Abstract
Lymphocyte depletion and immunosuppression are typical clinical characteristics of pigs infected with classical swine fever virus (CSFV). The apoptosis of virus-infected and bystander cells plays a role in the immunopathology of classical swine fever (CSF). Here, we offer the first evidence that autophagy is involved in apoptosis and death of T lymphocytes in the spleen of pigs infected with CSFV. Using immunohistochemical assays, we observed that more LC3II-positive cells appear in the T-cell zone of spleens. Spleen cell apoptosis was demonstrated using flow cytometry and TUNEL staining. Confocal immunofluorescence revealed that partial LC3II-positive cells were simultaneously TUNEL-positive. By cultivating spleen cells ex vivo, we demonstrated that the inhibition of autophagy by 3-MA treatment inhibited apoptosis and death of T lymphocytes caused by CSFV infection but did not have this effect on B lymphocytes. Further observations demonstrated that uninfected cells in the spleen were also undergoing autophagy in vivo. In summary, these results linked autophagy with the apoptosis and cell death of splenic T cells, providing a new outlook to understand the mechanism of T lymphocyte depletion and immunosuppression during CSF.
Introduction
Classical swine fever virus (CSFV) is an enveloped RNA virus belonging to the Pestivirus genus within the family Flaviviridae 1,2. CSFV typically causes hemorrhagic syndrome and immunosuppression associated with dengue and hepatitis C virus3. Monocytes and macrophages are the main targets for CSFV infection in vivo 4. However, it is curious that immunosuppression is mainly attributed to the depletion of bystander leukocyte populations, and hemorrhages are not caused by viral replication in endothelial cells5–7. These findings complicate the pathological mechanism of CSFV.
Apoptosis, a well-known type I programmed cell death, mediates the elimination of damaged, aberrant or infected cells in multicellular organisms8. Leukocyte depletion in the peripheral blood of CSFV-infected pigs is associated with apoptosis. An increase in the number of apoptotic lymphocytes appears as early as 1 day post infection (dpi), which appears before viremia9. In addition, apoptosis is also involved in pathological lesions in the thymus, spleen and lymph node of pigs infected by CSFV10. Curiously, CSFV does not cause the death of alveolar macrophages and even inhibits the apoptosis of primary porcine endothelial cells in in vitro studies7,11. These controversial results confuse the cell death mechanism of lymphocytes during CSFV infection and raise the possibility that other cell death mechanisms may be involved.
Autophagy is an evolutionarily conserved degradation process that maintains the metabolic balance and homeostasis of eukaryotes12,13. Autophagy-related (ATG) genes are involved in a multistep mechanism to regulate cytoplasmic cargo sequestration inside double-membrane vesicles and delivery to lysosomes for degradation14. Although autophagy is described as type II programmed cell death, unlike apoptosis, it occurs independently of the function of caspases in apoptosis pathways15,16. By contrast, autophagy can be induced to accomplish cell death when the apoptosis pathway is inhibited17. Moreover, common upstream signals can occasionally trigger both autophagy and apoptosis, resulting in cell death18. Previously, we demonstrated that CSFV induces autophagy to enhance viral replication and that the autophagy machinery was hijacked to inhibit the apoptosis of host cells19,20. However, whether autophagy occurs in host cells of CSFV-infected pigs remains unclear. The relationship between autophagy and cell death pathways during CSFV infection in vivo remains unknown.
The spleen, in which CSFV appears earlier and in which large numbers of viral particles reside, contains various types of macrophage and lymphocyte populations that are sorted by biological origin and behavior21–23. To uncover the possible mechanism of lymphocyte depletion during CSF, the association between autophagy and apoptosis in spleen cells of pigs infected with CSFV was investigated. The results showed that autophagy and apoptosis pathways were both activated in the spleen of CSFV-infected pigs using western blotting analysis. More LC3II-positive cells appeared in the T-cell zone of spleen paraffin sections. Confocal images revealed that partial LC3II-positive cells were stained by TUNEL. By cultivating spleen cells ex vivo, we offered direct evidence that apoptosis and death of T lymphocytes, but not B lymphocytes, can be prevented by the inhibition of autophagy. For the first time, we demonstrate that autophagy is involved in the apoptosis and death of T lymphocytes in the spleen of pigs infected with CSFV. This finding provides a new idea for exploring the mechanism of immunosuppression caused by CSFV infection.
Results
Pigs infected with the CSFV Shimen strain exhibited typical clinical symptoms
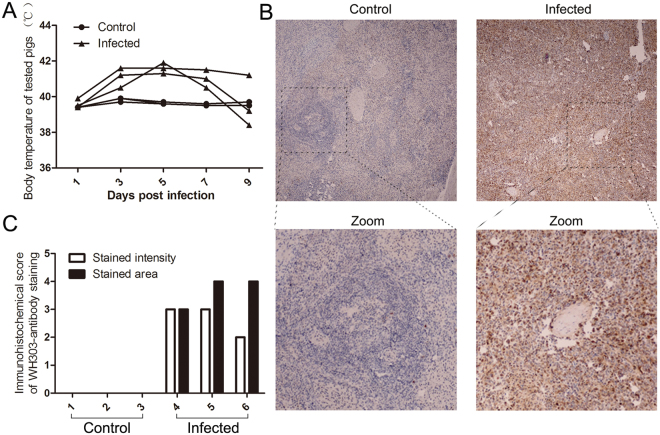
Pigs inoculated with the CSFV Shimen strain exhibited clinical symptoms of CSF at 3 to 5 dpi in our study. The body temperature of each animal was recorded every two days (Fig. 1A). All animals were sacrificed in accordance with animal ethics guidelines and approved protocols when severe signs appeared at 9 dpi. Typical spleen infarction was observed in CSFV-infected pigs (data not shown). To detect the distribution of CSFV antigen in the spleen, paraffin sections were stained with WH303 antibody, which reacts with E2 protein of CSFV24. The immunohistochemical images revealed that most spleen cells exhibited positive staining in infected groups (Fig. 1B and C). By contrast, control groups displayed negative staining (Fig. 1B and C).
Figure 1.
Body temperature of tested pigs and distribution of the CSFV antigen in the spleen. (A) Body temperature of tested pigs. 3 pigs were infected, and 3 pigs were used as controls. Each infected pig was intramuscularly injected with 105 TCID50 of Shimen strain CSFV. Body temperature was assessed every 2 days until 9 days post infection. (B) Location of CSFV in the spleen. Paraffin sections of spleens were stained with mouse monoclonal anti-CSFV E2 protein antibody (1:50) as described in Materials and Methods. The images were a representative immunocytochemistry result captured at magnifications under 20× objectives. (C) Immunocytochemistry score. 3 sections of each sample were prepared, and 3 random visual fields of each section were snapped. The score was evaluated by two different clinical doctors. Score of stained intensity: characteristics of positive-stained cells. Pale yellow, tan and sepia were scored as 1, 2, and 3, respectively. Score of stained area: the mean ratios of positive cells in each visual field. Scores of 1, 2, 3, and 4 were corresponded to 6~25%, 26~50%, 51~75% and >75%, respectively.
Increased apoptosis of spleen cells in CSFV-infected pigs
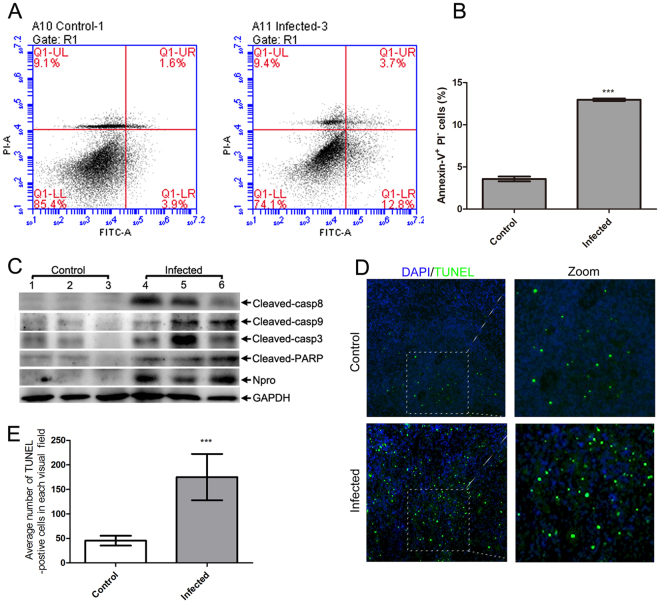
In vivo, apoptosis is associated with the immunopathology mechanism of CSF9,10. To confirm the involvement of apoptosis in spleen cells of pigs infected with CSFV, further research was carried out. Given that phagocytic removal of apoptotic cells is rapid25,26, the detection of apoptosis in vivo is difficult. Therefore, Annexin-V, which binds to phosphatidylserine exposed on the surface of early apoptotic cells, was introduced to evaluate cells that were programmed to die26. A representative example of flow cytometry detection of apoptosis in spleen cells is presented in Fig. 2A. Statistical analysis indicated that CSFV obviously increased the frequency of the early apoptotic cell population (Annexin V+ PI−) (Fig. 2B). To demonstrate that apoptotic signals were activated in spleen cells, hallmark apoptotic proteins were analyzed by immunoblotting. Cleaved caspase-8 and -9 are typically considered as extrinsic and intrinsic initiators, respectively. However, cleaved caspase-3 and PARP are considered as functional downstream effectors27. Our results demonstrated that CSFV-mediated up-regulation of cleaved caspase-3 and PARP levels were increased in spleen cells (Figs 2C and S1). In addition, we evaluated caspase-8 and caspase-9 expression to differentiate extrinsic and intrinsic apoptosis. Both initiators were initiated in the spleen cells of pigs infected with CSFV (Figs 2C and S1). To further confirm the presence of apoptotic spleen cells in situ, we stained DNA fragmentation of paraffin sections using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) method. An increased ratio of TUNEL-positive cells appeared in the CSFV-infected group when sections were inspected by immunofluorescence microscopy (Fig. 2D and E). Overall, our data demonstrated that CSFV infection caused the apoptosis of cells in the spleen.
Figure 2.
Apoptosis was activated by CSFV infection in the spleen. (A) Flow cytometry analysis of cell death in the spleen. Cell death was quantified using FITC-labeled Annexin V and PI. Q1-UL, another dead cell population that was FITC-Annexin V-negative and PI− positive; Q1-UR, a necrotic or end stage apoptotic cell population that was FITC-Annexin V- and PI− positive; Q1-LL, a cell population not undergoing apoptosis that was FITC-Annexin-V- and PI− negative; Q1-LR, an early apoptotic cell population that was FITC-Annexin V-positive and PI− negative. (B) Statistical analysis of the ratio of apoptosis (Annexin-V+ PI−) cells in the spleen (mean ± SD; n = 3; ***p < 0.001). (C) Western blotting revealed changes of apoptotic proteins. Cell lysates were prepared as described in Materials and Methods, and antibodies specific for the indicated proteins were used for western blotting analysis. (D) TUNEL staining demonstrated the existence of apoptotic cells in situ. The paraffin sections were stained with TUNEL as described in Materials and Methods. A representative image captured at magnifications under 20× objective was shown. (E) Statistical analysis of the number of TUNEL-positive cells in the spleen. 3 sections of each sample were prepared, and 3 random visual fields of each section were snapped. The number of apoptotic cells in each image was counted using Image-Pro Plus (version 6.0) (mean ± SD; n = 9; ***p < 0.001).
CSFV enhanced the autophagy pathway in spleen cells
Autophagy is involved in the replication of CSFV in vitro, as demonstrated in our previous study19. To clarify the effect of autophagy on splenic cells in pigs infected with CSFV, we first evaluated the expression of LC3II and SQSTM1, which were used as markers for assessing autophagy28. In contrast to the increased levels of LC3II, which indicate the formation of the autophagosome29, SQSTM1 exhibited reduced levels (Fig. 3A), reflecting the increased protein degradation of autolysosomes30. Simultaneously, we assessed changes in ATG5, which associates with membranes of precursor autophagosomes31. The results revealed that CSFV increased the expression of ATG5 in spleen cells (Fig. 3A). Moreover, increased levels of BECN1, which plays a role in the initiation of autophagy during CSFV infection in vitro 19, was detected in CSFV-infected spleen cells (Fig. 3A). To strengthen the occurrence of autophagy and to examine the spatial distribution of autophagic cells in the spleen of piglets infected with CSFV, paraffin sections were stained using a LC3II-specific antibody. As presented in Fig. 3B and C, CSFV increased the assessment score of the immunohistochemical assay. Notably, CSFV caused LC3II-positive cells to be mainly distributed around the splenic central arteriole (Fig. 3B, Zoom-2 and Zoom-3), which is a zone of T-lymphocyte predominance (T-cell zone) called the periarteriolar lymphoid sheath (PALS)32,33 (Fig. 3B and D). However, relatively few LC3II-positive cells were observed in the area with closely packed monomorphic small lymphocytes surrounding the PALS (Fig. 3B, Zoom-1 and Zoom-4), which is referred to the B-cell zone32,33 (Fig. 3B and D). Together, these results demonstrate the association between autophagy and CSFV infection in the spleen. To the best of our knowledge, this is the first data demonstrating that autophagy is activated by CSFV infection in vivo.
Figure 3.
CSFV induced autophagy in splenic cells. (A) Analysis of autophagy-related proteins by western blotting. Cell lysates were prepared as described in Materials and Methods, and antibodies specific for the indicated proteins were used for western blotting. The relative levels of target proteins were estimated by densitometry, and the ratios were calculated relative to GAPDH (mean ± SD; n = 3; *p < 0.05, ***p < 0.001). (B) Immunochemistry assay revealed the distribution of autophagic cells in the spleen. The paraffin sections were stained with LC3II antibody as described in Materials and Methods. Visual fields of each section were snapped under 20× objectives. (C) Immunocytochemistry score. 3 sections of each sample were prepared, and 3 random visual fields of each section were obtained. The score was evaluated by two different clinical doctors. The judging standard was the same as described in Fig. 1C. (D) Comparison of the mean optical staining density of T-cell and B-cell zones in the spleen. B-cell and T-cell zones in the spleen were defined according to the structure of the splenic central arteriole and the density of cell populations as described in Materials and Methods. Representative structures of B-cell zone (zoom-1 and zoom-4) and T-cell zone (zoom-2 and zoom-3) were presented in Fig. 3B. The mean optical density of B-cell and T-cell zones was analyzed using Image-Pro Plus (version 6.0) as described in Materials and Methods. The data represent the mean ± SD of 3 random fields of each section (**p < 0.01).
Autophagic cells partially underwent apoptosis in the spleen of pigs infected with CSFV
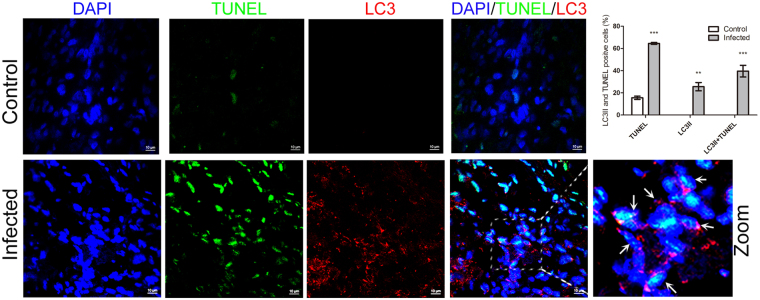
Our present data demonstrated that CSFV infection caused autophagy and apoptosis simultaneously in the spleen. Apoptosis is associated with the depletion of lymphocytes4,9,34. To clarify the relationship between autophagy and apoptosis of spleen cells in pigs infected with CSFV, frozen spleen sections were double stained with LC3II-antibody and TUNEL. Quantitative analysis of confocal images revealed that more apoptotic cells (~65%) appeared in CSFV-infected spleen compared with autophagic cells (~25%). Notably, partial LC3II-positive cells (~40%) were stained with TUNEL in CSFV-infected spleens (Fig. 4). This finding offered evidence that autophagy may participate in the apoptotic process in spleen cells of CSFV-infected pigs.
Figure 4.
Confocal microscopy showing the connection of autophagic and apoptotic cells. Frozen sections stained with TUNEL (green) were simultaneously immunostained with LC3II-antibody (red). Nuclei was stained with DAPI. In the zoomed images, the arrows indicated the colocalization of cells both undergoing autophagy and apoptosis. The average ratios of TUNEL-positive, LC3II-positive and double stained cells were based on at least 50 cells in each group (mean ± SD; n ≧ 50 cells; **p < 0.01, ***p < 0.001).
CSFV caused autophagy-mediated apoptosis and cell death in spleen T lymphocytes
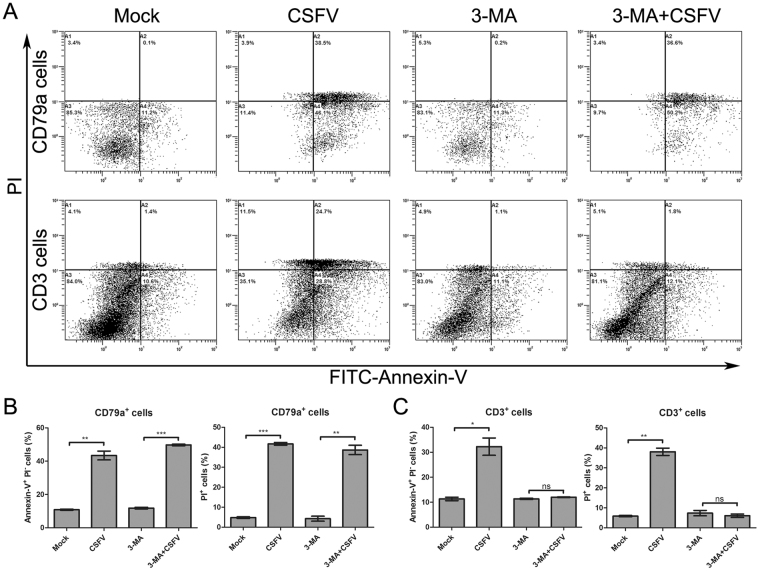
Based on the findings that LC3II-positive cells were predominant in the T-cell zone of the spleen (Fig. 3B and D) and that partial LC3II-positive cells were also TUNEL-positive in CSFV-infected spleens (Fig. 4), we assessed whether autophagy played a role in the apoptosis of T lymphocytes in the spleen of pigs infected with CSFV. To answer this question, we attempted to culture spleen cells ex vivo following the method described previously35. The drug 3-methyladenine (3-MA) was used to inhibit autophagy in cultured spleen cells. As shown in Fig. 5, CSFV infection not only increased early apoptosis (Annexin-V+) of CD79a+ and CD3+ cells but also increased cell death (PI+). However, the early apoptosis and death of CD3+ cells but not CD79a+ cells are obviously prevented by 3-MA (Fig. 5B and C). CD79a and CD3 are the special surface receptors of B and T lymphocytes, respectively36,37. According to these data, we hypothesized that autophagy resulted in apoptosis and death of T lymphocytes in the spleen of pigs infected with CSFV.
Figure 5.
Inhibition of autophagy reduced apoptosis and death of spleen CD3+ cells ex vivo. (A) Flow cytometry analysis of splenic cell death. Spleen cells were prepared and cultivated ex vivo as described in Materials and Methods. CSFV infection (MOI = 1) was conducted after cells were pretreated with 3-MA (5 mM) for 4 h. At 3 dpi, cultivated cells were stained with APC-conjugated antibody against CD79a and PE/Cy5-conjugated antibody against CD3 for cell type identification. Then, cells were stained with FITC-Annexin V and PI to analyze cell death. (B) Statistical analysis of apoptosis (Annexin-V+ PI−) and death (PI+) ratios of CD79a+ cells (mean ± SD; n = 3; **p < 0.01, ***p < 0.001). (C) Statistical analysis of apoptosis (Annexin-V+ PI−) and death (PI+) ratios of CD3+ cells (mean ± SD; n = 3; *p < 0.05, **p < 0.01).
Autophagy and apoptosis also occurred in bystander spleen cells
Previous reports demonstrated that the depleted leukocyte populations are mainly bystanders during CSFV infection in vivo 5,7, so we further examined whether autophagy and apoptosis in spleen cells were directly caused by CSFV infection. By staining E2 protein with WH303 antibody, confocal immunofluorescence of LC3II or TUNEL staining merged with the CSFV antigen were investigated. Most apoptotic cells were not infected with CSFV in vivo (Fig. 6A). However, it is worth noting that a small proportion of autophagic cells were not infected by CSFV, and that not all CSFV-infected cells were undergoing autophagy (Fig. 6B), in contrast to the observation in vitro 19.
Figure 6.
Confocal microscopy showing the colocalization of autophagic cells or apoptotic cells with CSFV. (A) Frozen sections were double stained with LC3II-antibody (green) and WH303-antibody (red). Nuclei was stained with DAPI. In the zoomed images, the arrows indicated autophagic cells infected with CSFV. The average ratios of LC3II-positive cells infected with CSFV were based on at least 50 cells in each field (mean ± SD; n ≧ 50 cells; *p < 0.05). (B) Frozen sections were double stained with LC3II-antibody (green) and WH303-antibody (red). Nuclei was stained with DAPI. In the zoomed images, the arrows indicated apoptotic cells infected with CSFV. The average ratios of TUNEL-positive cells infected with CSFV were based on at least 50 cells in each field (mean ± SD; n ≧ 50 cells; **p < 0.01).
Discussion
Leukopenia is a typical feature of piglets infected with CSFV, which may ultimately result in immunosuppression4,38,39. Although previous reports have linked apoptosis with the depletion of lymphocytes9,34, whether other cell death signals play a role in the process remains unknown. As the type II programmed cell death signal, autophagy can exert an independent or cooperative function with apoptosis to determine the fate of cells40. Continuous with our previous findings that CSFV induces autophagy to inhibit replication and apoptosis in host cells in vitro 19,20, we present evidence for the first time that autophagy participates in the apoptosis and death of T lymphocytes in the spleen of pigs infected with CSFV.
In the spleen, apoptosis is associated with pathological lesions caused by CSFV infection10. We sought to confirm the apoptosis of splenic cells and to explore the relationship between autophagy and apoptosis in the spleen of CSFV-infected pigs. An increased ratio of Annexin-V positive cells was detected using flow cytometry in the spleen. The results in the previous publication demonstrated that CSFV increased the percentage of cells with low mitochondrial transmembrane potential (ΔΨm) in peripheral blood lymphocytes9. Similar to Annexin-V, which binds to phosphatidylserine exposed on the surface of early apoptotic cells26, reduction of ΔΨm is also an early marker of apoptotic cells41. Previous findings demonstrated that CSFV inhibits apoptosis signals of vascular endothelial cells in vitro 11. In the present study, extrinsic and intrinsic apoptosis signals were activated using western blotting. The contrasting roles of apoptosis in vitro and in vivo have long been discussed9,11. Furthermore, TUNEL staining in situ revealed the frequent emergence of apoptotic cells in the spleen. This finding is consistent with the results demonstrating that TUNEL-positive cells are frequently observed in periarterial lymphatic sheaths (PALS) from 3 dpi10.
Previous in vitro studies demonstrated that virus-induced autophagy enhances the replication of particles and reduces apoptosis in host cells42,43. However, few reports have focused on autophagy and viruses in vivo. Whether autophagy is helpful for the existence of the virus in the host is unknown. The changes in autophagy markers (LC3II and SQSTM1) and the increased expression of autophagy-related genes, including ATG5 and BECN1, demonstrated that CSFV enhanced the formation of autophagy bulk and autophagy flux in splenic cells. These findings are consistent with those of a previous in vitro study showing that CSFV induced autophagy19. Activation of autophagy in CSFV-infected pigs demonstrated that viral immunopathology in vivo is related to autophagy or autophagy-related genes44–46. Moreover, examination of paraffin sections stained by LC3II-specific antibody demonstrated that autophagic cells were mainly scattered around PALS, which are mainly populated by T lymphocytes and referred to as the T-cell zone based on previous reports10. This observation links T cells with autophagy during CSFV infection in vivo.
As type II programmed cell death, autophagy is closely related to apoptosis. Autophagy and apoptosis can act as partners, antagonists or enablers to induce cell death in a complex manner18. Here, we first observed that autophagic cells were partially undergoing apoptosis simultaneously based on confocal images. This finding suggested that apoptotic cells in the spleen during CSFV infection might be partially caused by autophagy. By cultivating spleen cells ex vivo, we demonstrated that inhibition of autophagy by 3-MA treatment prevented apoptosis and death of T lymphocytes, but not B lymphocytes, caused by CSFV infection. Furthermore, we demonstrated that not all cells undergoing autophagy and apoptosis were infected with CSFV. Thus, autophagy and apoptosis signals in bystander cells were activated in vivo. These overall results were somewhat consistent with the finding that autophagy results in apoptosis of bystander CD4+ T lymphocytes during the development of AIDS47. By contrast, CSFV only induced autophagy in infected host cells to enhance viral replication19 and to reduce the apoptosis of infected cells in vitro 20. We hypothesized that the induction of autophagy in bystander cells in vivo may be attributed to complex stress inducers of autophagy, including starvation, cytokines, or pathogens42. For example, Type I interferons (IFN) are important proteins in the antiviral response, but evidence has highlighted their newly discovered function as inducers of autophagy48. Interestingly, up-regulation of IFN-stimulated genes is related to cell death in acute CSFV-infected piglets49. However, whether autophagy of bystander cells was induced by high levels of IFN remains to be clarified.
In conclusion, the present study offered the first data that apoptosis and death of T lymphocytes in the spleen are dependent on the activation of autophagy in CSFV-infected pigs. We explored a new approach for understanding the depletion of the T cell population during CSFV infection in vivo, which is associated with immunosuppression in pigs. Our findings may be helpful to clarify the pathogenesis of CSFV infection and to develop novel antiviral strategies. Chemical drugs target to ATG proteins may be potential modulators for treatment of T leukocytes depletion of pigs caused by CSFV infection. However, future studies should focus on how autophagy is involved in apoptosis and death of T cells and why B cells do not undergo autophagy in this context.
Materials and Methods
Antibodies
The following primary antibodies were used in the study: rabbit polyclonal anti-LC3B (Cell Signaling Technology, 2775), rabbit polyclonal anti-SQSTM1/p62 (Sigma, SAB2104334), rabbit polyclonal anti-BECN1 (ABclonal, A7353), rabbit polyclonal anti-ATG5 (ABclonal, A0203), mouse monoclonal anti-GAPDH (Beyotime, AG019), rabbit polyclonal anti-caspase-8 (Beyotime, AC056), rabbit polyclonal anti-caspase-9 (Beyotime, AC062), rabbit polyclonal anti-Caspase-3 (ABclonal, A2156), rabbit polyclonal anti-PARP (Beyotime, AP102) and mouse monoclonal anti-CSFV E2 (WH303) (JBT, 9011). Mouse polyclonal anti-CSFV Npro was kindly provided by Dr. Xinglong Yu (Veterinary Department, Hunan Agricultural University, China). The secondary antibodies used for immunoblotting analysis were HRP-conjugated goat anti-mouse IgG (Bioworld, BS12478), HRP-conjugated goat anti-rabbit IgG (Bioworld, BS13278). The secondary antibodies used for immunofluorescence including Dylight 488 goat anti-mouse IgG (EarthOx, E032210), Dylight 488 goat anti-rabbit IgG (EarthOx, E032220), Dylight 549 goat anti-mouse IgG (EarthOx, E032310) and Dylight 549 goat anti-rabbit IgG (EarthOx, E032320).
Virus
Blood stock of the virulent CSFV strain Shimen was propagated 1 passage in the swine kidney cell line PK-15. Virus titers were determined by end-point titration on PK-15 cells as previously described19. Briefly, cells cultivated in 96-well plates were fixed with 80% acetone at −20 °C for 30 min, and infected cells were detected using the immunofluorescence assay. The primary antibody was mouse anti-CSFV E2 antibody (WH303), and the secondary antibody was Dylight488-conjugated goat anti-mouse IgG. Virus titers were calculated according to Kaerber and expressed as 50% tissue culture infectious doses (TCID50) per milliliter.
Infection of pigs
A total of 6 animals (Tibet swine, 5 months old) bred at the specific pathogen-free (SPF) unit of the institute were assessed using ELISA and PCR (data not shown). 3 animals were randomly selected, and each one was infected with 105 TCID50 CSFV. The other 3 animals were intramuscularly injected with the same dose of saline water and used as controls. Examination of body temperature and clinical symptoms were conducted every 2 days. All animals were sacrificed at 9 dpi for the collection of spleens when severe clinical symptoms appeared in most infected animals. All experimental procedures were performed in accordance with animal ethics guidelines and approved by the Animal Care and Use Committee of South China Agriculture University, China. The issue number is 2014-12.
Culture and infection of spleen cells ex vivo
Spleen cells were prepared and cultivated following the methods previously described35. Briefly, the spleens of anti-CSFV antibody-negative pigs were removed in sterile conditions. The spleen tissue was cut, softly pressed and dispersed by passing through 70-μm cell strainers. Dispersed cells were washed twice in RPMI 1640 supplemented with 2% antibiotics. Approximately 2 × 106 cells were cultivated in a volume of 200 μl culture medium in 96-well U-bottom plates. The culture medium contained RPMI 1640 supplemented with 15% FBS (Gibco) and 1% antibiotics (Gibco). After 18 h, cells were pretreated with 5 mM 3-MA for 4 h followed by inoculating with CSFV at 1 multiplicity of infection for 1 h. Uninfected virus was removed by washing cells twice in RPMI 1640 supplemented with 2% antibiotics. Then, cells continued cultivating in complete medium containing 5 mM 3-MA for 3 days at 37 °C with 5% CO2.
Immunoblotting
After washing spleens samples with PBS three times, 50 to 60 mg small mass of each sample was incubated on ice with 1 ml RIPA lysis buffer (Beyotime, P0013B) containing 1 mM PMSF (Beyotime, ST506) and homogenized by Dounce Tissue Grinders. The precipitation was removed by centrifugation at 14,000 g for 10 min at 4 °C. Then, the supernatant was collected, and the protein concentration was quantified by the BCA protein assay kit (Thermo, 23227). Equal amounts of total proteins (20 μg) were separated by 12% SDS-PAGE gels and then electrotransferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, IPVH00010). Then, 5% nonfat milk dissolved in PBS containing 0.1% Tween 20 was used to block the membranes for 1 h at 25 °C. Membranes were incubated with the corresponding primary antibodies at 4 °C overnight and secondary antibodies conjugated to HRP at 37 °C for 1 h. The bands were detected using the ECL Plus kit (Beyotime, P0018) and visualized using the chemiluminescence imaging system (Fine-do × 6, Tanon). Protein blots were measured with Image-Pro Plus (version 6.0).
Immunohistochemistry
Samples of spleen were fixed in 10% phosphate-buffered formalin, embedded in paraffin, and sliced at 3 to 4 mm. Tissue sections were deparaffinized by 3 steps in xylol at 5 min per step; two steps of 5 min each in absolute ethanol; and one 4-min step in 95% ethanol, 85% ethanol and 70% ethanol each followed by 2 min in distilled water. Antigen retrieval with pH 8.0 EDTA buffer was conducted in the pressure cooker (5 min from the beginning of boiling). Afterwards, samples were incubated with 3% hydrogen peroxide for 8 min to block endogenous peroxidase activity. To examine autophagy activation, paraffin sections were stained with rabbit polyclonal anti-LC3II antibody (1:400). For the detection of cells infected with CSFV, mouse monoclonal anti-CSFV E2 protein antibody (1:50) was utilized. ChemMate EnVision kit (DAKO) was used to display the staining. After counterstaining with hematoxylin, 3 digital images for each section at 3456 × 2304-pixel resolution at magnifications under 20× objectives were captured (Olympus, BX43).
Evaluation of immunohistochemistry images
The score of immunohistochemistry images was judged by two different clinical doctors according to the criteria reported previously50. The staining pattern is not given a serially numerical score but is only assigned a broad category. The score of staining intensity is judged according to characteristics of positively stained cells. Pale yellow, tan and sepia were given scores of 1, 2 and 3, respectively. The score of the stained area was judged according to the ratio of positive cells. Scores of 1, 2, 3, and 4 corresponded to ratios of 6~25%, 26~50%, 51~75% and >75%, respectively.
Semi-quantitative evaluation of IHC staining by Image-Pro Plus (version 6.0) was conducted according to the method introduced in a previous report51. Briefly, after B-cell and T-cell zones were defined according to the structure of the splenic central arteriole and the packed density of the cell populations32,33, parameters, including area sum and IOD, were measured. The optical density of the area of interest was calibrated and established as follows: hue, 0 to 30; saturation, 0 to 255; intensity, 0 to 255. The mean optical staining density of B-cell and T-cell zones was calculated by IOD/area sum.
TUNEL staining
To analyze the distribution of cells undergoing apoptosis, paraffin sections of spleen were stained with TUNEL BrightGreen Apoptosis Detection Kit (Vazyme, A112-01/02/03) according to the manufacturer’s instructions. 3 digital images for each section at 3456 × 2304-pixel resolution at magnifications under 20× objectives were captured (Olympus, BX53).
FACS Analysis
For spleen tissues, partial spleen blocks were minced and passed through a 70-μm cell strainer. Dispersed tissues were washed in phosphate-buffered saline 3 times and then directly stained with FITC-conjugated Annexin-V and propidium iodide (PI) for 10 min to analyze apoptosis using the Annexin V-FITC Apoptosis Detection Kit I (Vazyme, A211-01/02) according to the manufacturer’s instructions.
For spleen cells cultivated ex vivo, cells washed in phosphate-buffered saline 3 times were respectively stained with APC-conjugated antibody against CD79a (Abnova, MAB4515) and PE/Cy5-conjugated antibody against CD3 (Abcam, ab25535) at 37 °C for 1 hour. After cells were washed in phosphate-buffered saline 3 times again, the Annexin V-FITC Apoptosis Detection Kit I was used to analyze apoptosis as described above.
Confocal microscopy
Spleen samples were sectioned (8 μm) on a cryostat. After fixation in 4% paraformaldehyde and permeabilization with 0.25% TritonX-100, cryostat sections were double-stained for the immunofluorescence assay. In brief, mouse monoclonal anti-CSFV E2 protein (1:50) and anti-mouse secondary antibodies conjugated to Dylight-549 (1:200) were used to determine CSFV infection. Anti-LC3B antibody (1:400) and then anti-rabbit secondary antibodies conjugated to Dylight-488 (1:200) or Dylight-549 (1:200) were stained to detect the formation of autophagy. To stain apoptotic cells, TUNEL BrightGreen Apoptosis Detection Kit (Vazyme, A112-01/02/03) was used according to the manufacturer’s instructions. Wherever indicated, nuclei were stained with DAPI (Beyotime, C1005). The fluorescence signals were visualized under a 100× oil objective using LSM710 confocal microscope (Leica).
Statistical analysis
Statistical analysis was performed utilizing Graph Pad Prism 5 software. Unpaired Student’s t-test was used to compare means of different groups. Value of P lesser than 0.05 were considered significant and that lesser than 0.001 were considered highly significant.
Electronic supplementary material
Acknowledgements
We are grateful to Dr Xinglong Yu for providing the anti-CSFV Npro antibody. This work was supported by grants from the Natural Science Foundation of China (Nos. U1405216, 31472200, and 31672590), the Science and Technology Program of Guangzhou, China (Nos 2015A050502044 and 2015B020230009), and the Key Program of National Research and Development Plan (Nos 2016YFD0500707, 2016YFD0500801).
Author Contributions
H.G. performed the spleen detection and drafted the manuscript. M.Z. participated in the design of the study. S.F. and J.Y. prepared materials for the experiments. J.L. participated in pig feeding. W.H. and H.X. helped to collect the spleens. J.C. conceived of the study and participated in its design and coordination. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Hongchao Gou and Mingqiu Zhao contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14082-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paton DJ, et al. A proposed division of the pestivirus genus using monoclonal antibodies, supported by cross-neutralisation assays and genetic sequencing. Vet Res. 1995;26:92. [PubMed] [Google Scholar]
- 2.Thiel HJ, Stark R, Weiland E, Rumenapf T, Meyers G. Hog cholera virus: molecular composition of virions from a pestivirus. J Virol. 1991;65:4705. doi: 10.1128/jvi.65.9.4705-4712.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange A, Blome S, Moennig V, Greiser-Wilke I. Pathogenesis of classical swine fever–similarities to viral haemorrhagic fevers: a review. Berl Munch Tierarztl Wochenschr. 2011;124:36. [PubMed] [Google Scholar]
- 4.Susa M, Konig M, Saalmuller A, Reddehase MJ, Thiel HJ. Pathogenesis of classical swine fever: B-lymphocyte deficiency caused by hog cholera virus. J Virol. 1992;66:1171. doi: 10.1128/jvi.66.2.1171-1175.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Summerfield A, Hofmann MA, McCullough KC. Low density blood granulocytic cells induced during classical swine fever are targets for virus infection. Vet Immunol Immunopathol. 1998;63:289. doi: 10.1016/S0165-2427(98)00108-1. [DOI] [PubMed] [Google Scholar]
- 6.Blome S, Meindl-Bohmer A, Nowak G, Moennig V. Disseminated intravascular coagulation does not play a major role in the pathogenesis of classical swine fever. Vet Microbiol. 2013;162:360. doi: 10.1016/j.vetmic.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Lee WC, Wang CS, Chien MS. Virus antigen expression and alterations in peripheral blood mononuclear cell subpopulations after classical swine fever virus infection. Vet Microbiol. 1999;67:17. doi: 10.1016/S0378-1135(99)00029-2. [DOI] [PubMed] [Google Scholar]
- 8.Benedict CA, Norris PS, Ware CF. To kill or be killed: viral evasion of apoptosis. Nat Immunol. 2002;3:1013. doi: 10.1038/ni1102-1013. [DOI] [PubMed] [Google Scholar]
- 9.Summerfield A, Knotig SM, McCullough KC. Lymphocyte apoptosis during classical swine fever: implication of activation-induced cell death. J Virol. 1998;72:1853. doi: 10.1128/jvi.72.3.1853-1861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato M, Mikami O, Kobayashi M, Nakajima Y. Apoptosis in the lymphatic organs of piglets inoculated with classical swine fever virus. Vet Microbiol. 2000;75:1. doi: 10.1016/S0378-1135(00)00198-X. [DOI] [PubMed] [Google Scholar]
- 11.Johns HL, et al. Classical swine fever virus infection protects aortic endothelial cells from pIpC-mediated apoptosis. J Gen Virol. 2010;91:1038. doi: 10.1099/vir.0.016576-0. [DOI] [PubMed] [Google Scholar]
- 12.Reggiori F. 1. Membrane origin for autophagy. Curr Top Dev Biol. 2006;74:1. doi: 10.1016/S0070-2153(06)74001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 15.Kirkegaard K, Taylor MP, Jackson WT. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol. 2004;2:301. doi: 10.1038/nrmicro865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu HJ, Pu JL, Krafft PR, Zhang JM, Chen S. The molecular mechanisms between autophagy and apoptosis: potential role in central nervous system disorders. Cell Mol Neurobiol. 2015;35:85. doi: 10.1007/s10571-014-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Kim SO, Li Y, Han J. Autophagy contributes to caspase-independent macrophage cell death. J Biol Chem. 2006;281:19179. doi: 10.1074/jbc.M513377200. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 19.Pei J, et al. Autophagy enhances the replication of classical swine fever virus in vitro. Autophagy. 2014;10:93. doi: 10.4161/auto.26843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pei J, et al. Absence of autophagy promotes apoptosis by modulating the ROS-dependent RLR signaling pathway in classical swine fever virus-infected cells. Autophagy. 2016;12:1738. doi: 10.1080/15548627.2016.1196318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terpstra C. Hog cholera: an update of present knowledge. Br Vet J. 1991;147:397. doi: 10.1016/0007-1935(91)90081-W. [DOI] [PubMed] [Google Scholar]
- 22.Narita M, Kawashima K, Shimizu M. Viral antigen and B and T lymphocytes in lymphoid tissues of gnotobiotic piglets infected with hog cholera virus. J Comp Pathol. 1996;114:257. doi: 10.1016/S0021-9975(96)80047-8. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Villamandos JC, et al. Morphological and immunohistochemical changes in splenic macrophages of pigs infected with classical swine fever. J Comp Pathol. 2001;125:98. doi: 10.1053/jcpa.2001.0487. [DOI] [PubMed] [Google Scholar]
- 24.Lin M, Lin F, Mallory M, Clavijo A. Deletions of structural glycoprotein E2 of classical swine fever virus strain alfort/187 resolve a linear epitope of monoclonal antibody WH303 and the minimal N-terminal domain essential for binding immunoglobulin G antibodies of a pig hyperimmune serum. J Virol. 2000;74:11619. doi: 10.1128/JVI.74.24.11619-11625.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Eijnde SM, et al. Cell surface exposure of phosphatidylserine during apoptosis is phylogenetically conserved. Apoptosis. 1998;3:9. doi: 10.1023/A:1009650917818. [DOI] [PubMed] [Google Scholar]
- 26.Zwaal RF, Comfurius P, Bevers EM. Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci. 2005;62:971. doi: 10.1007/s00018-005-4527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu G, Shi Y. Apoptosis signaling pathways and lymphocyte homeostasis. Cell Res. 2007;17:759. doi: 10.1038/cr.2007.52. [DOI] [PubMed] [Google Scholar]
- 28.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 30.Bjorkoy G, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizushima N, et al. A protein conjugation system essential for autophagy. Nature. 1998;395:395. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 32.van Krieken JH, Te VJ, Kleiverda K, Leenheers-Binnendijk L, van de Velde CJ. The human spleen; a histological study in splenectomy specimens embedded in methylmethacrylate. Histopathology. 1985;9:571. doi: 10.1111/j.1365-2559.1985.tb02841.x. [DOI] [PubMed] [Google Scholar]
- 33.Pabst R, Geisler R. The route of migration of lymphocytes from blood to spleen and mesenteric lymph nodes in the pig. Cell Tissue Res. 1981;221:361. doi: 10.1007/BF00216740. [DOI] [PubMed] [Google Scholar]
- 34.Summerfield A, et al. Depletion of CD4(+) and CD8(high +) T-cells before the onset of viraemia during classical swine fever. Vet Immunol Immunopathol. 2001;78:3. doi: 10.1016/S0165-2427(00)00248-8. [DOI] [PubMed] [Google Scholar]
- 35.Jekle A, et al. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J Virol. 2003;77:5846. doi: 10.1128/JVI.77.10.5846-5854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, et al. Molecular cloning and expression analysis of pig CD79alpha. Vet Immunol Immunopathol. 2008;125:368. doi: 10.1016/j.vetimm.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Gerner W, Kaser T, Pintaric M, Groiss S, Saalmuller A. Detection of intracellular antigens in porcine PBMC by flow cytometry: A comparison of fixation and permeabilisation reagents. Vet Immunol Immunopathol. 2008;121:251. doi: 10.1016/j.vetimm.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Moennig V, Plagemann PG. The pestiviruses. Adv Virus Res. 1992;41:53. doi: 10.1016/S0065-3527(08)60035-4. [DOI] [PubMed] [Google Scholar]
- 39.Paton DJ, Greiser-Wilke I. Classical swine fever–an update. Res Vet Sci. 2003;75:169. doi: 10.1016/S0034-5288(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 40.Seay MD, Dinesh-Kumar SP. Life after death: are autophagy genes involved in cell death and survival during plant innate immune responses? Autophagy. 2005;1:185. doi: 10.4161/auto.1.3.2080. [DOI] [PubMed] [Google Scholar]
- 41.Akbar AN, Salmon M, Savill J, Janossy G. A possible role for bcl-2 in regulating T-cell memory–a ‘balancing act’ between cell death and survival. Immunol Today. 1993;14:526. doi: 10.1016/0167-5699(93)90181-J. [DOI] [PubMed] [Google Scholar]
- 42.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh JE, Lee HK. Autophagy as an innate immune modulator. Immune Netw. 2013;13:1. doi: 10.4110/in.2013.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhai X, et al. Coxsackievirus B3 Induces Autophagic Response in Cardiac Myocytes in vivo. Biochemistry (Mosc) 2015;80:1001. doi: 10.1134/S0006297915080052. [DOI] [PubMed] [Google Scholar]
- 45.Park S, et al. Autophagy Genes Enhance Murine Gammaherpesvirus 68 Reactivation from Latency by Preventing Virus-Induced Systemic Inflammation. Cell Host Microbe. 2016;19:91. doi: 10.1016/j.chom.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu Q, et al. Homeostatic Control of Innate Lung Inflammation by Vici Syndrome Gene Epg5 and Additional Autophagy Genes Promotes Influenza Pathogenesis. Cell Host Microbe. 2016;19:102. doi: 10.1016/j.chom.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Espert L, et al. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest. 2006;116:2161. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmeisser H, Bekisz J, Zoon KC. New function of type I IFN: induction of autophagy. J Interferon Cytokine Res. 2014;34:71. doi: 10.1089/jir.2013.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renson P, et al. Acute induction of cell death-related IFN stimulated genes (ISG) differentiates highly from moderately virulent CSFV strains. Vet Res. 2010;41:7. doi: 10.1051/vetres/2009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamashita S, Taniguchi S, Hoshiai O, Tawara H, Yasuda K. Immunoperoxidase techniques with a combined use of PAP and ABC procedures: application to immunohistochemical studies using monoclonal antibodies. Okajimas Folia Anat Jpn. 1984;61:387. doi: 10.2535/ofaj1936.61.5_387. [DOI] [PubMed] [Google Scholar]
- 51.Wang CJ, et al. Survivin expression quantified by Image Pro-Plus compared with visual assessment. Appl Immunohistochem Mol Morphol. 2009;17:530. doi: 10.1097/PAI.0b013e3181a13bf2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.