Abstract
Spontaneous activity of serotonergic neurons of the dorsal raphe nucleus (DRN) regulates mood and motivational state. Potentiation of serotonergic function is one of the therapeutic strategies for treatment of various psychiatric disorders, such as major depression, panic disorder and obsessive-compulsive disorder. However, the control mechanisms of the serotonergic firing activity are still unknown. In this study, we examined the control mechanisms for serotonergic spontaneous activity and effects of chronic antidepressant administration on these mechanisms by using modified ex vivo electrophysiological recording methods. Serotonergic neurons remained firing even in the absence of glutamatergic and GABAergic ionotropic inputs, while blockade of L-type voltage dependent Ca2+ channels (VDCCs) in serotonergic neurons decreased spontaneous firing activity. L-type VDCCs in serotonergic neurons received gamma-aminobutyric acid B (GABAB) receptor-mediated inhibition, which maintained serotonergic slow spontaneous firing activity. Chronic administration of an antidepressant, citalopram, disinhibited the serotonergic spontaneous firing activity by weakening the GABAB receptor-mediated inhibition of L-type VDCCs in serotonergic neurons. Our results provide a new mechanism underlying the spontaneous serotonergic activity and new insights into the mechanism of action of antidepressants.
Introduction
The serotonergic system plays an important role in regulating a wide variety of brain functions, such as mood and cognition1. Among the serotonergic nuclei, the DRN regulates mood- and emotion-related behaviors, and the functional changes in this area are associated with various mental illnesses. DRN serotonergic neurons have slow and regular firing activity when recorded in vivo 2, suggesting that this tonic firing plays important roles for maintaining mood. Supporting this hypothesis, a growing body of evidence implicates that a change in the activity of DRN serotonergic neurons alters affection status3–5, while the mechanisms for modulating serotonergic activity are not fully uncovered.
Despite the fact that serotonergic neurons are tonically active when recorded in vivo, most of the previous ex vivo electrophysiological analyses used pharmacological and/or electrical stimulations to generate continuous firing because of the difficulty in maintaining the spontaneous activity of serotonergic neurons in acute brain slices2,6,7. In this context, it was widely believed that the excitatory inputs from another brain area, such as the prefrontal cortex and locus coeruleus, are necessary for the tonic firing activity of serotonergic neurons, while previous study suggests the existence of intrinsic pacemaker mechanisms in serotonergic neurons8. Until now, this contradiction between in vivo and ex vivo studies was not resolved.
Like most neurons, serotonergic neurons receive local GABAergic inhibitory inputs9. Recently, we investigated the local feedback circuit between DRN serotonergic neurons and GABAergic interneurons and found that continuous GABAergic inhibition maintains serotonergic activity10. GABAA receptor-mediated ionotropic inputs are well-studied, while several lines of evidence suggest that postsynaptic GABAB receptors may contribute to the modulation of serotonergic neurons4,11. Furthermore, chronic stress increases GABAergic neuronal activity and GABAB receptor expression in the DRN12,13. These observations indicate the possibility that GABAB receptor-mediated signaling contributes to the modulation of the baseline activity of serotonergic neurons, while little is known about its molecular mechanisms of GABAB receptor-mediated inhibition of serotonergic neurons.
Most of the clinically-used drugs for psychiatric disorders such as selective serotonin reuptake inhibitors (SSRIs) modulate the serotonergic function of the brain14, while the precise mechanisms of such serotonergic drugs remain to be elucidated. Antidepressants have a delayed onset of action, suggesting that chronic antidepressant treatment-induced cellular and synaptic changes are necessary for the therapeutic action15,16. Consistent with these reports, we previously showed that chronic treatment with antidepressants enhances serotonin release in vitro 17,18. These findings suggest the possibility that chronic treatment with antidepressant potentiates serotonergic activity.
In the present study, by using modified ex vivo electrophysiological recording method, we could record serotonergic spontaneous firing activity even without any stimulations. This spontaneous firing activity was mainly regulated by L-type voltage-dependent Ca2+ current, which was continuously inhibited by GABAB receptor-mediated signaling. Moreover, chronic administration of an antidepressant disinhibited the serotonergic spontaneous firing activity by weakening the GABAB receptor-mediated continuous inhibition. These results offer a new mechanism for the GABAergic inhibition of DRN serotonergic neurons, which was responsive to chronic antidepressant treatment.
Results
DRN serotonergic neurons spontaneously generate action potentials in ex vivo electrophysiological recordings
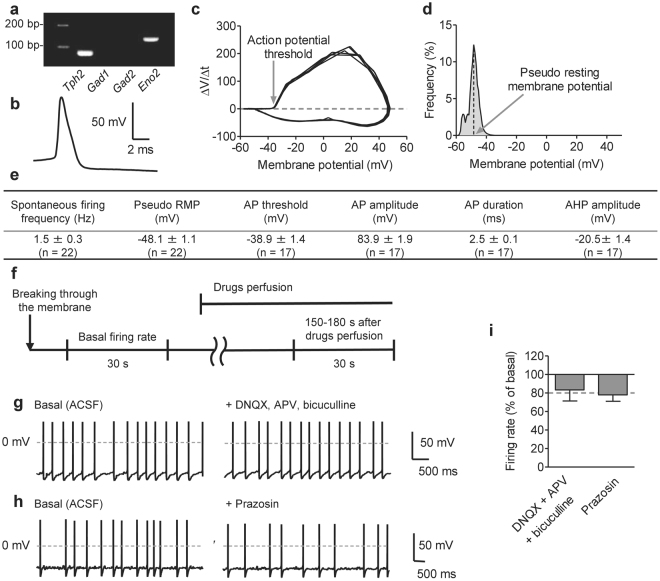
To examine control mechanisms for DRN serotonergic activity, we modified the recording method, which enables recording serotonergic spontaneous firing activity. While most of previous researches pointed out that serotonergic neurons are silent in ex vivo recordings2,6, recent study suggests that part of serotonergic neurons (~50%) showed spontaneous firing activity in “high quality” brain slices7. To increase spontaneously active serotonergic neurons, we prepared coronal brain slices with strictly controlled knife speed and vibration (see Methods) to avoid pressure-induced neuronal damage. Additionally, we used NMDG-based cutting solution, which are suitable for slicing adult brains19. By these modifications, we achieved recording spontaneous firing activity from more than 75% of DRN serotonergic neurons, which expressed Tph2 mRNA (Fig. 1a,e; Supplementary Fig. S1). Similar to previous reports2, serotonergic neurons showed wide action potential (AP) and large afterhyperpolarization (AHP) amplitude (Fig. 1b,e). To examine AP threshold and resting membrane potential (RMP) in spontaneously active serotonergic neurons, we used phase plane plot and voltage histogram, respectively20 (Fig. 1c,d). In our methods, most of firing characters were essentially similar to those reported previously7,21, while slight depolarization of RMP (−48.1 ± 1.1 mV) and low AP threshold (−38.9 ± 1.4 mV) were observed compared to the previous data (RMP; −56 ± 3.6 mV, AP threshold; −28 ± 1.1 mV)22 (Fig. 1e).
Figure 1.
Serotonergic neurons in the dorsal raphe nucleus (DRN) show spontaneous firing activity. (a) Representative cropped image of single-cell reverse transcription polymerase chain reaction after whole-cell recording. Tryptophan hydroxylase 2 (Tph2) mRNA was used as a marker of serotonergic neurons. Glutamate decarboxylase 1 and 2 mRNA (Gad1 and Gad2), markers of GABAergic neurons, were used as negative controls. Gamma-enolase mRNA (Eno2), a marker for neurons, was used as a positive control. Uncropped image was shown in Supplementary Fig. S1. (b) Representative trace of the action potential (AP) recorded from DRN serotonergic neurons. Serotonergic neurons showed a wide action potential and a long-lasting after hyperpolarization. (c) Representative phase plane plot of membrane potential vs. its derivative with respect to time (dV/dt). Five APs from one neuron was plotted. (d) Representative membrane voltage histogram. The higher voltage peak was considered as pseudo resting membrane potential (RMP). (e) Electrophysiological characters of 22 serotonergic neurons from 7 mice. Recordings were performed in normal ACSF condition without any drug or electrical stimulation. AHP; afterhyperpolarization. (f) Time course of recording the effects of drug perfusion. Spontaneous firing was recorded for 30 s before and after drug application, and changes in the firing rate were calculated. (g,h) Representative traces of the spontaneous firing before (left) and after (right) the application of DNQX (20 μM), APV (50 μM) and bicuculline (20 μM) (g) or prazosin (1 μM) (h). (i) The changes in the spontaneous firing rate before and after the application of DNQX (20 μM), APV (50 μM), and bicuculline (20 μM), or prazosin (1 μM). (DNQX + APV + bicuculline, n = 4 neurons from 3 mice, P = 0.2545 by paired t-test; prazosin, n = 3 neurons from 2 mice, P = 0.0855 by paired t-test). Data are presented as the mean ± S.E.M.
We next confirmed whether the spontaneous firing activity of serotonergic neurons depends on extrinsic synaptic inputs or intrinsic activity, we examined the contribution of major ionotropic inputs and noradrenergic α1 receptor2,4 (Fig. 1f). Bath application of glutamate and GABAA receptor antagonists (20 μM 6,7-dinitroquinoxaline-2,3-(1 H, 4 H)-dione [DNQX], 50 μM DL-(-)-2-amino-5-phosphonopentanoic acid [APV], and 20 μM bicuculline) slightly decreased but did not eliminate spontaneous firing activity of serotonergic neurons (Fig. 1g,i). Similarly, α1 receptor antagonist (1 μM prazosin) failed to abolish the spontaneous activity (Fig. 1h,i), suggesting that serotonergic spontaneous activity shown here mainly depended on intrinsic activity of serotonergic neurons.
L-type voltage-dependent calcium current is responsible for the spontaneous firing activity of DRN serotonergic neurons
In several types of spontaneously active neurons, such as dopaminergic neurons, the major factors for generating firing activity are T-type voltage-dependent calcium channels (VDCCs) and hyperpolarization-activated cyclic nucleotide–gated (HCN) channels23,24. Different from other pacemaker neurons, low-voltage activated (LVA) current and negative current injection-mediated voltage sag, which reflects the function of T-type VDCCs and HCN channels respectively, were subtle in serotonergic neurons (Supplementary Fig. S2b,c). Consistent with these observations, the blocking of T-type VDCCs (50 μM NiCl2) or HCN channels (20 μM ZD7288) did not decrease the serotonergic firing activity (Supplementary Fig. S2d).
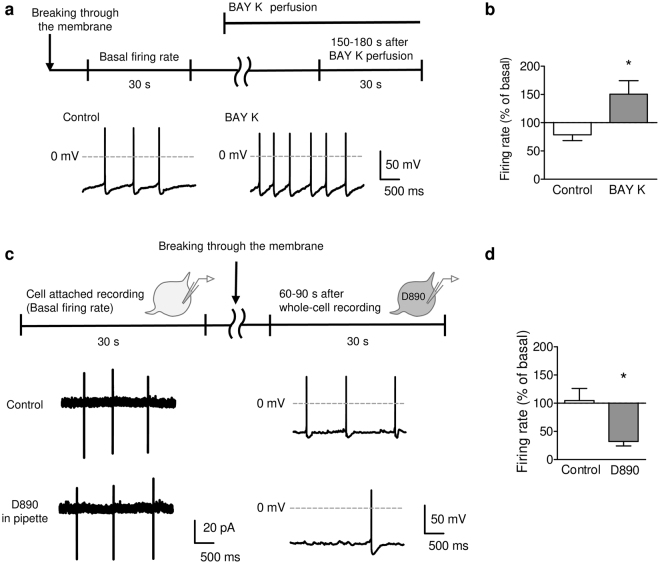
Recently, L-type VDCCs were recognized as machinery for generating spontaneous firing activity25. We next examined the involvement of L-type VDCCs in spontaneous firing activity. Blocking of L-type VDCCs with 10 μM nifedipine significantly decreased the spontaneous firing rate of serotonergic neurons (Supplementary Fig. S2a,d). On the contrary, bath application of an L-type VDCC activator, 1 μM (S)-(−)-Bay K 8644, significantly increased the spontaneous firing rate (Fig. 2a,b). As L-type VDCCs are widely expressed in the brain, we examined whether L-type VDCCs on serotonergic neurons or other neurons are critical for the spontaneous serotonergic activity. To test this issue, we performed intracellular application of a membrane-impermeable L-type VDCC blocker 0.5 mM D890 via a patch pipette, where L-type VDCCs are active in cell attached recordings and blocked after establishing whole-cell recordings. Intracellular application of D890 significantly decreased the spontaneous firing in whole-cell recordings compared to the basal firing rate in cell-attached recordings of the same neurons (Fig. 2c,d).
Figure 2.
L-type voltage-dependent Ca2+ channel (VDCC) participates in the spontaneous activity of DRN serotonergic neurons. (a) Representative traces of the spontaneous firing before (left) and after (right) the application of BAY K 8644 (BAY K; 1 μM). (b) The effect of BAY K 8644 (BAY K; 1 μM) on the spontaneous firing rate in serotonergic neurons. The average firing rate between 150–180 s after beginning the perfusion was compared to the basal firing rate (right). *P < 0.05 vs. Control. (Control, n = 4 neurons from 2 mice; BAY K, n = 6 neurons from 3 mice; P = 0.0496, Student’s t-test). (c) To block L-type VDCC current only in the recorded neurons, D890 (0.5 mM) was added to pipette solution, and the spontaneous firing was recorded for 30 s both in cell-attached (left) and whole-cell (right) configurations. (d) The average firing rate between 60–90 s after beginning the whole-cell recordings was compared to that in the cell-attached recordings. *P < 0.05 vs. Control. (Control, n = 4 neurons from 3 mice; D890, n = 4 neurons from 3 mice; P = 0.0190, Student’s t-test). Data are presented as the mean ± S.E.M.
GABAB receptor-mediated signaling inhibits the L-type VDCC-mediated spontaneous firing activity
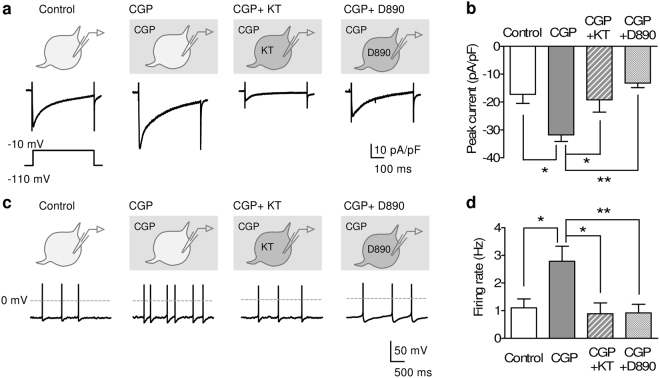
Both in previous in vivo recordings and our ex vivo recordings in this study, the firing rate of serotonergic neurons was slower than that of other spontaneously active neurons26,27. Thus, we hypothesized that serotonergic firing activity receives continuous inhibition. To test this hypothesis, we examined the inhibition mechanisms of L-type VDCC current in serotonergic neurons. Besides GABAA receptors, GABAB receptor is a key molecule that inhibits serotonergic neurons28. As expected, the pharmacological blocking of GABAB receptors (10 μM CGP52432) increased VDCC current (Fig. 3a,b). GABAB receptors are mainly coupled with Gi/o-type G protein and inhibit the activity of protein kinase A (PKA)29. GABAB receptor antagonist-induced increase in VDCC current was abolished by intracellular application of a PKA inhibitor (1 μM KT5720) or an L-type VDCC blocker (0.5 mM D890) (Fig. 3a,b), suggesting that GABAB receptors continuously inhibit L-type VDCC current by weakening PKA activity.
Figure 3.
GABAB receptors inhibit both L-type VDCC and pacemaker activity in DRN serotonergic neurons. (a,b) Representative traces (a) and peak current density (b) of high voltage activated (HVA) VDCC current in serotonergic neurons. The recordings were performed in the presence of DNQX (20 μM), APV (50 μM), bicuculline (20 μM), and tetrodotoxin (1 μM). HVA VDCC current was evoked by voltage step from −110 mV to −10 mV. CGP52432 (CGP; 10 μM) was bath-applied. KT5720 (KT; 1 μM) and D890 (0.5 mM) were applied through a patch pipette. *P < 0.05, **P < 0.01. (Control, n = 5 neurons from 2 mice; CGP, n = 8 neurons from 4 mice; CGP + KT, n = 8 neurons from 2 mice; CGP + D890, n = 7 neurons from 2 mice; one-way ANOVA; F(3, 24) = 6.767, P = 0.018; Tukey’s Multiple Comparison Test; Control vs. CGP, P < 0.05, CGP vs. CGP + KT, P < 0.05, CGP vs. CGP + D890, P < 0.01). (c,d) Representative traces (c) and average spontaneous firing rate (d) in serotonergic neurons. Recordings were performed in the presence of DNQX (20 μM), APV (50 μM), bicuculline (20 μM), WAY100635 (0.1 μM), and GR127935 (1 μM) to minimize the effects of presynaptic GABAB receptor inhibition. CGP52432 (CGP; 10 μM) was bath-applied. KT5720 (KT; 1 μM) and D890 (0.5 mM) were applied through a recording pipette. *P < 0.05, **P < 0.01. (Control, n = 8 neurons from 4 mice; CGP, n = 11 neurons from 2 mice; CGP + KT, n = 10 neurons from 2 mice; CGP + D890, n = 12 neurons from 2 mice; one-way ANOVA; F(3, 37) = 5.112, P = 0.0046; Tukey’s Multiple Comparison Test; Control vs. CGP, P < 0.05, CGP vs. CGP + KT, P < 0.05, CGP vs. CGP + D890, P < 0.01). Each representative trace shows the data from different cell. Data are presented as the mean ± S.E.M.
We further assessed the effects of GABAB receptor-mediated inhibition of spontaneous firing activity. To exclude the involvement of ionotropic inputs and presynaptic GABAB receptor-mediated change in serotonin release, we recorded the firing activity in the presence of antagonists of AMPA, NMDA, and GABAA receptors and 5-hydroxytryptamine 1 (5-HT1) autoreceptors (20 μM DNQX, 50 μM APV, 20 μM bicuculline, 0.1 μM WAY100635, and 1 μM GR127935, termed as “antagonist mix”). Application of the antagonist mix did not affect the spontaneous firing rate of serotonergic neurons (Supplementary Fig. S3). In the presence of the antagonist mix, CGP52432 significantly increased the firing rate of serotonergic neurons. The increasing effect of CGP52432 was blocked by the intracellular application of KT5720 or D890 (Fig. 3c,d). Each drug application had no significant effect on electrophysiological characteristics, except for L-type VDCC blocker-induced elongation of AP duration25 (Supplementary Table S1). These results indicate that GABAB receptor-mediated inhibition reduces the serotonergic spontaneous firing activity by inhibiting L-type VDCC current.
Chronic antidepressant increases spontaneous firing activity of DRN serotonergic neurons
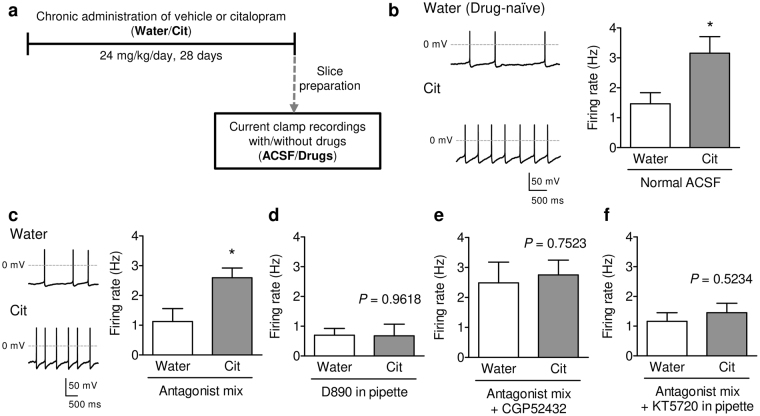
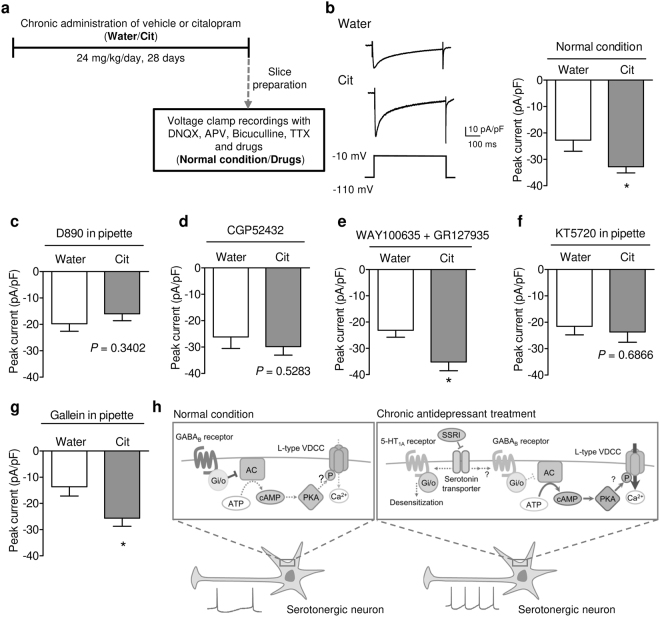
It is widely accepted that potentiation of serotonergic system is important for the therapeutic activity of antidepressants15–18, whereas the effects and mechanisms of action of antidepressants on the activity of serotonergic neurons are unclear. We thus examined whether chronic administration of SSRIs activates DRN serotonergic activity. Mice were treated with an SSRI, citalopram (24 mg/kg/day, in drinking water) for 28 days, and ex vivo whole-cell recordings were performed (Fig. 4a). Under normal ACSF condition, the spontaneous activity of DRN serotonergic neurons was significantly increased by chronic treatment with citalopram compared to the drug-naïve (water-drinking) group (Fig. 4b). To further assess whether the citalopram-induced increase in the spontaneous firing rate depends on the changes in synaptic inputs, autoinhibition, or serotonergic intrinsic activity, we examined the firing activity of DRN serotonergic neurons in the presence of the antagonist mix. Even in the presence of antagonist mix, the spontaneous firing activity of serotonergic neurons in the citalopram-treated group was significantly higher than that in the drug-naïve group (Fig. 4c).
Figure 4.
Chronic administration of citalopram increased the spontaneous firing rate of DRN serotonergic neurons. (a) Outline of recordings from citalopram-administrated mice. After chronic treatment with citalopram (Cit; 24 mg/kg/day) or its vehicle (Water) for 28 days, acute raphe slices were prepared, and whole-cell current clamp recordings were performed. (b) Representative traces (left) and average spontaneous firing rate (right) of DRN serotonergic neurons from drug-naïve (Water) and citalopram-treated (Cit) mice. *P < 0.05 vs. Water. (Water, n = 8 neurons from 4 mice; Cit, n = 13 neurons from 4 mice; Student’s t-test; P = 0.0491). (c) Representative traces (left) and average spontaneous firing rate (right) of DRN serotonergic neurons in the presence of the antagonist mix (20 μM DNQX, 50 μM APV, 20 μM Bicuculline, 0.1 μM WAY100635, and 1 μM GR127935). *P < 0.05 vs. Water. (Water, n = 8 neurons from 3 mice; Cit, n = 8 neurons from 4 mice; Student’s t-test; P = 0.0168). (d) The effects of intracellularly applied D890 on the spontaneous firing rate of DRN serotonergic neurons. P = 0.9618 vs. Water by Student’s t-test, Water, n = 13 neurons from 2 mice; Cit, n = 10 neurons from 2 mice. (e) The spontaneous firing rate of DRN serotonergic neurons in the presence of the antagonist mix and CGP52432. P = 0.7523 vs. Water by Student’s t-test, Water, n = 9 neurons from 2 mice; Cit, n = 12 neurons from 2 mice. (f) The effects of intracellularly applied KT5720 on the spontaneous firing rate of DRN serotonergic neurons in the presence of the antagonist mix. P = 0.5234 vs. Water by Student’s t-test, Water, n = 16 neurons from 2 mice; Cit, n = 22 neurons from 2 mice. Data are presented as the mean ± S.E.M.
As shown in Fig. 2d, intracellular application of D890 decreased spontaneous firing activity in both the drug-naïve and citalopram-treated groups, and in this condition, chronic citalopram-induced increase in spontaneous firing rate was not observed (Fig. 4d). Similarly, in the presence of the antagonist mix, bath application of nifedipine (10 μM) also blocked chronic citalopram-induced increase in spontaneous firing activity (Supplementary Fig. S7a).
Although one of the postulated mechanisms of action of SSRI on serotonergic neurons was decreasing 5-HT1A receptor-mediated autoinhibition15,30, SSRI also decreases GABAB receptor-mediated signaling11. Based on this observation, we hypothesized that chronic administration of citalopram disinhibits serotonergic neurons by decreasing the GABAB receptor-mediated inhibition. Consistent with the previous report11, GABAB receptor agonist (1 mM baclofen)-induced outward current was decreased in the citalopram-treated mice, indicating decreased GABAB receptor function in serotonergic neurons (Supplementary Fig. S4). As observed in Fig. 3d, blocking GABAB receptors increased the spontaneous activity of serotonergic neurons in the drug-naïve mice. On the other hand, blocking of GABAB receptors failed to increase firing rate in the citalopram-treated group, resulting in no difference between the drug-naïve and citalopram-treated groups (Fig. 4e). Furthermore, the intracellular application of KT5720 prevents chronic citalopram-induced increase in spontaneous activity, supporting the hypothesis that chronic citalopram activates serotonergic neurons through decreasing GABAB receptor-signaling and following increase in PKA activity (Fig. 4f).
Evidence suggests that GABAB receptor-mediated activation of G protein-coupled inwardly-rectifying K+ (GIRK) channels inhibits serotonergic neurons through hyperpolarization of the RMP31. However, there was no difference in pseudo RMP between the drug-naïve and citalopram-treated groups (Supplementary Table S2), indicating that chronic citalopram-induced activation of serotonergic neurons was not due to hyperpolarization of RMP. In addition, there was no positive correlation between spontaneous firing rate and pseudo RMP in “normal ACSF”, “Antagonist mix” and “Antagonist + CGP” recording conditions (Supplementary Fig. S5a,b,d). On the contrary, significant positive correlation between spontaneous firing rate and pseudo RMP was observed in “D890 in pipette” and “KT5720 in pipette” conditions, where activity of L-type VDCCs were low (Supplementary Fig. S5c,e). These results suggest that the value of RMP had little effect on spontaneous firing activity, at least when L-type VDCCs are normally active.
Chronic antidepressant activates L-type VDCCs in DRN serotonergic neurons
We next examined the effects of chronic administration of citalopram in L-type VDCC current in serotonergic neurons (Fig. 5a). Voltage-clamp recordings showed that high voltage activated (HVA) current was significantly increased by chronic citalopram administration, while low-voltage activated current was not affected (Fig. 5b, Supplementary Fig. S6). The citalopram-induced increase in HVA current was abolished by the intracellular application of D890 or bath application of nifedipine (Fig. 5c, Supplementary Fig. S7b), indicating that chronic citalopram increased L-type VDCC current. Similar to spontaneous activity, blocking GABAB receptors also increased VDCC current in the drug-naïve group but not in the citalopram-treated group, resulting in no difference between two groups (Fig. 5d). In the presence of nifedipine, CGP52432 did not increase HVA current in both groups, suggesting that the effect of CGP52432 in Fig. 5d was not due to the increase in other types of HVA VDCC current (Supplementary Fig. S7b). Although 5-HT1 autoreceptors are also Gi/o-coupled G protein-coupled receptors, bath application of 5-HT1 receptor antagonists had no effect on chronic citalopram-induced increase in VDCC current (Fig. 5e).
Figure 5.
Chronic administration of citalopram increased L-type VDCC current. (a) Outline of recordings from citalopram-administrated mice. After chronic treatment with citalopram (Cit; 24 mg/kg/day) or its vehicle (Water) for 28 days, acute raphe slices were prepared, and whole-cell voltage clamp recordings were performed. (b) Representative traces (left) and peak current (right) of high voltage activated (HVA) current in DRN serotonergic neurons from drug-naïve (Water) and citalopram-treated (Cit) mice. *P < 0.05 vs. Water. (Water, n = 8 neurons from 3 mice; Cit, n = 9 neurons from 4 mice; Student’s t-test; P = 0.0487). (c) Effects of intracellularly applied D890 on HVA current in DRN serotonergic neurons. P = 0.3402 vs. Water by Student’s t-test, Water, n = 8 neurons from 2 mice; Cit, n = 8 neurons from 2 mice. (d) Effects of bath-applied CGP52432 on high voltage activated (HVA) current in DRN serotonergic neurons. P = 0.5283 vs. Water by Student’s t-test, Water, n = 14 neurons from 4 mice; Cit, n = 11 neurons from 2 mice. (e) HVA VDCC current was recorded in the presence of WAY100635 and GR127935. *P < 0.05 vs. Water. (Water, n = 11 neurons from 2 mice; Cit, n = 12 neurons from 2 mice; Student’s t-test; P = 0.0107). (f) Effects of intracellularly applied KT5720 on HVA current in DRN serotonergic neurons. P = 0.6866 vs. Water by Student’s t-test, Water, n = 10 neurons from 2 mice; Cit, n = 12 neurons from 2 mice. (g) Effects of intracellularly applied gallein (20 μM) on HVA current in DRN serotonergic neurons. *P < 0.05 vs. Water. (Water, n = 12 neurons from 2 mice; Cit, n = 17 neurons from 2 mice; Student’s t-test; P = 0.0172). Data are presented as the mean ± S.E.M. (h) In normal condition, continuous GABAB receptor signaling inhibits PKA activation. Decreased PKA activity might cause inhibition of L-type VDCC activity and subsequently serotonergic firing activity. After chronic antidepressant treatment, postsynaptic GABAB signaling is decreased, resulting in activation of PKA and disinhibition of L-type VDCCs. Increasing Ca2+ current through L-type VDCCs accelerates serotonergic firing activity.
Moreover, consistent with current clamp data, intracellular KT5720 also diminished the increasing effect of citalopram on HVA current (Fig. 5f). However, intracellular application of gallein (20 μM)-induced inhibition of Gβγ signaling, which is another downstream signaling by GABAB receptors, did not affect the effect of chronic citalopram on VDCC current (Fig. 5g), indicating that disinhibition of PKA via decreasing GABAB receptor signaling might play a critical part in SSRI-induced increase in VDCC current (Fig. 5h).
Discussion
In this study, we determined the control mechanisms for serotonergic spontaneous firing activity. Furthermore, we found that chronic antidepressant treatment increased DRN serotonergic spontaneous activity by enhancing the spontaneous firing activity through a novel mechanism that weakens the GABAB receptor-mediated inhibition of L-type VDCCs (Fig. 5h).
In the central nervous system, several types of neurons such as the midbrain dopaminergic neurons show spontaneous firing activity26. By using modified ex vivo recordings, we found that the vast majority of DRN serotonergic neurons were spontaneously active. Our data also exhibited the capability of serotonergic neurons to generate spontaneous firing activity without excitatory glutamatergic inputs or noradrenergic inputs, which are thought to be the major driving force for the firing activity of serotonergic neurons.
It is widely accepted that noradrenergic signaling on serotonergic neurons maintain serotonergic tonic activity and in ex vivo slices weak α1 receptor signaling causes low serotonergic spontaneous activity2,32. In accordance, effect of α1 antagonist on spontaneous firing activity was slight in our recording method, suggesting that spontaneous activity shown here was not due to the increase in noradrenergic inputs. On the other hand, the significant contribution of α1 receptor signaling on in vivo serotonergic activity was well established33. Further study will be needed to reveal the involvement of intrinsic mechanisms shown in present study on serotonergic activity control in vivo.
Although T-type VDCCs and HCN channels are the well-known molecular basis for generating spontaneous firing activity24, growing evidence suggests that L-type VDCCs, especially Cav1.3, which has a low voltage threshold34 (−55 mV), also contribute to maintaining the spontaneous firing activity25. In Cav1.3 knockout mice, the spontaneous firing activity of ventral tegmental dopaminergic neurons is lower than that in wild-type mice, whereas the activation of L-type VDCC increases the burst firing of dopaminergic neurons35. Consistent with these reports, the present results showed that activation of L-type VDCC also increased DRN serotonergic neuronal activity. Considering that pseudo RMPs of most of spontaneously active serotonergic neurons in this report were higher than voltage threshold of Cav1.3, present results strongly indicate the involvement of L-type VDCCs on generation of spontaneous firing activity.
For generating APs, depolarizing Ca2+ current through L-type VDCCs at subthreshold membrane potential plays an essential role36, while Ca2+ influx-driven subsequent signals, such as activation of Ca2+-activated K+ channels, also controls cell excitability. Ca2+-activated SK and BK channels are functionally coupled with L-type VDCCs and activation of these channels contributes to the generation of AHP25,37. SK channel-induced AHP are known to slow down firing activity and blockade of SK channels increases burst-like activity in serotonergic neurons38. On the contrary, BK current-induced repolarization are required for spontaneous firing activity and L-type VDCC activator-induced increase in firing rate25,39. While present data suggested that increasing depolarizing current through L-type VDCCs accelerate AP generation, subsequent signalings such as Ca2+-activated K+ channels-induced repolarization might be involved in the maintenance of increased tonic firing activity.
It is well-known that the local GABAergic inhibition decreases serotonergic activity4,10. Besides ionotropic GABAA receptors, metabotropic GABAB receptors are also expressed in serotonergic neurons31. Whereas both serotonergic and GABAergic neurons in the DRN express GABAB receptors, our analysis with intracellular drug application suggest that the activation of postsynaptic GABAB receptors plays an essential role in the inhibition of serotonergic neurons. While GABAB receptor agonist-induced activation of GIRK channels and subsequent membrane hyperpolarization are well-studied11,31,40, GABAB antagonist-induced depolarization was subtle in present study, suggesting that inactivation of GIRK channels had little effects on GABAB antagonist-induced activation of serotonergic firing activity. However, additional researches will be required to investigate the downstream signalings of GABAB receptors and how those signalings control serotonergic activity.
In present study, we showed that inhibition of postsynaptic GABAB receptors in serotonergic neurons increased serotonergic activity. By contrast, previous evidences indicate that intra-DRN application of GABAB agonist increases serotonin and glutamate release by stimulation of presynaptic GABAB receptors41–43. Additionally, Milnar et al. have shown that co-treatment of GABAA and GABAB antagonists had no effect on the firing rate of phenylephrine-treated serotonergic neurons44. One of the reasons for this apparent discrepancy between previous reports and our results might be difference in recording condition. In the present experiments with GABAB antagonist, GABAergic and glutamatergic ionotropic inputs were also blocked, where these blockers might mask changes in glutamate and GABA release through inhibition of presynaptic GABAB receptors. Consequently, the effects of postsynaptic GABAB receptor-antagonism might become highlighted. Further assessment of selective inhibition of GABAB receptors on serotonergic neurons would be needed to elucidate GABAB receptor-mediated control of serotonergic intrinsic activity.
GABAB receptors interact with a variety of channels and modulate their function45. The interaction between GABAB receptors and P/Q-type VDCCs is widely accepted as the mechanism of action for GABAB receptor-mediated inhibition of neurotransmitter release29. Whereas, there is no consensus on whether and how GABAB receptors modulate L-type VDCCs because the effects of GABAB receptor activation on L-type VDCCs varies with maturation state of cells46,47. Evidence suggests that GABAB receptors couple to Gq proteins and activate L-type VDCC through PKC signaling during neonatal development48. In this study, we used adult mice where GABAB receptors might mainly couple to Gi proteins. Considering that PKA-mediated phosphorylation is one of the activation pathway of L-type VDCC49, present results indicate that GABAB antagonist activates L-type VDCCs through disinhibiting PKA.
Whereas an antidepressant-induced increase in synaptic serotonin level and subsequent stimulation of postsynaptic serotonin receptors in the projection areas plays an important role in antidepressant effects50,51, accumulating evidence indicates that the altered DRN serotonergic activity contributes to the pathology and treatment of mental disorders3–5. Under stress conditions, the activity of serotonergic neurons is decreased3,4, and thus, chronic treatment with an antidepressant might potentiate its therapeutic effects by disinhibiting serotonergic activity.
As widely accepted, acute SSRI administration decreases activity of serotonergic neurons by increasing local serotonin levels, while continuous increase in serotonin level desensitizes 5-HT1A autoreceptors and increases serotonin release15,52. Recent evidence indicates that dendritic serotonin release in DRN is mainly mediated by L-type VDCCs53, suggesting that chronic SSRI induced activation of L-type VDCC increases local serotonin release and might also facilitates disinhibition of 5-HT1A autoreceptors. While chronic SSRI-induced activation of serotonergic neurons were still observed in the presence of antagonist mix, that contains a 5-HT1A receptor antagonist, it is possible that chronic SSRI-induced activation of L-type VDCC further activate serotonergic neurons via disinhibiting autoreceptors. Additional research is required to identify the interaction between chronic SSRI-induced activation of L-type VDCCs and desensitization of autoreceptors.
The contribution of GABAB receptors to pathogenesis and treatment for mental disorders has long been discussed. Systemic administration of GABAB receptor antagonist shows serotonin-dependent antidepressant-like effect54,55. Furthermore, mice lacking GABAB1b, which preferentially exists as a postsynaptic GABAB receptor, acquire stress resilience12,56. Consistent with a previous study11, our findings suggest that down regulation of postsynaptic GABAB signaling in serotonergic neurons is essential for the antidepressant effect of SSRIs. On the contrary, upregulation of GABAB receptor-mediated signaling produces an antidepressant-like effect in the lateral habenula and hippocampus39,57. These discrepancies indicate that region-specific modulation of GABAB signaling is critical for therapeutic effects. In this situation, our finding of the GABAB receptor-L-type VDCC signaling-mediated modulation of serotonergic function provides a novel strategy for the treatment of psychiatric disorders.
Present results suggest that chronic inhibition of serotonin transporter (SERT) decreases GABAB receptor signaling in serotonergic neurons. Unlike 5-HT1A receptors, GABAB receptors do not internalize due to prolonged agonist exposure, and phosphorylation/dephosphorylation balance of GABAB receptors determines their membrane expression and complex formation58,59. Evidence suggests that the chronic but not acute administration of SSRI decreases the expression of several protein kinases60,61. In this context, one of the possible mechanisms that explain the missing link between SERT inhibition and decrease in GABAB receptor signaling is that chronic treatment with SSRI might reduce phosphorylation of GABAB receptors by decreasing kinase expression. Further research will be needed to determine chronic SERT inhibition-induced signalings in serotonergic neurons.
In conclusion, the current ex vivo electrophysiological investigations indicated that DRN serotonergic neurons possess spontaneous firing activity, in which L-type VDCC is a key modulator. This spontaneous activity received tonic inhibition through GABAB receptor-mediated inhibition of L-type VDCCs, and chronic administration of SSRI weakened this inhibition. Our findings provide a new mechanism for the regulation of serotonergic activity and raise the possibility that postsynaptic GABAB receptor-mediated inhibition of L-type VDCCs in serotonergic neurons might be a promising target for the treatment of psychiatric disorders.
Methods
Reagents
DL-2-Amino-5-phosphonopentanoic acid (DL-APV; a selective NMDA antagonist; Sigma-Aldrich, St-Louis, MO, USA), WAY100635 (a 5-HT1A antagonist; Abcam Biochemicals, Cambridge, UK), GR127935 (a selective 5-HT1B antagonist; Abcam Biochemicals), CGP52432 (a selective GABAB antagonist; Abcam Biochemicals), and tetrodotoxin (a selective voltage-dependent Na+ channel blocker; Sigma-Aldrich) were dissolved in water. 6,7-dinitroquinoxaline-2,3(1 H,4 H)-dione (DNQX; an AMPA (non-NMDA) antagonist; Tocris Bioscience, Bristol, UK), bicuculline (a selective GABAA antagonist; Enzo Life Science, Farmingdale, NY, USA), prazosin (an α1 receptor antagonist; Sigma-Aldrich), ZD7288 (a selective hyperpolarization-activated cyclic nucleotide–gated channel blocker; Cayman Chemical Company, Ann Arbor, MI, USA), (S)-(-)-Bay K 8644 (Bay K 8644; an L-type voltage-dependent Ca2+ channel (VDCC) activator; Santa Cruz Biotechnology, Santa Cruz, CA, USA), nifedipine (an L-type VDCC blocker; Wako Pure Chemical Industries, Osaka, Japan), KT5720 (a selective PKA inhibitor; Wako Pure Chemical Industries), and gallein (a selective Gβγ inhibitor; Sigma-Aldrich) were dissolved in dimethyl sulfoxide (DMSO). Baclofen (a selective GABAB agonist; Wako Pure Chemical Industries) was directly dissolved in artificial cerebrospinal fluid (ACSF). D890 (a quaternary derivative of methoxyverapamil acts as a membrane-impermeable L-type VDCC blocker; Abcam Biochemicals) was directly dissolved in the pipette solution. Stock solutions were stored at −20 °C until use and dissolved in ACSF or pipette solution for recording. The final concentration of DMSO in ACSF and pipette solution was lower than 0.05%.
Animals
All animal care and experimental procedures were conducted in accordance with the ethical guidelines of the Kyoto University Animal Research Committee and were approved by the Animal Research Committee, Graduate School of Pharmaceutical Sciences, Kyoto University (Approval number: 13–41). Male C57BL/6 J mice were purchased from Nihon SLC (Shizuoka, Japan) and singly housed at a constant ambient temperature of 24 ± 1 °C on a 12-h light-dark cycle with access to food and water ad libitum.
For chronic antidepressant treatment, citalopram hydrobromide (FWD Chemicals, Shanghai, China) was dissolved in drinking water (0.2 mg/mL) and administrated for 28 days. Water consumption was approximately 3–4 mL/day/mouse, resulting in average dose at 24 mg/kg/day. The drug containing drinking water was shielded from light and changed every 3–5 day.
Preparation of acute raphe slices for electrophysiological analysis
Male 11–12-week-old mice were deeply anesthetized with isoflurane and decapitated. The brains were rapidly collected in ice-cold cutting solution (composition in mM; 120 NMDG-Cl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgCl2, 15 D-glucose, and 1.3 ascorbic acid, pH 7.2). Coronal midbrain slices (200-μm thick) were prepared with a vibratome (VT1000S, Leica, Wetzlar, Germany). Knife speed and frequency were 0.025–0.05 mm/s and 60–70 Hz, respectively. Slices were recovered in oxygenated ACSF (composition in mM; 124 NaCl, 3 KCl, 26 NaHCO3, 1 NaH2PO4, 2.4 CaCl2, 1.2 MgCl2, and 10 D-glucose, pH 7.3) at 32 °C for at least 1 h before recording. After recovery, individual slices were transferred to a recording chamber with continuous perfusion of oxygenated ACSF at a flow rate of 1–2 mL/min. ACSF were warmed to keep the recording chamber at 27 ± 1 °C. Recordings were performed only within 4 hours after recovery.
Electrophysiological recordings
Electrophysiological recordings were performed as previously described10 with several modifications. Electrophysiological recordings were performed with an EPC9 amplifier (HEKA, Pfalz, Germany), and the data were recorded using Patchmaster software (HEKA). The resistance of the electrodes was 3–6 MΩ when filled with the internal solution (composition in mM; 140 K-gluconate, 5 KCl, 10 HEPES, 2 Na-ATP, 2 MgCl2, and 0.2 EGTA, pH 7.3 adjusted with KOH for current clamp recordings, and 120 CsMeSO4, 15 CsCl, 8 NaCl, 10 HEPES, 2 Na-ATP, 0.3 Na-GTP, 0.2 EGTA, 10 TEA-Cl, and 5 QX-314, pH 7.3 adjusted with CsOH for voltage clamp recordings). Individual neurons were visualized with a microscope equipped with a 40 × water-immersion objective lens (Carl Zeiss, Jena, Germany) and a CCD camera. The series resistance was compensated by 70% and maintained within 20 MΩ.
The spontaneous firing was examined in cell-attached or whole-cell current-clamp recordings. Cell-attached recordings were performed at a holding potential of 0 mV. In whole-cell current-clamp recordings, the current was held at 0 pA. For comparing the spontaneous activity between different neurons, spontaneous firing activity was recorded for 30 s after stabilization. To examine the change in spontaneous firing within a neuron, the average firing rate in the first 30 s was considered as the basal firing rate. VDCC current was recorded under a voltage-clamp condition in the presence of DNQX (20 μM), APV (50 μM), bicuculline (20 μM), and tetrodotoxin (1 μM), and was generated by depolarizing voltage steps from −110 mV to −40 mV or −10 mV. Membrane potential between recordings was held at −70 mV. Baclofen-induced current was recorded in the presence of DNQX (20 μM), APV (50 μM), and bicuculline (20 μM), and the holding potential was set at −50 mV.
Single-cell reverse transcription-polymerase chain reaction (RT-PCR)
Single-cell RT-PCR was performed as previously described10. After the whole-cell recording, the contents of the cell were aspirated into the recording pipette and harvested in a sampling tube. The collected samples were reverse-transcribed using a ReverTra Ace RT kit (TOYOBO, Tokyo, Japan) and amplified with Blend Taq (TOYOBO, Tokyo, Japan). The oligonucleotide primers used were 5′-TAGGCTTAGCGTCTCTGGGA-3′ and 5′-AAGGCCGAACTCGATTGTGA-3′ for Tph2; 5′-GGCCTGAAGATCTGTGGCTT-3′ and 5′-CAGAACCTTGGTGGAGCGAT-3′) for Gad1; 5′-ATGCAGAGCTGCAACCAGAT-3′ and 5′-GCCTCAAACCCAGTAGTCCC-3′ for Gad2; 5′-CCGCTGATCCTTCCCGATAC-3′ and 5′-CGACGTTGGCTGTGAACTTG-3′ for Eno2 as a neuronal marker. PCR products were analyzed using agarose gel electrophoresis. Only when Tph2 mRNA expression was detected, the data was used for analysis (Fig. 1a).
Statistics
All data are presented as the mean ± standard error of mean (S.E.M). Statistical analysis was performed by GraphPad Prism 5 (GraphPad, San Diego, CA, USA). Differences with P < 0.05 were considered significant. The differences between two groups were compared by two-tailed Student’s t-test. When comparing differences within the cell, two-tailed paired t-test was used for analysis. The differences between more than three groups were compared by one-way analysis of variance (ANOVA) with post hoc Tukey’s Multiple Comparison Test. When examining the time-course, two-way ANOVA for repeated measures was used for analysis. For correlation analysis, Pearson correlation coefficients were used.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Figure files.
Electronic supplementary material
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research (KAKENHI) from the Japanese Society for the Promotion of Science to K.N., H.S., T.N and S.K (16K15125, 25460098, 17H04008, 16H05091). We thank Dr. T. Momiyama (The Jikei University School of Medicine) for expert assistance in electrophysiological recording.
Author Contributions
N.A., K.N., T.N., and S.K. designed the project. N.A. performed the experiments. N.A., N.N., H. Kinoshita, H. Kawai, N.S. and H.S. analyzed the data. N.A., K.N., T.N., and S.K. wrote the manuscript. S.K. supervised the experiments and finalized the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-13599-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kazuki Nagayasu, Email: nagayasu@pharm.kyoto-u.ac.jp.
Shuji Kaneko, Email: skaneko@pharm.kyoto-u.ac.jp.
References
- 1.Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends. Cogn. Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- 3.Bambico FR, Nguyen NT, Gobbi G. Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur. Neuropsychopharmacol. 2009;19:215–228. doi: 10.1016/j.euroneuro.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Challis C, et al. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J. Neurosci. 2013;33:13978–13988. doi: 10.1523/JNEUROSCI.2383-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teissier A, et al. Activity of Raphé Serotonergic Neurons Controls Emotional Behaviors. Cell Rep. 2015;13:1965–1976. doi: 10.1016/j.celrep.2015.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mannoury la Cour C, et al. GABAB receptors in 5-HT transporter- and 5-HT1A receptor-knock-out mice: further evidence of a transduction pathway shared with 5-HT1A receptors. J. Neurochem. 2004;89:886–896. doi: 10.1111/j.1471-4159.2004.02367.x. [DOI] [PubMed] [Google Scholar]
- 7.Prinz A, Selesnew LM, Liss B, Roeper J, Carlsson T. Increased excitability in serotonin neurons in the dorsal raphe nucleus in the 6-OHDA mouse model of Parkinson’s disease. Exp. Neurol. 2013;248:236–245. doi: 10.1016/j.expneurol.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Burlhis TM, Aghajanian GK. Pacemaker potentials of serotonergic dorsal raphe neurons: Contribution of a low-threshold Ca2+ conductance. Synapse. 1987;1:582–8. doi: 10.1002/syn.890010611. [DOI] [PubMed] [Google Scholar]
- 9.Weissbourd B, et al. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron. 2014;83:645–662. doi: 10.1016/j.neuron.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asaoka N, et al. 2015. Olanzapine augments the effect of selective serotonin reuptake inhibitors by suppressing GABAergic inhibition via antagonism of 5-HT6 receptors in the dorsal raphe nucleus. Neuropharmacology. 2016;95:261–268. doi: 10.1016/j.neuropharm.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Cornelisse LN, et al. Reduced 5-HT1A- and GABAB receptor function in dorsal raphé neurons upon chronic fluoxetine treatment of socially stressed rats. J. Neurophysiol. 2007;98:196–204. doi: 10.1152/jn.00109.2007. [DOI] [PubMed] [Google Scholar]
- 12.O’Leary OF, et al. GABAB1 receptor subunit isoforms differentially regulate stress resilience. Proc. Natl. Acad. Sci. USA. 2014;111:15232–15237. doi: 10.1073/pnas.1404090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araki R, et al. Epigenetic regulation of dorsal raphe GABAB1a associated with isolation-induced abnormal responses to social stimulation in mice. Neuropharmacology. 2016;101:1–12. doi: 10.1016/j.neuropharm.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int. J. Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- 15.Hervás I, et al. Desensitization of 5-HT1A autoreceptors by a low chronic fluoxetine dose effect of the concurrent administration of WAY-100635. Neuropsychopharmacology. 2001;24:11–20. doi: 10.1016/S0893-133X(00)00175-5. [DOI] [PubMed] [Google Scholar]
- 16.Shishkina GT, Kalinina TS, Dygalo NN. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience. 2007;150:404–412. doi: 10.1016/j.neuroscience.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Nagayasu K, et al. Utility of organotypic raphe slice cultures to investigate the effects of sustained exposure to selective 5-HT reuptake inhibitors on 5-HT release. Br. J. Pharmacol. 2010;161:1527–1541. doi: 10.1111/j.1476-5381.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagayasu K, et al. Chronic effects of antidepressants on serotonin release in rat raphe slice cultures: high potency of milnacipran in the augmentation of serotonin release. Int. J. Neuropsychopharmacol. 2013;16:2295–2306. doi: 10.1017/S1461145713000771. [DOI] [PubMed] [Google Scholar]
- 19.Ting JT, Daigle TL, Chen Q, Feng G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol. Biol. 2014;1183:221–242. doi: 10.1007/978-1-4939-1096-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tateno T, Robinson HP. The mechanism of ethanol action on midbrain dopaminergic neuron firing: a dynamic-clamp study of the role of Ih and GABAergic synaptic integration. J. Neurophysiol. 2011;106:1901–1922. doi: 10.1152/jn.00162.2011. [DOI] [PubMed] [Google Scholar]
- 21.Calizo LH, et al. Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology. 2011;61:524–543. doi: 10.1016/j.neuropharm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espallergues J, et al. HDAC6 regulates glucocorticoid receptor signaling in serotonin pathways with critical impact on stress resilience. J Neurosci. 2012;32:4400–16. doi: 10.1523/JNEUROSCI.5634-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 24.Lambert RC, Bessaïh T, Crunelli V, Leresche N. The many faces of T-type calcium channels. Pflugers. Arch. 2014;466:415–423. doi: 10.1007/s00424-013-1353-6. [DOI] [PubMed] [Google Scholar]
- 25.Vandael DH, et al. Cav1.3 and BK channels for timing and regulating cell firing. Mol. Neurobiol. 2010;42:185–198. doi: 10.1007/s12035-010-8151-3. [DOI] [PubMed] [Google Scholar]
- 26.Neuhoff H, Neu A, Liss B, Roeper J. Ih channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J. Neurosci. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Padilla J, et al. Mitochondrial oxidant stress in locus coeruleus is regulated by activity and nitric oxide synthase. Nat. Neurosci. 2014;17:832–840. doi: 10.1038/nn.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Innis RB, Nestler EJ, Aghajanian GK. Evidence for G protein mediation of serotonin- and GABAB-induced hyperpolarization of rat dorsal raphe neurons. Brain Res. 1988;459:27–36. doi: 10.1016/0006-8993(88)90282-X. [DOI] [PubMed] [Google Scholar]
- 29.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol. Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 30.Geddes SD, et al. Time-dependent modulation of glutamate synapses onto 5-HT neurons by antidepressant treatment. Neuropharmacology. 2015;95:130–143. doi: 10.1016/j.neuropharm.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 31.Llamosas N, Ugedo L, Torrecilla M. Inactivation of GIRK channels weakens the pre- and postsynaptic inhibitory activity in dorsal raphe neurons. Physiol. Rep. 2017;5:e13141. doi: 10.14814/phy2.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bortolozzi A, Artigas F. Control of 5-hydroxytryptamine release in the dorsal raphe nucleus by the noradrenergic system in rat brain. Role of alpha-adrenoceptors. Neuropsychopharmacology. 2003;28:421–34. doi: 10.1038/sj.npp.1300061. [DOI] [PubMed] [Google Scholar]
- 33.Baraban JM, Aghajanian GK. Suppression of serotonergic neuronal firing by alpha-adrenoceptor antagonists: evidence against GABA mediation. Eur J Pharmacol. 1980;66:287–94. doi: 10.1016/0014-2999(80)90461-6. [DOI] [PubMed] [Google Scholar]
- 34.Xu W, Lipscombe D. Neuronal Cav1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, et al. Cav1.2 and Cav1.3 L-type calcium channels regulate dopaminergic firing activity in the mouse ventral tegmental area. J. Neurophysiol. 2014;112:1119–1130. doi: 10.1152/jn.00757.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putzier I, Kullmann PH, Horn JP, Levitan ES. Cav1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J. Neurosci. 2009;29:15414–15419. doi: 10.1523/JNEUROSCI.4742-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat. Rev. Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 38.Rouchet N, et al. SK channel blockade promotes burst firing in dorsal raphe serotonergic neurons. Eur. J. Neurosci. 2008;28:1108–1115. doi: 10.1111/j.1460-9568.2008.06430.x. [DOI] [PubMed] [Google Scholar]
- 39.Sausbier M, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc. Natl. Acad. Sci. USA. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lecca S, et al. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat. Med. 2016;22:254–261. doi: 10.1038/nm.4037. [DOI] [PubMed] [Google Scholar]
- 41.Abellán MT, Adell A, Honrubia MA, Mengod G, Artigas F. GABAB-RI receptors in serotonergic neurons: effects of baclofen on 5-HT output in rat brain. GABAB-RI receptors in serotonergic neurons: effects of baclofen on 5-HT output in rat brain.GABAB-RI receptors in serotonergic neurons: effects of baclofen on 5-HT output in rat brain. Neuroreport. 2000;11:941–5. doi: 10.1097/00001756-200004070-00009. [DOI] [PubMed] [Google Scholar]
- 42.Abellán MT, Jolas T, Aghajanian GK, Artigas F. Dual control of dorsal raphe serotonergic neurons by GABAB receptors. Electrophysiological and microdialysis studies. Synapse. 2000;36:21–34. doi: 10.1002/(SICI)1098-2396(200004)36:1<21::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi A, et al. Glutamate input in the dorsal raphe nucleus as a determinant of escalated aggression in male mice. J Neurosci. 2015;35:6452–63. doi: 10.1523/JNEUROSCI.2450-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mlinar B, et al. Nonexocytotic serotonin release tonically suppresses serotonergic neuron activity. J Gen Physiol. 2015;145:225–51. doi: 10.1085/jgp.201411330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwenk J, et al. Modular composition and dynamics of native GABAB receptors identified by high-resolution proteomics. Nat. Neurosci. 2016;18:233–242. doi: 10.1038/nn.4198. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Elsen F, Barnbrock A, Richter DW. Postnatal development of GABAB receptor-mediated modulation of voltage-activated Ca2+ currents in mouse brain-stem neurons. Eur. J. Neurosci. 1999;11:2332–2342. doi: 10.1046/j.1460-9568.1999.00655.x. [DOI] [PubMed] [Google Scholar]
- 47.Bray JG, Mynlieff M. Involvement of protein kinase C and protein kinase A in the enhancement of L-type calcium current by GABAB receptor activation in neonatal hippocampus. Neuroscience. 2011;179:62–72. doi: 10.1016/j.neuroscience.2011.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karls A, Mynlieff M. GABAB receptors couple to Gαq to mediate increases in voltage-dependent calcium current during development. J. Neurochem. 2015;135:88–100. doi: 10.1111/jnc.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao T, et al. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/S0896-6273(00)80358-X. [DOI] [PubMed] [Google Scholar]
- 50.Diaz SL, et al. 5-HT2B receptors are required for serotonin-selective antidepressant actions. Mol. Psychiatry. 2012;17:154–163. doi: 10.1038/mp.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samuels BA, et al. 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat. Neurosci. 2015;18:1606–1616. doi: 10.1038/nn.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guiard BP, Mansari ME, Murphy DL, Blier P. Altered response to the selective serotonin reuptake inhibitor escitalopram in mice heterozygous for the serotonin transporter: an electrophysiological and neurochemical study. Int J Neuropsychopharmacol. 2012;15:349–61. doi: 10.1017/S1461145711000484. [DOI] [PubMed] [Google Scholar]
- 53.Colgan LA, Cavolo SL, Commons KG, Levitan ES. Action potential-independent and pharmacologically unique vesicular serotonin release from dendrites. J Neurosci. 2012;32:15737–46. doi: 10.1523/JNEUROSCI.0020-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slattery DA, Desrayaud S, Cryan JF. GABAB receptor antagonist-mediated antidepressant-like behavior is serotonin-dependent. J. Pharmacol. Exp. Ther. 2005;312:290–296. doi: 10.1124/jpet.104.073536. [DOI] [PubMed] [Google Scholar]
- 55.Nowak G, et al. Antidepressant-like activity of CGP 36742 and CGP 51176, selective GABAB receptor antagonists, in rodents. Br. J. Pharmacol. 2006;149:581–590. doi: 10.1038/sj.bjp.0706845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vigot R, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfe SA, et al. FMRP regulates an ethanol-dependent shift in GABABR function and expression with rapid antidepressant properties. Nat. Commun. 2016;7:12867. doi: 10.1038/ncomms12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guetg N, et al. NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1. Proc. Natl. Acad. Sci. USA. 2010;107:13924–13929. doi: 10.1073/pnas.1000909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terunuma M, Pangalos MN, Moss SJ. Functional modulation of GABAB receptors by protein kinases and receptor trafficking. Adv. Pharmacol. 2010;58:113–122. doi: 10.1016/S1054-3589(10)58005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rausch JL, et al. Antidepressant effects on kinase gene expression patterns in rat brain. Neurosci. Lett. 2002;334:91–94. doi: 10.1016/S0304-3940(02)01106-0. [DOI] [PubMed] [Google Scholar]
- 61.Robison AJ, et al. Fluoxetine epigenetically alters the CaMKIIα promoter in nucleus accumbens to regulate ΔFosB binding and antidepressant effects. Neuropsychopharmacology. 2014;39:1178–1186. doi: 10.1038/npp.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Supplementary Figure files.