ABSTRACT
In this study, the effect of individual lecithin phospholipids on the antimicrobial properties of eugenol against Escherichia coli C600 was investigated. We tested five major phospholipids common in soy or egg lecithin (1,2-dihexadecanoyl-sn-glycero-3-phosphocholine [DPPC], 1,2-dioctadecanoyl-sn-glycero-3-phosphocholine [DSPC], 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine [DPPE], 1,2-dihexadecanoyl-sn-glycero-3-phosphate [sodium salt] [DPPA], and 1,2-dihexadecanoyl-sn-glycero-3-phospho-l-serine [DPPS]) and one synthetic cationic phospholipid (1,2-dioctadecanoyl-sn-glycero-3-ethylphosphocholine [18:0 EPC]). Among the six phospholipids, DPPC, DSPC, DPPE, DPPA, and the cationic 18:0 EPC showed critical synergistic concentrations that significantly improved the inactivation effect of eugenol against E. coli after 30 min of exposure. At the critical synergistic concentration, an additional ca. 0.4 to 1.9 log reduction (ca. 0.66 to 2.17 log CFU/ml reduction) in the microbial population was observed compared to eugenol-only (control) treatments (ca. 0.25 log reduction). In all cases, increasing the phospholipid amount above the critical synergistic concentration (which was different for each phospholipid) resulted in antimicrobial properties similar to those seen with the eugenol-only (control) treatments. DPPS did not affect the antimicrobial properties of eugenol at the tested concentrations. The critical synergistic concentration of phospholipids was correlated with their critical micelle concentrations (CMC).
IMPORTANCE Essential oils (EOs) are naturally occurring antimicrobials, with limited use in food due to their hydrophobicity and strong aroma. Lecithin is used as a natural emulsifier to stabilize EOs in aqueous systems. We previously demonstrated that, within a narrow critical-concentration window, lecithin can synergistically enhance the antimicrobial properties of eugenol. Since lecithin is a mixture of different phospholipids, we aimed to identify which phospholipids are crucial for the observed synergistic effect. This research studied the bioactivity of lecithin phospholipids, contributing to a rational design in using lecithin to effectively control foodborne pathogens in foods.
KEYWORDS: E. coli, eugenol, lecithin, monoterpenes, phospholipids
INTRODUCTION
In the United States alone, foodborne diseases cause ca. 48 million illnesses, ca. 128,000 hospitalizations, and ca. 3,000 death every year (1). Among all the foodborne disease agents, the World Health Organization (WHO) reports that pathogenic Escherichia coli significantly contributes to this burden (2). The Centers for Disease Control and Prevention (CDC) estimated the occurrence of 265,000 Shiga toxin-producing E. coli (STEC) infections, 3,600 U.S. hospitalizations, and 30 deaths every year (3).
Essential oils (EOs) extracted from plants (e.g., eugenol, carvacrol, thymol) continue to gain attention as natural antimicrobials due to their “green” and “clean label” image (4). EOs are generally recognized as safe (GRAS) but have found limited use in food systems due to their hydrophobicity and strong aroma. Many studies showed that, after encapsulation of EOs in emulsifier micelles or liposomes, both the stability and the antimicrobial properties of the EO improve (4–6). Donsì and coworkers (8, 9) found an increase in d-limonene antimicrobial activity against Lactobacillus delbrueckii and Saccharomyces cerevisiae under conditions of encapsulation in the emulsifiers Tween 20 and glycerol monooleate. Lecithin has been widely used in foods (e.g., infant formula and chocolate) as a natural emulsifier (7) to improve physical stability. However, previous reports showed that the antimicrobial properties of EO against E. coli were not enhanced when EOs were combined with lecithin (8, 9) or observed that water-insoluble surfactants (including lecithin) in nanoemulsions diminished the antimicrobial efficacy of EOs (10). In contrast, we previously reported that the antimicrobial properties of eugenol were significantly improved when lecithin was added at a critical low concentration (11). Nanoscale (<50-nm) aggregates were observed when eugenol was mixed with critical synergistic concentration of lecithin, and it was suggested that electrostatic interactions between charged phospholipids and the negatively charged bacterial cell membrane were responsible for the improved antimicrobial properties.
Lecithin is a mixture of various phospholipids, including phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidic acid (PA), and phosphatidylserine (PS) (7, 12). Due to the amphiphilic nature of these phospholipids, lamellar aggregates and other structures (e.g., hexagonally arranged rods and tubes) have been reported to form depending on concentration and temperature (13–15). Lecithin phospholipids differ in charged head groups (Table 1), lipid chain length, and degree of saturation (16), resulting in distinct molecular conformations, critical micelle concentrations (CMC), and phase transition behaviors (15, 17). Moreover, phospholipids are major components of bacterial cell membranes (18), and previous reports showed that the interaction between the bacterial cell and the essential oil depended on the cell membrane phospholipid composition (19). Therefore, we hypothesize that not all phospholipids in lecithin are responsible for the enhanced antimicrobial activity of essential oil against bacteria. The aim of this study was to identify the effect of six single phospholipids on the antimicrobial effect of eugenol against E. coli C600, leading to a rational use of phospholipid-based natural antimicrobial systems.
TABLE 1.
Chemical structure, highest inactivation, critical synergistic concentrations, and critical micelle concentrations of tested phospholipidsa
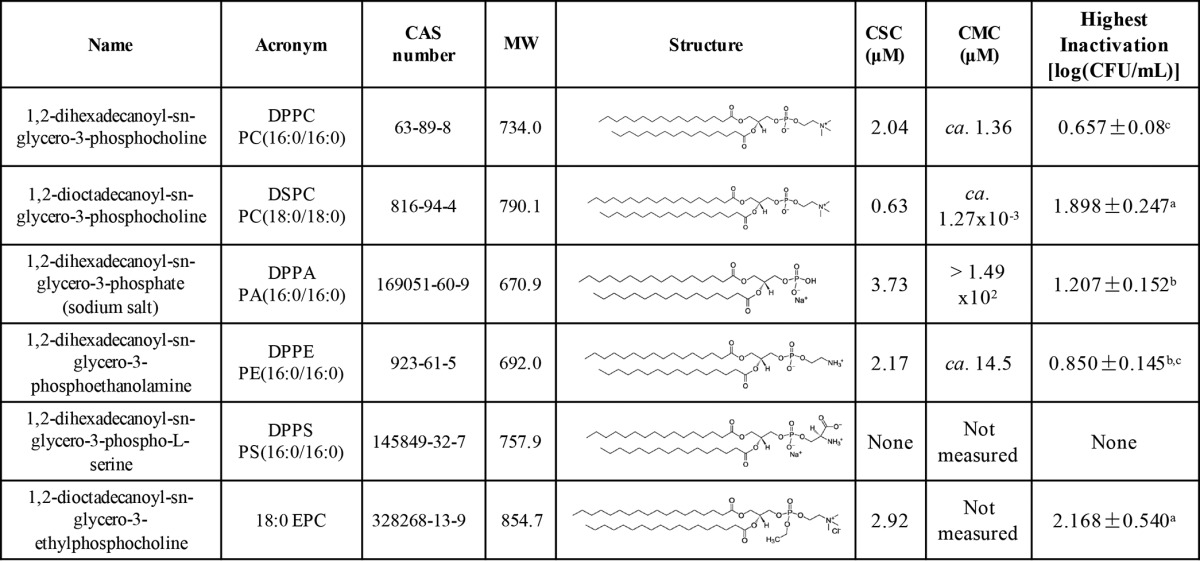
CAS, Chemical Abstracts Service; CMC, critical micelle concentration; CSC, critical synergistic concentration; MW, molecular weight. Highest inactivation is represented as means ± SDs (n = 3). Values followed by the same superscript letter are not significantly different using LSD analysis (α ≤ 0.05).
RESULTS
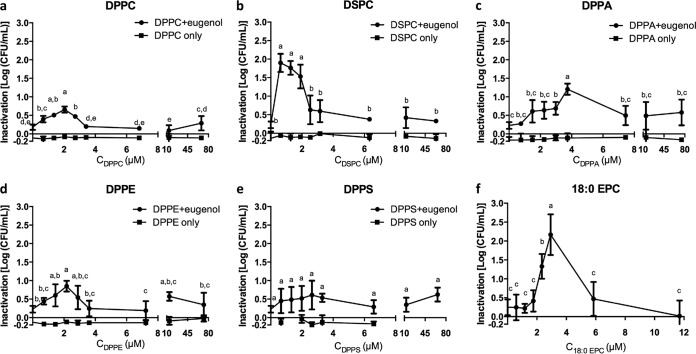
The inactivation of E. coli C600 after a 30-min exposure to a buffer system containing 0.043% (vol/vol) eugenol without phospholipid (control) or with various concentrations of each phospholipid is shown in Fig. 1a to e for 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dioctadecanoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-dihexadecanoyl-sn-glycero-3-phosphate (sodium salt) (DPPA), 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (DPPE), and 1,2-dihexadecanoyl-sn-glycero-3-phospho-l-serine (DPPS), respectively. Four phospholipids (DPPC, DSPC, DPPA, and DPPE) showed similar patterns of bioactivity; i.e., adding the phospholipid to a specific low critical concentration improved the bactericidal effect of eugenol. Beyond this critical synergistic concentration, the synergistic effect was diminished and the microbial inactivation was significantly reduced to levels not significantly different from those seen with eugenol-only control samples. DPPS was the only phospholipid that did not exhibit a critical concentration. When DPPS was added at concentrations in a 3 log range (from 0.66 μM to 66.97 μM), the inactivation effect of eugenol against E. coli was not significantly different from that seen with samples containing eugenol only (P = 0.576).
FIG 1.
Inactivation of E. coli C600 after 30 min of exposure to a constant eugenol concentration (0.043% [vol/vol]) and increasing levels of phospholipids (filled circles) and phospholipid-only controls (filled squares). All controls showed no inactivation effect. Means data followed by different letters are significantly different as determined using LSD analysis (α = <0.05). Error bars represent standard deviations of the means of results from three independent replicates.
The critical synergistic concentrations for bioactivity differed among the phospholipids. Adding 1.36 μM to 2.72 μM DPPC to the system significantly (P < 0.05) improved the antimicrobial effect of eugenol against E. coli C600 by an additional ca. 0.5 log cycle reduction. Similarly, an additional ca. 1.5 log cycle reduction in E. coli C600 (P < 0.05) was obtained with 0.63 to 1.90 μM DSPC added. For DPPE, the maximum additional microbial inactivation was reached when 2.17 μM DPPE was introduced to the system, and an additional 1 log cycle reduction was observed when 3.73 μM DPPA was added. Experiments were also conducted using 1,2-dioctadecanoyl-sn-glycero-3-ethylphosphocholine (18:0 EPC), a cationic derivative of DSPC. Figure 1f shows that adding 18:0 EPC at below 2.34 μM or above 2.93 μM did not change the antimicrobial properties of eugenol. However, at the critical synergistic concentrations of 2.34 μM to 2.93 μM, an additional ca. 1.9 log cycle inactivation of E. coli at the same eugenol concentration was found after 30 min of exposure to eugenol. Phospholipid-only experiments (no eugenol) were conducted at various concentrations as negative controls to establish the absence of any potential bioactive effect of the phospholipids on E. coli. In all cases, no significant effect of any phospholipid at any concentration on the final microbial population was observed, compared to the bacterial populations suspended in phosphate-buffered saline (PBS) for 30 min (Fig. 1a to e). On the basis of consistently negative results obtained with all tested phospholipids, we assumed the absence of an antimicrobial effect of 18:0 EPC and no control experiment (18:0 EPC only) was conducted (Fig. 1f).
Table 1 also shows the critical synergistic concentration and maximum additional antimicrobial effect caused by the various phospholipids tested in this study. The critical synergistic concentration depended on the type of phospholipid and the additional inactivation effect of mixing eugenol with the phospholipids at the critical synergistic concentrations of ca. 0.5 to 2 log CFU/ml.
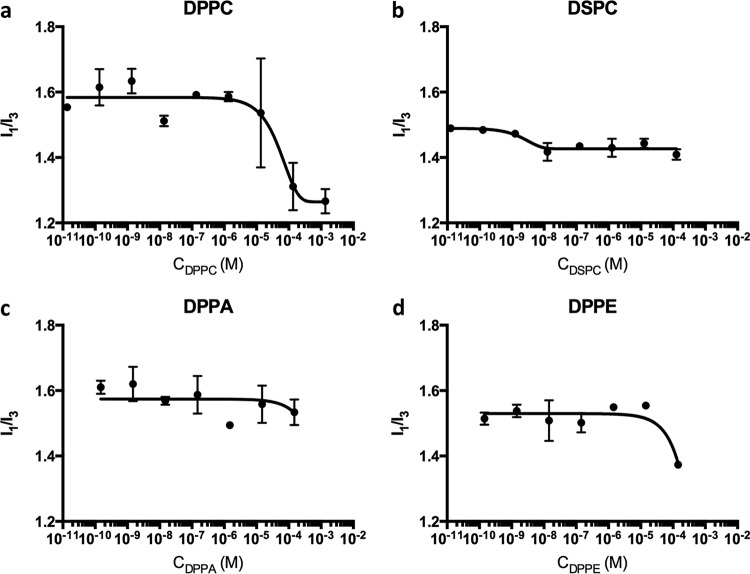
Pyrene was used as a probe to measure the CMC of DPPC, DSPC, DPPE, and DPPA, indicating their structure in PBS. The ratio of the first peak (I1) to the third peak (I3) in each spectrum was calculated. Figure 2a shows a decrease in the I1/I3 of pyrene when the DPPC was around 1.36 × 10−6 M, which may indicate the CMC of DPPC in PBS. Similarly, results in Fig. 2b indicated a decrease of I1/I3 started at ca. 1.27 × 10−9 M for DSPC. There was no clear change in I1/I3 over the tested DPPA concentrations (Fig. 2c). As shown in Fig. 2d, the I1/I3 of DPPE declined at around 1.45 × 10−5 M.
FIG 2.
Pyrene 1:3 ratio (I1/I3) versus phospholipids at different concentrations (C) in PBS buffer system. Error bars represent standard deviations of the means of results from two independent replicates.
DISCUSSION
Eugenol-only control samples showed a lower overall level of reduction (around 0.2 log CFU/ml) than was seen in our previous study (ca. 2 log CFU/ml [11]), suggesting lower eugenol antimicrobial power. However, the synergistic effect of lecithin was consistent with previous results; i.e., the synergistic effect was observed regardless of the antimicrobial power of the eugenol for the results shown in Fig. 1. Among the five natural phospholipids tested, DPPC, DSPC, DPPE, and DPPA showed a synergistic effect with eugenol at critical phospholipid concentrations. The enhanced antimicrobial potency of eugenol was diminished by adding phospholipids at a concentration above or below the critical synergistic concentrations. These results were consistent with our previous finding that using lecithin within a narrow window of concentrations improved the antimicrobial efficacy of eugenol against E. coli. The mechanism (or mechanisms) behind the observed inactivation patterns is not known. On the basis of previous cryo-transmission electron microscopy (cryo-TEM) observations and fluorescence studies, we proposed that the enhanced antimicrobial activity was due to the formation of nanoscale structures (diameter, <50 nm) when lecithin was added at critical synergistic concentrations. At the critical phospholipid concentration, the amphiphilic phospholipids self-assembled from monomers into small aggregates where eugenol could favorably partition. These small aggregates with large net surface area and containing positively charged phospholipid head groups would in turn enhance the interaction between eugenol and negatively charged E. coli cell membranes. Adding phospholipids at well above the critical synergistic concentration caused the formation of a large micron-scale network that could block the net contact between eugenol and target cell membranes and in turn diminish the enhanced antimicrobial properties.
Since phospholipids are amphiphilic molecules, they self-assemble into thermodynamically stable structures in aqueous systems. The CMC of phospholipids varies with both the phospholipid's head group and the acyl chain length (20–22). Several studies demonstrated the presence of temperature- and concentration-dependent architectures when phospholipids were suspended in water (23, 24). Even though the conformation of these phospholipids at critical synergistic concentrations was not determined, the CMC for DPPS, DSPC, DPPE, and DPPA was tested as an indicator for the formation of a specific structure leading to a potential synergistic effect with eugenol. Both surface tension (pendant drop) and pyrene fluorescent probe methods were used to determine the critical micelle concentration of the various phospholipids. However, great variability was found in the surface tension measurement data, probably due to the very low phospholipid concentration needed to synergistically improve the antimicrobial properties of eugenol (results not shown).
With increasing concentrations of each of the tested phospholipids, the I1/I3 ratio of pyrene fluorescence signal was expected to decrease as the CMC was approached, indicating the transferring of the pyrene probe into a more hydrophobic micellar environment than water (25, 26). As shown in Fig. 2, the CMC for DPPC, DSPC, DPPA, and DPPE were ca. 1.36 × 10−6 M, ca. 1.27 × 10−9 M, >1.49 × 10−4 M, and ca. 14.5 × 10−6 M, respectively. The CMC of DSCP was ca. 3 log cycles below that of DPPC, which may have been due to the longer hydrocarbon chains in DSPC (27). This was consistent with a previous report of a study by Smith and Tanford where the ln(CMC) linearly decreased with increases in the phosphatidylcholine hydrocarbon tail length. In their study, phosphatidylcholine with two 15-carbon acyl chains had a larger calculated ln(CMC) value (−24.6) than DPPC (16-carbon acyl chain; −25.5) (28). The CMC of DPPC in PBS obtained in this study differs from the CMC of DPPC in water (4.6 ± 0.5 × 10−10 M) reported previously (28). Since the ionic environment influences micelle formation in an aqueous solution (29, 30), the presence of PBS may explain the discrepancy between the observed and the reported critical micelle concentrations for DPPC. Negatively charged DPPA was expected to show a higher CMC consistent with more-hydrophilic phosphatides. For instance, the CMC of zwitterionic spin-labeled lauroylphosphatidylcholine (0.1 mM) is ca. 6 times lower than that of negatively charged spin-labeled lauroylphosphatidic acid (0.77 mM) in 0.1 M unbuffered NaCl solution (31).
With respect to the distinct differences in the CMC of tested phospholipids, it is reasonable to see different critical synergistic concentrations. Interestingly, the critical synergistic concentration correlated with the critical micelle concentration (Table 1). The critical synergistic concentration and the CMC were in the following order: DPPA>DPPE>DPPC>DSPC. The correlation between log (critical synergistic concentration) and log (CMC) was r = 0.976 (P < 0.01). This supported our hypothesis as discussed above. The synergistic effect of the combination of eugenol and phospholipids may be related to the specific structure formation that resulted from adding phospholipids at critical synergistic concentrations. However, for DPPE and DPPC, the critical synergistic concentrations were below their CMC. In contrast, for DSPC and DPPA, the critical synergistic concentrations were above their CMC. This indicates that there could be two or more mechanisms behind the enhanced antimicrobial property of eugenol seen when a phospholipid is used. When the critical synergistic concentrations were above the CMC, the enhanced antimicrobial property of eugenol may have been due to the encapsulation of eugenol into phospholipid micelles. However, with concentrations below the CMC, the phospholipid may form phospholipid-rich “patches” on the eugenol droplets as discussed in our previous study (11). The partition of phospholipids into the E. coli cell membrane (and therefore the modification of the membrane fluidity and permeability for hydrophilic compounds) is another mechanism that we plan to investigate (32).
However, critically thinking about the CMC study, we notice that the phase behavior of amphiphilic molecules such as phospholipids is dynamic and complicated. Recent studies suggested the occurrence of two or more CMC (33) or several critical aggregation concentrations (CAC) instead of a single CMC (34). For example, Burdíková and coworkers stated that after the first change in fluorescence signals indicating a CMC, further phase transitions from micelle to liposomes caused additional shifts in the I1/I3 fluorescence signal indicating further aggregation patterns or CAC (34). The CMC value determined in this study may indicate a specific phospholipid aggregation formation instead of simply micelle formation.
In contrast to DPPC, DSPC, DPPE, and DPPA, DPPS did not exhibit a synergistic effect on the antimicrobial properties of eugenol against E. coli. The reason is not clear. It is possible that the two negatively charged groups in the polar head of DPPS caused electrostatic repulsion, blocking potential absorption by negatively charged E. coli cell membranes. Even though the overall net charge of DPPS is the same as that of DPPA, a previous study showed that zwiterionic phospholipids in water expose a charged polar head group outside the self-assembled phospholipid bilayers (35). As a consequence, zwiterionic phospholipid could favorably bind ions with an opposite charge. Therefore, two negatively charged groups on the polar head of DPPS would potentially cause strong repulsion between eugenol-DPPS aggregates and E. coli cell membranes, diminishing the antimicrobial effect.
The antimicrobial potency of eugenol with a stable cationic phospholipid (18:0 EPC) was tested to further elucidate the effect of charge on the phospholipid synergistic effect. 18:0 EPC has the same acyl chain length and head group as DSPC. The only difference is that 18:0 EPC does not have a negatively charged -O- head group; thus, it carries positive charges only. We hypothesized that 18:0 EPC at a critical synergistic concentration would induce a significantly higher antimicrobial effect of eugenol against E. coli than DSPC. However, as Table 1 shows, the synergistic antimicrobial effects seen with 18:0 EPC and DSPC were not significantly different, so the number of negatively charged groups was not important in changing the electrostatic interactions between aggregates and between eugenol-phospholipid and bacterial cells. The electrostatic attraction between eugenol-phospholipid aggregates and E. coli cells may not play a major role in determining the synergistic effect.
Furthermore, that maximal inactivation effects achieved by adding DPPC, DSPC, DPPE, DPPA, or 18:0 EPC to the eugenol-in-water system were not the same. What drew our attention was that, interestingly, eugenol with two phospholipids (DSPC and 18:0 EPC) containing 18 carbon acyl chains showed significantly higher levels of antimicrobial properties against E. coli. This was consistent with observations by Donsì and coworkers, who concluded that the increase in the level of essential oil antimicrobial activity depended on both the concentration and the type of the emulsifiers (9).
Conclusion.
According to our findings, not all phospholipids in lecithin synergistically enhance the antimicrobial effect of eugenol. Among all the mechanisms suggested to be responsible for the enhanced antimicrobial effect of eugenol that results from adding a critical low concentration of phospholipids, the electrostatic interaction between eugenol-phospholipid aggregates and E. coli cells may not be the main mechanism of action, as DSPC and cationic 18:0 EPC showed similar levels of synergistic action. In terms of designing a more effective antimicrobial delivery system, the most important factor is suggested to be the chain length of phospholipids, as (DSPC) at a 18:0 ratio showed an increased level of synergistic action in comparison to DPPC (16:0). However, more-sensitive techniques need to be used to characterize the eugenol-phospholipid structures as well as their interactions with E. coli cells to elucidate the mechanisms behind the observed synergistic effect. Until now, we were limited by the detection limit of many structure characterization techniques due to the very low concentration of phospholipids leading to synergism.
MATERIALS AND METHODS
Phospholipids.
From the myriad phospholipids in lecithin, we selected DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine), DPPE (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine), DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine), DPPA (1,2-dipalmitoyl-sn-glycero-3-phosphate sodium salt), and DPPS (1,2-dipalmitoyl-sn-glycero-3-phospho-l-serine sodium salt) (Avanti, Alabaster, AL; Table 1) to conduct further experiments on the basis of their being the major components in both soy and egg lecithin. Cationic 18:0 EPC (1,2-distearoyl-sn-glycero-3-ethylphosphocholine chloride salt) was also obtained from Avanti Polar Lipids, Inc., to study the effect of the phospholipid charge on the synergistic effect in combination with eugenol.
Bacterial strain.
E. coli C600 (36) was obtained from the Yale E. coli Genetic Stock Center. It is a prophage-cured derivative of E. coli K-12 (37) that showed a positive response to eugenol-lecithin buffered systems in a previous study (11). After growth in tryptic soy broth (TSB; Difco, Sparks, MD) under aerobic conditions at 37°C for 24 h, a glycerol stock was made by mixing 1-ml culture aliquots with 0.3 ml 50% (vol/vol) glycerol–water. The stock was stored in a 2-ml centrifuge tube at −80°C until needed for experiments. To make a working culture, the stock culture was grown in TSB under the same conditions as previously described until the optical density at 610 nm (OD610) reached ca. 0.9, as monitored using a DU730 spectrophotometer (Beckman Coulter, Pasadena, CA).
Eugenol-phospholipid preparations.
The procedure to prepare the eugenol-phospholipid samples followed our previous method (11) with minor modifications. Phospholipid stock solutions (10 mg/100 ml) were prepared by boiling 10 mg of each phospholipid in 100 ml of phosphate-buffered saline (PBS; pH ca. 7.20) for 7 min and then homogenizing (Ultra-Turrax IKA T-18 basic; Staufen, Germany) at 11,000 rpm for 1 min. The solution was then cooled to room temperature, and sterile deionized water was added to bring the solution back to 100 ml. The stock solution was subsequently diluted in PBS to reach the final phospholipid concentration, ranging from 0 to 80 μM. A 0.043% (vol/vol) eugenol concentration (previously shown to reduce the population of E. coli C600 at least 0.2 log CFU/ml after a 30-min exposure [11]) dispersed in PBS-phospholipid was prepared by suspending 43 μl eugenol (Acros Oganics, Fairlawn, NJ) in 99 ml dilute phospholipid solution, followed by rotary homogenization (Ultra-Turrax IKA T-18 basic; Staufen, Germany) at 11,000 rpm for 3 min.
Quantification of bactericidal activity of eugenol-lecithin mixtures.
After mixing a 1-ml working culture (without washing) with 99 ml eugenol-phospholipid mixtures (initial inoculum concentration ca. 6 log CFU/ml; “phospholipid concentration” refers to the concentration reached after adding 1 ml working culture), one sample was taken immediately (t = 0 min) to determine the original inoculum concentration and another after 30 min (t = 30 min) of incubation at 37°C in a shaking incubator (N1570; Sheldon Manufacturing, Inc., OR) at a speed of 300 rpm to determine the final concentration. No appreciable phase separation was observed in any sample during the time span of the treatments. Each sample (0.1 ml) taken at t = 0 min and t = 30 min was serially diluted, plated onto two separate tryptic soy agar plates (TSA; Difco, Sparks, MD), grown overnight at 37°C, and counted. Inactivation (log CFU counts per milliliter) was calculated using equation 1 (see below), and the results were plotted versus each phospholipid concentration. Similar experiments were conducted without eugenol (phospholipid only) as controls. Three independent trials were conducted at each phospholipid concentration with one replication per trial (measured on duplicate plates) to quantify the bactericidal activity.
| (1) |
Critical micelle concentration of phospholipids.
Pyrene was used as a fluorescent probe to measure the CMC of DPPC, DSPC, DPPA, and DPPE, following previously reported methods with modifications (25, 38). Serial dilutions were done with respect to the target, and phospholipid concentrations ranging from 10−11 M to 10−2 M and 2 μM pyrene (from a stock solution of 0.4 mM pyrene–ethanol) were added to the phospholipid-PBS solution. Fluorescence measurements were conducted using a Fluorolog-3 spectrofluorometer (Horiba Scientific, Edison, NJ) with the excitation wavelength set at 331 nm and a fixed scan time at 0.8 s. The emission spectra were obtained in a wavelength range of 341 to 450 nm. The ratio of the first vibrational peak intensity (I1; around 372 nm) to the third peak intensity (I3 at around 383 nm) was used as a parameter to indicate the polarity of pyrene's environment (25). Two independent samples (replicates) were prepared for each phospholipid concentration to measure the fluorescence intensity.
Statistical analysis.
For all microbial studies, the inactivation (log CFU count per milliliter) seen after 30 min of exposure to eugenol–single-phospholipid treatment was the response variable. All experiments were analyzed as completely randomized experimental designs with three replicates, except for the CMC experiments, where two replicates were used. Means were separated using least significant difference (LSD) analysis (α = 0.05) when analysis of variance (ANOVA) showed significant treatment effects by the use of Minitab V. 15 (Minitab, State College, PA).
ACKNOWLEDGMENT
We thank Marissa Saladin from the Pennsylvania State University for kindly helping with the fluorescence study.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2011. Estimates of foodborne illness in the United States. http://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html Accessed November 2016.
- 2.World Health Organization. 2015. WHO estimates of the global burden of foodborne diseases. WHO, Geneva, Switzerland. [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2014. Reports of selected E. coli outbreak investigations. https://www.cdc.gov/ecoli/outbreaks.html.
- 4.Burt S. 2004. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Gaysinsky S, Davidson PM, Bruce BD, Weiss J. 2005. Growth inhibition of Escherichia coli O157: H7 and Listeria monocytogenes by carvacrol and eugenol encapsulated in surfactant micelles. J Food Prot 68:2559–2566. doi: 10.4315/0362-028X-68.12.2559. [DOI] [PubMed] [Google Scholar]
- 6.Gaysinsky S, Davidson PM, Bruce BD, Weiss J. 2005. Stability and antimicrobial efficiency of eugenol encapsulated in surfactant micelles as affected by temperature and pH. J Food Prot 68:1359–1366. doi: 10.4315/0362-028X-68.7.1359. [DOI] [PubMed] [Google Scholar]
- 7.Van Nieuwenhuyzen W. 1981. The industrial uses of special lecithins: a review. J Am Oil Chem Soc 58:886–888. doi: 10.1007/BF02659651. [DOI] [Google Scholar]
- 8.Donsì F, Annunziata M, Vincensi M, Ferrari G. 2012. Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J Biotechnol 159:342–350. doi: 10.1016/j.jbiotec.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Donsì F, Annunziata M, Sessa M, Ferrari G. 2011. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT Food Sci Technol 44:1908–1914. doi: 10.1016/j.lwt.2011.03.003. [DOI] [Google Scholar]
- 10.Chang Y, McLandsborough L, McClements DJ. 2012. Physical properties and antimicrobial efficacy of thyme oil nanoemulsions: influence of ripening inhibitors. J Agric Food Chem 60:12056–12063. doi: 10.1021/jf304045a. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Dudley EG, Davidson PM, Harte F. 2017. Critical concentration of lecithin enhances the antimicrobial activity of eugenol against Escherichia coli. Appl Environ Microbiol 83:e03467-16. doi: 10.1128/AEM.03467-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rydhag L, Wilton I. 1981. The function of phospholipids of soybean lecithin in emulsions. J Am Oil Chem Soc 58:830–837. doi: 10.1007/BF02665591. [DOI] [Google Scholar]
- 13.Siegel DP. 1986. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. II. Implications for membrane-membrane interactions and membrane fusion. Biophys J 49:1171–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polozova AI, Dubachev GE, Simonova TN, Barsukov LI. 1995. Temperature-induced micellar-lamellar transformation in binary mixtures of saturated phosphatidylcholines with sodium cholate. FEBS Lett 358:17–22. doi: 10.1016/0014-5793(94)01378-E. [DOI] [PubMed] [Google Scholar]
- 15.Schurtenberger P, Peng Q, Leser M, Luisi P-L. 1993. Structure and phase behavior of lecithin-based microemulsions: a study of the chain length dependence. J Colloid Interface Sci 156:43–51. doi: 10.1006/jcis.1993.1078. [DOI] [Google Scholar]
- 16.Koivusalo M, Haimi P, Heikinheimo L, Kostiainen R, Somerharju P. 2001. Quantitative determination of phospholipid compositions by ESI-MS: effects of acyl chain length, unsaturation, and lipid concentration on instrument response. J Lipid Res 42:663–672. [PubMed] [Google Scholar]
- 17.Sun W, Tristram-Nagle S, Suter R, Nagle J. 1996. Structure of gel phase saturated lecithin bilayers: temperature and chain length dependence. Biophys J 71:885–891. doi: 10.1016/S0006-3495(96)79290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mileykovskaya E, Dowhan W. 2000. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J Bacteriol 182:1172–1175. doi: 10.1128/JB.182.4.1172-1175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cristani M, D'Arrigo M, Mandalari G, Castelli F, Sarpietro MG, Micieli D, Venuti V, Bisignano G, Saija A, Trombetta D. 2007. Interaction of four monoterpenes contained in essential oils with model membranes: implications for their antibacterial activity. J Agric Food Chem 55:6300–6308. doi: 10.1021/jf070094x. [DOI] [PubMed] [Google Scholar]
- 20.Heerklotz H, Epand RM. 2001. The enthalpy of acyl chain packing and the apparent water-accessible apolar surface area of phospholipids. Biophys J 80:271–279. doi: 10.1016/S0006-3495(01)76012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh D. 1990. CRC handbook of lipid bilayers. CRC Press, Boca Raton, FL. [Google Scholar]
- 22.Kleinschmidt JH, Tamm LK. 2002. Structural transitions in short-chain lipid assemblies studied by 31 P-NMR spectroscopy. Biophys J 83:994–1003. doi: 10.1016/S0006-3495(02)75225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel D. 1986. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal amphiphile phases. III. Isotropic and inverted cubic state formation via intermediates in transitions between Lα and HII phases. Chem Phys Lipids 42:279–301. [DOI] [PubMed] [Google Scholar]
- 24.Harlos K, Eibl H. 1981. Hexagonal phases in phospholipids with saturated chains: phosphatidylethanolamines and phosphatidic acids. Biochemistry 20:2888–2892. doi: 10.1021/bi00513a027. [DOI] [PubMed] [Google Scholar]
- 25.Goddard ED, Turro NJ, Kuo PL, Ananthapadmanabhan KP. 1985. Fluorescence probes for critical micelle concentration. Langmuir 1:352–355. doi: 10.1021/la00063a015. [DOI] [PubMed] [Google Scholar]
- 26.Aguiar J, Carpena P, Molina-Bolıìvar JA, Carnero Ruiz C. 2003. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J Colloid Interface Sci 258:116–122. doi: 10.1016/S0021-9797(02)00082-6. [DOI] [Google Scholar]
- 27.Marsh D, King MD. 1986. Prediction of the critical micelle concentrations of mono-and di-acyl phospholipids. Chem Phys Lipids 42:271–277. doi: 10.1016/0009-3084(86)90086-1. [DOI] [PubMed] [Google Scholar]
- 28.Smith R, Tanford C. 1972. The critical micelle concentration of l-α-dipalmitoylphosphatidylcholine in water and water/methanol solutions. J Mol Biol 67:75–83. doi: 10.1016/0022-2836(72)90387-7. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y-X, Tan R-C, Li Y-L, Yang Y-Q, Yu L, He Q-C. 2001. Effect of salts on the formation of C8-lecithin micelles in aqueous solution. J Colloid Interface Sci 236:28–34. doi: 10.1006/jcis.2000.7278. [DOI] [PubMed] [Google Scholar]
- 30.Maiti K, Mitra D, Guha S, Moulik SP. 2009. Salt effect on self-aggregation of sodium dodecylsulfate (SDS) and tetradecyltrimethylammonium bromide (TTAB): physicochemical correlation and assessment in the light of Hofmeister (lyotropic) effect. J Mol Liq 146:44–51. doi: 10.1016/j.molliq.2009.01.014. [DOI] [Google Scholar]
- 31.King MD, Marsh D. 1987. Head group and chain length dependence of phospholipid self-assembly studied by spin-label electron spin resonance. Biochemistry 26:1224–1231. doi: 10.1021/bi00379a004. [DOI] [PubMed] [Google Scholar]
- 32.Keweloh H, Diefenbach R, Rehm H-J. 1991. Increase of phenol tolerance of Escherichia coli by alterations of the fatty acid composition of the membrane lipids. Arch Microbiol 157:49–53. doi: 10.1007/BF00245334. [DOI] [PubMed] [Google Scholar]
- 33.Danino D, Abezgauz L, Portnaya I, Dan N. 2016. From discs to ribbons networks: the second critical micelle concentration in nonionic sterol solutions. J Phys Chem Lett 7:1434–1439. doi: 10.1021/acs.jpclett.6b00266. [DOI] [PubMed] [Google Scholar]
- 34.Burdíková J, Mravec F, Pekař M. 2016. The formation of mixed micelles of sugar surfactants and phospholipids and their interactions with hyaluronan. Colloid Polym Sci 294:823–831. doi: 10.1007/s00396-016-3840-8. [DOI] [Google Scholar]
- 35.Fukuma T, Higgins MJ, Jarvis SP. 2007. Direct imaging of lipid-ion network formation under physiological conditions by frequency modulation atomic force microscopy. Phys Rev Lett 98:106101. doi: 10.1103/PhysRevLett.98.106101. [DOI] [PubMed] [Google Scholar]
- 36.Appleyard RK. 1954. Segregation of new lysogenic types during growth of a doubly lysogenic strain derived from Escherichia coli K12. Genetics 39:440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pósfai G, Plunkett G, Fehér T, Frisch D, Keil GM, Umenhoffer K, Kolisnychenko V, Stahl B, Sharma SS, De Arruda M. 2006. Emergent properties of reduced-genome Escherichia coli. Science 312:1044–1046. doi: 10.1126/science.1126439. [DOI] [PubMed] [Google Scholar]
- 38.Basu Ray G, Chakraborty I, Moulik SP. 2006. Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J Colloid Interface Sci 294:248–254. doi: 10.1016/j.jcis.2005.07.006. [DOI] [PubMed] [Google Scholar]