ABSTRACT
Lactobacillus brevis is an obligatory heterofermentative lactic acid bacterium that produces high levels of acetate, which improve the aerobic stability of silages against deterioration caused by yeasts and molds. However, the mechanism involved in acetate accumulation has yet to be elucidated. Here, experimental evidence indicated that aerobiosis resulted in the conversion of lactate to acetate after glucose exhaustion in L. brevis ATCC 367 (GenBank accession number NC_008497). To elucidate the conversion pathway, in silico analysis showed that lactate was first converted to pyruvate by the reverse catalytic reaction of lactate dehydrogenase (LDH); subsequently, pyruvate conversion to acetate might be mediated by pyruvate dehydrogenase (PDH) or pyruvate oxidase (POX). Transcriptional analysis indicated that the pdh and pox genes of L. brevis ATCC 367 were upregulated 37.92- and 18.32-fold, respectively, by oxygen and glucose exhaustion, corresponding to 5.32- and 2.35-fold increases in the respective enzyme activities. Compared with the wild-type strain, the transcription and enzymatic activity of PDH remained stable in the Δpox mutant, while those of POX increased significantly in the Δpdh mutant. More lactate but less acetate was produced in the Δpdh mutant than in the wild-type and Δpox mutant strains, and more H2O2 (a product of the POX pathway) was produced in the Δpdh mutant. We speculated that the high levels of aerobic acetate accumulation in L. brevis ATCC 367 originated mainly from the reuse of lactate to produce pyruvate, which was further converted to acetate by the predominant and secondary functions of PDH and POX, respectively.
IMPORTANCE PDH and POX are two possible key enzymes involved in aerobic acetate accumulation in lactic acid bacteria (LAB). It is currently thought that POX plays the major role in aerobic growth in homofermentative LAB and some heterofermentative LAB, while the impact of PDH remains unclear. In this study, we reported that both PDH and POX worked in the aerobic conversion of lactate to acetate in L. brevis ATCC 367, in dominant and secondary roles, respectively. Our findings will further develop the theory of aerobic metabolism by LAB.
KEYWORDS: Lactobacillus brevis, aerobic acetate accumulation, pyruvate dehydrogenase, pyruvate oxidase
INTRODUCTION
Lactic acid bacteria (LAB) are widely distributed on plant surfaces, playing key roles in the ensiling of green forages (1). LAB convert water-soluble carbohydrates into lactate, leading to a decrease in pH, which prevents the growth of undesirable microorganisms (2). When the silo is opened for feeding, air penetrates the ensiled material and promotes the growth of aerobic spoilage yeasts and molds, subsequently causing aerobic deterioration or fungal toxin production (3). Several strategies have been developed to enhance the aerobic stability of silages, including the use of carefully selected LAB inoculants and organic acid additives (4–8). Heterofermentative LAB species, such as Lactobacillus buchneri, Lactobacillus brevis, and Lactobacillus parafarraginis, are the most frequently used bacterial silage starters (9–12). Several features make them attractive organisms for the production of silages, including high resistance to stressors such as ethanol or oxygen and the ability to convert lactate to acetate under aerobic conditions (13, 14). The aerobic stability of silage exposed to air was directly correlated with the amount of acetate present; acetate is considered the most effective agent for uncoupling cell membranes, and it has inhibitory effects on the growth of spoilage microorganisms (15, 16).
As the first anti-aerobic deterioration metabolite, abundant acetate is not accumulated during the fermentation process of heterofermentative LAB (17). L. buchneri metabolizes free sugars to lactate, ethanol, and CO2 via the phosphoketolase pathway, but no acetate accumulates under anaerobic conditions (18). Recently, a novel metabolic pathway for the conversion of lactate to acetate in L. buchneri, in which lactate oxidase (LOX) or lactate dehydrogenase (LDH) and pyruvate oxidase (POX) were involved, was proposed (13). Similar aerobic acetate formation was observed in heterofermentative Lactobacillus spicheri (19). However, genomic evidence indicates that Lactobacillus reuteri and Lactobacillus fermentum lack the lox and pox genes, and their aerobic pyruvate conversion pathways are under investigation (19, 20). Metabolic net prediction indicates that pyruvate dehydrogenase (PDH) might be an alternative to POX for aerobic pyruvate-acetate conversion in LAB. However, the implication of PDH under aerobic conditions remains controversial, because several researchers reported that the pdh gene was transcriptionally activated when oxygen was present but others reported the opposite results (19, 21, 22). Therefore, it is now thought that most industrially relevant lactobacilli can use the POX pathway (20), but PDH effects on aerobic acetate production are inconclusive for lactobacilli.
L. brevis is a heterofermentative LAB. It is a commonly used silage inoculant that enhances the aerobic stability of various ensiled forages exposed to air. The mechanism of the enhancement of aerobic stability depends on the heterofermentative metabolism of the bacterium and its capacity to form high levels of acetate upon aerobic exposure (15). However, the detailed pathway involved in the aerobic acetate accumulation has yet to be fully understood. In addition, to manufacture silage inoculant, it is valuable to produce large amounts of biomass during culture preparation. Therefore, the aims of this work were to characterize the aerobic growth and metabolism of L. brevis ATCC 367 and to identify the pathway involved in the conversion of lactate to acetate and the harvest of large amounts of biomass. The explanation of the acetate production mechanism may be useful for the development of new inoculants for silage.
RESULTS
Effects of aerobic conditions on glucose consumption, growth performance, and metabolite production of L. brevis ATCC 367.
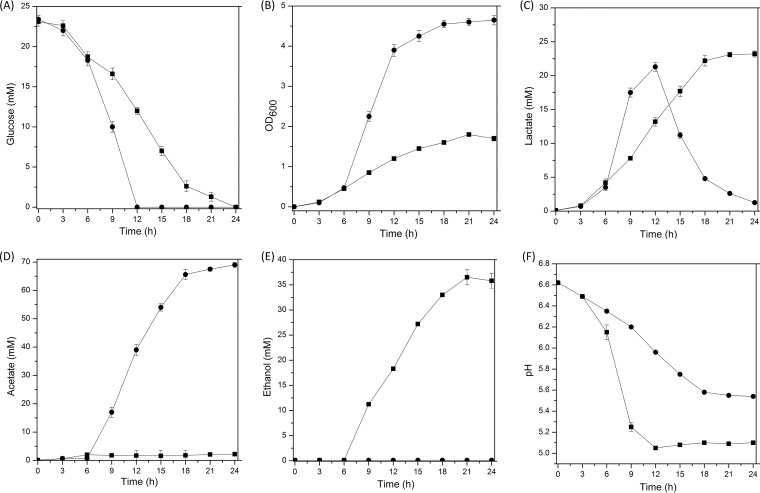
To determine the acetate accumulation under aerobic cultivation conditions, the growth and metabolism of L. brevis ATCC 367 were compared under anaerobic and aerobic conditions. As Fig. 1A shows, glucose was exhausted after 12 h under aerobic conditions, while about 50% of the glucose was left in the anaerobic cultures. Aerobic growth shortened the logarithmic phase and increased the final cell density by 2.74-fold, compared with anaerobic growth (Fig. 1B).
FIG 1.
Glucose consumption (A), growth performance (B), metabolite production (C to E), and pH (F) of L. brevis ATCC 367 under anaerobic (squares) and aerobic (circles) conditions. In all panels, the values are means ± standard deviations of three independent experiments.
When grown anaerobically, L. brevis ATCC 367 produced mainly lactate and ethanol, with trace amounts of acetate (Fig. 1C to E). Aerobic cultivation reduced the production of lactate and ethanol and resulted in substantial formation of acetate, leading to a higher pH value (Fig. 1F). Interestingly, after glucose was exhausted in the aerobic cultures, conversion of lactate to acetate was observed (Fig. 1C and D). The decrease in lactate levels was accompanied by the constant accumulation of acetate, while this phenomenon was not observed under anaerobic conditions.
In silico analysis of aerobic pathways in L. brevis ATCC 367.
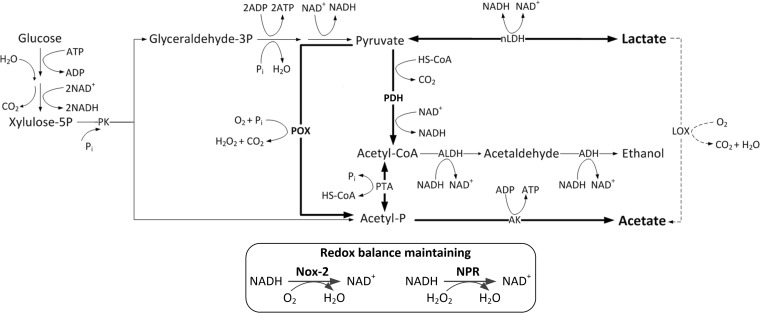
To investigate the oxygen-inducible conversion pathway from lactate to acetate, in silico analysis based on the annotation of the L. brevis ATCC 367 genome (GenBank accession number NC_008497) was performed, which allowed identification of all putative enzymes involved in aerobic conversion, i.e., pyruvate oxidase (POX), pyruvate dehydrogenase complex (PDH complex), H2O-forming NADH oxidase (NOX-2), and NADH peroxidase (NPR). However, genes for pyruvate formate lyase (PFL), lactate oxidase (LOX), and H2O2-forming NADH oxidase (NOX-1) were absent. The putative aerobic metabolism net is presented in Fig. 2. Under aerobic conditions, lactate could be transformed into pyruvate through only one possible pathway, the reverse reaction catalyzed by NADH-dependent lactate dehydrogenase (nLDH), accompanied by the regeneration of NADH. Then, pyruvate could be degraded into either acetyl coenzyme A (acetyl-CoA), by the action of the PDH complex, or acetyl-Pi, by the action of POX, along with production of H2O2. The acetyl-CoA and acetyl-Pi would subsequently be converted into acetate by acetate kinase (AK), generating extra ATP. The intercellular redox balance could be maintained by the actions of NOX-2 and NPR. The H2O2 produced in the POX pathway could be degraded by NPR.
FIG 2.
In silico analysis of pyruvate metabolism in L. brevis ATCC 367. nLDH, NADH-dependent lactate dehydrogenase (GenBank accession number LVIS_RS14045); POX, pyruvate oxidase (GenBank accession number LVIS_RS13065); PDH complex, pyruvate dehydrogenase complex (which is composed of pyruvate dehydrogenase [PDH] [GenBank accession number LVIS_RS18410], dihydrolipoyl transacetylase [DLAT], and dihydrolipoyl dehydrogenase [DLD]); PTA, phosphotransacetylase (GenBank accession number LVIS_RS14835); AK, acetate kinase (GenBank accession number LVIS_RS12175); ALDH, acetaldehyde dehydrogenase (GenBank accession number LVIS_RS12125); ADH, alcohol dehydrogenase; NOX-2, H2O-forming NADH oxidase (GenBank accession number LVIS_RS13170); NPR, NADH peroxidase (GenBank accession number LVIS_B09); PK, phosphoketolase (GenBank accession number LVIS_RS13895); HS-CoA, coenzyme A.
According to the predicted net, the PDH complex and POX were key enzymes involved in the conversion of lactate to acetate. Thus, the deduced amino acid sequences of PDH and POX of L. brevis ATCC 367 were aligned with those of Lactobacillus plantarum WCFS1 (GenBank accession number NC_004567), L. buchneri CD034 (GenBank accession number NC_018610), and L. spicheri DSM 15429 (GenBank accession number NZ_AZFC00000000), for which metabolic pathways for aerobic acetate accumulation have been proposed (13, 16, 19). Sequence alignments (see Fig. S1 and S2 in the supplemental material) showed that the PDHs from the four lactobacilli had greater homology (63.98% to 93.26% identities), while the POXs were more diversified (40.00% to 86.20% identities).
Inactivation of pdh and pox genes in L. brevis ATCC 367.
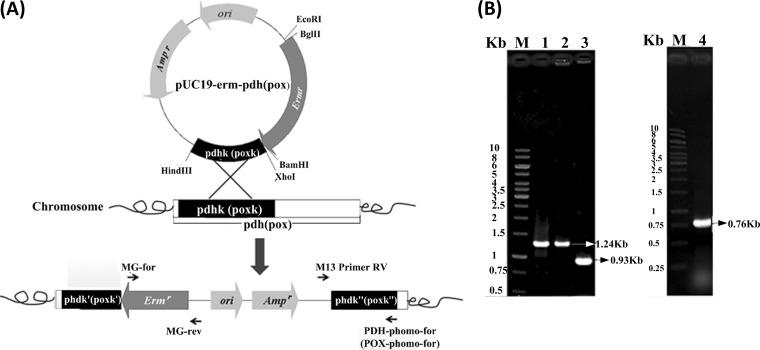
To confirm the roles of PDH and POX in the aerobic growth of L. brevis ATCC 367, the pdh and pox genes were inactivated by integration of the vectors pUC19-erm-pdh and pUC19-erm-pox, respectively (Fig. 3A). The inactivation of these two genes was verified by PCR amplification (Fig. 3B). Two mutant strains were generated, i.e., L. brevis ATCC 367 Δpdh (Δpdh mutant) and L. brevis ATCC 367 Δpox (Δpox mutant).
FIG 3.
Schematic representation of inactivation of pdh (or pox) in L. brevis ATCC 367 (A) and agarose gel electrophoresis of PCR products for verification (B). In panel A, pdhk and poxk indicate the homogeneous pdh and pox fragments, respectively. In panel B, the lanes were as follows: lanes M, 1-kb DNA ladder; lane 1, erm fragment (positive control); lane 2, PCR product of the erm fragment with genomic DNA of the Δpdh mutant as the template, using the primers MG-for and MG-rev; lane 3, PCR product with genomic DNA of the Δpdh mutant as the template, using the primers M13 Primer RV and PDH-phomo-for; lane 4, PCR product with genomic DNA of the Δpox mutant as the template, using the primers M13 Primer RV and PDH-phomo-for. The sizes of objective DNA bands are indicated by the corresponding numbers.
Transcriptional levels of pdh and pox genes in L. brevis ATCC 367 and mutants.
To identify changes in the expression of genes involved in the aerobic conversion of lactate to acetate, the transcriptional levels of pdh and pox were analyzed (Table 1). In the early growth phase, the transcriptional levels of pdh were stable in both anaerobic and aerobic cultures. After glucose was exhausted (late growth phase), however, the pdh gene was upregulated 37.92-fold in aerobic cultures, compared with anaerobic cultures. Similarly, in the late growth phase, the transcriptional level of pox was induced to increase 18.32-fold under aerobic conditions, compared with anaerobic conditions. In the Δpdh mutant, transcription of the pox gene was significantly increased 2.24-fold (P = 0.002), compared with the wild-type strain, in the late growth phase of aerobic cultures. However, the transcriptional level of the pdh gene in the Δpox mutant did not change significantly under aerobic conditions, with a 1.07-fold increase (P = 0.08), compared with the wild-type strain.
TABLE 1.
Differential transcription of pdh and pox in L. brevis ATCC 367 and Δpdh and Δpox mutants at different growth phases under anaerobic and aerobic conditions
| Gene and growth phasea | Relative gene expressionb |
|||||
|---|---|---|---|---|---|---|
| Wild type |
Δpdh |
Δpox |
||||
| Anaerobic | Aerobic | Anaerobic | Aerobic | Anaerobic | Aerobic | |
| pdh | ||||||
| Early | 1.00 ± 0.00 | 0.99 ± 0.02 | Null | Null | 1.00 ± 0.00 | 0.99 ± 0.04 |
| Late | 1.03 ± 0.01 | 38.04 ± 0.85c,d | Null | Null | 0.98 ± 0.02 | 40.78 ± 0.82c,d |
| pox | ||||||
| Early | 1.00 ± 0.05 | 1.27 ± 0.05 | 1.00 ± 0.01 | 1.02 ± 0.04 | Null | Null |
| Late | 1.09 ± 0.03 | 18.87 ± 0.78c,d | 1.04 ± 0.04 | 42.21 ± 0.45c,d | Null | Null |
pdh, pyruvate dehydrogenase gene; pox, pyruvate oxidase gene.
Relative gene expression was calculated using 16S rRNA as the housekeeping gene and the early growth phase under anaerobic conditions as the reference condition. The strains were as follows: wild type, L. brevis ATCC 367; Δpdh, L. brevis ATCC 367 Δpdh; Δpox, L. brevis ATCC 367 Δpox.
Significant difference (P < 0.05) in relative gene expression among different grow conditions in the same growth phase.
Significant difference (P < 0.05) in relative gene expression among different growth phases under the same growth conditions.
Enzymatic activities of PDH and POX in L. brevis ATCC 367 and mutants.
The PDH activities in the wild-type strain were similar in aerobic and anaerobic cultures in the early growth phase, while the PDH activity in the wild-type strain increased 5.32-fold in aerobic cultures in the late growth phase. POX activity was not detected in anaerobically cultivated wild-type cells, but the activity was 1.06 ± 0.31 U per mg of total protein in aerobically cultivated wild-type cells in the early growth phase and increased 2.35-fold in the late growth phase. Under aerobic conditions, the POX activity of the Δpdh mutant was increased 1.80-fold (P = 0.02), compared with that of the wild-type strain, in the late growth phase. The PDH activity of the Δpox mutant did not differ significantly from that of the wild-type strain in both early and late stages of growth, under both aerobic and anaerobic conditions (Fig. 4).
FIG 4.
Specific activities of PDH (A) and POX (B) of the L. brevis ATCC 367, Δpdh mutant, and Δpox mutant strains under anaerobic and aerobic conditions. POX activity was not detected in the anaerobic cultures in the early growth phase and the late growth phase (n.d.). The pdh gene was inactivated; therefore, the PDH activity in the Δpdh mutant was null. The pox gene was inactivated; therefore, the POX activity in the Δpox mutant was null. Early, early growth phase; Late, late growth phase. The values are means ± standard deviations of three independent experiments. Statistically significant difference is indicated by an asterisk (*, P < 0.05).
Effects of inactivation of pdh and pox genes on aerobic growth of L. brevis.
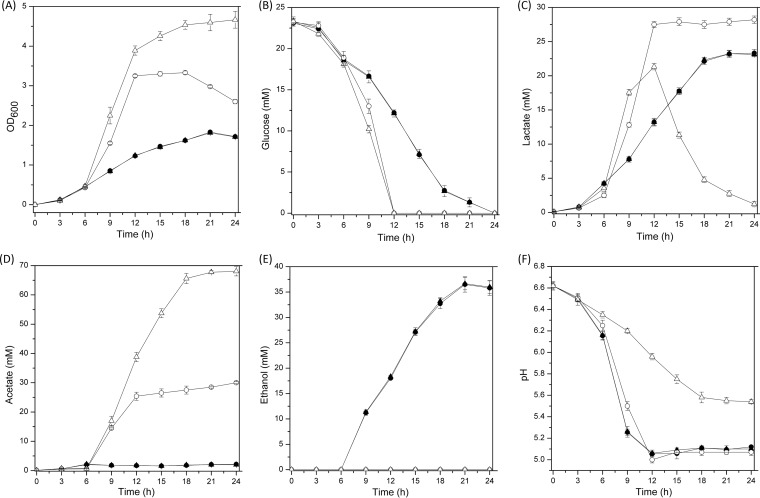
Under anaerobic conditions, there was no difference in growth among the L. brevis ATCC 367 and Δpdh and Δpox mutant strains. Moreover, levels of glucose consumption and metabolite production were similar among L. brevis ATCC 367 and the two mutant strains (Fig. 1 and 5). Under aerobic conditions, levels of growth, lactate production, and acetate production were similar between the wild-type strain and the Δpox mutant but the Δpdh mutant showed weak growth ability, with the final cell density being decreased by 37.78% and no conversion of lactate to acetate (Fig. 1 and 5).
FIG 5.
Comparison of growth performance (A), glucose consumption (B), metabolite production (C to E), and pH (F) between the Δpdh mutant (circles) and the Δpox mutant (triangles) under anaerobic (closed symbols) and aerobic (open symbols) conditions. The curves for the two mutant strains under anaerobic conditions mostly overlap. In all panels, the values are means ± standard deviations of three independent experiments.
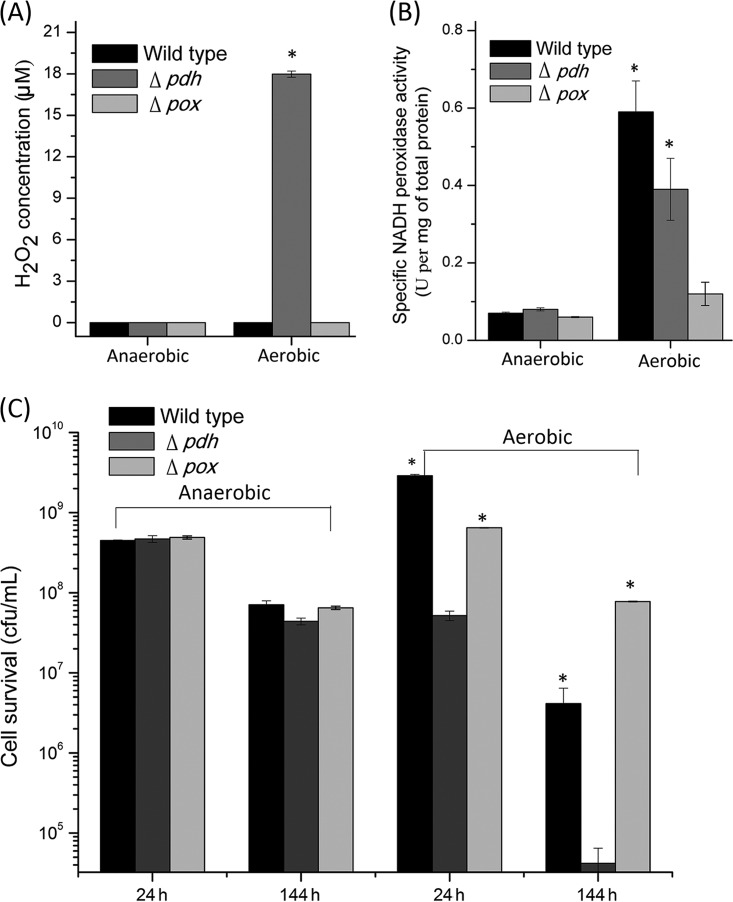
H2O2 accumulation and cell survival.
To confirm that POX was involved in the aerobic conversion of lactate to acetate, one of the products of the POX pathway, H2O2, was detected in 24-h cultures of the wild-type and Δpdh and Δpox mutant strains cultivated under both aerobic and anaerobic conditions. The results showed that anaerobiosis yielded no H2O2 (Fig. 6A). Under aerobic conditions, no H2O2 was produced in the wild-type and Δpox mutant strains, while 17.98 ± 0.22 μM H2O2 was produced in the Δpdh mutant strain. To evaluate the degradation of H2O2, the activity of NPR was determined. The anaerobic cultures exhibited very low specific activities, below 0.08 ± 0.004 U per mg of total protein. Under aerobic conditions, the activity was 0.12 ± 0.03 U per mg of total protein for the Δpox mutant but was significantly increased in the wild-type and Δpdh mutant strains, with 0.59 ± 0.08 U per mg of total protein (P = 0.002) and 0.39 ± 0.08 U per mg of total protein (P = 0.006), respectively (Fig. 6B).
FIG 6.
H2O2 accumulation (A), NADH peroxidase activity (B), and cell survival during 144 h of storage at 4°C (C) of the wild-type L. brevis ATCC 367, Δpdh mutant, and Δpox mutant strains after 24 h of anaerobic or aerobic cultivation. The values are means ± standard deviations of three independent experiments. Statistically significant differences are indicated by asterisks (*, P < 0.05).
To verify H2O2 production, the long-term survival of L. brevis ATCC 367 and the two mutants was determined after anaerobic and aerobic cultivation. As shown in Fig. 6C, anaerobic cultures of the three strains showed 10-fold decreases in viable cells after 144 h of storage at 4°C. In contrast, the cell viability of aerobic cultures of the Δpdh mutant decreased 103-fold after 144 h of storage at 4°C, while the viability of the wild-type and Δpox mutant strains decreased 102- and 10-fold, respectively.
DISCUSSION
L. brevis is a heterofermentative bacterium and is gaining attention as a new silage starter because of its ability to produce high levels of acetate (15). Here, the detailed pathway of aerobic acetate accumulation through a second oxidation of lactate was identified in L. brevis ATCC 367. We found that PDH played the predominant role in the aerobic conversion of pyruvate to acetate and POX had a secondary role. L. brevis, having both functional PDH and POX under aerobic conditions, was different from the other lactobacilli reported previously (20).
Aerobiosis had positive effects on L. brevis ATCC 367 growth, as observed by the 3-fold increase in biomass, compared with anaerobic cultivation. Therefore, aerobic cultivation contributed to an energetically favorable lifestyle of L. brevis. The extra energy provided in aerobic growth might be produced in three ways, as follows. (i) Oxygen repressed the expression of alcohol dehydrogenase (20) and thus shifted ethanol production to acetate. (ii) When glucose levels were substantial, pyruvate was channeled to acetyl-CoA followed by acetate production, instead of reduction of pyruvate to lactate (23, 24). (iii) After glucose was exhausted, lactate was reoxidized to pyruvate, which was finally converted to acetate (the lactate-to-acetate pathway) (25). Moreover, to maintain the intracellular redox balance, oxygen was used as an electron acceptor to regenerate NAD+ through NADH oxidase, which had a higher affinity for NADH than did lactate dehydrogenase and alcohol dehydrogenase (26–28). In these three pathways, acetate resulted from dephosphorylation of acetyl-Pi by the action of acetate kinase (AK), accompanied by ATP generation (20). Our data demonstrated that, under aerobic conditions, L. brevis ATCC 367 converted part of pyruvate to acetate in the logarithmic growth phase when glucose was present (Fig. 1, 6 h to 12 h) and redirected metabolism toward production of acetate at the expense of lactate after glucose was exhausted (Fig. 1, 12 h to 24 h).
According to the predicted aerobic conditions of cultivation shown in Fig. 2, the aerobic conversion of lactate to acetate could be mediated either by PDH or by POX (13, 16, 20). Previous reports confirmed that POX was the key enzyme for aerobic accumulation of acetate in homofermentative LAB and some heterofermentative LAB, while PDH did not show a prominent effect on acetate production (20, 29–31). In L. brevis ATCC 367, however, the increased ranges of the transcriptional level of the pdh gene and the enzymatic activity of PDH were both higher than those of POX under aerobic conditions after glucose was exhausted. Moreover, the inactivation of pox had little influence on the transcription of pdh and enzymatic activity of PDH, while inactivation of pdh significantly improved the transcription of pox and enzymatic activity of POX. These results suggested that PDH dominated the aerobic acetate accumulation pathway and POX was at a disadvantage in the competition with PDH during aerobic cultivation. In addition, levels of anaerobic growth and metabolite production of the wild-type, Δpdh mutant, and Δpox mutant strains were similar, indicating that erythromycin had little influence on the growth of the mutants and inactivation of pdh or pox did not influence anaerobic metabolic characteristics. Under aerobic conditions, however, inactivation of pdh resulted in smaller final biomass amounts of the Δpdh mutant than of the wild-type strain (Fig. 1 and 5), while the Δpox mutant maintained a growth profile similar to that of the wild-type strain, implying that the aerobic conversion of lactate to acetate still occurred and provided extra ATP to support cell growth in the Δpox mutant. Moreover, the Δpdh mutant lost the phenomenon of aerobic conversion of lactate to acetate, indicating that the inactivation of pdh blocked the main conversion pathway from lactate to acetate. These results strongly suggested that PDH rather than POX dominated aerobic acetate generation after glucose exhaustion. We hypothesized that aerobic conversion of lactate to acetate was a strain-specific characteristic.
The transcription of pox and enzymatic activity of POX suggested that the POX pathway participated in the aerobic conversion of lactate to acetate, while inactivation of the pox gene in the Δpox mutant did not change the growth, lactate production, and acetate production, compared with the wild-type strain. It was unclear whether the POX pathway was involved in aerobic acetate accumulation. However, H2O2, the product of the oxidation of pyruvate by POX, was detected. H2O2 in the aerobic cultures of the Δpdh mutant suggested that the POX pathway was workable. However, H2O2 was not detected in the aerobic cultures of the wild-type strain; it might be degraded by the activity of NPR, with contents below the detection limit. As a type of reactive oxygen species (ROS), H2O2 can influence the long-term survival of LAB (32). During long-term storage, the lower viability of the wild-type strain, compared with that of the Δpox mutant, suggested that trace H2O2 (below the detection limit) existed in the aerobic cultures of the wild-type strain. The results indicated that H2O2 was produced during the aerobic cultivation of L. brevis ATCC 367. Also, because the H2O2-forming NADH oxidase gene was absent in the genome of L. brevis ATCC 367, the most likely source of oxidative stress by H2O2 originated from the action of POX in aerobic cultures of L. brevis ATCC 367. Consequently, we speculated that POX also worked for aerobic conversion of lactate to acetate in L. brevis ATCC 367, secondary to the role of PDH.
In conclusion, this study demonstrated the predominant role of PDH and the secondary role of POX in the aerobic conversion of lactate to acetate in L. brevis ATCC 367. Our findings will further develop the aerobic growth theory of LAB and provide a new strategy to develop inoculants for improvement of the aerobic stability of silages against deterioration caused by yeasts and molds.
MATERIALS AND METHODS
Strains, media, and growth conditions.
L. brevis ATCC 367 and its derivatives were cultivated at 37°C in De Man-Rogosa-Sharpe (MRS) medium containing 0.5% glucose, under two different conditions, i.e., (i) anaerobiosis, with static cultures in 100-ml Erlenmeyer flasks containing 50 ml medium, and (ii) aerobiosis, with aerated cultures in 300-ml Erlenmeyer flasks containing 50 ml medium, agitated on a rotary shaker at 200 rpm. Escherichia coli DH5α was grown aerobically at 37°C in Luria-Bertani (LB) medium. When appropriate, the following antibiotics were added to the medium: ampicillin (100 μg/ml for E. coli) and erythromycin (250 μg/ml for E. coli or 5 μg/ml for L. brevis). Cell growth was monitored by the change in the optical density at 600 nm (OD600). Three independent cultivations were carried out for each growth condition (anaerobiosis and aerobiosis).
In silico analysis of genes involved in cultivation of L. brevis ATCC 367 under aerobic conditions.
The whole-genome sequence of L. brevis ATCC 367 is available at GenBank under accession number NC_008497 (33). Data on genes involved in aerobic cultivation, including those encoding NADH-dependent lactate dehydrogenase, pyruvate oxidase, pyruvate dehydrogenase complex, pyruvate formate lyase, phosphotransacetylase, acetate kinase, acetaldehyde dehydrogenase, alcohol dehydrogenase, lactate oxidase, H2O2-forming NADH oxidase, H2O-forming NADH oxidase, and NADH peroxidase, were retrieved. The deduced amino acid sequences of PDH and POX of L. brevis ATCC 367 were aligned with those of L. plantarum WCFS1 (GenBank accession number NC_004567), L. buchneri CD034 (GenBank accession number NC_018610), and L. spicheri DSM 15429 (GenBank accession number NZ_AZFC00000000) using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo).
Inactivation of pyruvate dehydrogenase and pyruvate oxidase genes in L. brevis ATCC 367.
The primers used in this study are listed in Table 2. Molecular cloning techniques were performed using standard methods (34). Taq DNA polymerase, restriction enzymes, and T4 DNA ligase were used according to the instructions of the manufacturer (TaKaRa, Tokyo, Japan).
TABLE 2.
Plasmids and primers used in this study
| Primer name | Sequence (5′ to 3′)a | Useb |
|---|---|---|
| MG-for | ACAAGATCTTATGGACAGTTGCGGA | Cloning of Erm |
| MG-rev | TATGGATCCAGCTACCAAGACGAAG | Cloning of Erm |
| PDH-phomo-for | ATCTCTAGAGGCTGACCCATACAAGCA | Cloning of pdhk |
| PDH-phomo-rev | CATAAGCTTCCACAATATCCATCCCATC | Cloning of pdhk |
| POX-phomo-for | ATTTCTAGAACCGTGGAAGGACTTTATTTG | Cloning of poxk |
| POX-phomo-rev | TTCAAGCTTCGTTTCACCAGTCGCAATC | Cloning of poxk |
| M13 Primer RV | CAGGAAACAGCTATGAC | Verification of gene inactivation |
| PDH-RT-for | CAAATGATTCAACACGGGACT | Quantitative PCR |
| PDH-RT-rev | TAAGCAAAGGCGACACGAT | Quantitative PCR |
| POX-RT-for | GCCTGGTGCGACCCATCTA | Quantitative PCR |
| POX-RT-rev | TGGCGTTTCATCCATTTCCTG | Quantitative PCR |
| 16S-RT-for | CGGCGTATTAGTTAGTTGGTG | Quantitative PCR |
| 16S-RT-rev | TTCCCTACTGCTGCCTCCC | Quantitative PCR |
Restriction sites in the primer sequences are underlined.
Erm, erythromycin resistance gene; pdhk, homogeneous fragment of pdh; poxk, homogeneous fragment of pox.
Inactivation of the pdh and pox genes was carried out using a single-crossover integration strategy (35). An erythromycin resistance gene was PCR amplified from the vector pMG36e (36) by using the primers MG-for and MG-rev and then was subcloned into the BamHI site of pUC19, resulting in the suicide-integration vector pUC-erm. Subsequently, a DNA fragment containing pdhk (or poxk) was PCR amplified inside pdh (or pox) from genomic DNA of L. brevis ATCC 367 by using the primers PDH-phomo-for and PDH-phomo-rev (or POX-phomo-for and POX-phomo-rev) and then was subcloned into the XbaI and HindIII sites of pUC19-erm, generating the integration vector pUC19-erm-pdh (or pUC19-erm-pox).
The integration vectors were electroporated into competent cells of L. brevis ATCC 367 according to a method described previously (37). Transformants were selected on MRS agar containing 5 μg/ml erythromycin, and the inactivation of pdh (or pox) was verified by PCR amplification with the primers M13 Primer RV and PDH-phomo-for (or M13 Primer RV and POX-phomo-for), generating two mutants, L. brevis ATCC 367 Δpdh and L. brevis ATCC 367 Δpox.
Transcriptional analysis of pdh and pox genes in wild-type and mutant strains.
Cultures of L. brevis ATCC 367, L. brevis ATCC 367 Δpdh, and L. brevis ATCC 367 Δpox under both conditions of cultivation were harvested in the early growth phase (6 h) or the late growth phase (11 h). Total RNA was isolated with a RNAsimple total RNA kit (Tiangen, Beijing, China), according to the manufacturer's protocols. Reverse transcription (RT) was performed with random 6-mers and an oligo(dT) primer, using the PrimeScript RT reagent kit (TaKaRa). Real-time PCR was performed with SYBR Premix Ex TaqII (TaKaRa), applying the protocol for the real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The 16S rRNA gene transcript was used as the housekeeping gene and amplified with the primers 16S-RT-for and 16S-RT-rev. The pdh and pox transcripts were PCR amplified with the primers PDH-RT-for and PDH-RT-rev and the primers POX-RT-for and POX-RT-rev, respectively. The relative transcription levels for pdh and pox were estimated with the ΔΔCT method (38). Three technical replicates of the gene transcription analysis were performed for each experiment.
Determination of PDH, POX, and NPR activities in wild-type and mutant strains.
Cells grown under both types of conditions were harvested from 200 ml (4 × 50 ml) of cultures by centrifugation at 6,000 × g for 3 min and were washed twice with precooled 50 mM potassium phosphate buffer (pH 7.2 for PDH, pH 6.5 for POX, and pH 7.0 for NPR analysis). The cells were suspended in 4 ml of potassium phosphate buffer and disrupted by sonication (JY92-IIN sonicator; Scientz, Ningbo, China) on ice (400 W; sonication for 4 s and intermission for 6 s for 90 cycles). The supernatant was recovered by centrifugation at 12,000 × g for 10 min at 4°C. Protein concentrations were determined using the Bradford protein assay, with bovine serum albumin as a standard.
PDH activity was assayed as described previously (21, 39). Briefly, the total 100-μl assay mixture contained 5 mM pyruvate, 5 mM MgCl2, 0.1 mg/liter lipoamide dehydrogenase, 0.3 mM dithiothreitol, 0.6 mM iodonitrotetrazolium chloride, 0.2 mM coenzyme A, 0.2 mM thiamine pyrophosphate, and 5 mM NAD+ in 50 mM potassium phosphate buffer (pH 7.2). The reaction was initiated by the addition of 10 μl of cell extract and was monitored by measuring absorption changes at 500 nm, using a SpectraMax 190 microplate reader (Molecular Devices Corp.), at 37°C for 20 min at 1-min intervals. For assays of POX activity, the 100-μl assay mixture contained 40 mM pyruvate, 5 mM MgCl2, 0.03 mM flavin adenine dinucleotide (FAD), 3 mM 4-aminoantipyrine, 10 mM 2-hydroxy-3,5-dichlorobenzene sulfonate, and 0.4 U/ml horseradish peroxidase in 50 mM potassium phosphate buffer (pH 6.5). The reaction was initiated by the addition of 10 μl of cell extract and was monitored by measuring absorption changes at 546 nm, using a SpectraMax 190 microplate reader (Molecular Devices Corp.), at 37°C for 20 min at 1-min intervals. For all measurements, cell extracts were heated at 100°C for 10 min for use as negative controls. The specific activity of enzymes was expressed as the generation of 1 nmol colored product by 1 mg total protein per minute.
The NADH consumed by NPR was determined spectrophotometrically by measuring the initial rate of NADH oxidation at 25°C, as described previously (23). The total 200-μl NPR assay mixture consisted of 0.4 mM NADH, 0.03 mM FAD, and 1.5 mM H2O2 in 50 mM potassium phosphate buffer (pH 7.0). The reaction was initiated by the addition of 10 μl of cell extract and was monitored by measuring the decrease in absorbance at 340 nm. For all measurements, cell extracts were heated at 100°C for 10 min for use as negative controls. One unit of enzyme was defined as the amount that catalyzed the oxidation at 25°C of 1 μM NADH to NAD+ per minute. Three technical replicates were performed for each experiment.
Analytical methods for metabolites.
Glucose, acetate, and lactate levels in the culture supernatants of L. brevis ATCC 367 and the two mutant strains cultivated under both types of conditions were analyzed by the following method. One milliliter of 50-ml anaerobic and aerobic cultures was centrifuged at 6,000 × g for 3 min to remove cells. The supernatant obtained was diluted 20-fold and filtered through a 0.22-μm membrane. A total of 20 μl of the dilution was subjected to high-performance liquid chromatography (HPLC) (Shimazu, Kyoto, Japan) using an Aminex HPX-87H column (300 mm by 7.8 mm; Bio-Rad) at a column temperature of 55°C, with 5 mM sulfuric acid as the mobile phase and a flow rate of 0.4 ml/min.
H2O2 concentrations in the 24-h aerobic culture supernatants of L. brevis ATCC 367 and the two mutant strains were measured using a H2O2 quantified analysis kit (Sangon Biotech, Shanghai, China), by standard procedures. Three technical replicates were performed for each experiment.
Determination of cell survival.
L. brevis ATCC 367, L. brevis ATCC 367 Δpdh, and L. brevis ATCC 367 Δpox were cultivated under anaerobic and aerobic conditions for 24 h. The cultures were then transferred to 4°C, and cell viability was examined after 144 h of storage. Cell survival was calculated by determination of numbers of CFU, as described previously (40). Briefly, cultures were serially diluted 10-fold, and the dilution time (Tdilution) of the original sample was 0. For objective dilution, 5-μl samples were pipetted onto a plate containing 1.5% agar medium. The plates were air dried and then incubated until colonies were visible, with an average size of 200 to 500 μm; the colony number of every drop (Ncolony) was counted. The CFU concentration (number of CFU per milliliter) was calculated by using the following equation: CFU/ml = 10Tdilution × 200 × Ncolony.
Statistical analysis.
Statistical analysis was performed using unpaired two-tailed Student's t tests. P values of <0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by Public Service Sectors (Agriculture) Special and Scientific Research Projects (grant 201503134), the National Natural Science Foundation of China (grant 31471715), and the National Science Foundation for Young Scientists of China (grant 31400077).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01659-17.
REFERENCES
- 1.Barrangou R, Lahtinen SJ, Ibrahim F, Ouwehand AC. 2011. Genus Lactobacillus, p 77–91. In Lahtinen S, Ouwehand AC, Salminen S, von Wright A (ed), Lactic acid bacteria: microbiological and functional aspects, 4th ed. CRC Press, Boca Raton, FL. [Google Scholar]
- 2.Schmidt RJ, Kung L Jr. 2010. The effects of Lactobacillus buchneri with or without a homolactic bacterium on the fermentation and aerobic stability of corn silages made at different locations. J Dairy Sci 93:1616–1624. doi: 10.3168/jds.2009-2555. [DOI] [PubMed] [Google Scholar]
- 3.Eikmeyer FG, Heinl S, Marx H, Pühler A, Grabherr R, Schlüter A. 2015. Identification of oxygen-responsive transcripts in the silage inoculant Lactobacillus buchneri CD034 by RNA sequencing. PLoS One 10:e0134149. doi: 10.1371/journal.pone.0134149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driehuis F, Oude Elferink SJWH, Van Wikselaar PG. 2001. Fermentation characteristics and aerobic stability of grass silage inoculated with Lactobacillus buchneri, with or without homofermentative lactic acid bacteria. Grass Forage Sci 56:330–343. doi: 10.1046/j.1365-2494.2001.00282.x. [DOI] [Google Scholar]
- 5.Santos AO, Ávila CLS, Pinto JC, Carvalho BF, Dias DR, Schwan RF. 2016. Fermentative profile and bacterial diversity of corn silages inoculated with new tropical lactic acid bacteria. J Appl Microbiol 120:266–279. doi: 10.1111/jam.12980. [DOI] [PubMed] [Google Scholar]
- 6.Gulfam A, Guo G, Tajebe S, Chen L, Liu QH, Yuan XJ, Bai YF, Saho T. 2017. Characteristics of lactic acid bacteria isolates and their effect on the fermentation quality of Napier grass silage at three high temperatures. J Sci Food Agric 97:1931–1938. doi: 10.1002/jsfa.7998. [DOI] [PubMed] [Google Scholar]
- 7.Lay CL, Mounier J, Vasseur V, Weill A, Blay GL, Barbier G, Coton E. 2016. In vitro and in situ screening of lactic acid bacteria and propionibacteria antifungal activities against bakery product spoilage molds. Food Control 60:247–255. doi: 10.1016/j.foodcont.2015.07.034. [DOI] [Google Scholar]
- 8.Acosta Aragón Y, Jatkauskas J, Vrotniakienė V. 2012. The effect of a silage inoculant on silage quality, aerobic stability, and meat production on farm scale. ISRN Vet Sci 2012:345927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eikmeyer FG, Köfinger P, Poschenel A, Jünemann S, Zakrzewski M, Heinl S, Mayrhuber E, Grabherr R, Pühler A, Schwab H. 2013. Metagenome analyses reveal the influence of the inoculant Lactobacillus buchneri CD034 on the microbial community involved in grass ensiling. J Biotechnol 167:334–343. doi: 10.1016/j.jbiotec.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Liu QH, Yang FY, Zhang JG, Shao T. 2014. Characteristics of Lactobacillus parafarraginis ZH1 and its role in improving the aerobic stability of silages. J Appl Microbiol 117:405–416. doi: 10.1111/jam.12530. [DOI] [PubMed] [Google Scholar]
- 11.Daniel JLP, Checolli M, Zwielehner J, Junges D, Fernandes J, Nussio LG. 2015. The effects of Lactobacillus kefiri and L. brevis on the fermentation and aerobic stability of sugarcane silage. Anim Feed Sci Technol 205:69–74. doi: 10.1016/j.anifeedsci.2015.04.015. [DOI] [Google Scholar]
- 12.Holzer M, Mayrhuber E, Danner H, Braun R. 2003. The role of Lactobacillus buchneri in forage preservation. Trends Biotechnol 21:282–287. doi: 10.1016/S0167-7799(03)00106-9. [DOI] [PubMed] [Google Scholar]
- 13.Heinl S, Grabherr R. 2017. Systems biology of robustness and flexibility: Lactobacillus buchneri—a show case. J Biotechnol 257:61–69. doi: 10.1016/j.jbiotec.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Hu W, Schmidt RJ, McDonell EE, Klingerman CM, Kung L Jr. 2009. The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J Dairy Sci 92:3907–3914. doi: 10.3168/jds.2008-1788. [DOI] [PubMed] [Google Scholar]
- 15.Danner H, Holzer M, Mayrhuber E, Braun R. 2003. Acetic acid increases stability of silage under aerobic conditions. Appl Environ Microbiol 69:562–567. doi: 10.1128/AEM.69.1.562-567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorquet F, Goffin P, Muscariello L, Baudry JB, Ladero V, Sacco M, Kleerebezem M, Hols P. 2004. Characterization and functional analysis of the poxB gene, which encodes pyruvate oxidase in Lactobacillus plantarum. J Bacteriol 186:3749–3759. doi: 10.1128/JB.186.12.3749-3759.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johanningsmeier SD, McFeeters RF. 2013. Metabolism of lactic acid in fermented cucumbers by Lactobacillus buchneri and related species, potential spoilage organisms in reduced salt fermentations. Food Microbiol 35:129–135. doi: 10.1016/j.fm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Skinner-Nemec KA, Leathers TD. 2008. Lactobacillus buchneri strain NRRL B-30929 converts a concentrated mixture of xylose and glucose into ethanol and other products. J Ind Microbiol Biotechnol 35:75–81. doi: 10.1007/s10295-007-0267-8. [DOI] [PubMed] [Google Scholar]
- 19.Ianniello RG, Zheng J, Zotta T, Ricciardi A, Gänzle MG. 2015. Biochemical analysis of respiratory metabolism in the heterofermentative Lactobacillus spicheri and Lactobacillus reuteri. J Appl Microbiol 119:763–775. doi: 10.1111/jam.12853. [DOI] [PubMed] [Google Scholar]
- 20.Zotta T, Parente E, Ricciardi A. 2017. Aerobic metabolism in the genus Lactobacillus: impact on stress response and potential applications in the food industry. J Appl Microbiol 122:857–869. doi: 10.1111/jam.13399. [DOI] [PubMed] [Google Scholar]
- 21.Lopez de Felipe F, Gaudu P. 2009. Multiple control of the acetate pathway in Lactococcus lactis under aeration by catabolite repression and metabolites. Appl Microbiol Biotechnol 82:1115–1122. doi: 10.1007/s00253-009-1897-8. [DOI] [PubMed] [Google Scholar]
- 22.Larsen N, Moslehi-Jenabian S, Werner BB, Jensen ML, Garrigues C, Vogensen FK, Jespersen L. 2016. Transcriptome analysis of Lactococcus lactis subsp. lactis during milk acidification as affected by dissolved oxygen and the redox potential. Int J Food Microbiol 226:5–12. doi: 10.1016/j.ijfoodmicro.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Quatravaux S, Remize F, Bryckaert E, Colavizza D, Guzzo J. 2006. Examination of Lactobacillus plantarum lactate metabolism side effects in relation to the modulation of aeration parameters. J Appl Microbiol 101:903–912. doi: 10.1111/j.1365-2672.2006.02955.x. [DOI] [PubMed] [Google Scholar]
- 24.McLeod A, Zagorec M, Champomier-Vergès MC, Naterstad K, Axelsson L. 2010. Primary metabolism in Lactobacillus sakei food isolates by proteomic analysis. BMC Microbiol 10:120. doi: 10.1186/1471-2180-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johanningsmeier SD, McFeeters RF. 2015. Metabolic footprinting of Lactobacillus buchneri strain LA1147 during anaerobic spoilage of fermented cucumbers. Int J Food Microbiol 215:40–48. doi: 10.1016/j.ijfoodmicro.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Geueke B, Riebel B, Hummel W. 2003. NADH oxidase from Lactobacillus brevis: a new catalyst for the regeneration of NAD. Enzyme Microb Technol 32:205–211. doi: 10.1016/S0141-0229(02)00290-9. [DOI] [Google Scholar]
- 27.Berezina OV, Jurgens G, Zakharova NV, Shakulov RS, Yarotsky SV, Granström TB. 2013. Evaluation of carbon and electron flow in Lactobacillus brevis as a potential host for heterologous 1-butanol biosynthesis. Adv Microbiol 3:450–461. doi: 10.4236/aim.2013.35061. [DOI] [Google Scholar]
- 28.Jänsch A, Freiding S, Behr J, Vogel RF. 2011. Contribution of the NADH-oxidase (Nox) to the aerobic life of Lactobacillus sanfranciscensis DSM20451T. Food Microbiol 28:29–37. doi: 10.1016/j.fm.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Jensen NB, Melchiorsen CR, Jokumsen KV, Villadsen J. 2001. Metabolic behavior of Lactococcus lactis MG1363 in microaerobic continuous cultivation at a low dilution rate. Appl Environ Microbiol 67:2677–2682. doi: 10.1128/AEM.67.6.2677-2682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hertzberger RY, Pridmore RD, Gysler C, Kleerebezem M, Teixeira de Mattos MJ. 2013. Oxygen relieves the CO2 and acetate dependency of Lactobacillus johnsonii NCC 533. PLoS One 8:e57235. doi: 10.1371/journal.pone.0057235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goffin P, Muscariello L, Lorquet F, Stukkens A, Prozzi D, Sacco M, Kleerebezem M, Hols P. 2006. Involvement of pyruvate oxidase activity and acetate production in the survival of Lactobacillus plantarum during the stationary phase of aerobic growth. Appl Environ Microbiol 72:7933–7940. doi: 10.1128/AEM.00659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezaïki L, Cesselin B, Yamamoto Y, Vido K, van West E, Gaudu P, Gruss A. 2004. Respiration metabolism reduces oxidative and acid stress to improve long-term survival of Lactococcus lactis. Mol Microbiol 53:1331–1342. doi: 10.1111/j.1365-2958.2004.04217.x. [DOI] [PubMed] [Google Scholar]
- 33.Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koonin E, Pavlov A, Pavlova N, Karamychev V, Polouchine N, Shakhova V, Grigoriev I, Lou Y, Rohksar D, Lucas S, Huang K, Goodstein DM, Hawkins T, Plengvidhya V, Welker D, Hughes J, Goh Y, Benson A, Baldwin K, Lee JH, Diaz-Muniz I, Dosti B, Smeianov V, Wechter W, Barabote R, Lorca G, Altermann E, Barrangou R, Ganesan B, Xie Y, Rawsthorne H, Tamir D, Parker C, Breidt F, Broadbent J, Hutkins R, O'Sullivan D, Steele J, Unlu G, Saier M, Klaenhammer T, Richardson P, Kozyavkin S, Weimer B, Mills D. 2006. Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci U S A 103:15611–15616. doi: 10.1073/pnas.0607117103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 35.Leenhouts KJ, Kok J, Venema G. 1989. Campbell-like integration of heterologous plasmid DNA into the chromosome of Lactococcus lactis subsp. lactis. Appl Environ Microbiol 55:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van de Guchte M, van der Vossen JM, Kok J, Venema G. 1989. Construction of a lactococcal expression vector: expression of hen egg white lysozyme in Lactococcus lactis subsp. lactis. Appl Environ Microbiol 55:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aukrust TW, Brurberg MB, Nes IF. 1995. Transformation of Lactobacillus by electroporation. Methods Mol Biol 47:201–208. [DOI] [PubMed] [Google Scholar]
- 38.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Risse B, Stempfer G, Rudolph R, Möllering H, Jaenicke R. 1992. Stability and reconstitution of pyruvate oxidase from Lactobacillus plantarum: dissection of the stabilizing effects of coenzyme binding and subunit interaction. Protein Sci 1:1699–1709. doi: 10.1002/pro.5560011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C, Xin Y, Wang Y, Guo T, Lu S, Kong J. 2015. Identification of a novel dye-decolorizing peroxidase, EfeB, translocated by a twin-arginine translocation system in Streptococcus thermophilus CGMCC 7179. Appl Environ Microbiol 81:6108–6119. doi: 10.1128/AEM.01300-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.