ABSTRACT
Alcohol abuse impairs immune defense. To study the effect of chronic-plus-binge alcohol exposure on the granulopoietic response, acute alcohol intoxication (intraperitoneal injection of 5 g alcohol/kg body weight) was introduced to mice chronically fed on the Lieber-DeCarli low-fat liquid alcohol diet for 5 weeks. Bacteremia was induced by intravenous injection of Escherichia coli. Bacteremia caused a remarkable increase in marrow lin− c-kit+ Sca-1+ cells. Activation of cell proliferation supported the increase in marrow lin− c-kit+ Sca-1+ cells. Alcohol administration inhibited this activation of lin− c-kit+ Sca-1+ cells. The bone marrow of pair-fed control mice receiving intraperitoneal saline stored a large number of mature granulocytes expressing a high level of Gr1 (Gr1hi cells). The proportion of Gr1hi cells and the total number of Gr1+ cells were markedly reduced in the bone marrow, along with an increase in the ratio of Gr1+ granulocytes in peripheral white blood cells following bacteremia. E. coli infection stimulated proliferation of granulopoietic precursor cells, resulting in a marked increase in the ratio of immature Gr1lo cells in the bone marrow. Alcohol administration itself triggered marrow release of Gr1+ cells, resulting in reduction of the marrow granulocyte reserve with an elevation of granulocytes in the circulation. Alcohol also impaired activation of granulopoietic precursor proliferation following bacteremia. Alcohol disrupted lipopolysaccharide (LPS)-TLR4-ERK1/2-cyclin D1 signaling and inhibited upregulation of Sca-1 and C/EBPβ expression by lineage-negative marrow cells in response to bacteremia. These results indicate that chronic-plus-binge alcohol exposure inhibits the granulopoietic response by disrupting key cell signaling for hematopoietic precursor cell activation and commitment to granulocyte lineage development.
KEYWORDS: Alcohol, bacteremia, cell signaling, granulocytes, granulopoietic response, stem cells
INTRODUCTION
Primitive hematopoietic precursor cells, particularly hematopoietic stem cells (HSCs), in the adult bone marrow constitute an important component of the host defense system through actively participating in the granulopoietic response to serious bacterial infection (1, 2). The majority of these rare event precursors are maintained in a quiescent state under normal conditions (3). Only a small proportion of them enter into cell cycling for self-renewal and/or proliferation on a regular basis. The homeostasis of HSC quiescence, self-renewal, proliferation, and differentiation in the marrow niche environment secures a sufficient pool of HSCs while supporting the development of all blood cell types in the body. During bacterial infection, the equilibrium of hematopoiesis is altered. Bone marrow shifts its hematopoietic activity toward enhancing production of granulocytes. This granulopoietic response is imperative for reinforcing host immune defense against invading pathogens. Our recent studies have revealed that pattern recognition receptor ligands (such as lipopolysaccharides [LPS]) released by microbes and inflammatory mediators (such as tumor necrosis factor-α [TNF-α], gamma interferon [IFN-γ], and interleukin-6 [IL-6]) generated from host tissue cells evoke rapid activation of HSCs (1, 4). The marrow pool of lineage-negative (lin−), stem cell growth factor receptor-positive (c-kit+), stem cell antigen-1-positive (Sca-1+) cells (LKS cells, a population enriched with HSCs) in mice is substantially expanded, with a significant increase in their proliferative activity during bacteremia. These activated primitive hematopoietic precursor cells also undergo functional reprograming to enhance their commitment to granulocyte lineage differentiation.
Alcohol is the most frequently abused substance, and it injures the bone marrow and impairs host immune defense against bacterial infection (5, 6). Alcohol dependence is an independent risk factor for developing serious infections, including sepsis, septic shock, and pulmonary infections (7, 8). Alcoholic patients frequently show abnormalities of granulocyte levels and functional activities in the systemic circulation (9–11). Bone marrow samples from alcohol-abusing patients exhibit a significant reduction in mature granulocytes with vacuolization of myeloid progenitor cells (12). In the clinic, it has long been recognized that the occurrence of serious bacterial infection in alcoholic patients is frequently preceded by an episode (or episodes) of very heavy drinking (13), indicating that chronic-plus-binge drinking can be a highly risky pattern of alcohol abuse. Observations of alcoholic liver injury have also demonstrated that chronic-plus-binge alcohol consumption disrupts the homeostasis of granulocytes in the peripheral circulation and organ tissues (14). In the present study, we determined defects of the granulopoietic response to bacteremia in a murine model of chronic-plus-binge alcohol consumption. Our focus was on delineating the negative impact of chronic-plus-binge alcohol exposure on the host immune defense at the level of the hematopoietic precursor cell compartment.
RESULTS
Chronic-plus-binge alcohol administration impaired marrow primitive hematopoietic precursor cell activation and the granulopoietic response to bacteremia.
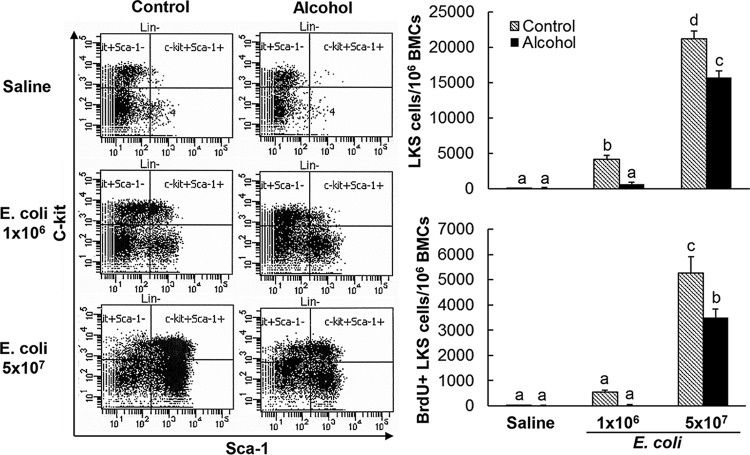
As shown in Fig. 1, in pair-fed control mice receiving intravenous (i.v.) saline, the number of bone marrow lin− c-kit+ Sca-1+ cells was very small (around 0.01% of total nucleated bone marrow cells [BMCs]). Bacteremia caused a remarkable increase in the number of lin− c-kit+ Sca-1+ cells in the bone marrow (approximately 46- and 233-fold increases in mice at 24 h following Escherichia coli i.v. challenge with 1 × 106 and 5 × 107 CFU/mouse, respectively). Bacteremia also caused activation of lin− c-kit+ Sca-1+ cell proliferation, as reflected by their substantial increase in 5-bromo-2-deoxyuridine (BrdU) incorporation, which supported the marked increase in the marrow pool of lin− c-kit+ Sca-1+ cells. Chronic-plus-binge alcohol administration did not affect the number of marrow lin− c-kit+ Sca-1+ cells and their activity of proliferation in mice without bacteremia. However, such alcohol treatment significantly inhibited the increase in marrow lin− c-kit+ Sca-1+ cells and their activation of proliferation at 24 h following i.v. challenge with E. coli (P < 0.05).
FIG 1.
Effect of chronic-plus-binge alcohol administration on changes in bone marrow lin− c-kit+ Sca-1+ cells in mice challenged i.v. with saline or E. coli for 24 h. Control, i.p. saline to mice on control diet; Alcohol, acute alcohol intoxication to mice on chronic alcohol diet; Saline, i.v. saline; E. coli 1 × 106, 1 × 106 CFU E. coli/mouse i.v.; E. coli 5 × 107, 5 × 107 CFU E. coli/mouse i.v. The data are presented as means and SEM; n = 5. Bars with different letters in each graph are statistically significantly different (P < 0.05).
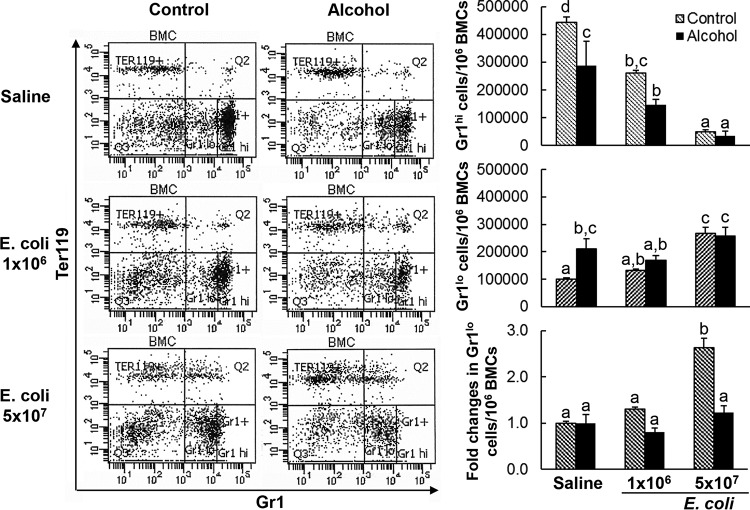
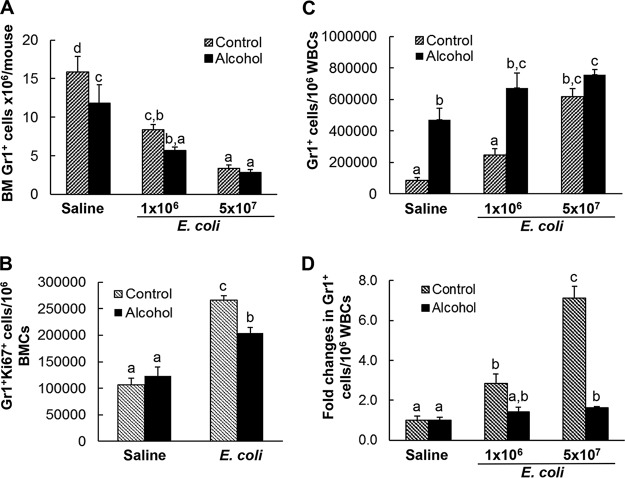
During granulocyte development in the bone marrow, expression of granulocyte differentiation antigen 1 (Gr1) by cells in the granulocyte lineage increases with cell maturation. In pair-fed control mice receiving i.v. saline, the bone marrow stored a large number of mature granulocytes expressing the high level of Gr1 (Gr1hi cells) (Fig. 2). Bacteremia resulted in a marked reduction of Gr1hi cells in the bone marrow. The total number of Gr1+ cells in the bone marrow was also significantly reduced following systemic infection with E. coli (Fig. 3A) (P < 0.05). Concomitantly, the ratio of Gr1-positive granulocytes in peripheral white blood cells (WBCs) was substantially increased (Fig. 3C). These changes suggest the active release of mature granulocytes from the bone into the systemic circulation in response to bacteremia. Systemic E. coli infection enhanced proliferation of granulocyte precursor cells in the bone marrow, as reflected by the significant increase in the number of cycling cells positive for Ki67 antigen in the marrow pool of Gr1+ cells (Fig. 3B) (P < 0.05). This granulopoietic response resulted in an increase in the ratio of immature Gr1lo cells in the bone marrow Gr1+ cell population (Fig. 2).
FIG 2.
Effect of chronic-plus-binge alcohol administration on changes in the bone marrow granulocyte (Gr1+) population in mice challenged i.v. with saline or E. coli for 24 h. Control, i.p. saline to mice on control diet; Alcohol, acute alcohol intoxication to mice on chronic alcohol diet; Saline, i.v. saline; E. coli 1 × 106, 1 × 106 CFU E. coli/mouse i.v.; E. coli 5 × 107, i.v. 5 × 107 CFU E. coli/mouse. The data are presented as means and SEM; n = 5. Bars with different letters in each graph are statistically significantly different (P < 0.05).
FIG 3.
(A, C, and D) Effect of chronic-plus-binge alcohol administration on changes in the total number of bone marrow granulocytes (A), the ratio of granulocytes in blood WBCs (C), and the fold increase in the ratio of granulocytes in blood WBCs (D) in mice challenged i.v. with saline or E. coli for 24 h; n = 5. (B) Effect of chronic-plus-binge alcohol administration on the change in Gr1+ Ki67+ cells in mice challenged i.v. with saline or 5 × 107 CFU E. coli/mouse for 24 h; n = 4 to 6. The data are presented as means and SEM. Bars with different letters in each panel are statistically significantly different (P < 0.05).
Chronic-plus-binge alcohol administration impaired these activities in mice during the granulopoietic response to systemic E. coli infection. Alcohol treatment inhibited the increase in marrow lin− c-kit+ Sca-1+ cells and impaired activation of lin− c-kit+ Sca-1+ cell proliferation following bacteremia (Fig. 1). Chronic-plus-binge alcohol administration itself triggered release of mature granulocytes from the bone marrow into the systemic circulation in mice receiving i.v. saline. In animals with chronic-plus-binge alcohol administration, the marrow pool of Gr1hi cells and the total number of Gr1+ cells decreased significantly in the absence of systemic E. coli infection (Fig. 2 and 3A) (P < 0.05). This reduction in the Gr1hi cell population was associated with an increase in the ratio of Gr1lo cells in BMCs. Nevertheless, chronic-plus-binge alcohol administration did not alter the number of cycling granulopoietic precursor cells positive for Ki67 antigen expression in the marrow pool of Gr1+ cells (Fig. 3B). In the meantime, the ratio of Gr1+ granulocytes in WBCs in the systemic circulation was significantly increased (P < 0.05). Chronic-plus-binge alcohol administration inhibited bacteremia-induced enhancement of granulocyte precursor cell proliferation (Fig. 3B) and expansion of the Gr1lo cell pool in the bone marrow (Fig. 2). The bacteremia-induced reduction of total Gr1+ cells in the bone marrow tended to be more severe in mice with chronic-plus-binge alcohol administration (Fig. 3A). Chronic-plus-binge alcohol administration also impaired further increase in the ratio of Gr1+ granulocytes in peripheral WBCs following systemic E. coli infection (Fig. 3C and D).
In vitro alcohol exposure impaired hematopoietic precursor cell proliferation in response to LPS stimulation.
LPS, the cell wall component of Gram-negative bacteria, is a major pathogen-associated molecular pattern ligand commonly serving as a proximal stimulant for hematopoietic precursor activation during bacterial infection. As shown in Fig. 4, in vitro culture of mouse primary bone marrow lin− c-kit+ cells (containing both lin− c-kit+ Sca-1+ cells and lin− c-kit+ Sca-1− cells) with LPS for 72 h significantly increased the proliferative activity (as reflected in cell BrdU incorporation) of c-kit+ Sca-1+ precursor cells (3.3-fold increase; P < 0.05) and differentiated Gr1+ cells (6.6-fold increase; P < 0.05) in the culture system. Alcohol exposure during the entire 72-h period of cell culture did not show any significant effect on the baseline activity of cell proliferation in both cell populations. However, alcohol treatment in vitro caused profound inhibition of LPS-stimulated activation of c-kit+ Sca-1+ precursor cell proliferation in the culture system. This alcohol exposure also inhibited LPS-stimulated increase in BrdU incorporation in the differentiated Gr1+ cell population in the culture system in an alcohol dose-dependent manner.
FIG 4.
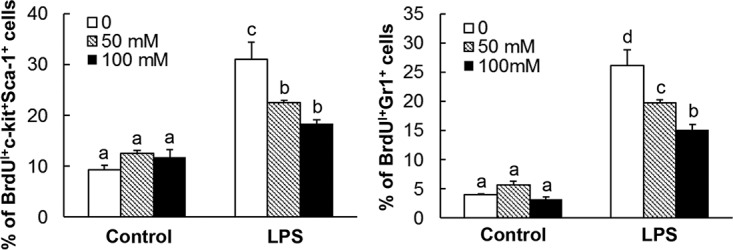
Alcohol exposure inhibited LPS-stimulated increase in BrdU incorporation into hematopoietic cells. The data are presented as means and SEM; n = 5. Bars with different letters in each graph are statistically significantly different (P < 0.05).
Chronic-plus-binge alcohol administration impaired activation of the ERK-cyclin D1 signaling pathway in bone marrow cells in response to bacteremia.
The ERK-cyclin D1 pathway plays an important role in the regulation of hematopoietic precursor cell proliferation. As shown in Fig. 5, the level of ERK1/2 phosphorylation in nucleated bone marrow cells of pair-fed control mice receiving i.v. saline was low. Chronic-plus-binge alcohol administration did not affect the baseline level of ERK1/2 phosphorylation in mice receiving i.v. saline. Bacteremia caused a rapid activation of the ERK signal pathway, as reflected by a marked increase in the level of ERK1/2 phosphorylation (a 6.4-fold increase in the ratio of phospho-ERK1/2 to total ERK1/2 content in cells [P < 0.05] and a 2.5-fold increase in the ratio of phospho-ERK1/2 to β-actin content in cells [P < 0.05]) in nucleated bone marrow cells at the early stage of systemic E. coli infection. Chronic-plus-binge alcohol administration caused profound inhibition of ERK1/2 phosphorylation in nucleated bone marrow cells in response to bacteremia.
FIG 5.
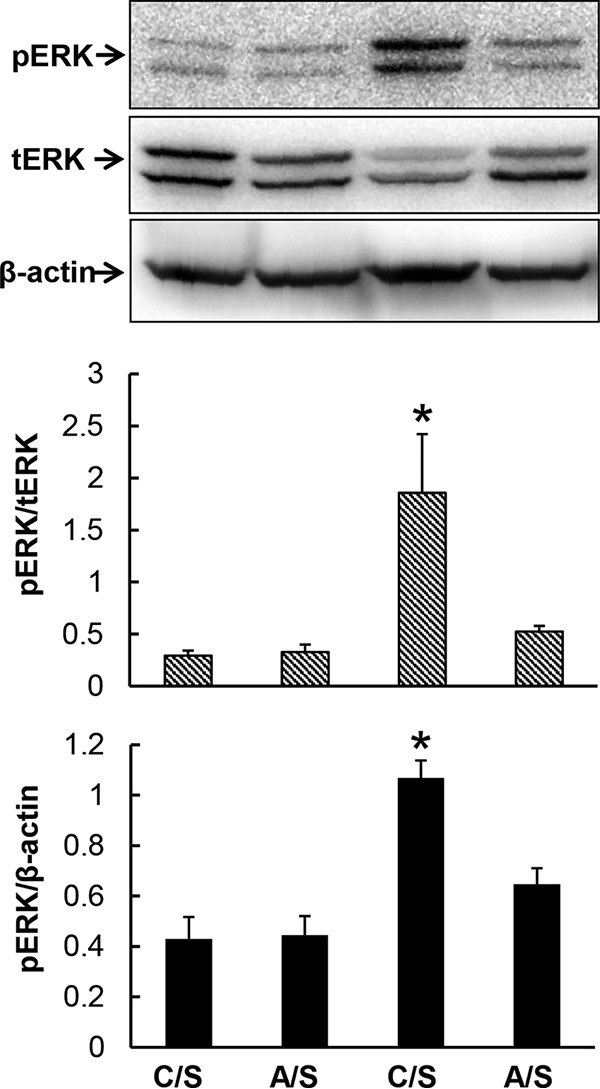
Chronic-plus-binge alcohol administration impaired ERK activation in marrow cells 8 h following bacteremia. C, i.p. saline to mice on control diet; A, acute alcohol intoxication to mice on chronic alcohol diet; S, i.v. saline; E, 5 × 107 CFU E. coli/mouse i.v. The data are presented as means and SEM; n = 4. *, P < 0.05 compared with other groups in the same graph.
Translocation of activated ERK1/2 from the cytoplasm into the nucleus promotes cyclin D1 expression by cells. D cyclins provide a strong force for S-phase entry during cell cycling. Consistent with the alteration of ERK1/2 phosphorylation in nucleated bone marrow cells, bacteremia caused significant upregulation of cyclin D1 mRNA expression (2.3-fold increase; P < 0.05) by nucleated bone marrow cells at 24 h following infection (Fig. 6A). Chronic-plus-binge alcohol administration did not affect the baseline level of cyclin D1 mRNA expression by bone marrow cells in mice receiving i.v. saline. However, chronic-plus-binge alcohol administration significantly inhibited bacteremia-induced upregulation of cyclin D1 mRNA expression by nucleated bone marrow cells (P < 0.05). Similarly, cyclin D1 protein expression in nucleated bone marrow cells was significantly increased (2-fold increase; P < 0.05) at 24 h following bacteremia (Fig. 6B). Although chronic-plus-binge alcohol administration did not affect the baseline level of cyclin D1 protein expression by bone marrow cells in mice receiving i.v. saline, alcohol administration significantly inhibited bacteremia-induced upregulation of cyclin D1 protein expression by nucleated bone marrow cells (P < 0.05).
FIG 6.
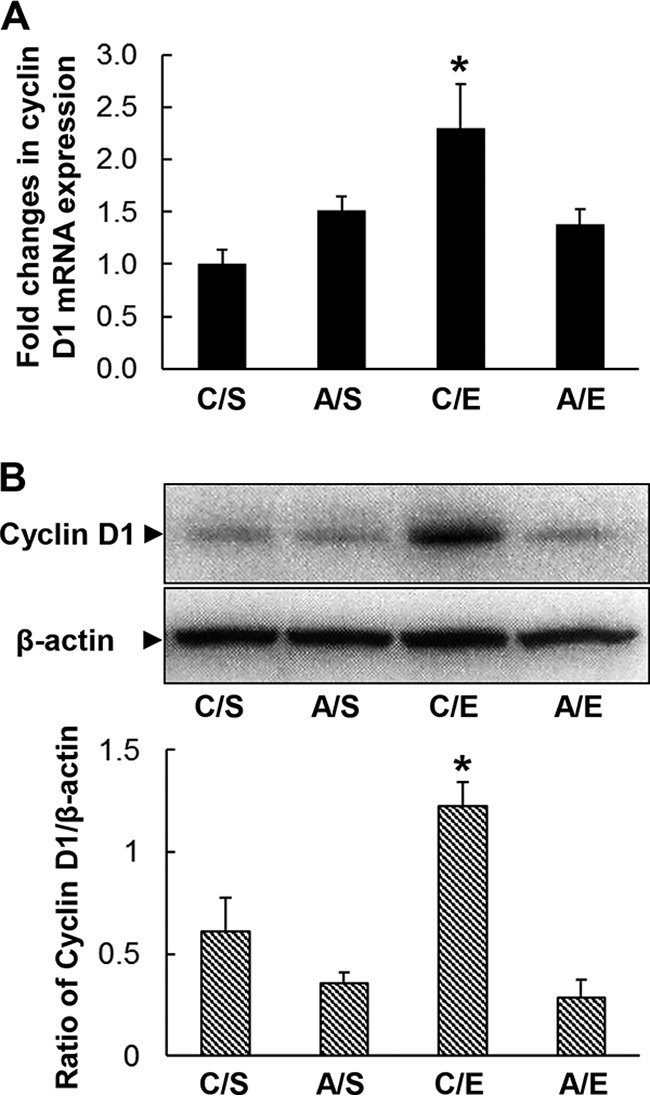
Chronic-plus-binge alcohol administration impaired cyclin D1 mRNA (A) and protein (B) expression by marrow cells 24 h following bacteremia. C, i.p. saline to mice on control diet; A, acute alcohol intoxication to mice on chronic alcohol diet; S, i.v. saline; E, 5 × 107 CFU E. coli/mouse i.v. The data are presented as means and SEM; n = 4 to 6. *, P < 0.05 compared with other groups in the same graph.
Chronic-plus-binge alcohol administration disrupted Sca1, CEBPβ, and CEBPα mRNA expression by lineage-negative bone marrow cells in response to bacteremia.
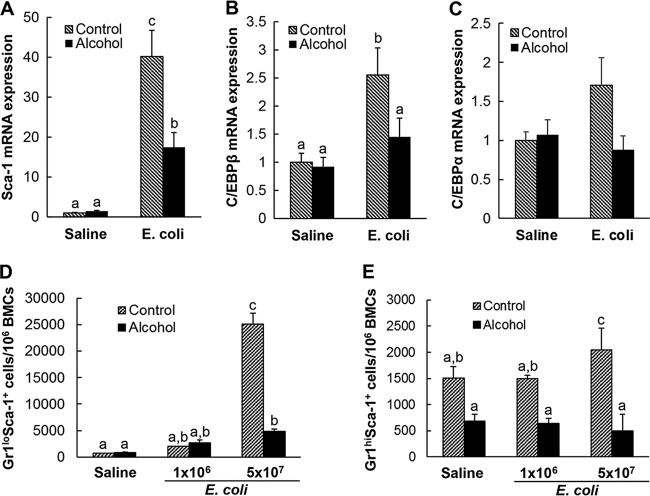
Bacteremia not only caused expansion of the lin− c-kit+ Sca-1+ cell population in bone marrow, but also markedly enhanced cell surface expression of Sca-1 by marrow lineage-negative precursor cells (Fig. 1). Real-time reverse transcription (RT)-PCR demonstrated that this enhancement of Sca-1 expression was primarily regulated at the transcriptional level (Fig. 7A). The baseline expression of Sca-1 mRNA by lineage-negative bone marrow cells was low in pair-fed control mice receiving i.v. saline. Twenty-four hours following bacteremia, Sca-1 mRNA expression by lineage-negative bone marrow cells was substantially increased (40.2-fold increase; P < 0.05). Chronic-plus-binge alcohol administration did not affect the baseline expression of Sca-1 by lineage-negative marrow cells in mice receiving i.v. saline. However, it profoundly inhibited the bacteremia-induced upregulation of Sca-1 mRNA expression by lineage-negative cells in the bone marrow. In the meantime, the expression of C/EBPβ mRNA by lineage-negative bone marrow cells was significantly upregulated (2.6-fold increase; P < 0.05) in response to bacteremia (Fig. 7B). Chronic-plus-binge alcohol administration did not affect the baseline expression of C/EBPβ mRNA by lineage-negative marrow cells but inhibited the bacteremia-induced upregulation of C/EBPβ mRNA by lineage-negative cells in the bone marrow. Systemic infection with E. coli for 24 h tended to upregulate C/EBPα mRNA expression by lineage-negative bone marrow cells, although this effect did not reach statistical significance (Fig. 7C). We also determined Sca-1, C/EBPβ, and C/EBPα mRNA expression by lineage-negative bone marrow cells in mice with and without chronic-plus-binge alcohol administration at 12 h post-i.v. challenge with either saline or E. coli (5 × 107 CFU/mouse). We did not observe any difference with statistical significance among all the treatment groups at that time point (data not shown). Along with the upregulation of Sca-1 expression by lineage-negative bone marrow cells, the ratio of Gr1lo Sca-1+ granulopoietic progenitors in BMCs was markedly increased following systemic E. coli infection (Fig. 7D). Bacteremia also tended to increase the ratio of Gr1hi Sca-1+ cells in BMCs (Fig. 7E). Chronic-plus-binge alcohol administration suppressed this bacteremia-induced increase in the ratio of Gr1lo Sca-1+ and Gr1hi Sca-1+ cells in BMCs.
FIG 7.
(A to C) Effect of chronic-plus-binge alcohol administration on changes in Sca-1 (A), C/EBPβ (B), and C/EBPα (C) gene expression by bone marrow lineage-negative cells 24 h following bacteremia. The data are presented as means and SEM; n = 3 to 5. Control, i.p. saline to mice on control diet; Alcohol, acute alcohol intoxication to mice on chronic alcohol diet; Saline, i.v. saline; E. coli, 5 × 107 CFU E. coli/mouse i.v. (D and E) Effect of chronic-plus-binge alcohol administration on changes in bone marrow Gr1lo Sca-1+ (D) and Gr1hi Sca-1+ (E) cell populations in mice challenged i.v. with saline or E. coli for 24 h. Control, i.p. saline to mice on control diet; Alcohol, acute alcohol intoxication to mice on chronic alcohol diet; Saline, i.v. saline; E. coli 1 × 106, 1 × 106 CFU E. coli/mouse i.v.; 5 × 107 E. coli, 5 × 107 CFU E. coli/mouse i.v. The data are presented as means and SEM; n = 5. Bars with different letters in each panel are statistically significantly different (P < 0.05).
DISCUSSION
In response to systemic E. coli infection, the lin− c-kit+ Sca-1+ cell population in bone marrow was markedly increased. This increase in the marrow pool of lin− c-kit+ Sca-1+ cells was more robust in mice challenged with the high dose (5 × 107 CFU/mouse) of E. coli. In the meantime, the activity of proliferation in marrow lin− c-kit+ Sca-1+ cells was significantly increased following bacteremia. These results are consistent with observations from our previous studies (1, 4, 15). We have observed a marked increase in the marrow lin− c-kit+ Sca-1+ cell population in mice either with E. coli infection in the bloodstream (1, 4) or with Streptococcus pneumoniae infection in the lung (15). Therefore, activation of primitive hematopoietic precursor cells bearing the lin− c-kit+ Sca-1+ marker commonly occurs during the host response to serious bacterial infection. Chronic-plus-binge alcohol administration significantly inhibited the bacteremia-induced activation of marrow lin− c-kit+ Sca-1+ cells in our current study. Chronic-plus-binge alcohol administration attenuated the increase in the marrow pool of lin− c-kit+ Sca-1+ cells and restricted the enhancement of lin− c-kit+ Sca-1+ cell proliferation in response to E. coli bacteremia. Bone marrow lin− c-kit+ Sca-1+ cells are enriched with hematopoietic stem cells, which give rise to all types of blood cells (16). The marked expansion of the marrow lin− c-kit+ Sca-1+ cell population, particularly the significant enhancement of lin− c-kit+ Sca-1+ cell proliferation, during the granulopoietic response is critical for initiating and supporting the increase in granulocyte production by the bone marrow in response to systemic bacterial infection. Chronic-plus-binge alcohol administration inhibited the activation of marrow lin− c-kit+ Sca-1+ cells, which may exert a negative impact on the granulopoietic response to severe bacterial infection.
Under normal circumstances, bone marrow possesses an enormous capacity for storing granulocytes with mature morphology before releasing them into the circulation. It is estimated that the bone marrow reserves approximately120 million granulocytes in a naive adult mouse, while neutrophils in the circulatory system are less than 2.5 million in the same animal (17). In our current study, flow cytometric analysis demonstrated that a large portion of bone marrow nucleated cells were positive for Gr1 in control mice receiving i.v. saline. Among Gr1+ cells, the majority exhibited the Gr1hi phenotype, indicating that they were morphologically mature granulocytes. An interesting observation in this study was that chronic-plus-binge alcohol administration itself triggered the release of mature granulolcytes from the marrow storage pool into the systemic circulation in the absence of bacterial infection. The proportion of Gr1hi granulocytes in BMCs and the total number of marrow Gr1+ cells were significantly reduced, with a substantial increase in the ratio of Gr1+ granulocytes in WBCs in the bloodstream in mice with chronic-plus-binge alcohol administration. In this study, flow cytometric gates for sample acquisition and data analysis defined a fixed range of fluorescence intensities for cells belonging to both the Gr1lo and Gr1hi subpopulations in bone marrow samples. Therefore, changes in the mean fluorescent intensity (MFI) of cells (a parameter reflecting the level of Gr1 antigen expression by cells) within each defined cell subpopulation in mice with different treatments were not a consideration in the experimental design. Specifically, the Gr1lo cell subpopulation had a lower MFI than the Gr1hi cell subpopulation. Alcohol treatment caused bone marrow release of granulocytes, mainly from the Gr1hi cell subpopulation. The concomitantly increased blood granulocytes essentially carried the Gr1hi phenotype in mice with chronic-plus-binge alcohol administration in the absence of E. coli challenge. Due to this increase in the proportion of Gr1lo cells in the marrow total granulocyte population following alcohol treatment, a corresponding reduction of MFI in the marrow total granulocyte population occurred. A recent study revealed a significant increase in blood neutrophil counts in both clinical patients and experimental animals with chronic-plus-binge alcohol exposure (18). This chronic-plus-binge alcohol-induced marrow release of granulocytes appears to primarily contribute to the development of inflammation in peripheral organ tissues without any validated significance in enhancing host defense. The mechanisms by which chronic-plus-binge alcohol administration triggers the release of granulocytes from the bone marrow remain to be elucidated. The CXCL12/CXCR4 axis plays a critical role in marrow retention of granulocytes (17, 19). Alcohol administration has been shown to exert influence on CXCL12/CXCR4 signaling (20). Further investigation of altered signaling regulation of granulocyte retention in or release from the bone marrow in hosts with chronic-plus-binge alcohol exposure will provide deeper insight into the underlying mechanisms.
E. coli bacteremia caused a substantial decrease in the proportion of Gr1hi granulocytes in BMCs and the total number of Gr1+ cells in the bone marrow, which was accompanied by a concomitant increase in the ratio of Gr1+ granulocytes in WBCs in the systemic circulation. This mobilization of granulocytes from the bone marrow storage pool into the circulation was further enhanced in animals challenged with the high dose of E. coli, suggesting an increase in the effort of bone marrow to produce granulocytes to reinforce host immune defense against serious bacterial infection. Chronic-plus-binge alcohol administration restricted further increase in circulating Gr1+ granulocytes in response to E. coli bacteremia. This negative effect at least partially resulted from the defective marrow reserve of granulocytes due to the release of the phagocytes from the bone marrow into the systemic circulation caused by chronic-plus-binge alcohol administration itself. The restriction of bone marrow granulocyte production by chronic-plus-binge alcohol administration was much more manifest in mice challenged with the high dose of E. coli.
Along with mobilization of Gr1hi granulocytes from the bone marrow, the proportion of Gr1lo immature cells in the granulocyte lineage was increased in the bone marrow of mice with systemic E. coli infection. The Ki67 antigen is expressed in the nuclei of cells actively cycling (21). Our results showed that Ki67 antigen-expressing cells in the granulocyte lineage were significantly increased in response to E. coli bacteremia, indicating that the activation of hematopoietic precursor cell proliferation continued after their commitment to granulocyte lineage development. Obviously, the combined efforts of enhanced cell proliferation at both stages of non-lineage-committed primitive hematopoietic precursor cells and lineage-committed granulopoietic progenitor cells contribute to the enhancement of granulocyte production by the bone marrow during the host defense response to bacteremia. Chronic-plus-binge alcohol administration inhibited the increase in Gr1+ Ki67+ cells in the bone marrow in response to systemic E. coli infection. In combination with its negative effect on activation of lin− c-kit+ Sca-1+ cell proliferation, chronic-plus-binge alcohol administration blocked the increase in Gr1lo immature cells in the bone marrow following bacteremia. This defect in activation of hematopoietic precursor cell proliferation impairs the sustained effort of enhancing granulocyte production by the bone marrow in response to systemic E. coli infection. The decrease in the total number of Gr1+ granulocytes in the bone marrow following bacteremia tended to be more severe in mice with chronic-plus-binge alcohol administration.
Hematopoietic precursor cells, including HSCs, express pattern recognition receptors and certain types of cytokine receptors (22, 23). LPS (the ligand for Toll-like receptor 4 [TLR4]) released from inoculated E. coli cells served as the most proximal stimulant to host cells in our experimental model. Binding of LPS to TLR4 on the surfaces of hematopoietic cells may activate the ERK1/2 pathway through activation of the Ras-c–Raf–mitogen-activated protein kinase ERK kinase 1/2 (MEK1/2) cascades (24). Phosphorylation of ERK1/2 leads to its nuclear translocation, where it activates several ERK targets (including transcription factors, such as Elk-1) and induces expression of the first class G1 cyclin, cyclin D. Upregulation of cyclin D–cyclin-dependent kinase 4/6 (CDK4/6) activity promotes expression of gene products required for S phase entry during cell proliferation by enhancing the function of E2F transcription factors (25, 26). Culture of primary lin− c-kit+ cells with LPS stimulated c-kit+ Sca-1+ cell proliferation in the culture system. Primary lin− c-kit+ cells also underwent differentiation to give rise to Gr1+ cells during culture in our current study. LPS stimulated BrdU incorporation into Gr1+ cells in the culture system, which represents the activation of cell proliferation, along with their differentiation during culture. Alcohol exposure inhibited the LPS-induced activation of c-kit+ Sca-1+ cell proliferation and BrdU incorporation into Gr1+ cells. Our previous studies showed that TLR4 knockout blocks activation of lin− c-kit+ Sca-1+ cell proliferation in the bone marrow in response to E. coli bacteremia (2). These observations indicate that the LPS/TLR4 signaling system plays a critical role in mediating bacteremia-induced activation of lin− c-kit+ Sca-1+ cell proliferation during the granulopoietic response. Alcohol impairs this activation of lin− c-kit+ Sca-1+ cell proliferation by disrupting LPS/TLR4 signaling. In the current study, we further observed impairment of signaling activation downstream of LPS/TLR4 engagement in mice administered chronic-plus-binge alcohol. Chronic-plus-binge alcohol administration blocked the increase in ERK1/2 phosphorylation and upregulation of cyclin D1 expression at both the mRNA and protein levels in bone marrow cells following bacteremia.
Sca-1 is an 18-kDa, glycophosphatidylinositol (GPI)-anchored cell surface protein belonging to the lymphocyte activation protein-6 (Ly6) family (27). Neither natural ligand to Sca-1 nor the intracellular signaling pathway directly associated with Sca-1 has yet been identified. Along with the expansion of the lin− c-kit+ Sca-1+ cell population in the bone marrow, Sca-1 expression at both the mRNA and protein levels by lineage-negative bone marrow cells was substantially increased. Our previous studies have shown that upregulation of Sca-1 expression is associated with the enhancement of hematopoietic precursor cell commitment to granulocyte lineage development in response to serious bacterial infection (1, 28, 29). Sca-1 evidently plays a critical role in maintaining the marrow pool of Gr1+ granulocytes during the granulopoietic response to bacteremia (2). The total number of Gr1+ granulocytes in the bone marrow diminishes in Sca-1-null mice following systemic E. coli infection. Sca-1 appears to function like a coreceptor. Engagement of Sca-1 with cross-linked antibodies promotes LPS-stimulated Sca-1 gene expression, as well as other granulopoietic gene expression, by hematopoietic precursor cells (2). In this study, E. coli bacteremia caused a significant increase in Sca-1 gene expression, along with marked upregulation of Sca-1 protein expression, by lineage-negative bone marrow cells. Chronic-plus-binge alcohol administration inhibited the enhancement of Sca-1 and CEBPβ gene expression during the host response to systemic E. coli infection. At the protein level, alcohol treatment also attenuated the increase in Sca-1 expression by marrow lin− c-kit+ cells in response to the E. coli challenge (Fig. 1). In addition, bacteremia caused a marked increase in Sca-1 expression by cells in the granulocyte lineage compartment (Gr1+) in the bone marrow. This bacteremia-induced increase in penetration of Sca-1 expression into the lineage-committed granulopoietic cell population in the bone marrow was impaired by chronic-plus-binge alcohol administration. We tried determining C/EBP-β and C-EBP-α protein expression by primitive hematopoietic precursor cells using flow cytometry in combination with the intracellular/intranuclear staining technique. Our measurement was unsuccessful because signals from these transcriptional factors in the targeted cell subtypes were too weak to conduct any conclusive comparisons. Therefore, it remains to be elucidated if alcohol exposure would exert a similar effect on C/EBP-β and C-EBP-α protein expression in hematopoietic stem/progenitor cells.
In summary, our current investigation demonstrates that chronic-plus-binge alcohol administration exerts a profoundly negative impact on activation of hematopoietic precursor cells during the granulopoietic response to E. coli bacteremia. Chronic-plus-binge alcohol exposure disrupts the activation of LPS-TLR4-ERK1/2-cyclin D1 signaling and inhibits the upregulation of key granulopoietic factor expression in hematopoietic precursor cells following systemic infection with E. coli.
MATERIALS AND METHODS
Animals.
Male BALB/c mice (6 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). All the animals were housed in specific-pathogen-free facilities with a 12-h light/dark cycle. Approvals from the Institutional Animal Care and Use Committees of Northeast Ohio Medical University and Michigan State University, in adherence with National Institutes of Health guidelines, were obtained prior to initiation of all experiments.
Chronic alcohol feeding was provided to mice by maintaining them on the Lieber-DeCarli low-fat liquid alcohol diet (LED supplies 36% of calories as ethanol; 710261; Dyets, Bethlehem, PA) for 5 days and standard laboratory diet plus 20% alcohol in drinking water for 2 days per week for a total of 5 weeks (30). Pair-fed control animals were fed the isocaloric Lieber-DeCarli low-fat liquid control diet (LCD) (Dyets; 710028) and standard laboratory diet with drinking water utilizing the same schedule. The median alcohol concentration in the blood (from random morning samples) was 8.5 mM (39 mg/dl) (30) in animals fed on this protocol of chronic alcohol consumption. At the end of the 5-week feeding on an alcohol diet, acute alcohol intoxication was produced in the mice via intraperitoneal (i.p.) injection with 20% alcohol in saline at a dose of 5 g alcohol/kg body weight. Blood alcohol levels achieved in this model are in the ranges of 106.3 to 132.8, 87.7 to 122.4, and 48.4 to 61.4 mM, respectively, at 90 min, 3 h, and 6 h after alcohol administration (4, 28). (In clinical settings, blood alcohol levels in patients with alcohol intoxication can range from <17 mM to >87 mM [31]). Pair-fed mice on the isocaloric Lieber-DeCarli low-fat liquid control diet received an equal volume of saline i.p. Thirty minutes after i.p. injection of either alcohol or saline, bacteremia was induced in the mice by injection (i.v. via the penile vein) of live E. coli bacteria (strain E11775 from the American Type Culture Collection, Rockville, MD; ∼1 × 106 or ∼5 × 107 CFU in 100 μl of saline/mouse to induce different severities of infection) under isoflurane anesthesia. Controls were injected with an equal volume of pyrogen-free saline. In a subset of experiments, animals received an i.v. injection of BrdU (BD Biosciences, San Diego, CA; 1 mg in 100 μl of phosphate-buffered saline [PBS]/mouse) 24 h before termination of the experiment. Animals were sacrificed at different time points, as indicated in each figure legend. Upon sacrifice, a heparinized blood sample was obtained by cardiac puncture. WBCs were prepared by lysing erythrocytes in each blood sample with red blood cell (RBC) lysis solution (Qiagen Sciences, Germantown, MD) and then washing with RPMI 1640 medium containing 2% bovine serum albumin (BSA). Femurs and tibias were collected, and BMCs were flushed with a total volume of 2 ml RPMI 1640 medium (Life Technologies, Grand Island, NY) containing 2% BSA (HyClone Laboratories, Logan, UT) through a 23-gauge needle. The bone marrow cells were filtered through a 70-μm nylon mesh (Sefar America Inc., Kansas City, MO). Contaminating erythrocytes in bone marrow cell samples were lysed with RBC lysis solution (Qiagen Sciences). After washing with RPMI 1640 medium containing 2% BSA, the remaining nucleated BMCs were quantified under a light microscope with a hemocytometer.
Preparation of bacteria.
For each experiment, a frozen stock culture of E. coli was added to tryptic soy broth and incubated for 18 h at 37°C in an orbital shaker. Bacteria were collected and washed twice with PBS. A suspension of bacteria in PBS at a concentration of 1 × 109 CFU/ml was prepared based on its optical density at 600 nm. Actual numbers of viable bacteria were verified by standard plate counts of the bacterial suspensions on MacConkey agar plates following overnight incubation at 37°C.
Flow cytometric analysis.
Flow cytometric analysis of cells was conducted as previously described (2, 29). Briefly, nucleated bone marrow cells or white blood cells suspended in RPMI 1640 medium containing 2% BSA (1 × 106 cells in 100 μl medium) were added with a mixed panel of biotinylated anti-mouse lineage markers (10 μg/ml of each antibody against CD3e [clone 145-2C11], CD45R/B220 [clone RA3-6B2], CD11b [Mac-1; clone M1/70], and TER-119 [clone TER-119]), with or without Gr1 (Ly-6G/Ly-6C; clone RB6-8C5), or isotype control antibodies (clones A19-3, R35-95, and A95-1) (BD Biosciences). Following incubation for 15 min at 4°C, fluorochrome-conjugated streptavidin, anti-mouse c-kit (CD117; clone 2B8), and anti-Sca-1 (Ly-6A/E; clone D7), with or without anti-Gr1 (BD Biosciences), or the matched isotype control antibodies (clones A95-1 and R35-95) were added to the incubation system at a final concentration of 10 μg/ml for each agent. Samples were further incubated in the dark for 15 min at 4°C. The antibody-stained cells were then washed with cold PBS containing 2% BSA. To measure cell BrdU incorporation, cells were further processed using a BD BrdU flow kit (BD Biosciences). To measure Ki67 antigen expression by cells, cells were further processed to make them both cell membrane and nuclear membrane permeable for antibody using the procedure (without the step of DNA digestion with DNase) provided with the BD BrdU flow kit (BD Biosciences). The permeabilized cells were incubated with 10 μg/ml of fluorocome-conjugated Ki67 antibody (Santa Cruz Biotechnology, Inc., Dallas, TX) in the dark for 20 min at room temperature. At the end of the staining procedure, the cells were suspended in 0.5 ml of PBS containing 1% paraformaldehyde. Analysis of the cell phenotypes, BrdU incorporation, and Ki67 antigen expression was performed on a FACSAria III with FACSDiva software (Becton Dickinson, San Jose, CA). Cell populations of interest were gated based on their markers or marker combinations. Depending on the cell types being analyzed, the number of cells acquired in each sample was in the range of 5,000 to 300,000.
Sorting and culture of bone marrow lin− c-kit+ Sca-1− cells.
Nucleated bone marrow cells were suspended in StemSpan serum-free expansion medium (SSSFEM) (StemCell Technologies, Vancouver, BC, Canada). The staining procedure for cell surface makers was similar to that described above, except that the amount of antibodies used for each sample was increased proportionally. Sorting of marrow lin− c-kit+ cells was performed using the MACS MicroBeads isolation procedure (Miltenyi Biotec Inc., San Diego, CA). The purity of the sorted cell population achieved 90%.
For phenotypic analysis, sorted marrow lin− c-kit+ cells from healthy mice were plated in a 96-well tissue culture plate at 5 × 104 cells per well in a total volume of 100 μl SSSFEM containing 10% mouse plasma. The cells were cultured in the absence or presence of LPS (E. coli 0111:B4; 10 ng/ml; Sigma-Aldrich Co. LLC, St. Louis, MO) with or without different concentrations (50 and 100 mM) of alcohol at 37°C in an atmosphere of 5% CO2 for 72 h. BrdU (final concentration, 10 μM) was added to culture media 4 h prior to the termination of cell culture. At the end of culture, the cells were stained for surface markers and BrdU incorporation using specific fluorochrome-conjugated antibodies as previously described. Flow cytometric analysis of live (propidium iodide-negative) cells was conducted on a FACSAria III flow cytometer.
Western blot analysis.
Western blot analysis of phospho-ERK and cyclin D1 protein expression by cells was performed using a protocol reported previously (29, 32) with minor modifications. Protein was extracted from nucleated bone marrow cells with lysis buffer (10 mM Tris-HCl buffer containing 1% Triton X-100, 5 mM EDTA, 50 mM NaCl, 30 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride [PMSF], 50 mM sodium fluoride, 5 mg/ml aprotinin, 5 mg/ml pepstatin, and 5 mg/ml leupeptin, pH 7.6). The protein concentration was determined with a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Rockford, IL). Thirty micrograms of protein sample was resolved using an SDS-12% PAGE ready gel (Bio-Rad Laboratories, Hercules, CA) and transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories). The membrane was blocked with 5% milk in Tris-buffered saline with Tween 20 (TBST) buffer and hybridized sequentially with the primary antibody against phospho-ERK1/2 (anti-mouse phospho-p44/42 MAPK Thr202/Tyr204) or cyclin D1 (Cell Signaling Technology) and the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody (Cell Signaling Technology). Determination of the bound antibody was conducted using the ECL Western blotting detection system (GE Healthcare, Piscataway, NJ) and imaged using the Kodak Gel Logic 2200 imaging system (Carestream Molecular Imaging, Rochester, NY) with Kodak molecular imaging software. The membrane was stripped and then reprobed sequentially with rabbit anti-mouse total ERK1/2 or anti-β-actin antibody (Cell Signaling Technology) and the corresponding HRP-conjugated goat anti-rabbit IgG to determine total ERK1/2 or anti-β-actin content, respectively. Semiquantification was performed using ImageJ software. The data are presented as the normalized intensity ratio of the detected protein band versus the loading reference (total ERK1/2 or β-actin) band in the same sample.
Real-time RT-PCR determination.
Total RNA samples were prepared from total nucleated bone marrow cells and marrow lineage-negative cells (isolated using the MACS MicroBeads isolation procedure [Miltenyi Biotec Inc.]) using an RNeasy Plus minikit (Qiagen, Valencia, CA) and procedures provided by the manufacturer. Real-time RT-PCR analysis of mRNA expression by cells was performed as reported previously (33). Each RNA sample was subjected to 2-step real-time RT-PCR using an iScript Reverse Transcription Supermix kit and an SsoFast EvaGreen Supermix kit (Bio-Rad, Hercules, CA), respectively, on the CFX96 real-time system (Bio-Rad). The amplification primer pairs were as follows: cyclin D1, forward primer, 5′-TGCCTGCCAGGAACAGATTG, and reverse primer 5′-AGCCTCTTCCTCCACTTCCC; Sca-1, forward primer 5′-GTTTGCTGATTCTTCTTGTGGCCC, and reverse primer 5′-ACTGCTGCCTCCTGAGTAACAC; CEBPβ, forward primer 5′-AAGAAGACGGTGGACAAGCTGA, and reverse primer 5′-TTGTGCTGCGTCTCCAGGTT; CEBPα, forward primer 5′-GCGGGCAAAGCCAAGAAGTC, and reverse primer, 5′-TGTCACTGGTCAACTCCAGCAC; 18S rRNA, forward primer 5′-ATTCGAACGTCTGCCCTATAA, and reverse primer 5′-GTCACCCGTGGTCACCATG.
The sets of primers for murine cyclin D1, Sca-1, CEBPβ, CEBPα, and 18S rRNA were designed using Primer Express software (Life Technologies). The expression of cyclin D1, Sca-1, CEBPβ, and CEBPα mRNAs was determined by normalizing the cycle threshold (CT) numbers of their individual mRNAs with that of 18S rRNA in each sample. Changes in cyclin D1, Sca-1, CEBPβ, and CEBPα mRNA expression by cells from groups with different treatments are expressed as fold alterations over the baseline expression by cells from the corresponding control group.
Statistical analysis.
The data are presented as means and standard errors of the mean (SEM). The sample size is indicated in each figure legend. Statistical analysis was conducted using one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test. Differences with P values of <0.05 were accepted as statistically significant.
ACKNOWLEDGMENTS
This investigation was supported by NIH grants R01AA022816 and R01AA019676.
We have no financial conflicts of interest.
REFERENCES
- 1.Zhang P, Nelson S, Bagby GJ, Siggins RII, Shellito JE, Welsh DA. 2008. The lineage-c-Kit+Sca-1+ cell response to Escherichia coli bacteremia in Balb/c mice. Stem Cells 26:1778–1786. doi: 10.1634/stemcells.2007-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi X, Siggins RW, Stanford WL, Melvan JN, Basson MD, Zhang P. 2013. Toll-like receptor 4/stem cell antigen 1 signaling promotes hematopoietic precursor cell commitment to granulocyte development during the granulopoietic response to Escherichia coli bacteremia. Infect Immun 81:2197–2205. doi: 10.1128/IAI.01280-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaves CJ. 2015. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood 125:2605–2613. doi: 10.1182/blood-2014-12-570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang P, Welsh DA, Siggins RW II, Bagby GJ, Raasch CE, Happel KI, Nelson S. 2009. Acute alcohol intoxication inhibits the lineage-c-kit+ Sca-1+ cell response to Escherichia coli bacteremia. J Immunol 182:1568–1576. doi: 10.4049/jimmunol.182.3.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heermans EH. 1998. Booze and blood: the effects of acute and chronic alcohol abuse on the hematopoietic system. Clin Lab Sci 11:229–232. [PubMed] [Google Scholar]
- 6.Zhang P, Bagby GJ, Happel KI, Raasch CE, Nelson S. 2008. Alcohol abuse, immunosuppression, and pulmonary infection. Curr Drug Abuse Rev 1:56–67. doi: 10.2174/1874473710801010056. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien JM Jr, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, Lemeshow S, Douglas IS. 2007. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med 35:345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- 8.Gacouin A, Legay F, Camus C, Volatron AC, Barbarot N, Donnio PY, Thomas R, Le Tulzo Y. 2008. At-risk drinkers are at higher risk to acquire a bacterial infection during an intensive care unit stay than abstinent or moderate drinkers. Crit Care Med 36:1735–1741. doi: 10.1097/CCM.0b013e318174dd75. [DOI] [PubMed] [Google Scholar]
- 9.MacGregor RR, Louria DB. 1997. Alcohol and infection. Curr Clin Top Infect Dis 17:291–315. [PubMed] [Google Scholar]
- 10.Kim HY, Chang Y, Park JY, Ahn H, Cho H, Han SJ, Oh S, Kim D, Jung YJ, Kim BG, Lee KL, Kim W. 2016. Characterization of acute-on-chronic liver failure and prediction of mortality in Asian patients with active alcoholism. J Gastroenterol Hepatol 31:427–433. doi: 10.1111/jgh.13084. [DOI] [PubMed] [Google Scholar]
- 11.Breitmeier D, Becker N, Weilbach C, Albrecht K, Scheinichen D, Panning B, Schneider U, Jüttner B. 2008. Ethanol-induced malfunction of neutrophils respiratory burst on patients suffering from alcohol dependence. Alcohol Clin Exp Res 32:1708–1713. doi: 10.1111/j.1530-0277.2008.00748.x. [DOI] [PubMed] [Google Scholar]
- 12.Yeung KY, Klug PP, Lessin LS. 1988. Alcohol-induced vacuolization in bone marrow cells: ultrastructure and mechanism of formation. Blood Cells 13:487–502. [PubMed] [Google Scholar]
- 13.Dorff GJ, Rytel MW, Farmer SG, Scanlon G. 1973. Etiologies and characteristic features of pneumonias in a municipal hospital. Am J Med Sci 266:349–358. doi: 10.1097/00000441-197311000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Bertola A, Park O, Gao B. 2013. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology 58:1814–1823. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raasch CE, Zhang P, Siggins RW II, LaMotte LR, Nelson S, Bagby GJ. 2010. Acute alcohol intoxication impairs the hematopoietic precursor cell response to pneumococcal pneumonia. Alcohol Clin Exp Res 34:2035–2043. doi: 10.1111/j.1530-0277.2010.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. 1992. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood 80:3044–3050. [PubMed] [Google Scholar]
- 17.Furze RC, Rankin SM. 2008. Neutrophil mobilization and clearance in the bone marrow. Immunology 125:281–288. doi: 10.1111/j.1365-2567.2008.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, He Y, Zhou Z, Ramirez T, Gao Y, Gao Y, Ross RA, Cao H, Cai Y, Xu M, Feng D, Zhang P, Liangpunsakul S, Gao B. 2017. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47phox-oxidative stress pathway in neutrophils. Gut 66:705–715. doi: 10.1136/gutjnl-2016-311861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eash KJ, Means JM, White DW, Link DC. 2009. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 113:4711–4719. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil-Bernabe P, Boveda-Ruiz C, D'Alessandro-Gabazza C, Toda M, Miyake Y, Mifuji-Moroka R, Iwasa M, Morser J, Gabazza EC, Takei Y. 2011. Atherosclerosis amelioration by moderate alcohol consumption is associated with increased circulating levels of stromal cell-derived factor-1. Circ J 75:2269–2279. doi: 10.1253/circj.CJ-11-0026. [DOI] [PubMed] [Google Scholar]
- 21.Brown DC, Gatter KC. 2002. Ki67 protein: the immaculate deception? Histopathology 40:2–11. doi: 10.1046/j.1365-2559.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 22.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. 2006. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinstry WJ, Li CL, Rasko JE, Nicola NA, Johnson GR, Metcalf D. 1997. Cytokine receptor expression on hematopoietic stem and progenitor cells. Blood 89:65–71. [PubMed] [Google Scholar]
- 24.Guha M, Mackman N. 2001. LPS induction of gene expression in human monocytes. Cell Signal 13:85–94. doi: 10.1016/S0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 25.Torii S, Yamamoto T, Tsuchiya Y, Nishida E. 2006. ERK MAP kinase in G cell cycle progression and cancer. Cancer Sci 97:697–702. doi: 10.1111/j.1349-7006.2006.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chambard JC, Lefloch R, Pouysségur J, Lenormand P. 2007. ERK implication in cell cycle regulation. Biochim Biophys Acta 1773:1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Gumley TP, McKenzie IF, Sandrin MS. 1995. Tissue expression, structure and function of the murine Ly-6 family of molecules. Immunol Cell Biol 73:277–296. doi: 10.1038/icb.1995.45. [DOI] [PubMed] [Google Scholar]
- 28.Melvan JN, Siggins RW, Bagby GJ, Stanford WL, Welsh DA, Nelson S, Zhang P. 2011. Suppression of the stem cell antigen-1 response and granulocyte lineage expansion by alcohol during septicemia. Crit Care Med 39:2121–2130. doi: 10.1097/CCM.0b013e31821e89dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melvan JN, Siggins RW, Stanford WL, Porretta C, Nelson S, Bagby GJ, Zhang P. 2012. Alcohol impairs the myeloid proliferative response to bacteremia in mice by inhibiting the stem cell antigen-1/ERK pathway. J Immunol 188:1961–1969. doi: 10.4049/jimmunol.1102395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason CM, Dobard D, Zhang P, Nelson S. 2004. Alcohol exacerbates murine pulmonary tuberculosis. Infect Immun 72:2556–2563. doi: 10.1128/IAI.72.5.2556-2563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majori S, Ricci G, Marchiori F, Bocchi M, Zannoni M. 2015. Prevalence of acute alcohol intoxication in Borgo Trento Hospital Emergency Department (Verona). J Prev Med Hyg 56:E196–E202. [PMC free article] [PubMed] [Google Scholar]
- 32.Siggins RW, Melvan JN, Welsh DA, Bagby GJ, Nelson S, Zhang P. 2011. Alcohol suppresses the granulopoietic response to pulmonary Streptococcus pneumoniae infection with enhancement of STAT3 signaling. J Immunol 186:4306–4313. doi: 10.4049/jimmunol.1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi X, Chang CC, Basson MD, Upham BL, Wei LX, Zhang P. 2014. Alcohol disrupts human liver stem/progenitor cell proliferation and differentiation. J Stem Cell Res Ther 4:205. doi: 10.4172/2157-7633.1000205. [DOI] [PMC free article] [PubMed] [Google Scholar]