Abstract
Background
Cell-assisted lipotransfer is a novel technique for fat grafting. This study aimed to investigate the clinical efficacy of cell-assisted lipotransfer technology compared with conventional fat grafting.
Methods
According to PRISMA guidelines, related articles in PubMed, Embase and Cochrane library were systematically searched. Studies focusing on fat survival rate and/or patient satisfaction rate for fat grafting alone versus cell-assisted lipotransfer were retrieved. Estimated fat survival and patient satisfaction rates were pooled. Subgroup analysis was stratified by the transplant site. Publication bias was conducted. Furthermore, the stability of results was assessed by sensitivity analysis.
Results
Nine articles were included in the meta-analysis. Significant heterogeneity was observed among individual studies for fat survival rate assessment (I 2 = 98.3%, P < 0.001). The fat survival rate was significantly higher in the cell-assisted lipotransfer group than in the control group [weighted mean difference = 25.85, 95% confidence interval 5.39–46.31; P = 0.013]. Notably, results remained unchanged in the sensitivity analyses. No significant difference was found in the patient satisfaction rate between the cell-assisted lipotransfer and control groups [odds ratio = 3.69, 95% confidence interval 0.73–18.53; P = 0.113]. In subgroup analysis, a significantly higher patient satisfaction rate was found in cell-assisted lipotransfer fat graft group in the face (odds ratio = 18.85, 95% confidence interval 9.03, 28.68; P < 0.001) and arm (odds ratio = 64.60, 95% confidence interval 58.79, 70.41; P < 0.001) than in the controls. Finally, no significant publication bias was found (P = 0.371).
Conclusion
This study suggests that cell-assisted lipotransfer is superior to conventional lipoinjection with improved fat survival rate. However, the long-term efficacy should be evaluated in further studies.
Keywords: Fat grafting, Cell-assisted lipotransfer, Fat survival rate, Patient satisfaction rate, Meta-analysis
Background
Fat grafting was first reported in 1989, but the use of this technology was restricted due to its unpredictability and low graft survival rate [1, 2]. In 2008, a new technology based on adipose tissue-derived stromal cells (ADSCs) was reported by Yoshimura and colleagues [3]. Subsequently, cell-assisted lipotransfer (CAL) has become increasingly popular with the development of autologous fat harvesting, processing, reinjection and storage. Although autologous fat is biocompatible, nonimmunogenic and easily obtained, the fat resorption rate is found to be unstable, ranging from 20 to 80% [4]. However, the best technology for handling adipose tissue remains controversial.
Several clinical studies with favourable and unfavourable results using CAL compared with conventional lipoinjection have been reported. For example, Zhao et al. asserted that bone marrow-derived mesenchymal stem cell-assisted fat graft was more effective and safe for soft tissue than conventional fat grafting, based on high patient satisfaction and low complication rate [5]. However, Peltoniemi et al. reported similar survival rates for patients who underwent cell enrichment and water-assisted lipotransfer [6].
A meta-analysis that supported the superior clinical efficacy of CAL has been reported [7]. Subsequently, several clinical studies confirmed these findings [8, 9]. Moreover, although no study has discussed this issue, the transplant site may be one of the factors influencing the efficacy of fat grafting [10, 11]. Thus, we conducted a meta-analysis to investigate whether CAL could improve fat survival and patient satisfaction rates. We also performed a subgroup analysis stratified by the transplant site.
Methods
Search strategy
The meta-analysis was performed according to PRISMA guidelines. Related articles in PubMed, Embase and the Cochrane library were systematically searched with no language restriction. Articles published before 20 April 2017and containing the following terms were included in the study: (“fat graft” or “fat transplantation” or “lipotransfer” or “lipofilling” or “lipografts” or “autologous fat”) AND (“SVF” or “stem cell” or “ADSC” or “ASC” or “ADRC” or “cell-assisted” or “progenitor-enriched” or “cell-enhanced”).Additionally, to include more available research for meta-analysis, the reference lists of the included articles were also searched.
Study selection
The eligibility of each study was independently assessed by two investigators following the inclusion criteria: (1) the study subjects were patients who had undergone soft tissue reconstruction or filling, (2) studies assessed the clinical efficacy of autologous CAL, (3) patients in the control group were treated with fat grafting alone and (4) fat survival rate and/or patient satisfaction rate were assessed in the studies.
We excluded the following studies: (1) those in which the outcomes, including fat survival and patient satisfaction rates, were not provided or could not be calculated and (2) those studies that did not involve clinical research, such as reviews, letters and conference abstracts. If the same patients were included in more than one study, the latest reference would be included in the meta-analysis.
Data extraction
Data extraction was independently conducted by two investigators following a pre-designed extraction form. The following information would be extracted: the first author’s name, publication year, study area, follow-up period, age, BMI, sample size, intervention strategies and research outcome. The extracted information would be checked by each other after the data extraction work had been completed, and any inconsistencies would be resolved through discussion.
Statistical analysis
Patient satisfaction and fat survival rates were transformed into estimates of the odds ratio (OR) with its 95% confidence interval (95% CI) and weighted mean difference (WMD) with its 95% CI, respectively. Cochran’s Q statistic and I 2 test were used to analyse heterogeneity among individual studies [12]. If significant heterogeneity was identified (P < 0.05 or I 2 > 50%), random effects model would be used to calculate the combined effect value. Otherwise, fixed effects model would be used to combine the data.
Subgroup analysis was performed upon stratification by the transplant site. Publication bias was assessed using Egger’s test. The stability of the results was also confirmed by sensitivity analysis. All statistical analyses were performed using Stata11.0 software (Stata Corporation, College Station, TX).
Results
Literature search
A flow chart of the literature search is shown in Fig. 1. According to a pre-designed selection strategy, a total of 3617 articles were originally identified in PubMed (n = 1375), Embase (n = 2206) and the Cochrane library (n = 36). After removing duplicated articles, 2643 articles were left. A total of 2619 of these articles were then removed after reviewing the titles and/or abstracts. After reading the full text, 15 more articles were removed, and no article was included upon a manual search. Finally, nine articles were included in the meta-analysis [5, 6, 8, 9, 13–17].
Fig. 1.
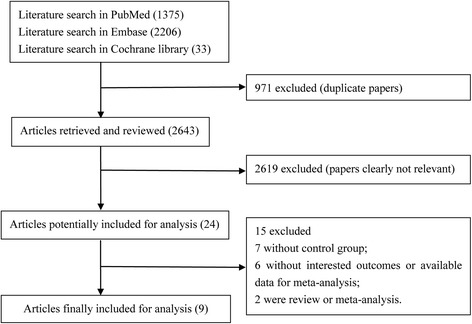
The flow chart of study selection
Characteristics of included studies
The baseline characteristics of the included studies are shown in Table 1. The nine studies were reported between 2011 and 2016. The regions of the included studies widely varied, including the USA, Brazil, China, Finland and Denmark. Three transplant sites were included in the meta-analysis, which were the face, breast and arm. The follow-up period was 4–36 months. Patients in the study by Zhao et al. were treated with a bone marrow-derived mesenchymal stem cell fat graft [5], and patients in the other studies were treated with a stromal vascular fraction fat graft [6, 8, 9, 13–17]. Patients in two studies underwent two or three fat grafts [13, 16], whereas patients in the other studies underwent a single one [5, 6, 8, 9, 14, 15, 17].
Table 1.
The baseline characteristics of included studies
| Study, year, country | Recipient sites | Follow-up (months) | VMM | No. of operations | Group | Number | Age, years | BMI | Gender, M/F | FSR, % | Satisfactory |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissiani LA, 2016, Brazil | Breast | 36 | MRI | 1 | SVF fat | 11 | 43–58 | 23.5–30.9 | 0/11 | 78.8 (74.9) | NR |
| 1 | Fat | 8 | 36–69 | 20.8–32.4 | 0/8 | 51.4 (18.4) | NR | ||||
| Sasaki GH, 2015, USA | Face | 12 | Photography | 1 | SVF fat | 9 | 65.5 (52–77) | 22.0 (21.0–30.8) | NR | 72.9 (50.0) | NR |
| 1 | Fat | 92 | 60.5 (58–63) | 22.0 (19.9–24.2) | 38.3 (12.9) | NR | |||||
| Zhao JH, 2014, China | Face | 14 | NA | 1 | BMSC fat | 10 | 25 (20–35) | NR | 3/7 | NR | 10 |
| 1 | Fat | 26 | 24 (18–38) | NR | 8/18 | NR | 24 | ||||
| Tanikawa DY, 2013, Brazil | Face | 6 | CT | 1 | SVF fat | 7 | 12.1 ± 2.2 | < 25 | 2/5 | 88.0 (13.0) | 7 |
| 1 | Fat | 7 | 18.7 ± 7.6 | < 25 | 3/4 | 54.0 (20.0) | 3 | ||||
| Peltoniemi HH, 2013, Finland | Breast | 6 | MRI | 1 | SVF fat | 10 | 51 (29–58) | 23.4 (20.3–32.5) | 0/10 | 50.0 (10.0) | NR |
| 1 | Fat | 8 | 39 (33–63) | 23.4 (20.3–25.9) | 0/8 | 54.0 (7.0) | NR | ||||
| Li J, 2013, China | Face | 6 | CT | 1 | SVF fat | 26 | 29.5 ± 6.8 | NR | 0/26 | 64.8 (10.2) | NR |
| 1 | Fat | 12 | 29.1 ± 6.0 | NR | 0/12 | 46.4 (9.3) | NR | ||||
| Kolle SF, 2013, Denmark | Arm | 4 | MRI | 1 | SVF fat | 10 | 28.4 ± 8.9 | 24.7 ± 2.0 | NR | 80.9 (6.0) | NR |
| Fat | 10 | 16.3 (7.2) | NR | ||||||||
| Chang Q, 2013, China | Face | 18 | CT | 2 in 30%, 3 in 20% | SVF fat | 10 | 27.5 (19–35) | NR | 8/12 | 68.3 (1.7) | NR |
| Fat | 10 | 58.5 (1.3) | NR | ||||||||
| Sterodimas A, 2011, Brazil | Face | 18 | NA | 1 | SVF fat | 10 | 43.9 (22–72) | NR | 3/7 | NR | 9 |
| 2 in 30%, 3 in 40% | Fat | 10 | 46.2 (25–70) | 2/8 | NR | 9 |
BMSC, bone marrow-derived mesenchymal stem cells; CT, computed tomographic; F, female; FSR, fat survival rate; M, male; MRI, magnetic resonance imaging; NR, not reported; NA, not available; SVF, stromal vascular fraction; US, ultrasound; VMM, volumetric measurement method
Meta-analysis of fat survival and patient satisfaction rates
As shown in Fig. 2a, fat survival rate was reported in seven studies [6, 8, 9, 13–15, 17]. Significant heterogeneity in this variable was observed among individual studies (I 2 = 98.3%, P < 0.001), and the random effects model was used to pool estimates of fat survival rate. The fat survival rate was significantly higher in the CAL group than in the control group (WMD = 25.85, 95% CI 5.39, 46.31; P = 0.013). The results of sensitivity analysis are shown in Fig. 2b and revealed that the results after removing each article remained unchanged, suggesting the stability of the meta-analysis.
Fig. 2.
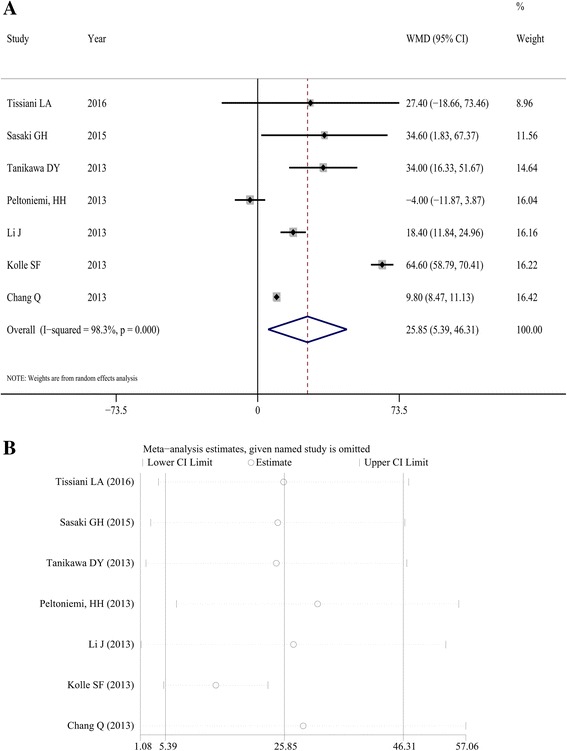
Comparison between cell-assisted lipotransfer (CAL) and conventional fat grafting regarding fat survival rate. a Forest map for fat survival rate comparison between CAL and conventional fat grafting. b Sensitivity analysis
As shown in Fig. 3, patient satisfaction rate was reported in three studies [5, 16, 17]. Heterogeneity among individual studies was not statistically significant (I 2 = 0.0%, P = 0.383), and the fixed effects model was used to pool data on patient satisfaction rate. No significant difference was found in patient satisfaction rate between the CAL and control groups (OR = 3.69, 95% CI 0.73, 18.53; P = 0.113).
Fig. 3.
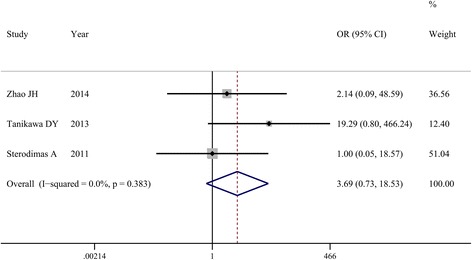
Comparison between cell-assisted lipotransfer (CAL) and conventional fat grafting regarding patient satisfaction rate
Subgroup analysis
Subgroup analysis for patient satisfaction rate was stratified by the transplant site, and the results for the subgroup analysis are shown in Table 2. No significant difference was found between the CAL and control groups regarding breast fat transfer (OR = 3.16, 95% CI − 22.66, 28.99; P = 0.810). A significantly higher patient satisfaction rate was found in the CAL group for face fat graft than in the control group (OR = 18.85, 95% CI 9.03, 28.68; P < 0.001). CAL was associated with a significantly higher patient satisfaction rate for arm fat graft (OR = 64.60, 95% CI 58.79, 70.41; P < 0.001).
Table 2.
Subgroup analysis for fat survival rate
| Subgroup | N | WMD (95% CI) | P a | Ph | I 2 (%) |
|---|---|---|---|---|---|
| Breast | 2 | 3.16 (− 22.66, 28.99) | 0.810 | 0.188 | 42.3 |
| Face | 4 | 18.85 (9.03, 28.68) | < 0.001 | 0.001 | 80.5 |
| Arm | 1 | 64.60 (58.79, 70.41) | < 0.001 | – | – |
N number of study, WMD weighted mean difference, P a, P value of association, Ph, P value of heterogeneity, CI confidence interval
Publication bias
Publication bias was analysed based on data for fat survival rate. No significant publication bias was found using Egger’s test (P = 0.371).
Discussion
This study attempted to systematically investigate the clinical efficacy of CAL technology compared with conventional fat grafting. In total, nine articles were included in the meta-analysis. This study demonstrated that fat survival rate was significantly higher for patients using CAL. Although no significant difference was found in patient satisfaction rate between the CAL and control groups, a significantly higher patient satisfaction rate was found among patients who underwent CAL fat graft in the face and arm than in the controls. Thus, we suggest that CAL is superior to conventional lipoinjection with improved fat survival rate. However, the long-term efficacy should be evaluated in a further study.
Human adipose tissue was recommended as an ideal source of autologous cells because it is plentiful and easily obtained. Graft take rate and volume retention could be highly improved using CAL, which transforms poor-adipose derived stromal cells fat grafts into enriched ones [3]. Although the mechanism associated with fat graft survival remains unclear, lack of adequate neovascularisation has been recognised as one of the reasons for graft loss. Previous evidence supported that CAL had the ability on adipogenesis and angiogenesis in the adipose repair process [18, 19]. By pooling data from previous clinical data, we proved that CAL was superior to conventional lipoinjection with improved fat survival rate. However, the precise mechanism of CAL on improving fat survival rate should be further studied.
Although a significant improvement in fat survival rate in CAL fat enrichment was demonstrated in the meta-analysis, significant heterogeneity in this variable among the studies should not be ignored. It is known that fat survival rate is affected by the method of obtaining, isolating and preparing cells in different clinical settings [20, 21]. This would make it difficult to compare studies according to different techniques used in the surgery. Moreover, after transfer, the amount of fibrosis induced and the number of viable fat cells were reported to be the main factors for the clinical longevity of the correction [22–24]. Additionally, the survival of fat cell grafts would also be affected by the anatomic site and the mobility and vascularity of the recipient tissue [25]. Fat graft results would also depend on the background of patients, technique used and surgeon’s expertise. However, the above factors could not be fully balanced among individual studies in the meta-analysis, which may explain the significant heterogeneity. For the technology of fat grafting, further technical and outcome standardisation is thus required.
No significant difference was found between CAL and conventional fat grafting in breast fat transfer. Patients were more satisfied with CAL in the arm and face than with conventional fat grafting. No recent study has provided evidence regarding the fat transfer site that is superior. However, it should be recommended that clinical efficacy be assessed based on transplant site.
The strengths of this meta-analysis are as follows: First, the clinical efficacy of CAL or fat grafting alone was quantitatively analysed based on case-control studies. Second, although significant heterogeneity was found for data on fat survival rate, the findings of sensitivity analysis guaranteed the stability of the results. Third, no significant publication bias was found in the meta-analysis.
Some limitations should also be noted in the meta-analysis. First, only four out of nine included studies were randomised controlled trials. Although the quality of included studies was fit for the meta-analysis, the type of research design might limit the strength of the conclusion [9, 14, 16, 17]. Moreover, fat grafts were randomly injected into the posterior part of the right and left upper arms in the study by Kølle et al. [14]. Therefore, analysis of the quality of the included studies could not be performed in the meta-analysis. Second, the number of included studies was small; therefore, further study is needed to verify the current conclusion by incorporating more randomised controlled trials with high quality.
Conclusion
Numerous methods have been proposed to enhance the survival of fat grafts, but no definitive treatment protocol is available. CAL offers new perspectives for improving fat graft survival. In summary, this study suggests that CAL is superior to conventional lipoinjection with improved fat survival rate. However, its long-term clinical efficacy should be evaluated in a further study.
Acknowledgements
None
Funding
None
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the PubMed, Embase and Cochrane library.
Abbreviations
- 95% CI
95% confidence interval
- ADSCs
Adipose tissue-derived stromal cells
- CAL
Cell-assisted lipotransfer
- OR
Odds ratio
- WMD
Weighted mean difference
Authors’ contributions
YWg selected the studies, extracted the data, performed the meta-analysis and wrote the manuscript. YWu designed the study and the extracted data. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu Wang, Email: FennyYW88@163.com.
Yanfei Wu, Phone: +86-0573-83643658, Email: wang029165@163.com.
References
- 1.Billings E, Jr, May JW., Jr Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg. 1989;83:368–381. doi: 10.1097/00006534-198902000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Gir P, Brown SA, Oni G, Kashefi N, Mojallal A, Rohrich RJ. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130:249–258. doi: 10.1097/PRS.0b013e318254b4d3. [DOI] [PubMed] [Google Scholar]
- 3.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthet Plast Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiryaki T, Findikli N, Tiryaki D. Staged stem cell-enriched tissue (SET) injections for soft tissue augmentation in hostile recipient areas: a preliminary report. Aesthet Plast Surg. 2011;35:965–971. doi: 10.1007/s00266-011-9716-x. [DOI] [PubMed] [Google Scholar]
- 5.Jianhui Z, Chenggang Y, Binglun L, Yan H, Li Y, Xianjie M, Yingjun S, Shuzhong G. Autologous fat graft and bone marrow-derived mesenchymal stem cells assisted fat graft for treatment of Parry-Romberg syndrome. Ann Plast Surg. 2014;73(Suppl 1):S99–103. doi: 10.1097/SAP.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 6.Peltoniemi HH, Salmi A, Miettinen S, Mannerstrom B, Saariniemi K, Mikkonen R, Kuokkanen H, Herold C. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: a prospective comparative study. J Plast Reconstr Aesthet Surg. 2013;66:1494–1503. doi: 10.1016/j.bjps.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y, Wang J, Li H, Liang X, Bae J, Huang X, Li Q. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:44e–57e. doi: 10.1097/PRS.0000000000001981. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki GH. The safety and efficacy of cell-assisted fat grafting to traditional fat grafting in the anterior mid-face: an indirect assessment by 3D imaging. Aesthet Plast Surg. 2015;39:833–846. doi: 10.1007/s00266-015-0533-5. [DOI] [PubMed] [Google Scholar]
- 9.Tissiani LA, Alonso N. A prospective and controlled clinical trial on stromal vascular fraction enriched fat grafts in secondary breast reconstruction. Stem Cells Int. 2016;2016:2636454. doi: 10.1155/2016/2636454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma ZW, Liu LD, Li K, Zhang YJ, Dong JH. Improvement of graft function and animal survival by fat emulsion in liver transplant rats. Colloids Surf B Biointerfaces. 2007:54, 25–32. doi: 10.1016/S0927-7765(06)00170-6. [DOI] [PubMed]
- 11.Patrick CM, Hayashi PH, Kozlowski T, Greene KG, Semelka RC, Barritt AS. Focal fat masquerading as malignancy in the liver graft of a post-transplant patient. Dig Dis Sci. 2011;56:3382–3385. doi: 10.1007/s10620-011-1739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Q, Li J, Dong Z, Liu L, Lu F. Quantitative volumetric analysis of progressive hemifacial atrophy corrected using stromal vascular fraction-supplemented autologous fat grafts. Dermatol Surg. 2013;39:1465–1473. doi: 10.1111/dsu.12310. [DOI] [PubMed] [Google Scholar]
- 14.Kolle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M, Rasmussen BS, Talman ML, Thomsen C, Dickmeiss E, Drzewiecki KT. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382:1113–1120. doi: 10.1016/S0140-6736(13)61410-5. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Gao J, Cha P, Chang Q, Liao Y, Liu C, Li K, Lu F. Supplementing fat grafts with adipose stromal cells for cosmetic facial contouring. Dermatol Surg. 2013;39:449–456. doi: 10.1111/dsu.12058. [DOI] [PubMed] [Google Scholar]
- 16.Sterodimas A, de Faria J, Nicaretta B, Boriani F. Autologous fat transplantation versus adipose-derived stem cell-enriched lipografts: a study. Aesthet Surg J. 2011;31:682–693. doi: 10.1177/1090820X11415976. [DOI] [PubMed] [Google Scholar]
- 17.Tanikawa DY, Aguena M, Bueno DF, Passos-Bueno MR, Alonso N. Fat grafts supplemented with adipose-derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast Reconstr Surg. 2013;132:141–152. doi: 10.1097/PRS.0b013e3182910a82. [DOI] [PubMed] [Google Scholar]
- 18.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider S, Unger M, van Griensven M, Balmayor ER. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur J Med Res. 2017:22–17. doi: 10.1186/s40001-017-0258-9. [DOI] [PMC free article] [PubMed]
- 20.Sommer B, Sattler G. Current concepts of fat graft survival: histology of aspirated adipose tissue and review of the literature. Dermatol Surg. 2000;26:1159–1166. doi: 10.1046/j.1524-4725.2000.00278.x. [DOI] [PubMed] [Google Scholar]
- 21.Morgani SM, Brickman JM. Survival of the fattest. elife. 2013;2:e01760. doi: 10.7554/eLife.01760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Contreras GA, Thelen K, Schmidt SE, Strieder-Barboza C, Preseault CL, Raphael W, Kiupel M, Caron J, Lock AL. Adipose tissue remodeling in late-lactation dairy cows during feed-restriction-induced negative energy balance. J Dairy Sci. 2016;99:10009–10021. doi: 10.3168/jds.2016-11552. [DOI] [PubMed] [Google Scholar]
- 23.Mottillo EP. Adipose tissue remodeling during endurance training. Exerc Sport Sci Rev. 2016;44:3. doi: 10.1249/JES.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 24.Kakudo N, Tanaka Y, Morimoto N, Ogawa T, Kushida S, Hara T, Kusumoto K. Adipose-derived regenerative cell (ADRC)-enriched fat grafting: optimal cell concentration and effects on grafted fat characteristics. J Transl Med. 2013;11:254. doi: 10.1186/1479-5876-11-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaoutzanis C, Xin M, Ballard TN, Welch KB, Momoh AO, Kozlow JH, Brown DL, Cederna PS, Wilkins EG. Autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76:270–275. doi: 10.1097/SAP.0000000000000561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the PubMed, Embase and Cochrane library.


