 687 | 111 | |
| 9 | 76 | F | MCNS | 10.7 | 3.9 | 0.9 | 34.8 | 0.73 | 58 | 746 | 154 | 36 | |
| 10 | 65 | M | MCNS | 3.7 | 4.8 | 1.4 | 11.6 | 1.01 | 58 | 542 | 546 | 144 | |
| 11 | 81 | F | FSGS | 7.0 | 5.5 | 2.8 | 25.5 | 0.75 | 56 | 541 | 165 | 45 | |
| 12 | 78 | F | MCNS | 4.8 | 4.4 | 1.8 | 11.1 | 0.81 | 52 | 366 | 218 | 87 | |
| 13 | 75 | M | MCNS | 10.7 | 3.6 | 1.1 | 33.5 | 1.10 | 51 | 422 | 244 | 33 | |
| 14 | 89 | F | Not done | 4.2 | 4.7 | 2.4 | 16.4 | 0.81 | 50 | 687 | 203 | 58 | |
| 15 | 70 | M | MN | 8.0 | 5.6 | 2.9 | 18.9 | 1.20 | 47 | 633 | 241 | 43 | |
| 16 | 60 | M | Not done | Thrombocytosis | 11.0 | 4.5 | 1.9 | 18.7 | 1.46 | 40 | 866 | 224 | 40 |
| 17 | 60 | M | FSGS | 18.2 | 3.5 | 0.9 | 37.2 | 1.72 | 33 | 467 | 412 | 114 | |
| 18 | 81 | M | MN | 5.7 | 4.2 | 1.8 | 25.4 | 1.61 | 33 | 604 | 224 | 74 | |
| 19 | 87 | F | Not done | MN suspected | 6.5 | 4.6 | 1.8 | 24.2 | 1.20 | 33 | 734 | 529 | 21 |
| 20 | 71 | F | Not done | MCNS suspected | 12.3 | 4.4 | 1.4 | 78.0 | 1.38 | 30 | 362 | 314 | 123 |
| 21 | 86 | M | Not done | MCNS suspected | 3.9 | 4.1 | 0.8 | 27.9 | 1.76 | 29 | 1590 | 488 | 39 |
| 22 | 71 | M | Not done | RA, bucillamine | 7.5 | 5.1 | 2.1 | 45.8 | 1.97 | 27 | 883 | 60 | 104 |
| 23 | 54 | M | Not done | DM | 12.0 | 3.9 | 1.5 | 16.8 | 2.56 | 22 | 311 | 156 | 38 |
| 24 | 76 | F | Not done | 17.3 | 5.5 | 1.0 | 20.5 | 2.02 | 19 | 2755 | 414 | 42 | |
| 25 | 76 | M | Not done | AL amyloidosis | 4.6 | 4.1 | 1.8 | 46.0 | 3.57 | 14 | 647 | 48 | 19 |
| 26 | 77 | F | Crescentic GN | 3.6 | 5.4 | 1.8 | 45.7 | 3.77 | 10 | 1290 | 285 | 42 | |
| 27 | 82 | M | Not done | DM | 7.8 | 5.4 | 2.4 | 69.8 | 5.12 | 9 | 1022 | 192 | 58 |
| 28 | 74 | F | Crescentic GN | 4.8 | 5.5 | 2.0 | 70.9 | 4.13 | 9 | 1146 | 362 | 52 | |
| 29 | 70 | F | Not done | MPO-ANCA | 3.9 | 5.3 | 1.9 | 69.3 | 5.64 | 6 | 2285 | 636 | 108 |
| Median | 71 | 7.0 | 4.8 | 1.8 | 21.9 | 1.2 | 47 | 715 | 264 | 74 | |||
| 25% | 61 | 4.6 | 4.4 | 1.4 | 13.5 | 0.75 | 27 | 542 | 203 | 42 | |||
| 75% | 77 | 10.7 | 5.4 | 2.1 | 37.2 | 1.97 | 65 | 1020 | 412 | 104 | |||
MCNS minimal change nephrotic syndrome, endo-GN endocapillary proliferative glomerulonephritis, MN membranous nephropathy, FSGS focal segmental glomerulosclerosis, RA rheumatoid arthritis, DM diabetes mellitus, AL amyloid light chain, crescentic GN crescentic glomerulonephritis, MPO-ANCA myeloperoxidase anti-neutrophil cytoplasmic antibody, U-protein urinary protein
We enrolled 29 patients with nephrotic syndrome and 6 patients without urinary protein or acid–base disturbances as a control group. All patients were Japanese. They were admitted to Aichi Medical University Hospital between August 2014 and August 2015. The diagnosis of nephrotic syndrome was based on massive proteinuria and hypoalbuminemia with or without edema. The study population included 14 men and 15 women, with a median age of 71 years [interquartile range (IQR), 61–77]. Sixteen patients had pathological diagnoses based on kidney biopsy: 7 patients with minimal change nephrotic syndrome (MCNS), 2 with focal segmental glomerulosclerosis (FSGS), 3 with membranous nephropathy (MN), 2 with endocapillary proliferative glomerulonephritis (endo-PGN), and 2 with crescentic glomerulonephritis (crescentic GN). The remaining 13 patients did not undergo kidney biopsy due to comorbidities or refusal to provide consent. Based on clinical and laboratory findings, these patients were suspected of having the following diagnoses: MCNS (n = 2), MN (n = 3; 1 with primary MN and 2 with MN secondary to malignancy and bucillamine), lupus nephritis (n = 1), essential thrombocytosis (n = 1), diabetes mellitus (n = 2), systemic amyloidosis (amyloid light chain type) (n = 1), anti-myeloperoxidase (MPO) antineutrophil cytoplasmic antibody (ANCA)-associated nephritis (n = 1), and no primary disease (n = 1). The median levels of urinary protein, serum total protein (TP), and serum Alb were 7.0 g/day (IQR, 4.6–10.7), 4.8 g/dL (4.4–5.4), and 1.8 g/dL (1.4–2.1), respectively. The median blood urea nitrogen (BUN) and creatinine (Cr) levels and estimated glomerular filtration rate (eGFR) were 21.9 mg/dL (13.5–37.2), 1.2 mg/dL (0.75–1.97), and 47.0 ml/min/1.72 m2 (27–65), respectively. The median immunoglobulin G (IgG), IgA, and IgM concentrations were 715 mg/dL (542–1020), 264 mg/dL (203–412), and 74 mg/dL (42–104), respectively (Table 1).
Methods
Serum and urine samples were obtained from all patients with nephrotic syndrome before steroid and diuretic administration.
Laboratory examinations included TP (g/dL), Alb (g/dL), BUN (mg/dL), Cr (mg/dL), eGFR (mL/min/1.73 m2), Na (mEq/L), K (mEq/L), Cl (mEq/L), Ca (mg/dL), phosphate (P) (mg/dL), magnesium (Mg) (mg/dL), lactic acid (lactate) (mmol/L), IgG (mg/dL), IgA (mg/dL), IgM (mg/dL), plasma osmolarity (mOsm/L), arterial blood gas analysis, plasma renin activity (PRA) (ng/mL/h), and plasma aldosterone concentration (PAC) (pg/mL). Urinary examination was performed to evaluate daily urinary protein (g/day), urinary protein concentration (mg/dL), urine osmolarity (mOsm/L), Na (mEq/L), K (mEq/L), Cl (mEq/L), and Cr (mg/dL).
The anion gap (AG) in blood was calculated using the formula Na − (Cl + HCO3). The normal range was 12 ± 2 mEq/L. Corrected AG (cAG) was calculated using the formula [AG + 2.5 × (4.4 − measured albumin (g/dL))] according to the methods of Nguyen et al. [8] and Rastegar [9].
CO2/HCO3 method (traditional Boston model)
Initially, we defined acidemia or alkalemia based on a pH of 7.40. Metabolic or respiratory disturbances, and acidosis or alkalosis, were determined using the CO2/HCO3 method. In respiratory alkalosis, we diagnosed acute or chronic respiratory alkalosis by comparing actual HCO3 to estimated HCO3 using Kellum’s compensatory formula [10]. Thus, estimated HCO3 = 0.2 × (actual PaCO2) + 17 in acute respiratory alkalosis and estimated HCO3 = 0.5 × (actual PaCO2) + 5 in chronic respiratory alkalosis. When there was a discrepancy between actual and estimated HCO3, we determined if additional metabolic acidosis or alkalosis was present.
Physicochemical method (Stewart model)
We first evaluated several formulas reported by Nguyen et al. in 2009 [8], Rastegar in 2009 [9], Kishen et al. in 2014 [11], and Agrafiotis et al. in 2014 [12]. The Stewart model includes the following parameters: carbon dioxide (PaCO2); total concentration of non-volatile weak acids (ATOT), which is the sum of all buffer pairs (mostly weak acids) that move toward equilibrium with a dissociated anion [A−] according to its dissociation constant (e.g., albumin with its net negative charge under physiological conditions); and strong ion difference (SIG). The formulas for ATOT reported by Nguyen et al. [8] and Kishen et al. [11] were incorrect. The formula should be ATOT = Alb (g/dL) × 10 × (0.123 × pH − 0.631) + P (mg/dL) × 0.3229 × (0.309 × pH – 0.625) according to Rastegar [9], or ATOT = Alb (g/dL) × 10 × (0.1204 × pH − 0.625) + P (mg/dL) × 0.3229 × (0.309 × pH − 0.469) according to Agrafiotis et al. [12]. Regarding the apparent strong ion difference (SIDa), the values for Ca2+, Mg2+, and lactate are negligible. The formula described for effective strong ion difference (SIDe) by Nguyen et al. and Kishen et al. (SIDe = [HCO3 −] + ATOT) was incorrect because of errors in their formulas for ATOT. The units for ATOT, SIDa, SIDe, and SIG should be mEq/L, but not mmol/L as described by Kishen et al. [HCO3 −] in SIDe is calculated as 2.46 × 10−8 × PCO2/10(−pH) according to Kellum [13] or as 0.0301 × PCO2 × 10(pH−6.1) according to Agrafiotis et al. [12]. Ultimately, we decided to use the formulas from Agrafiotis et al. [12]:
ATOT (mEq/L) = [Alb (g/dL)] × 10 × (0.1204 × pH − 0.625) + [P (mg/dL)] × 0.3229 × (0.309 × pH − 0.469).
SIDa (mEq/L) = [Na+] + [K+] − [Cl−].
SIDe (mEq/L) = [HCO3 −] + ATOT = [0.0301 × PCO2 × 10(pH−6.1)] + ATOT.
SIG (mEq/L) = SIDa − SIDe.
Relationships between albumin and ATOT and between cAG and SIG
We created scatter plots with albumin or cAG on the x-axis and ATOT or SIG on the y-axis. We also derived correlation coefficients using Excel (Microsoft Corporation, Redmond, WA).
Relationship between eGFR and SIG or (SIG − lactate)
We created scatter plots with eGFR on the x-axis, and SIG or (SIG − lactate) on the y-axis. (SIG − lactate) represents unknown non-volatile acids other than lactate. We also derived correlation coefficients using Excel (Microsoft Corporation).
Relationship between IgG and ATOT, SIDa, SIDe, and SIG
We created scatter plots with IgG on the x-axis and ATOT, SIDa, SIDe, or SIG on the y-axis. We also derived correlation coefficients using Excel (Microsoft Corporation).
Results
Serum electrolytes (Table 2)
Table 2.
Summary of laboratory data in the nephrotic syndrome and control groups
| Unit | Nephrotic syndrome group | Control group | |||
|---|---|---|---|---|---|
| Median | 25–75% | Median | 25–75% | ||
| Albumin | g/dL | 1.8 | 1.4–2.1 | 4.7 | 4.5–4.8 |
| Na | mEq/L | 140 | 137–142 | 142 | 140–145 |
| K | mEq/L | 4.1 | 3.7–4.5 | 4.7 | 4.5–4.8 |
| Cl | mEq/L | 107 | 104–110 | 105 | 103–105 |
| Na-Cl | mEq/L | 33 | 31–34 | 37 | 37–39 |
| Na + K-Cl | mEq/L | 36.4 | 35.0–38.0 | 42.7 | 41.9–43.3 |
| Ca | mg/dL | 7.7 | 7.1–8.1 | 9.6 | 9.4–9.8 |
| cCa | mg/dL | 9.8 | 9.6–10.0 | 9.6 | 9.4–9.8 |
| P | mg/dL | 3.8 | 3.3–4.2 | 3.4 | 3.0–3.8 |
| Mg | mg/dL | 1.9 | 1.7–2.0 | 2.1 | 2.0–2.2 |
| Lactate | mmol/L | 1.3 | 1.0–1.9 | 0.9 | 0.8–1.0 |
| pH | 7.43 | 7.42–7.45 | 7.40 | 7.39–7.41 | |
| PaCO2 | Torr | 34.9 | 30.8–40.0 | 40.4 | 39.4–41.1 |
| PaO2 | Torr | 84.2 | 70.3–98.8 | 89.5 | 87.5–90.1 |
| HCO3 | mEq/L | 23.7 | 21.1–26.0 | 25.1 | 24.2–25.3 |
| A-aDO2 | Torr | 33.9 | 28.4–53.6 | 10.8 | 9.6–11.4 |
| AG | mEq/L | 8.6 | 7.0–10.8 | 13.5 | 13.0–14.5 |
| cAG | mEq/L | 15.8 | 13.6–17.4 | 13.1 | 12.5–13.5 |
| ATOT | mEq/L | 7.13 | 6.00–7.83 | 14.48 | 13.73–14.57 |
| SIDa | mEq/L | 36.4 | 35.0–38.0 | 42.7 | 41.9–43.3 |
| SIDe | mEq/L | 29.9 | 27.1–32.9 | 38.7 | 38.5–39.3 |
| SIG | mEq/L | 6.6 | 4.4–8.2 | 3.9 | 3.4–5.1 |
| Posm | mOsm/L | 287 | 284–294 | nd | nd |
| Uosm | mOsm/L | 466 | 296–592 | nd | nd |
| U-Na | mEq/L | 60 | 37–111 | nd | nd |
| U-K | mEq/L | 31.1 | 20.2–51.5 | nd | nd |
| U-Cl | mEq/L | 61 | 31–101 | nd | nd |
| Urinary AG | mEq/L | 38.9 | 29.0–55.0 | nd | nd |
cCa corrected Ca, AG anion gap, cAG corrected anion gap, A TOT nonvolatile weak acids, SIDa apparent strong ion difference, SIGc corrected strong ion gap, Posm plasma osmolarity, Uosm urinary osmolarity, U-Na urinary Na, U-K urinary K, U-Cl urinary Cl
The median serum concentrations of Na, K, and Cl were 140 mEq/L (IQR, 137–142), 4.1 (3.7–4.5), and 107 mEq/L (104–110), respectively. The concentration of serum K was slightly lower than in the control group [4.7 mEq/L (4.5–4.8)]. The median actual Ca concentration was significantly lower at 7.7 mg/dL (7.1–8.1); however, the median Ca concentration corrected for serum albumin was within the normal range at 9.8 mg/dL (9.6–10.0). The median P and Mg levels were 3.8 (3.3–4.2) and 1.9 mg/dL (1.7–2.0), respectively. The median lactate level was 1.3 mmol/L (1.0–1.9), which slightly higher than in the control group [0.9 mmol/L (0.8–1.0)]. The median IgG, IgA, and IgM concentrations were 715 (542–1020), 264 (203–412), and 74 mg/dL (42–104), respectively.
Traditional model (Boston model)
The median pH was slightly alkalotic at 7.43 (IQR, 7.42–7.45) (Tables 2, 3); 25 of 29 patients had a pH of more than 7.40, while 4 had a pH of less than 7.40. The median PaCO2 was 34.9 Torr (30.8–40.0). Twenty-two patients had PaCO2 levels of less than 40 Torr, while 7 had levels between 40 and 44.7 Torr. The median A-aDO2 was 33.9 Torr [28.4–53.6]. Given that the normal limit of A-aDO2 is less than 20 Torr, 26 of 29 patients (90%) in the high age group had impaired blood gas diffusion. The median PaO2 was 84.2 Torr [70.3–99.8]. These results suggest that hyperventilation occurs in most nephrotic patients. Since we routinely exclude pulmonary embolism and infection using laboratory data and chest computed tomography, hypoalbuminemia due to nephrotic syndrome seems to induce pulmonary interstitial edema, which in turn increases A-aDO2. Hyperventilation then occurs in order to maintain PaO2 levels. Primary respiratory alkalosis is essential in patients with nephrotic syndrome. The median HCO3 concentration was 23.7 mEq/L [21.1–26.0]. In cases of primary respiratory alkalosis, HCO3 concentrations should be decreased as a result of compensatory mechanisms. Actual HCO3 concentrations that are higher than expected suggest the coexistence of metabolic alkalosis. In nephrotic syndrome, the analysis of acid–base disturbances using the Boston method is limited because it requires the use of Kellum’s compensatory formula for respiratory alkalosis [10] or the concept of cAG.
Table 3.
Blood gas analysis
| Patient | pH | PaCO2 | PaO2 | HCO3 | A-aDO2 | AG | cAG | Lactate |
|---|---|---|---|---|---|---|---|---|
| Torr | Torr | mEq/L | Torr | mEq/L | mEq/L | mmol/L | ||
| 1 | 7.43 | 34.0 | 70.3 | 22.1 | 46.8 | 8.9 | 16.7 | 1.3 |
| 2 | 7.48 | 25.7 | 110.6 | 18.9 | 16.9 | 11.1 | 17.4 | 0.6 |
| 3 | 7.46 | 34.1 | 128.3 | 23.7 | 79.9 | 8.3 | 14.1 | 1.2 |
| 4 | 7.44 | 38.7 | 82.8 | 26.0 | 28.4 | 6.0 | 12.5 | 2.1 |
| 5 | 7.45 | 36.4 | 106.6 | 24.8 | 7.5 | 5.2 | 11.5 | 1.0 |
| 6 | 7.45 | 35.0 | 47.3 | 24.0 | 68.6 | 7.0 | 10.8 | 1.6 |
| 7 | 7.43 | 37.4 | 83.3 | 24.4 | 29.6 | 8.6 | 13.6 | 0.9 |
| 8 | 7.44 | 35.1 | 98.3 | 23.5 | 17.4 | 7.5 | 16.0 | 3.3 |
| 9 | 7.55 | 27.9 | 66.6 | 24.2 | 58.1 | 10.8 | 19.6 | 2.4 |
| 10 | 7.43 | 43.2 | 84.2 | 28.2 | 21.4 | 7.8 | 15.3 | 1.8 |
| 11 | 7.34 | 34.9 | 85.5 | 21.2 | 30.5 | 11.8 | 15.8 | 4.2 |
| 12 | 7.43 | 41.3 | 75.7 | 28.0 | 32.3 | 6.0 | 12.5 | 0.9 |
| 13 | 7.42 | 40.8 | 67.2 | 25.9 | 41.4 | 5.1 | 13.4 | 1.5 |
| 14 | 7.56 | 29.4 | 92.2 | 26.1 | 30.7 | 9.9 | 14.9 | 1.3 |
| 15 | 7.42 | 42.1 | 83.6 | 26.8 | 23.4 | 9.2 | 13.0 | 1.0 |
| 16 | 7.36 | 40.0 | 56.0 | 22.4 | 53.6 | 9.6 | 15.9 | 2.6 |
| 17 | 7.47 | 33.1 | 80.4 | 23.7 | 37.8 | 7.3 | 16.1 | 1.4 |
| 18 | 7.45 | 36.9 | 84.8 | 24.9 | 28.7 | 7.1 | 13.6 | 0.9 |
| 19 | 7.43 | 43.9 | 99.5 | 28.6 | 5.2 | 7.4 | 13.9 | 3.8 |
| 20 | 7.42 | 41.3 | 59.3 | 26.1 | 48.7 | 9.9 | 17.4 | 1.5 |
| 21 | 7.49 | 28.4 | 114.4 | 21.1 | 100.9 | 6.9 | 15.9 | 1.2 |
| 22 | 7.42 | 32.0 | 94.1 | 20.6 | 25.5 | 13.4 | 19.2 | 1.7 |
| 23 | 7.43 | 44.6 | 62.7 | 29.4 | 41.2 | 5.6 | 12.9 | 1.0 |
| 24 | 7.45 | 30.8 | 52.4 | 20.9 | 68.7 | 6.1 | 14.6 | 1.2 |
| 25 | 7.45 | 31.9 | 125.4 | 21.9 | 55.1 | 10.1 | 16.6 | 1.2 |
| 26 | 7.48 | 26.5 | 77.9 | 19.3 | 48.6 | 13.7 | 20.2 | 1.0 |
| 27 | 7.29 | 31.5 | 87.9 | 14.9 | 32.3 | 14.1 | 19.1 | 0.9 |
| 28 | 7.32 | 28.7 | 111.5 | 14.1 | 73.0 | 16.9 | 22.9 | 1.9 |
| 29 | 7.40 | 21.5 | 98.8 | 13.1 | 33.9 | 16.9 | 23.2 | 1.9 |
| Median | 7.43 | 34.9 | 84.2 | 23.7 | 33.9 | 8.6 | 15.8 | 1.3 |
| 25% | 7.42 | 30.8 | 70.3 | 21.1 | 28.4 | 7.0 | 13.6 | 1.0 |
| 75% | 7.45 | 40.0 | 98.8 | 26.0 | 53.6 | 10.8 | 17.4 | 1.9 |
AG anion gap, cAG corrected anion gap
Serum AG (Tables 2, 3)
The median AG was 8.6 mEq/L (IQR, 7.0–10.8), which was significantly lower than the normal range of 12 ± 2 mEq/L. The median cAG, which adjusts for serum albumin using the formula cAG = AG + 2.5 × (4.4 − measured albumin (g/dL)), was 15.8 mEq/L (13.6–17.4). Twelve patients had cAG values within the normal range of 11.0–15.1 mEq/L, 16 patients had increased levels greater than 15.1 mEq/L, and only one patient had a slightly decreased level of 10.8 mEq/L (Table 3).
PRA and PAC (Table 4)
Table 4.
Plasma and urine osmolarity, urinary electrolytes, plasma renin activity, and plasma aldosterone concentration
| Patient | Posm | Uosm | U-Na | U-K | U-Cl | Urinary AG | Renin | P-Ald |
|---|---|---|---|---|---|---|---|---|
| mOsm/L | mOsm/L | mEq/L | mEq/L | mEq/L | ng/mL/h | pg/mL | ||
| 1 | 276 | 810 | 20 | 54.8 | 46 | 28.8 | 19.0 | 618.0 |
| 2 | 283 | 722 | 80 | 52.0 | 96 | 36.0 | 0.6 | 10.0 |
| 3 | 284 | 499 | 152 | 19.0 | 141 | 30.0 | 1.1 | 39.1 |
| 4 | 287 | 528 | 117 | 34.6 | 90 | 61.6 | 0.1 | 10.0 |
| 5 | 282 | 592 | 127 | 46.2 | 141 | 32.2 | 0.2 | 40.1 |
| 6 | 281 | 290 | 53 | 22.0 | 49 | 26.0 | 13.0 | 191.0 |
| 7 | 293 | 602 | 155 | 28.4 | 133 | 50.4 | 0.4 | 70.1 |
| 8 | 290 | 818 | 42 | 68.4 | 108 | 2.4 | 16.0 | 309.0 |
| 9 | 298 | 749 | 81 | 39.2 | 81 | 39.2 | 18.0 | 77.4 |
| 10 | 285 | 417 | 39 | 20.1 | 31 | 28.1 | 1.5 | 67.8 |
| 11 | 306 | 432 | 113 | 17.0 | 101 | 29.0 | 0.8 | 101.0 |
| 12 | 285 | 244 | 114 | 100.0 | 159 | 55.0 | 0.6 | 10.0 |
| 13 | 294 | 813 | 36 | 61.9 | 39 | 58.9 | 2.6 | 45.0 |
| 14 | 286 | 466 | 105 | 32.3 | 87 | 50.3 | 0.7 | 27.9 |
| 15 | 294 | 475 | 85 | 31.1 | 72 | 44.1 | 1.6 | 50.4 |
| 16 | 282 | 328 | 138 | 26.9 | 126 | 38.9 | 0.5 | 19.2 |
| 17 | 293 | 560 | 56 | 30.1 | 21 | 65.1 | 0.4 | 10.0 |
| 18 | 286 | 484 | 37 | 51.5 | 22 | 66.5 | 0.9 | 33.6 |
| 19 | 301 | 392 | 111 | 32.3 | 86 | 57.3 | 0.8 | 10.0 |
| 20 | 332 | 796 | 18 | 72.3 | 12 | 78.3 | 0.7 | 16.4 |
| 21 | 281 | 453 | 26 | 42.9 | 25 | 43.9 | 0.5 | 32.1 |
| 22 | 287 | 474 | 62 | 24.5 | 46 | 40.5 | 13.0 | 85.6 |
| 23 | 291 | 296 | 109 | 14.7 | 110 | 13.7 | 0.4 | 10.0 |
| 24 | 267 | 264 | 33 | 27.4 | 31 | 29.4 | 0.4 | 26.7 |
| 25 | 297 | 275 | 33 | 16.6 | 16 | 33.6 | 2.9 | 20.6 |
| 26 | 285 | 248 | 48 | 20.2 | 40 | 28.2 | 0.2 | 53.1 |
| 27 | 310 | 431 | 35 | 57.1 | 25 | 67.1 | 1.2 | 50.8 |
| 28 | 302 | 274 | 41 | 19.1 | 24 | 36.1 | 0.3 | 59.1 |
| 29 | 286 | 196 | 60 | 15.8 | 61 | 14.8 | 0.1 | 72.4 |
| Median | 287 | 466 | 60 | 31.1 | 61 | 38.9 | 0.7 | 40.1 |
| 25% | 284 | 296 | 37 | 20.2 | 31 | 29.0 | 0.4 | 19.2 |
| 75% | 294 | 592 | 111 | 51.5 | 101 | 55.0 | 1.6 | 70.1 |
P-Ald plasma aldosterone concentration, Posm plasma osmolarity, Uosm urinary osmolarity, U-Na urinary Na, U-K urinary K, U-Cl urinary Cl
PRA values were within the normal range (0.3–2.9 ng/mL/h) in 19 patients, below normal in 5 patients, and above normal in 5 patients. PAC was within the normal range (30–160 pg/mL) in 15 patients. Hypoaldosteronism was observed in 11 patients, and 3 patients had hyperreninemic hyperaldosteronism. These results demonstrated that hypoaldosteronism and normoaldosteronism are the predominant conditions in nephrotic syndrome.
Stewart model (Table 5)
Table 5.
Data from the Stewart model
| Patient | pH | PaCO2 | ATOT | Delta ATOT (to alkalosis) | SIDa | Delta SIDa (to acidosis) | Normal AG metabolic | SIDe | SIG | Increased AG acidosis | Lactate | SIG-lactate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Torr | 14.09 | 42.7 | 38.9 | 1.4–7.0 | mmol/L | mEq/L | ||||||
| 1 | 7.43 | 34.00 | 6.16 | 7.93 | 34.8 | 7.9 | Neutral | 28.1 | 6.7 | 1.3 | 5.4 | |
| 2 | 7.48 | 25.70 | 7.69 | 6.40 | 34.1 | 8.6 | Acidosis | 26.4 | 7.7 | AG+ | 0.6 | 7.0 |
| 3 | 7.46 | 34.10 | 7.69 | 6.40 | 35.8 | 6.9 | Acidosis | 31.2 | 4.6 | 1.2 | 3.5 | |
| 4 | 7.44 | 38.70 | 7.13 | 6.96 | 36.5 | 6.2 | Alkalosis | 32.8 | 3.7 | 2.1 | 1.6 | |
| 5 | 7.45 | 36.40 | 7.47 | 6.62 | 34.4 | 8.3 | Acidosis | 31.9 | 2.5 | 1.0 | 1.5 | |
| 6 | 7.45 | 35.00 | 9.49 | 4.60 | 35.2 | 7.5 | Acidosis | 33.2 | 2.0 | 1.6 | 0.4 | |
| 7 | 7.43 | 37.40 | 8.60 | 5.49 | 37.1 | 5.6 | Acidosis | 32.8 | 4.3 | 0.9 | 3.5 | |
| 8 | 7.44 | 35.10 | 5.02 | 9.07 | 35.1 | 7.6 | Alkalosis | 28.2 | 6.9 | 3.3 | 3.6 | |
| 9 | 7.55 | 27.90 | 4.91 | 9.18 | 38.0 | 4.7 | Alkalosis | 28.7 | 9.3 | AG+ | 2.4 | 6.8 |
| 10 | 7.43 | 43.20 | 5.02 | 9.07 | 40.6 | 2.1 | Alkalosis | 33.0 | 7.6 | AG+ | 1.8 | 5.8 |
| 11 | 7.34 | 34.90 | 8.93 | 5.16 | 37.3 | 5.4 | Acidosis | 27.1 | 10.2 | AG+ | 4.2 | 6.0 |
| 12 | 7.43 | 41.30 | 6.67 | 7.42 | 37.9 | 4.8 | Alkalosis | 33.1 | 4.8 | 0.9 | 3.9 | |
| 13 | 7.42 | 40.80 | 5.25 | 8.84 | 35.3 | 7.4 | Alkalosis | 30.9 | 4.4 | 1.5 | 2.9 | |
| 14 | 7.56 | 29.40 | 8.91 | 5.18 | 39.7 | 3.0 | Alkalosis | 34.7 | 5.0 | 1.3 | 3.7 | |
| 15 | 7.42 | 42.10 | 10.44 | 3.65 | 40.3 | 2.4 | Alkalosis | 37.0 | 3.3 | 1.0 | 2.3 | |
| 16 | 7.36 | 40.00 | 7.83 | 6.26 | 36.5 | 6.2 | Neutral | 29.9 | 6.6 | 2.6 | 4.0 | |
| 17 | 7.47 | 33.10 | 3.89 | 10.20 | 34.5 | 8.2 | Alkalosis | 27.2 | 7.3 | AG+ | 1.4 | 6.0 |
| 18 | 7.45 | 36.90 | 6.84 | 7.25 | 36.4 | 6.3 | Alkalosis | 31.5 | 4.9 | 0.9 | 4.0 | |
| 19 | 7.43 | 43.90 | 7.04 | 7.05 | 39.5 | 3.2 | alkalosis | 35.4 | 4.1 | 3.8 | 0.4 | |
| 20 | 7.42 | 41.30 | 7.05 | 7.04 | 41.1 | 1.6 | Alkalosis | 32.9 | 8.2 | AG+ | 1.5 | 6.7 |
| 21 | 7.49 | 28.40 | 4.24 | 9.85 | 31.7 | 11.0 | Acidosis | 25.3 | 6.4 | 1.2 | 5.2 | |
| 22 | 7.42 | 32.00 | 7.77 | 6.32 | 38.9 | 3.8 | Alkalosis | 28.1 | 10.8 | AG+ | 1.7 | 9.1 |
| 23 | 7.43 | 44.60 | 6.00 | 8.09 | 38.5 | 4.2 | Alkalosis | 35.0 | 3.5 | 1.0 | 2.5 | |
| 24 | 7.45 | 30.80 | 5.20 | 8.89 | 30.7 | 12.0 | Acidosis | 25.8 | 4.9 | 1.2 | 3.6 | |
| 25 | 7.45 | 31.90 | 7.27 | 6.82 | 36.5 | 6.2 | Alkalosis | 29.0 | 7.5 | AG+ | 1.2 | 6.4 |
| 26 | 7.48 | 26.50 | 6.69 | 7.40 | 36.0 | 6.7 | Alkalosis | 25.9 | 10.1 | AG+ | 1.0 | 9.1 |
| 27 | 7.29 | 31.50 | 8.89 | 5.20 | 35.0 | 7.7 | Acidosis | 23.6 | 11.4 | AG+ | 0.9 | 10.4 |
| 28 | 7.32 | 28.70 | 8.53 | 5.56 | 36.4 | 6.3 | Acidosis | 22.8 | 13.6 | AG+ | 1.9 | 11.7 |
| 29 | 7.40 | 21.50 | 7.64 | 6.45 | 33.9 | 8.8 | Acidosis | 20.6 | 13.3 | AG+ | 1.9 | 11.4 |
| Median | 7.43 | 34.90 | 7.13 | 6.96 | 36.4 | 6.3 | 29.9 | 6.6 | 1.3 | |||
| 25% | 7.42 | 30.80 | 6.00 | 6.26 | 35.0 | 4.7 | 27.1 | 4.4 | 1.0 | |||
| 75% | 7.45 | 40.00 | 7.83 | 8.09 | 38.0 | 7.7 | 32.9 | 8.2 | 1.9 |
Control group mean values: ATOT, 14.09; SIDa, 42.7; SIG, 4.2; lactate, 0.9
In the control group, the median ATOT, SIDa, SIDe, and SIG values were 14.48 (IQR, 13.73–14.57), 42.7 (41.9–43.3), 38.7 (38.5–39.3), and 3.9 mEq/L (3.4–5.1), respectively. The mean ± SD for ATOT, SIDa, SIDe, and SIG were 14.09 ± 0.90 (normal range: 12.29–15.90), 42.7 ± 1.4 (normal range: 39.9–45.5), 38.5 ± 1.2 (normal range: 36.1–41.0), and 4.2 ± 1.4 (normal range: 1.4–7.0), respectively.
PaCO2 (Torr)
The median PaCO2 level was 34.9 Torr (IQR, 30.8–40.0). PaCO2 was less than 40 Torr in 21 of 29 patients (76%). Low PaCO2 levels, such as those observed here, contribute to the development of alkalosis (Fig. 1).
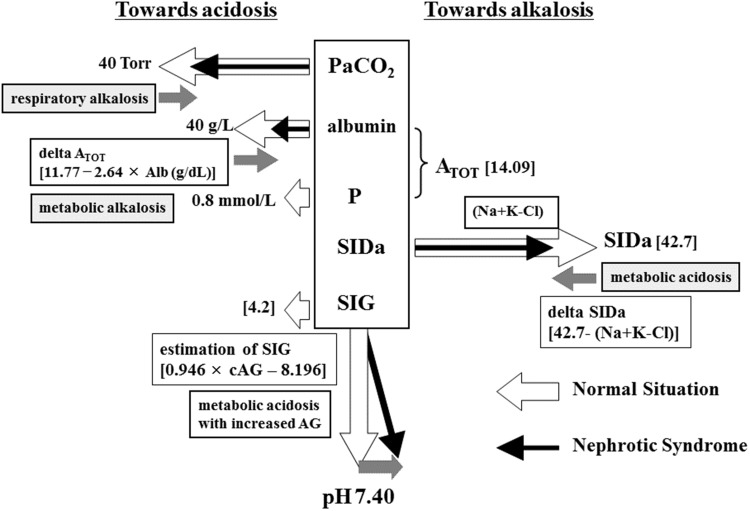
Fig. 1.
Determination of pH in the Stewart model. In the Stewart model, PaCO2, ATOT, and SIGc have powers toward acidosis. On the other hand, SIDa has a power toward alkalosis. In healthy individuals, pH should be 7.40. In nephrotic syndrome, patients are alkalotic due to decreased levels of PaCO2, decreased ATOT due to hypoalbuminemia, and decreased SIDa resulting from hyperchloremia due to hyporeninemic hypoaldosteronism
ATOT (mEq/L)
ATOT represents the total concentration of non-volatile weak acids. It depends on albumin and phosphate levels. The median ATOT was 7.13 (mEq/L) (IQR, 6.00–7.83), because hypoalbuminemia secondary to nephrotic syndrome directly interferes with ATOT values. The median delta ATOT, calculated by [14.09 (mean value of control group) − ATOT], was 6.96 (6.26–8.09). This lower value of ATOT contributed to metabolic alkalosis (Fig. 1).
SIDa (mEq/L)
SIDa reflects the likelihood of alkalosis. The median SIDa value, calculated by [Na + K − Cl], was 36.4 (mEq/L) (IQR, 35.0–38.0), which was lower than the mean value in the control group (42.7 mEq/L). The median delta SIDa, calculated by [42.7 (mean value of control group) − SIDa], was 6.3 (mEq/L) (4.7–7.7). Lower SIDa among nephrotic patients suggests a power to metabolic acidosis (Fig. 1). In individual patients, delta ATOT (alkalosis) greater than delta SIDa (acidosis) results in metabolic alkalosis. Inversely, when delta SIDa is greater than delta ATOT, the patient has metabolic acidosis with a normal anion gap.
SIDe (mEq/L)
The median SIDe was 29.9 mEq/L (IQR, 27.1–32.9), which was lower than the mean value in the control group (38.5 mEq/L).
SIG (mEq/L)
SIG reflects the accumulation of various non-volatile acids, including lactate or uremic substances. Increased AG suggests metabolic acidosis with an increased anion gap. The median SIG was 6.6 mEq/L (IQR, 4.4–8.2), which was higher than 3.9 mEq/L (3.4–5.1) in the control group. The mean ± SD in the control group was 4.2 ± 1.4 mEq/L (normal range: 1.4–7.0).
Lactate (mmol/L = mEq/L) and SIG (mEq/L)
The median lactate was 1.3 mmol/L (IQR, 1.0–1.9), higher than 0.9 mmol/L (0.8–1.0) in the control group. The mean was 0.9 mmol/L (normal range: 0.8–1.0). Lactate levels greater than 1.0 mmol/L were observed in 20 of 29 patients (69%), and 12 out of 29 patients had metabolic acidosis with an increased anion gap. (SIG − lactate) represents the accumulation of non-volatile acids except for lactate. Seven of 12 patients had mainly lactate accumulation. Three had accumulation of lactate and non-volatile acids such as uremic toxins. Two of 12 patients had accumulation of non-volatile acids except for lactate.
Relationship between serum albumin and ATOT
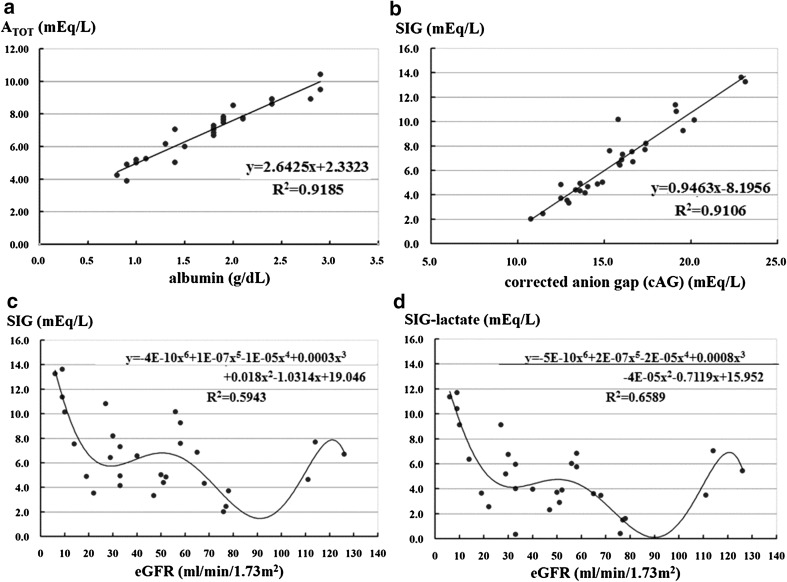
There was a strong significant relationship between serum albumin and ATOT: ATOT = 2.6425 × Alb + 2.3323 (R 2 = 0.91851) (Fig. 2a). We can easily calculate and estimate ATOT using serum albumin alone. When the serum albumin level is 4.4 g/dL, ATOT will be 14.0, a normal value (14.09). If the albumin level is 1.5 g/dL, ATOT will be 6.30. In addition, delta ATOT, which represents the power to metabolic alkalosis, is calculated using the formula 11.77 − 2.64 × Alb (g/dL) (Fig. 1).
Fig. 2.
Relationship between albumin, ATOT, eGFR, and SIG. a Relationship between albumin and ATOT: ATOT = 2.6425 × Alb + 2.3323 (R 2 = 0.91851). Delta ATOT is calculated as 11.77 − 2.64 × Alb (g/dL). This number indicates the power to alkalosis. b Relationship between cAG and SIG: SIG = 0.9463 × cAG − 8.1956 (R2 = 0.91057). c Relationship between eGFR and SIG: SIG = − 4E-10(eGFR)6 + 1E−07(eGFR)5 − 1E−05(eGFR)4 + 0.0003(eGFR)3 + 0.018(eGFR)2 − 1.0314(eGFR) + 19.046 (R2 = 0.5943). d Relationship between eGFR and SIG − lactate: (SIG-lactate) = − 5E−10 (eGFR)6 + 2E-07 (eGFR)5 − 2E−05 (eGFR)4 + 0.0008 (eGFR)3 − 4E−05 (eGFR)2 − 0.7119 (eGFR) + 15.952 (R 2 = 0.6589) (c, d). Six out of 9 patients with eGFR less than 29 mL/min had metabolic acidosis with an increased anion gap. Five out of 6 patients had accumulation of lactate and unknown non-volatile acids (i.e., uremic toxins). One patient had increased levels of lactate alone. On the other hand, increased lactate levels were observed in patients with eGFR greater than 30 mL/min, who have metabolic acidosis and an increased anion gap
Relationship between cAG and SIG
We estimated SIG using the formula SIG = 0.9463 × cAG − 8.1956 (R 2 = 0.91057) (Fig. 2b). After calculating delta cAG [cAG − 12], the formula can be rewritten as SIG = 0.9463 × delta cAG + 3.1605 (R² = 0.91057).
Relationship between eGFR and SIG or (SIG − lactate)
The relationship between eGFR and SIG is as follows: SIG = − 4E−10(eGFR)6 + 1E−07(eGFR)5 − 1E−05(eGFR)4 + 0.0003(eGFR)3 + 0.018(eGFR)2 − 1.0314(eGFR) + 19.046 (R 2 = 0.5943). The relationship between eGFR and (SIG − lactate) is as follows: (SIG − lactate) = − 5E−10(eGFR)6 + 2E−07(eGFR)5 − 2E−05(eGFR)4 + 0.0008(eGFR)3 − 4E−05(eGFR)2 − 0.7119(eGFR) + 15.952 (R 2 = 0.6589) (Fig. 2c, d). Six of 9 patients with eGFR less than 29 mL/min/1.72 m2 had metabolic acidosis with an increased anion gap. Five out of 6 patients had accumulation of lactate and unknown non-volatile acids (i.e., uremic toxins). One patient had increased lactate levels alone. On the other hand, patients with eGFR greater than 30 mL/min/1.72 m2 who had metabolic acidosis with an increased anion gap had increased lactate levels.
Relationship between IgG and ATOT, SIDa, SIDe, and SIG
Levels of IgG, a positively charged protein, might influence acid–base balance. The relationship between IgG and ATOT, SIDa, SIDe, and SIG can be expressed as ATOT = −0.0002 × (IgG) + 7.2633 (R² = 0.0073), SIDa = − 0.0029 × (IgG) + 39.076 (R² = 0.4337), SIDe = − 0.0046 × (IgG) + 33.834 (R² = 0.4105), and SIG = 0.0017 × (IgG) + 5.242 (R² = 0.0943), respectively. In other words, IgG levels have a negligible influence in the Stewart model.
Discussion
This study revealed 4 specific findings about acid–base disturbances in nephrotic syndrome. First, primary respiratory alkalosis occurs due to respiratory dysfunction, with elevated A-aDO2 caused by pulmonary interstitial edema due to hypoalbuminemia. Second, the comparison between delta ATOT and delta SID helps to determine whether metabolic alkalosis or metabolic acidosis with a normal anion gap is present. Third, we can estimate ATOT using serum albumin, SIDa as Na + K − Cl, and SIG using cAG. Fourth, 90% of patients had hyporeninemic or normoreninemic hypoaldosteronism, not hyperreninemic hyperaldosteronism. The above findings suggest that hyperchloremic metabolic acidosis in nephrotic syndrome is due to hyporeninemic hypoaldosteronism, with a shift of K into the intracellular space due to alkalemia.
The Boston model is easy for beginners to understand and use to analyze acid–base disturbances. However, without the use of compensatory formulas described by Kellum [10], this model is limited in the setting of complex conditions such as respiratory abnormalities and 2 or more concurrent metabolic abnormalities. It is very hard not only for beginners, but also for nephrologists to understand and calculate the effects of compensatory mechanisms. On the other hand, the Stewart model is theoretically superior to the Boston model in complex situations because it is based on physicochemical data. Changes after treatment such as supplementation of solution or albumin can be evaluated and be estimated using the Stewart model. The present study revealed that the formula from Agrafiotis et al. [12] is the most reliable; several manuscripts including those by Nguyen et al. [8], Restegar [9], and Kishen et al. [11], contained incorrect formulas. A lack of consensus on formulas for the Stewart model limits its use. Masevicius and Dubin [14] claimed that the Stewart approach is cumbersome, requires more determinations and calculations, and is more time-consuming and expensive. They only need to continue with the proper use of the Boston model. However, this study suggested that a combination of the Boston and Stewart models would be important to consider in complicated cases such as mixed metabolic disorders and hypoalbuminemia.
In the Steward model, the first step in the approach to analyzing acid–base disturbances in nephrotic syndrome is calculating delta ATOT and delta SIDa. The second step is comparing delta ATOT and delta SIDa values. When delta ATOT (14.1 − measured ATOT) is greater than delta SIDa (49.2 − measured SIDa), we can predict the presence of metabolic alkalosis. Inversely, when delta ATOT is smaller than delta SIDa, metabolic acidosis with a normal anion gap is likely to be present. Thus, we can easily distinguish between metabolic alkalosis and metabolic acidosis using delta ATOT and delta SIDa values. This study also demonstrated a strong relationship between ATOT and serum albumin and between SIG and cAG. We can estimate ATOT and delta ATOT using the following formulas: ATOT = 2.6425 × Alb + 2.3323 (R 2 = 0.91851) and delta ATOT = 11.77 − 2.64 × Alb (g/dL) (power to alkalosis). Since SIDa is calculated as (Na + K − Cl), the formula for delta SIDa is (49.2 − measured SIDa), which indicates the power to acidosis. In addition, we can identify another metabolic acidosis with an increased anion gap using the formula SIG = 0.9463 × cAG − 8.1956 (R 2 = 0.91057). (more than 7.0). The reliability of the estimated formula is 89.7% (26/29) in metabolic alkalosis or acidosis with a normal anion gap and 86.2% (25/29) in metabolic acidosis with an increased anion gap (data not shown). Our formulas will help [who?] understand mixed acid–base disturbances in nephrotic syndrome and renal failure.
The present study revealed that patients with eGFR less than 29 mL/min/1.72 m2 mainly have accumulation of lactate and uremic toxins. On the other hand, patients with eGFR greater than 30 mL/min/1.72 m2 have metabolic acidosis with an increased anion gap as a result of increased lactate levels alone. Systemic edema due to nephrotic syndrome from MCNS or FSGS may influence the production of lactate.
Regarding the mechanism of edema in nephrotic syndrome, the underfill and overfill hypotheses have been proposed [15]. According to the underfill hypothesis, the renin–angiotensin–aldosterone system is activated by decreased intravascular blood volume, which in turn induces sodium retention and causes edema. However, the present study demonstrated that only 10% of patients have hyperreninemic hyperaldosteronism, while 90% had hypoaldosteronism or normoaldosteronism. These data support the overfill hypothesis [16, 17]. Fluid management should involve administration of albumin and loop diuretics. Anti-aldosterone drugs such as spironolactone and eplerenone should be avoided. Recently, acetazolamide and hydrochlorothiazide followed by furosemide have been reported to be more effective for the treatment of refractory nephrotic edema [18]. However, the influence of these treatments on acid–base disturbances in nephrotic syndrome should be further studied because acetazolamide induces metabolic acidosis by increasing urinary excretion of Na+ and HCO3 −.
Conclusion
We showed that patients with nephrotic syndrome have primary respiratory alkalosis, decreased ATOT due to hypoalbuminemia (power to metabolic alkalosis), decreased SIDa (power to metabolic acidosis), and increased SIG, suggesting that they accumulate non-volatile acids such as lactate, uremic toxins, or other acids. Using both the Stewart and Boston methods facilitates the analysis of complex acid–base disturbances in primary and secondary nephrotic syndrome.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies were conducted (Aichi Medical University 14-164) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Schwartz WB, Relman AS. A critique of the parameters used in the evaluation of acid-base disorders. “Whole-blood buffer base” and “standard bicarbonate” compared with blood pH and plasma bicarbonate concentration. N Engl J Med. 1963;268:1382–1388. doi: 10.1056/NEJM196306202682503. [DOI] [PubMed] [Google Scholar]
- 2.Adrogué HJ, Gennari FJ, Galla JH, Madias NE. Assessing acid-base disorders. Kidney Int. 2009;76:1239–1247. doi: 10.1038/ki.2009.359. [DOI] [PubMed] [Google Scholar]
- 3.Siggaard-Andersen O. The van Slyke equation. Scand J Clin Lab Investig Suppl. 1977;146:15–20. doi: 10.3109/00365517709098927. [DOI] [PubMed] [Google Scholar]
- 4.Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore) 1977;56:38–54. doi: 10.1097/00005792-197756010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61:1444–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz I, Kraut J, Ornekian V, Nguyen MK. Acid-base analysis: a critique of the Stewart and bicarbonate-centered approaches. Am J Physiol Renal Physiol. 2008;294:F1009–F1031. doi: 10.1152/ajprenal.00475.2007. [DOI] [PubMed] [Google Scholar]
- 7.Seifter JL. Integration of acid-base and electrolyte disorders. N Engl J Med. 2014;371:1821–1831. doi: 10.1056/NEJMra1215672. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen BV, Vincent JL, Hamm JB, et al. The reproducibility of Stewart parameters for acid-base diagnosis using two central laboratory analyzers. Anesth Analg. 2009;109:1517–1523. doi: 10.1213/ANE.0b013e3181b62664. [DOI] [PubMed] [Google Scholar]
- 9.Rastegar A. Clinical utility of Stewart’s method in diagnosis and management of acid-base disorders. Clin J Am Soc Nephrol. 2009;4:1267–1274. doi: 10.2215/CJN.01820309. [DOI] [PubMed] [Google Scholar]
- 10.Kellum JA. Determinants of blood pH in health and disease. Crit Care. 2000;4:6–14. doi: 10.1186/cc644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishen R, Honoré PM, Jacobs R, et al. Facing acid-base disorders in the third millennium - the Stewart approach revisited. Int J Nephrol Renovasc Dis. 2014;7:209–217. doi: 10.2147/IJNRD.S62126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrafiotis M, Keklikoglou I, Papoti S, et al. Effect of the independent acid base variales on anion gap variation in cardiac surgical patients: a Stewart-Figge approach. Sci World J. 2014 (Article ID 907521). [DOI] [PMC free article] [PubMed]
- 13.Kellum JA. Clinical review: reinification of acid-base physiology. Crit Care. 2005;9:500–507. doi: 10.1186/cc3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masevicius FD, Dubin A. Has Stewart approach improved our ability to diagnose acid-base disorders in critically ill patients? World J Crit Care Med. 2015;4:62–70. doi: 10.5492/wjccm.v4.i1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphreys MH, Valentin JP, Qiu C, Ying WZ, Muldowney WP, Gardner DG. Underfill and overflow revisited: mechanisms of nephrotic edema. Trans Am Clin Climatol Assoc. 1993;104:47–59. [PMC free article] [PubMed] [Google Scholar]
- 16.Doucet A, Favre G, Deschênes G. Molecular mechanism of edema formation in nephrotic syndrome: therapeutic implications. Pediatr Nephrol. 2007;22:1983–1990. doi: 10.1007/s00467-007-0521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen RF, Buhl KB, Jensen BL, et al. Remission of nephrotic syndrome diminishes urinary plasmin content and abolishes activation of ENaC. Pediatr Nephrol. 2013;28:1227–1234. doi: 10.1007/s00467-013-2439-2. [DOI] [PubMed] [Google Scholar]
- 18.Fallahzadeh MA, Dormanesh B, Fallahzadeh MK, et al. Acetazolamide and hydrochlorothiazide followed by furosemide versus frosemide and hydrochlorohtiazide followed by furosemide for the treatment of adults with nephrotic edema: a randomized trial. Am J Kidney Dis. 2017;69:420–427. doi: 10.1053/j.ajkd.2016.10.022. [DOI] [PubMed] [Google Scholar]