ABSTRACT
Soil is a complex niche, where survival of microorganisms is at risk due to the presence of antimicrobial agents. Many microbes chemically modify cytotoxic compounds to block their deleterious effects. Streptothricin is a broad-spectrum antibiotic produced by streptomycetes that affects Gram-positive and Gram-negative bacteria alike. Here we identify the SatA (for streptothricin acetyltransferase A, formerly YyaR) enzyme of Bacillus subtilis as the mechanism used by this soil bacterium to detoxify streptothricin. B. subtilis strains lacking satA were susceptible to streptothricin. Ectopic expression of satA+ restored streptothricin resistance to B. subtilis satA (BsSatA) strains. Purified BsSatA acetylated streptothricin in vitro at the expense of acetyl-coenzyme A (acetyl-CoA). A single acetyl moiety transferred onto streptothricin by SatA blocked the toxic effects of the antibiotic. SatA bound streptothricin with high affinity (Kd [dissociation constant] = 1 μM), and did not bind acetyl-CoA in the absence of streptothricin. Expression of B. subtilis satA+ in Salmonella enterica conferred streptothricin resistance, indicating that SatA was necessary and sufficient to detoxify streptothricin. Using this heterologous system, we showed that the SatA homologue from Bacillus anthracis also had streptothricin acetyltransferase activity. Our data highlight the physiological relevance of lysine acetylation for the survival of B. subtilis in the soil.
IMPORTANCE Experimental support is provided for the functional assignment of gene products of the soil-dwelling bacilli Bacillus subtilis and Bacillus anthracis. This study focuses on one enzyme that is necessary and sufficient to block the cytotoxic effects of a common soil antibiotic. The enzyme alluded to is a member of a family of proteins that are broadly distributed in all domains of life but poorly studied in B. subtilis and B. anthracis. The initial characterization of the enzyme provides insights into its mechanism of catalysis.
KEYWORDS: Bacillus anthracis, Bacillus subtilis, acetyltransferases, antibiotic resistance, lysine acetylation, metabolic stress, streptothricin, Gcn5-related acetyltransferases
INTRODUCTION
Soil is a complex microbial niche occupied by prokaryotes and eukaryotes alike (1). This environment contains plant- and microbe-derived chemicals that compromise the survival of microorganisms (1–4). Such antimicrobial agents exert strong selective pressures for microbes to evolve means to neutralize or become resistant to them. The soil bacterium Bacillus subtilis survives its environment by diverse means such as sporulation, its natural competency to acquire genetic material, and through the synthesis of enzymes that can detoxify antimicrobials. Streptothricin is one of many antibiotics present in the soil, and it is synthesized by several species of streptomycetes. Streptothricin was discovered by Waksman and Woodruff in 1942 (5), but its chemical structure was reported almost 2 decades later (6, 7). Structurally, streptothricins have a common structure, consisting of streptolidine, a gulosamine moiety, and a β-lysine chain that varies from 1 to 7 units (Fig. 1) (8).
FIG 1.
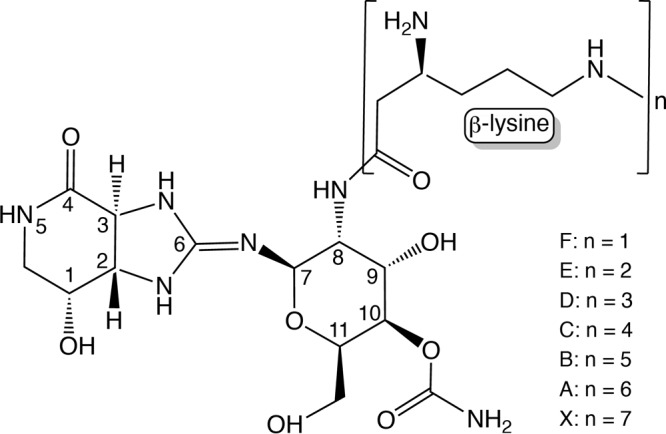
Structure of streptothricin. Streptothricins are made up of a streptolidine, a gulosamine moiety, and a β-lysine chain that varies from 1 to 7 units. Streptothricins A to F and X reflect the length of β-Lys residues.
Streptothricins are active against Gram-positive and Gram-negative bacteria as well as against fungi; however, their cytotoxic properties prevent their clinical or veterinary use (5, 8, 9). The presence of streptothricin results in the mistranslation of mRNAs (10) and its inhibitory effect increases as a function of the length of the β-lysine chain (11). Streptothricin inhibits protein synthesis by preventing binding of charged tRNAs to the A-site of the ribosome and blocking translocation of the peptidyl-tRNA to the P-site (12, 13). Streptothricin resistance can be caused by structural modification of the antibiotic. For example, in Streptomyces albulus, the protein SttH hydrolyzes the amine bond of the streptolidine, inactivating the antibiotic (14). An alternative mechanism of streptothricin inactivation is via the acetylation of the amino group of β-lysine, a mechanism first described in 1983 (15). Streptothricin acetyltransferases that block the toxic effects of the antibiotic do so by monoacetylation of the beta amino group of the first β-lysyl moiety of streptothricin. These type of enzymes have been described in Campylobacter coli BE/G4 (16), Streptomyces lavendulae (17), Streptomyces noursei (18, 19), Streptomyces rochei (20), and Escherichia coli (21), but not in Bacillus subtilis. We focused this study of streptothricin acetylation on B. subtilis in an effort to identify targets of Gcn5-related N-acetyltransferases (GNATs, PF00583) in this bacterium.
GNATs are found in all domains of life. These enzymes catalyze the transfer of acetyl groups onto varied substrates, including proteins, small molecules, tRNAs, and aminoglycoside antibiotics (22–26), allowing cells to cope with a variety of environmental and cellular stressors. The functions of the vast majority of putative GNATs are not supported by experimental data.
The B. subtilis genome may contain up to 30 genes encoding putative GNATs, but only a few of these proteins have functional assignments (27–29). Results from bioinformatics analysis suggested that the B. subtilis yyaR gene encodes a putative protein with 35% similarity (23% identity) to the streptothricin acetyltransferase (StaT) from S. lavendulae. Here we report that YyaR acetylates streptothricin and that acetylation blocks the toxic effect of the antibiotic. We also show that expression of yyaR in Salmonella enterica (a heterologous host) confers streptothricin resistance. We used S. enterica to determine whether or not a putative streptothricin acetyltransferase in the related organism Bacillus anthracis could also acetylate streptothricin. The crystal structure of the B. anthracis protein has been reported (RCSB PDB 3PP9), but experimental evidence validating the proposed annotation has not been published. Our data highlight the physiological importance of lysine acetylation in the detoxification of antimicrobial agents in the Gram-positive bacterium B. subtilis, and possibly in B. anthracis.
RESULTS AND DISCUSSION
Streptothricin inhibits growth of B. subtilis, and resistance to low levels of antibiotic depends on the function of the SatA protein.
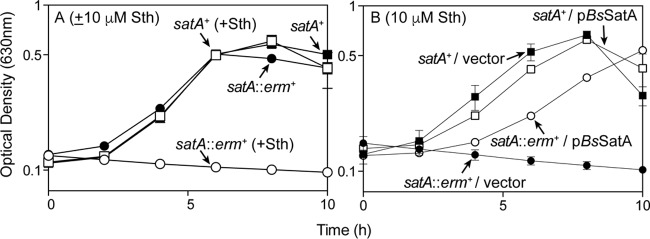
Growth of Bacillus subtilis 168 (here B. subtilis) was negatively affected by streptothricin, with an MIC of ≥60 μM (81 μg/ml; see Fig. S1 in the supplemental material), an MIC that was higher than the reported MIC for B. subtilis ATCC-6633 (2.4 μM; 3.2 μg/ml) (30). Comparisons of the primary amino acid sequence of the B. subtilis YyaR protein (here SatA) with that of the bona fide streptothricin acetyltransferase StaT enzyme from Streptomyces lavendulae suggested that yyaR encoded a putative streptothricin acetyltransferase (see Fig. S2 in the supplemental material). To test whether or not B. subtilis SatA (BsSatA) could acetylate streptothricin, B. subtilis strains carrying the satA+ allele (strain JE9142) or the null allele satA1::erm+ (strain JE20317) were grown in the presence or absence of streptothricin (Fig. 2). In the absence of streptothricin, both satA+ and satA strains grew equally well in minimal medium (Fig. 2A, solid squares and solid circles, respectively). In contrast, in the presence of streptothricin (10 μM), growth of the satA1::erm+ strain was arrested, while the satA+ strain grew to full density (Fig. 2A, open circles and open squares, respectively). These results indicated that the absence of the satA+ gene product led to streptothricin-induced growth arrest. Additionally, ectopic expression of satA+ restored streptothricin resistance to the satA1::erm+ strain, while growth of the satA1::erm+ strain carrying the vector without a gene cloned into it was inhibited when the medium was supplemented with streptothricin (10 μM; Fig. 2B, open circles and closed circles, respectively). These results supported the conclusion that BsSatA was somehow responsible for the observed resistance to streptothricin.
FIG 2.
Streptothricin inhibits growth of B. subtilis strains lacking satA. (A) Wild-type (satA+, squares) and satA (circles) strains were grown in minimal medium, either lacking streptothricin (closed symbols) or challenged with 10 μM streptothricin (open symbols). (B) Wild-type (satA+, squares) and satA (circles) strains carrying an empty cloning vector (closed symbols) or expressing the satA+ allele (open symbols) were grown in minimal medium containing 10 μM streptothricin. The following strains were analyzed: JE9142 (satA+), JE20317 (satA1::erm+), JE23688 (satA+ amyE::pBS1ClacZ), JE23690 (satA+ amyE::PsatA-satA), JE23689 (satA1::erm+ amyE::pBS1ClacZ), and JE23691 (satA::erm+ amyE::PsatA-satA). Error bars are present, although in many cases the deviations were too small to be visible.
BsSatA acetylates streptothricin.
To test whether or not BsSatA could directly acetylate streptothricin, the satA+ allele was cloned and overexpressed in E. coli and the overproduced protein was isolated to 81% purity (see Fig. S3 in the supplemental material). N-terminally hexahistidine (H6)-tagged BsSatA (H6-BsSatA) was purified as described in Materials and Methods, was not detagged, and was used in in vitro acetylation activity assays, also described below. Components of the reaction mixture were resolved on thin-layer chromatography (TLC) silica plates, and label distribution was visualized by phosphor imaging. Based on data shown in Fig. 3, we concluded that H6-BsSatA catalyzed the transfer of acetyl moieties from [1-14C]-acetyl coenzyme A ([1-14C]-AcCoA) onto streptothricin in a 1:1 ratio. The observed acetylation was enzymatic, since streptothricin was not acetylated in reaction mixtures devoid of H6-BsSatA (Fig. 3A).
FIG 3.
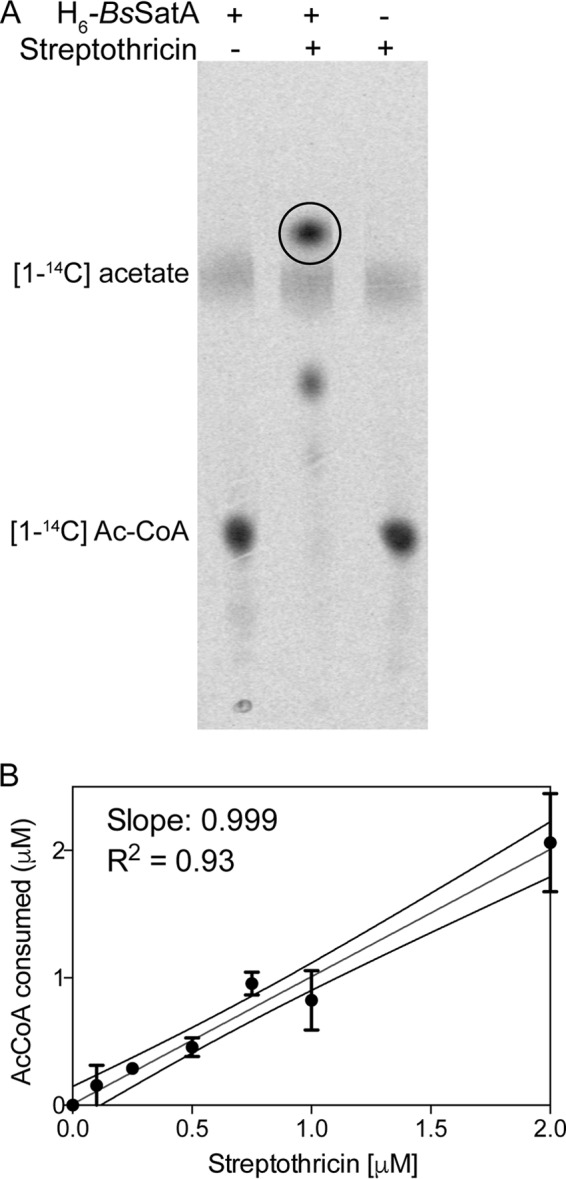
SatA acetylates streptothricin once. (A) Reaction mixtures of [1-14C]acetyl-CoA, streptothricin, and H6-BsSatA were incubated at 37°C for 1 h and then spotted onto a thin-layer chromatography (TLC) silica plate. The plate was developed for 1 to 2 h, and label distribution was visualized using a phosphor imager. (B) Quantification of the amount of acetylated streptothricin as a function of the amount of AcCoA consumed using a continuous spectrophotometric assay.
Other streptothricin acetyltransferases have been shown to monoacetylate the first β-lysyl moiety of streptothricin, leading to its inactivation (17, 31). To determine the number of acetyl moieties transferred by H6-BsSatA to streptothricin, we measured free thiols in reaction mixtures that contained purified H6-BsSatA, acetyl coenzyme A (AcCoA), and various streptothricin concentrations. By comparing the amount of AcCoA consumed as a function of the concentration of streptothricin, a slope of 1 (r2 = 0.93) was obtained (Fig. 3B). These data indicated that H6-BsSatA acetylated streptothricin at a single location per molecule, a conclusion that was consistent with data from other streptothricin acetyltransferases (17, 31).
H6-BsSatA poorly acetylates aminoglycoside antibiotics.
The substrate specificity of H6-BsSatA was not restricted to streptothricin (see Fig. S4 in the supplemental material). Other antibiotics that also interfere with ribosome function (e.g., neomycin and kanamycin) were incubated with H6-BsSatA and [1-14C]-AcCoA. As shown in Fig. S4, kanamycin and neomycin were acetylated by H6-BsSatA, albeit to a lesser extent than streptothricin. Quantification of the intensity of the signals showed that the enzyme acetylated neomycin 35%, and kanamycin 14%, relative to streptothricin (Fig. S4). These results indicated that neomycin and kanamycin were not the preferred substrates. Of note, the Nat protein from S. noursei can also weakly acetylate kanamycin and neomycin, though the observed level of acetylation did not confer resistance (18). A comparison of the primary sequence of BsSatA with that of S. noursei Nat showed that the sequences were only 24% identical (Fig. S2). Whether the specificity of BsSatA can be modified by mutation remains to be determined.
Acetylation of streptothricin blocks its toxic effects.
To determine whether or not acetylation affected the inhibitory effect of streptothricin, we incubated H6-BsSatA with increasing amounts of streptothricin for 60 min at 37°C. After incubation, a sample (5 μl) from each reaction mixture was spotted onto a layer of soft agar seeded with B. subtilis satA+ (strain JE9142). After overnight incubation at 37°C, zones of inhibition were measured (Fig. 4). Samples from reaction mixtures containing only streptothricin generated visible zones of inhibition. However, zones of inhibition were not observed when samples from reaction mixtures containing streptothricin and H6-BsSatA were tested. This suggested that acetylated streptothricin lacked antimicrobial activity, thereby allowing the cells to grow in the presence of streptothricin. Heat-inactivated H6-BsSatA failed to provide protection, and the diameter of the zone of clearance around the application spot matched that of a reaction mixture containing 50 μM streptothricin but devoid of H6-BsSatA. These results showed that acetyl-streptothricin had lost antimicrobial activity.
FIG 4.
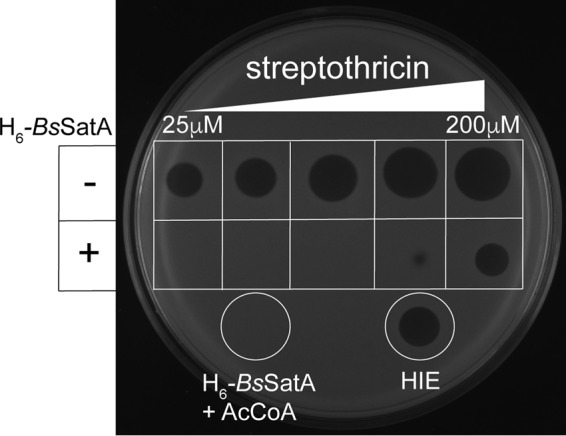
SatA detoxifies streptothricin over a range of concentrations. Soft agar (0.5%, wt/vol) seeded with a satA+ strain was layered on rich medium agar plates. Various concentrations of streptothricin were spotted. In the top row, in each area, 5-μl volumes of streptothricin at (from left to right) 25 μM, 50 μM, 100 μM, 150 μM, and 200 μM were spotted. In the bottom row, the same concentration of streptothricin with 3 μM H6-BsSatA was spotted. Zones of inhibition were not observed when H6-BsSatA was added to reaction mixtures containing 25 μM, 50 μM, or 100 μM streptothricin. However, heat-inactivated enzyme (HIE) did not detoxify streptothricin (50 μM, white circle on right). A sample from a reaction mixture containing H6-BsSatA and AcCoA but devoid of streptothricin was spotted as a negative control and no zone of inhibition was observed (white circle on left).
SatA protein from either B. subtilis or B. anthracis is necessary and sufficient to confer streptothricin resistance.
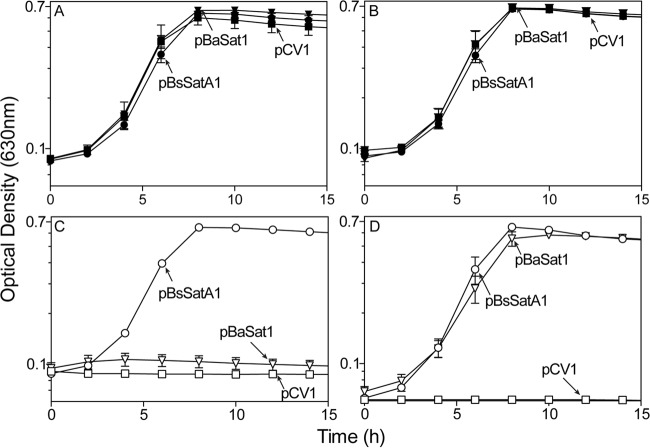
To determine whether or not BsSatA function was necessary and sufficient to detoxify streptothricin, we expressed the B. subtilis satA+ allele in Salmonella enterica and assessed streptothricin resistance. In the presence of streptothricin, a S. enterica strain expressing satA+ in trans (strain JE22334) grew, while the strain carrying the cloning vector (strain JE22263) did not (Fig. 5C).
FIG 5.
BsSatA and BaSatA impart streptothricin resistance to Salmonella enterica. Strains carrying a plasmid encoding BsSatA (pBsSATA1, circles), BaSatA (pBaSAT1, triangles) and those carrying the cloning vector (pCV1, squares) were grown in minimal medium, either lacking streptothricin (closed symbols; panels A and B) or challenged with 10 μM streptothricin (open symbols; panels C and D). l-(+)-Arabinose was present in the medium at 500 μM as inducer in panels B and D. The following strains were analyzed: JE22263 (ΔmetE2702 ara-9/pCV1), JE22334 (ΔmetE2702 ara-9/pBsSATA1), and JE24022 (ΔmetE2702 ara-9/pBaSAT1). Error bars are present, although in many cases they are too small to be visible.
Recently, a structure of a putative streptothricin acetyltransferase from Bacillus anthracis Ames (BaSatA) was solved (PDB 3PP9), but its function was not investigated. Given the homology between BsSatA and BaSatA we tested whether or not this protein could also confer streptothricin resistance to strains of S. enterica. The satA (GFG-5714) gene from Bacillus anthracis Ames was expressed in a S. enterica strain and tested for resistance to streptothricin. When challenged with 10 μM streptothricin, the strain carrying the B. anthracis satA+ allele (strain JE24022) failed to grow (Fig. 5C). B. anthracis satA+ allele was cloned into an arabinose-inducible promoter to be able to turn on its expression by the addition of inducer to the medium. Upon the addition of 500 μM l-(+)-arabinose, the strain expressing the B. anthracis satA+ allele (strain JE24022) became resistant to streptothricin (Fig. 5D). This indicated that SatA (formerly GFG-5714) from B. anthracis was a bona fide streptothricin acetyltransferase, and that the enzyme acetyltransferase activity associated with BsSatA and BaSat was necessary and sufficient to detoxify streptothricin.
Biologically active BsSatA is a dimer.
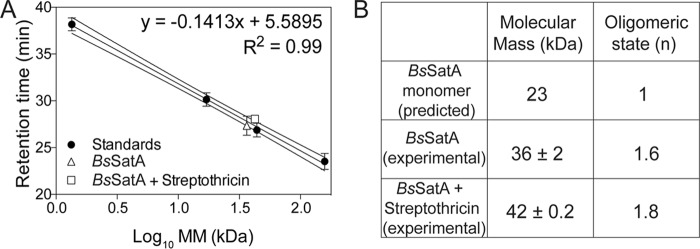
The oligomeric state of H6-BsSatA was analyzed by gel permeation using fast protein liquid chromatography (FPLC). Commercially available molecular mass standards were used to generate a plot of molecular mass (kDa) as a function of retention time. Under the conditions tested, H6-BsSatA had a retention time consistent with an ∼36 kDa protein (Fig. 6). Since the hypothetical molecular mass of H6-BsSatA is approximately 23 kDa, it was inferred that BsSatA behaved as a dimer in solution. A dimer would be consistent with the reported oligomeric state of the streptothricin acetyltransferase from Bacillus anthracis Ames, as shown by its three-dimensional crystal structure (PDB 3PP9).
FIG 6.
BsSatA is a dimer in solution. (A) H6-BsSatA retention data for gel permeation analysis. The retention time of H6-BsSatA was compared to times of a mixture of standards used to generate a calibration curve. Standards used were γ-globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa), and vitamin B12 (1.35 kDa), which were purchased from Bio-Rad Laboratories. (B) Calculated molecular masses and oligomers for BsSatA and BsSatA with streptothricin, indicating that BsSatA was likely a dimer in solution.
Kinetic parameters of BsSatA-dependent streptothricin acetylation.
We determined the kinetic parameters of BsSatA using a continuous spectrophotometric assay for the detection of free sulfhydryl groups (32, 33) with some modifications (34). The low Km of BsSatA for streptothricin (1 μM, Table 1) was consistent with that for StaT from Streptomyces lavendulae (2.3 μM) (17). Notably, the Km of BsSatA for AcCoA (107 μM) was slightly higher than that for StaT (69 μM) (17) (Table 1).
TABLE 1.
Kinetic and binding parameters
| Substrate | Km (μM) | kcat (s−1) | kcat/Km (M−1 s−1) | Kd (M) |
|---|---|---|---|---|
| Streptothricin | 1 ± 0.2 | 5 ± 0.6 | 5 × 106 | 1.28 × 10−6 |
| AcCoA | 107 ± 40 | 9 ± 2 | 8 × 104 | NDa |
ND, not determined.
Affinity of BsSatA for its substrates and inferred order of binding.
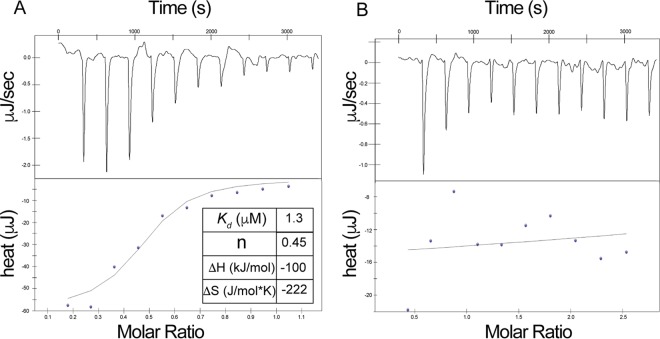
We used isothermal titration calorimetry (ITC) to quantify the affinity of BsSatA for AcCoA and streptothricin. Streptothricin bound to BsSatA with a Kd (dissociation constant) of 1.3 μM (Fig. 7A; Table 1), indicating a high affinity for that substrate. The high affinity of BsSatA for streptothricin was consistent with the need to detoxify this antimicrobial as quickly as possible to avert protein synthesis inhibition. Notably, in the absence of streptothricin BsSatA did not bind to AcCoA (Fig. 7B). On the basis of this information, we surmised that BsSatA undergoes a conformational change upon streptothricin binding. Such a change appears to be needed for AcCoA to bind to the enzyme. It is possible that this binding mechanism provides a way for the cell to conserve AcCoA until the toxic compound is detected.
FIG 7.
Binding of substrates to BsSatA. ITC was used to analyze the binding of streptothricin and AcCoA to BsSatA. (A and B) Binding isotherms for streptothricin or AcCoA to BsSatA. The top panels are the raw data from consecutive injections of ligand represented as heat release over injection time. The bottom panels are binding isotherms obtained by integration of the raw data. Data were acquired with a TA Instruments NanoITC, and the data were analyzed with NanoAnalyze 1.2 software. The streptothricin titration (A) displayed saturation, while AcCoA titration (B) showed no binding of AcCoA to BsSatA.
Conclusion.
The SatA (formerly YyaR) protein of B. subtilis, is a member of the Gcn5-related acetyltransferase superfamily of proteins (PF00583) that acetylates streptothricin, at the expense of AcCoA. BsSatA acetylates streptothricin once, blocking the cytotoxic effects of the antibiotic. BsSatA activity is necessary and sufficient to confer resistance to streptothricin. The high affinity of BsSatA for streptothricin reflects the need to detoxify this antibiotic quickly and efficiently. Collectively, the data reported herein support the conclusion that B. subtilis SatA is a bona fide streptothricin acetyltransferase. On the basis of our findings, we propose changing the name of the yyaR gene and its gene product to satA and SatA, respectively, for streptothricin acetyltransferase A. By extension, we suggest that the B. anthracis gene GFG-5714 be renamed satA.
MATERIALS AND METHODS
Culture media, growth conditions, and chemicals.
Lysogeny broth (LB; Difco) (35) was used as rich medium for Bacillus subtilis strains. Spizizen minimal medium supplemented with tryptophan (20 μg/ml) was used to grow B. subtilis under defined conditions (36). Nutrient broth (NB; Difco) supplemented with NaCl (85 mM) was used as rich medium for Salmonella enterica strains. No-carbon E (NCE) minimal medium supplemented with trace minerals (37), l-methionine (100 μM), and magnesium sulfate (1 mM) was used to grow S. enterica strains (38). Glycerol (22 mM) was used as the sole carbon and energy source in all cases. All strains were grown at 37°C with shaking (180 rpm). When added to the medium, antibiotics were present at the following concentrations: ampicillin (Fisher Scientific), 100 μg ml−1; and erythromycin (Sigma-Aldrich), 1 μg ml−1. HEPES buffer, tris(2-carboxyethyl)phosphine hydrochloride (TCEP), isopropyl-β-d-1-thiogalactopyranoside (IPTG), and dithiothreitol (DTT) were purchased from Gold BioTechnology; streptothricin sulfate was purchased from G Biosciences.
Bacterial strains.
B. subtilis strains used in these studies were derivatives of B. subtilis subsp. subtilis strain 168. Strains JE9142 (trpC2) and JE20317 (trpC2 satA1::erm+) were purchased from the Bacillus Genetic Stock Center (Columbus, OH). Other B. subtilis strains were constructed during the course of this work. S. enterica strains were derivatives of S. enterica subsp. enterica serovar Typhimurium strain LT2 and were constructed during the course of this work. All strains and plasmids used are listed in Table 2.
TABLE 2.
List of strains and plasmids used in this study
| Strain or plasmid | Genotype | Reference or sourcea |
|---|---|---|
| B. subtilis strains | ||
| JE9142 | trpC2 | BGSC (1A1)b |
| JE20317 | trpC2 satA1::erm+ | BKE40740b (44) |
| JE23688 | trpC2 amyE::pBS1C-lac | |
| JE23689 | trpC2 satA1::erm amyE::pBS1ClacZ | |
| JE23690 | trpC2 amyE:: PsatA-satA+ | |
| JE23691 | trpC2 satA1::erm amyE::PsatA-satA+ | |
| S. enterica strains | ||
| JE22263 | ΔmetE2702 ara-9/pCV1 | |
| JE22334 | ΔmetE2702 ara-9/pBsSATA1 | |
| JE24022 | ΔmetE2702 ara-9/pBaSAT1 | |
| E. coli strains | ||
| C41(λDE3) | ompT hsdS(rB− mB−) gal (λDE3) | 43 |
| Plasmids | ||
| pCV1 | ParaBAD arabinose-inducible cloning vector | 40 |
| pBsSATA1 | B. subtilis satA+ cloned into the BspQI sites of pCV1 | |
| pBaSAT1 | B. anthracis satA+ cloned into the BspQI sites of pCV1 | |
| pTEV16 | TEV protease-cleavable, N-terminal His6 tag overexpression vector | 40 |
| pBsSATA2 | B. subtilis satA+ cloned into the BspQI sites of pTEV16 | |
| pTEV5 | TEV protease-cleavable, N-terminal His6 tag overexpression vector | 42 |
| pBaSAT2 | B. anthracis sat+ cloned into EcoRI and SmaI sites of pTEV5 | GenScript |
| pBS1ClacZ | Integration and promoter-less lacZ-reporter vector. The plasmid contains ∼900 bp upstream and ∼500 bp downstream of amyE homology for integration into the chromosomal amyE locus | 41 |
| pBsSATA3 | B. subtilis satA+ with 265 bp upstream of the initiation codon cloned into the EcoRI and SpeI sites of pBS1ClacZ |
Unless otherwise stated, strains and plasmids were constructed during the course of this work.
B. subtilis strain number used by the BGSC (Bacillus Genetic Stock Center, The Ohio State University).
Plasmid construction.
All primers used in this study were synthesized by Integrated DNA Technologies (Coralville, IA) and are listed in Table 3. The satA (yyaR) gene was amplified from B. subtilis subsp. subtilis 168 genomic DNA using Phusion polymerase (TA 57°C) following manufacturer's instructions, and cloned into vectors using the BspQI (New England BioLabs) cloning method (39). The satA+ allele was cloned into both pCV1 (40), an l-(+)-arabinose-inducible complementation vector, and pTEV16 (40), an IPTG-inducible overproduction vector that fused a H6 tag to the N terminus of the protein. For the B. subtilis integration vector pBS1ClacZ (41), the satA+ allele and 265 bp upstream of it were amplified from B. subtilis 168 genomic DNA and cloned into the EcoRI and SpeI (ThermoFisher Scientific) restriction sites of pBS1ClacZ. The B. anthracis Ames satA (formerly GFG-5714) gene was codon-optimized for E. coli and synthesized by GenScript Biotech Corporation (China) into the EcoRI and SmaI sites of plasmid pTEV5 (42), an IPTG-inducible overproduction vector that fused a H6 tag to the N terminus of the protein. For cloning into pCV1, the B. anthracis satA gene was amplified from pBaSAT2 and cloned using the BspQI cloning method as described above. The sequences of vectors were verified (Georgia Genomics Facility, University of Georgia, Athens, GA).
TABLE 3.
List of primers used in this study
| Primer | Sequence |
|---|---|
| pBAD BsSatA Forward | NNGCTCTTCNTTCATGATCATGAAAATGACTC |
| pBAD BsSatA Reverse | NNGCTCTTCNTTATTAAAATTTATAGTACC |
| pTEV16 BsSatA Forward | NNGCTCTTCNAGCATGATCATGAAAATGACTC |
| pBAD BaSat Forward | NNGCTCTTCNTTCATGAGCCTGCTGATCCG |
| pAD BaSat Reverse | NNGCTCTTCNTTATTAGCTGTCAAAGTGCAGATACC |
| BsSatA −265 EcoRI_F pLac | GTTGTTGAATTCGGGTTGTGGGCGAGTTGAAGG |
| BsSatA STOP SpeI_R plac | GTTGTTACTAGTTAAAATTTATAGTACCAAAAAATCGC |
Growth analyses.
S. enterica strains were grown at 37°C as described above and challenged with streptothricin (10 μM). B. subtilis strains were also grown at 37°C in Spizizen glycerol minimal medium (36) and also challenged with 10 μM streptothricin.
All growth analyses were performed in 96-well microtiter dishes, with each strain grown under identical conditions in triplicate. Each well contained 198 μl of minimal medium inoculated with 1% (vol/vol) of an overnight culture for S. enterica strains, or 3% (vol/vol) of an overnight culture for B. subtilis strains. All starter cultures were grown for 14 to 16 h on rich medium at 37°C in an innova43 (New Brunswick Scientific) gyratory incubator shaking at 180 rpm. Cell density was monitored at 630 nm using a computer-controlled BioTek ELx808 absorbance plate reader (BioTek Instruments). Readings were acquired every 30 min with continuous shaking. Data were analyzed using the GraphPad Prism 4 software package (GraphPad Software).
Purification of His6-tagged B. subtilis SatA protein.
H6-BsSatA was overproduced in E. coli strain C41(λDE3) cells (43) carrying plasmid pBsSATA2 (satA+). Cells were grown in 1.5 liters of Terrific Broth (Cold Spring Harbor Laboratory protocols) at 37°C to an optical density at 600 nm (OD600) of 0.3 to 0.4. At this cell density, isopropyl-β-d-1-thiogalactopyranoside (IPTG; Sigma) was added to a final concentration of 500 μM and cultures were incubated overnight at 15°C in a 2.8-liter flask in an Innova 44 (New Brunswick Scientific) gyratory shaker at 160 rpm. Cells were harvested by centrifugation at 6,000 × g for 15 min, and the cell paste was frozen at −80°C until use. Cell paste was resuspended in binding buffer containing HEPES buffer (50 mM, pH 7.5 at 4°C) containing NaCl (500 mM) and imidazole (20 mM), 1 μg ml−1 lysozyme, 25 μg ml−1 DNase, and 0.5 mM phenylmethylsulfonyl fluoride (PMSF; Fisher Scientific). Cells were broken by sonication with a 550 sonic dismembrator (Fisher Scientific) for 4 cycles at an amplitude setting of 60 for 30 s on ice for 2 s on, followed by 2 s off. Cellular debris was removed by centrifugation at 40,000 × g for 45 min using an Avanti J-25I floor centrifuge with a JA-25.25 rotor (Beckman Coulter).
The clarified cell extract was loaded onto a 5-ml nickel-nitrilotriacetic acid (Ni-NTA) affinity column (HisPur; ThermoFisher Scientific) and purified as described elsewhere (23). Briefly, the column was washed with 10 bed volumes of binding buffer (HEPES, 50 mM, pH 7.5) containing NaCl (500 mM) and imidazole (20 mM), followed by six bed volumes of wash buffer (HEPES 50 mM, pH 7.5) containing NaCl (500 mM) and imidazole (40 mM). Protein was eluted off the column with six bed volumes of elution buffer (HEPES 50 mM, pH 7.5) containing NaCl (500 mM) and a high imidazole concentration (500 mM). Fractions of eluted protein were pooled and dialyzed against HEPES buffer (50 mM, pH 7.5 at 4°C) with decreasing amounts of NaCl down to 150 mM. Purified protein was flash frozen in liquid nitrogen and stored at −80°C until use. The protein was 81% homogeneous (Fig. S4).
In vitro acetylation assay and thin-layer chromatography.
Reaction mixtures (25 μl) containing HEPES buffer (50 mM, pH 7 at 24°C), TCEP (1 mM), [1-14C]-AcCoA (20 μM; specific radioactivity = 56.8 mCi/mmol), BsSatA (3 μM; extinction coefficient = 4.58 × 104 liter · mol−1 · cm−1 obtained through http://web.expasy.org/protparam/), and streptothricin (150 μM) were incubated at 37°C for 1 h. Reaction mixtures lacking enzyme or substrate were used as controls. Samples from reaction mixtures were spotted onto a Whatman PE SIL G/UV silica gel thin-layer chromatography (TLC) plate (Whatman Ltd.), and developed in a preequilibrated chamber with a mobile phase of 15% (wt/vol) KH2PO4. TLC plates were developed for 1 to 2 h, dried, and radioactivity distribution was visualized using a phosphor imager after 16 h of exposure. A Typhoon Trio+ Variable Mode imager (GE Health Life Sciences) with ImageQuant v5.2 software was used to image the phosphor screen.
Streptothricin resistance bioassay.
The B. subtilis satA+ strain (JE9142) was grown overnight at 37°C in rich medium for 14 to 16 h. A sample (100 μl) of starter culture was added to 3 ml of soft agar and poured onto a rich medium plate. Reaction mixtures containing HEPES buffer (50 mM, pH 7), TCEP (1 mM), AcCoA (400 μM), and various concentrations of streptothricin (5 to 200 μM) were incubated at 37°C for 1 h with or without BsSatA (3 μM). A sample (5 μl) of each reaction mixture was spotted onto satA+ (JE9142)-containing plates, which were incubated overnight at 37°C. Sensitivity to streptothricin was determined by the diameter of zones of inhibition. As a negative control, BsSatA was heated to 95°C for 5 min and added to a reaction mixture containing streptothricin (50 μM).
Gel permeation FPLC analysis.
H6-BsSatA protein (125 μg) was injected onto a Superose 12 10/300 gel filtration column connected to an ÄKTApurifier fast protein liquid chromatography (FPLC) system. The column was equilibrated with HEPES buffer (50 mM, pH 7.5) containing NaCl (150 mM). A mixture of standards was applied to the column to generate a calibration curve. A flow rate of 0.5 ml · min−1 was used to develop the column, and elution was monitored at 280 nm. Data analysis was performed using UNICORN 4.11 software (GE Healthcare Life Sciences).
Determination of kinetic parameters.
The kinetic parameters for the BsSatA reaction were determined using a continuous spectrophotometric assay that employed 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB; Ellman's reagent) to measure the release of coenzyme A (CoA) from AcCoA at 412 nm (32–34). Briefly, reaction mixtures (100 μl) contained HEPES (50 mM, pH 7), DTNB (0.3 mM), and H6-BsSatA (0.5 μg), saturating conditions on one of the substrates and varying concentrations of the second substrate. Reaction mixtures containing H6-BsSatA and AcCoA but no streptothricin were used as control to correct for background. When streptothricin was added to the mixture, AcCoA levels were kept at 100 μM and streptothricin was varied from 100 nM to 2.5 μM. When the concentration of AcCoA varied, the streptothricin level was kept at 10 μM, and acetyl-CoA was varied from 25 μM to 200 μM.
Reaction mixtures were incubated at 25°C and readings were taken every 5 s over 5 min using a SpectraMax Plus 384 microplate spectrophotometer (Molecular Devices). Reactions were performed in technical triplicates and biological duplicates. The molar extinction coefficient used for the concentration of the TNB2- anion was 14,150 M−1 · cm−1 (Sigma-Aldrich). The initial velocity versus substrate concentration was graphed using Prism 6 (GraphPad) software. Data were fitted to the Michaelis-Menten equation to determine the apparent Km and kcat for BsSatA. Catalytic efficiency (kcat/Km) was also calculated.
To determine the level of acetylation of streptothricin, reaction mixtures were prepared as described above with AcCoA (500 μM) and various streptothricin concentrations. Reaction mixtures were incubated for 1 h and the final absorbance at 412 nm was used to determine the amount of AcCoA consumed as a function of the concentration of streptothricin added to the reaction mixtures.
Isothermal titration calorimetry.
H6-BsSatA was dialyzed extensively against HEPES buffer (25 mM, pH 7.5; 150 mM NaCl), and binding assays were performed using a Nano ITC isothermal titration calorimeter (TA Instruments). AcCoA or streptothricin was used as titrant; these compounds were solubilized in the final protein dialysate. Protein was present at 40 μM in the sample cell and streptothricin (250 μM) or AcCoA (650 μM) was present in the injection syringe. All samples were degassed for 20 min at 25°C before use. Injections (2.4 μl each) were made every 5 min into the chamber containing H6-BsSatA under constant stirring at 350 rpm. ITC experiments were performed at 25°C. ITC data were analyzed using NanoAnalyze software (TA Instruments).
Supplementary Material
ACKNOWLEDGMENTS
The authors do not have a conflict of interest to declare.
This work was supported by HHS grant R01 GM062203 from the National Institutes of Health to J.C.E.-S.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01590-17.
REFERENCES
- 1.Handelsman J, Rondon MR, Brady SF, Clardy J, Goodman RM. 1998. Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chem Biol 5:R245–R249. doi: 10.1016/S1074-5521(98)90108-9. [DOI] [PubMed] [Google Scholar]
- 2.Baltz RH. 2007. Antimicrobials from actinomycetes: back to the future. Microbe 2:125–131. [Google Scholar]
- 3.Ananda Baskaran S, Venkitanarayanan K. 2014. Plant-derived antimicrobials reduce E. coli O157:H7 virulence factors critical for colonization in cattle gastrointestinal tract in vitro. Biomed Res Int 2014:212395. doi: 10.1155/2014/212395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molaei A, Lakzian A, Haghnia G, Astaraei A, Rasouli-Sadaghiani M, Teresa Ceccherini M, Datta R. 2017. Assessment of some cultural experimental methods to study the effects of antibiotics on microbial activities in a soil: an incubation study. PLoS One 12:e0180663. doi: 10.1371/journal.pone.0180663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waksman SA, Woodruff HB. 1942. Streptothricin, a new selective bacteriostatic and bactericidal agent active against Gram-negative bacteria. Proc Soc Exp Biol Med 49:207–210. doi: 10.3181/00379727-49-13515. [DOI] [Google Scholar]
- 6.Johnson AW, Westley JW. 1962. Streptothricin group of antibiotics. Part I. The general structural pattern. J Chem Soc 0:1642–1655. doi: 10.1039/JR9620001642. [DOI] [Google Scholar]
- 7.Van Tamelen EE, Dyer JR, Whaley HA, Carter HE, Whitfield GB Jr. 1961. Constitution of the streptolin-streptothricin group of Streptomyces antibiotics. J Am Chem Soc 83:4295–4296. doi: 10.1021/ja01481a051. [DOI] [Google Scholar]
- 8.Ji Z, Wei S, Zhang J, Wu W, Wang M. 2008. Identification of streptothricin class antibiotics in the early-stage of antibiotics screening by electrospray ionization mass spectrometry. J Antibiot (Tokyo) 61:660–667. doi: 10.1038/ja.2008.93. [DOI] [PubMed] [Google Scholar]
- 9.Robinson HJ, Graessle OE, Smith DG. 1944. Studies on the toxicity and activity of streptothricin. Science 99:540–542. doi: 10.1126/science.99.2583.540. [DOI] [PubMed] [Google Scholar]
- 10.Haupt I, Hubener R, Thrum H. 1978. Streptothricin F, an inhibitor of protein synthesis with miscoding activity. J Antibiot (Tokyo) 31:1137–1142. doi: 10.7164/antibiotics.31.1137. [DOI] [PubMed] [Google Scholar]
- 11.Jonah J, Rychlik I. 1983. Streptothricin F, p 238–247. In Hahn FE. (ed), Antibiotics IV: modes and mechanisms of microbial growth inhibitors, vol 6 Springer-Verlag, Berlin, Germany. [Google Scholar]
- 12.Haupt I, Jonak J, Rychlik I, Thrum H. 1980. Action of streptothricin F on ribosomal functions. J Antibiot (Tokyo) 33:636–641. doi: 10.7164/antibiotics.33.636. [DOI] [PubMed] [Google Scholar]
- 13.Seidel L, Haupt I. 1982. Isolation of streptothricin resistant mutants from E coli K12, strain A19. Z Allg Mikrobiol 22:133–137. (In German.) [DOI] [PubMed] [Google Scholar]
- 14.Hamano Y, Matsuura N, Kitamura M, Takagi H. 2006. A novel enzyme conferring streptothricin resistance alters the toxicity of streptothricin D from broad-spectrum to bacteria-specific. J Biol Chem 281:16842–16848. doi: 10.1074/jbc.M602294200. [DOI] [PubMed] [Google Scholar]
- 15.Keeratipibul S, Sugiyama M, Nomi R. 1983. Mechanism of resistance to streptothricin of a producing microorganism. Biotechnol Lett 5:441–446. doi: 10.1007/BF00132225. [DOI] [Google Scholar]
- 16.Jacob J, Evers S, Bischoff K, Carlier C, Courvalin P. 1994. Characterization of the sat4 gene encoding a streptothricin acetyltransferase in Campylobacter coli BE/G4. FEMS Microbiol Lett 120:13–17. doi: 10.1111/j.1574-6968.1994.tb07000.x. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T, Horinouchi S, Uozumi T, Beppu T. 1987. Purification and biochemical characterization of streptothricin acetyltransferase coded by the cloned streptothricin-resistance gene of Streptomyces lavendulae. J Antibiot (Tokyo) 40:1016–1022. doi: 10.7164/antibiotics.40.1016. [DOI] [PubMed] [Google Scholar]
- 18.Krugel H, Fiedler G, Haupt I, Sarfert E, Simon H. 1988. Analysis of the nourseothricin-resistance gene (nat) of Streptomyces noursei. Gene 62:209–217. doi: 10.1016/0378-1119(88)90559-8. [DOI] [PubMed] [Google Scholar]
- 19.Krugel H, Fiedler G, Smith C, Baumberg S. 1993. Sequence and transcriptional analysis of the nourseothricin acetyltransferase-encoding gene nat1 from Streptomyces noursei. Gene 127:127–131. doi: 10.1016/0378-1119(93)90627-F. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Moreno MA, Vallin C, Malpartida F. 1997. Streptothricin biosynthesis is catalyzed by enzymes related to nonribosomal peptide bond formation. J Bacteriol 179:6929–6936. doi: 10.1128/jb.179.22.6929-6936.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tietze E, Brevet J, Tschape H. 1987. Relationships among the streptothricin resistance transposons Tn1825 and Tn1826 and the trimethoprim resistance transposon Tn7. Plasmid 18:246–249. doi: 10.1016/0147-619X(87)90067-9. [DOI] [PubMed] [Google Scholar]
- 22.Starai VJ, Escalante-Semerena JC. 2004. Identification of the protein acetyltransferase (Pat) enzyme that acetylates acetyl-CoA synthetase in Salmonella enterica. J Mol Biol 340:1005–1012. doi: 10.1016/j.jmb.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Hentchel KL, Escalante-Semerena JC. 2015. In Salmonella enterica, the Gcn5-related acetyltransferase MddA (formerly YncA) acetylates methionine sulfoximine and methionine sulfone, blocking their toxic effects. J Bacteriol 197:314–325. doi: 10.1128/JB.02311-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikeuchi Y, Kitahara K, Suzuki T. 2008. The RNA acetyltransferase driven by ATP hydrolysis synthesizes N4-acetylcytidine of tRNA anticodon. EMBO J 27:2194–2203. doi: 10.1038/emboj.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheverton AM, Gollan B, Przydacz M, Wong CT, Mylona A, Hare SA, Helaine S. 2016. A salmonella toxin promotes persister formation through acetylation of tRNA. Mol Cell 63:86–96. doi: 10.1016/j.molcel.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright GD, Ladak P. 1997. Overexpression and characterization of the chromosomal aminoglycoside 6′-N-acetyltransferase from Enterococcus faecium. Antimicrob Agents Chemother 41:956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner JG, Grundy FJ, Henkin TM, Escalante-Semerena JC. 2006. Control of acetyl-coenzyme A synthetase (AcsA) activity by acetylation/deacetylation without NAD+ involvement in Bacillus subtilis. J Bacteriol 188:5460–5468. doi: 10.1128/JB.00215-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolridge DP, Martinez JD, Stringer DE, Gerner EW. 1999. Characterization of a novel spermidine/spermine acetyltransferase, BltD, from Bacillus subtilis. Biochem J 340:753–758. doi: 10.1042/bj3400753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forouhar F, Lee IS, Vujcic J, Vujcic S, Shen J, Vorobiev SM, Xiao R, Acton TB, Montelione GT, Porter CW, Tong L. 2005. Structural and functional evidence for Bacillus subtilis PaiA as a novel N1-spermidine/spermine acetyltransferase. J Biol Chem 280:40328–40336. doi: 10.1074/jbc.M505332200. [DOI] [PubMed] [Google Scholar]
- 30.Jonak J, Rychlik I. 1983. Streptothricin F, p 238–247. In Hahn FE. (ed), Antibiotics IV: modes and mechanisms of microbial growth inhibitors, vol 6 Springer-Verlag, Berlin, Germany. [Google Scholar]
- 31.Zähringer U, Voigt W, Seltmann G. 1993. Nourseothricin (streptothricin) inactivated by a plasmid pIE636 encoded acetyl transferase of Escherichia coli: location of the acetyl group. FEMS Microbiol Lett 110:331–334. doi: 10.1111/j.1574-6968.1993.tb06344.x. [DOI] [PubMed] [Google Scholar]
- 32.Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 33.Eyer P, Worek F, Kiderlen D, Sinko G, Stuglin A, Simeon-Rudolf V, Reiner E. 2003. Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal Biochem 312:224–227. doi: 10.1016/S0003-2697(02)00506-7. [DOI] [PubMed] [Google Scholar]
- 34.Thao S, Escalante-Semerena JC. 2011. Biochemical and thermodynamic analyses of Salmonella enterica Pat, a multidomain, multimeric N(epsilon)-lysine acetyltransferase involved in carbon and energy metabolism. mBio 2:e00216-11. doi: 10.1128/mBio.00216-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertani G. 2004. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol 186:595–600. doi: 10.1128/JB.186.3.595-600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spizizen J. 1958. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci U S A 44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balch WE, Wolfe RS. 1976. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl Environ Microbiol 32:781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berkowitz D, Hushon JM, Whitfield HJ Jr, Roth J, Ames BN. 1968. Procedure for identifying nonsense mutations. J Bacteriol 96:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galloway NR, Toutkoushian H, Nune M, Bose N, Momany C. 2013. Rapid cloning for protein crystallography using type IIS restriction enzymes. Crys Growth Des 13:2833–2839. doi: 10.1021/cg400171z. [DOI] [Google Scholar]
- 40.VanDrisse CM, Escalante-Semerena JC. 2016. New high-cloning-efficiency vectors for complementation studies and recombinant protein overproduction in Escherichia coli and Salmonella enterica. Plasmid 86:1–6. doi: 10.1016/j.plasmid.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radeck J, Kraft K, Bartels J, Cikovic T, Durr F, Emenegger J, Kelterborn S, Sauer C, Fritz G, Gebhard S, Mascher T. 2013. The Bacillus BioBrick Box: generation and evaluation of essential genetic building blocks for standardized work with Bacillus subtilis. J Biol Eng 7:29. doi: 10.1186/1754-1611-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocco CJ, Dennison KL, Klenchin VA, Rayment I, Escalante-Semerena JC. 2008. Construction and use of new cloning vectors for the rapid isolation of recombinant proteins from Escherichia coli. Plasmid 59:231–237. doi: 10.1016/j.plasmid.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miroux B, Walker JE. 1996. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol 260:289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- 44.Koo BM, Kritikos G, Farelli JD, Todor H, Tong K, Kimsey H, Wapinski I, Galardini M, Cabal A, Peters JM, Hachmann AB, Rudner DZ, Allen KN, Typas A, Gross CA. 2017. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Syst 4:291–305.e7. doi: 10.1016/j.cels.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.