Abstract
Oocyte cryopreservation is the technique of choice for the long-term storage of female gametes. However, it induces an irreversible loss of oocyte viability and function. We examined the effects of vitrification and a Rho-associated coiled-coil containing protein kinase 1 (ROCK1) inhibitor (ROCKi) on the meiotic and developmental competence of feline oocytes. We examined the expression of LIM kinase (LIMK) 1 and 2, with and without ROCKi treatment. Cumulus oocyte complexes (COCs) were matured in vitro with 0, 10, 20, and 40 µM ROCKi. The oocytes were subsequently assessed for maturation rate and embryo development following in vitro fertilization. We repeated the COC experiment, but vitrified and warmed the COCs prior to culture. We detected LIMK1 and LIMK2 expression in feline oocytes, which could be downregulated by ROCKi treatment. The ROCKi at 10 µM affected neither meiotic nor developmental competence (P > 0.05, versus control). However, high concentrations of ROCKi during maturation induced meiotic arrest at metaphase I. Appropriate concentrations of ROCKi significantly improved the normal fertilization rate of vitrified warmed oocytes (49.4 ± 3.4%) compared with that of the control (42.8 ± 8.6%, P < 0.05). The ROCKi also significantly improved the embryo cleavage rate (36.1 ± 3.8%) as compared with the non-treated control (27.4 ± 2.5%, P < 0.05). Thus, this study revealed that the main mediators of the ROCK cascade (LIM kinases) are expressed in feline oocytes. The ROCKi (10 µM) did not compromise the meiotic or developmental competence of feline oocytes. In addition, 10 µM ROCKi improved the cytoplasmic maturation of vitrified–warmed oocytes as indicated by their fertilization competence.
Keywords: Domestic cat, Embryo development, Oocyte, ROCK inhibitor, Vitrification
Oocyte cryopreservation is the method of choice for genetic preservation. This technique has predominantly been used in human and veterinary medicine for addressing the premature loss of fertility due to gonadal-toxic cancer therapy and for the genetic banking of desired animals, such as valuable or endangered species [1]. However, successful oocyte cryopreservation has been hampered by oocyte structural complexity and the limited availability of samples for study. Oocytes are the largest cells in the body, with a sophisticated structure and low membrane permeability to water and cryoprotectants [2]. As a result, freezing and thawing frequently result in irreversible cryodamage at the cellular and subcellular levels. Although several types of cryoinjuries have been identified, such as disruption of cell organelles [3, 4], the cytoskeleton [5,6,7,8], and the plasma membrane [7, 9, 10], the exact mechanisms that cause cell injury remain largely unknown. Freezing techniques including controlled-rate cryopreservation and vitrification are frequently used for oocyte cryopreservation. However, extensive intracellular ice formation renders irreversible cell damage to mammalian oocytes during cooling at a suboptimal rate [2, 3, 6, 9]. Therefore, a novel approach for ice-free cryopreservation by means of vitrification has been increasingly used. Vitrification is beneficial as it is simple and less time-consuming compared to controlled-rate freezing. However, high cryoprotectant concentrations and extremely high rates of freezing are required for this method [2, 3, 11]. These factors contribute to the poor quality of oocytes after vitrification, as the high cryoprotectant concentration is frequently toxic and can cause excessive osmotic stress [6, 11]. This method of cryopreservation has also been reported to activate apoptotic pathways, including intrinsic and extrinsic mechanisms [7].
In the domestic cat, information regarding the cryopreservability of immature oocytes is limited and overall success is currently poor [12,13,14,15]. The types of cryoinjury that occur during freezing and thawing are also poorly characterized. Of the potential cryoinjuries, cryopreservation-induced apoptosis and cell death have been reported [16,17,18,19,20,21]. Rho-associated coiled-coil containing protein kinase 1 (ROCK1) is a downstream target of the small GTP-binding protein Rho, which has been identified as a central regulator of apoptosis via activation of the lipase modulator (LIM) kinases and cofilin. These elements act synergistically to balance actin cytoskeleton dynamics and functions [5, 8, 22]. The cell cytoskeleton plays an essential role during oocyte maturation [5, 8, 22, 23], and preservation of its structure and function is essential for oocyte developmental competence following cryopreservation [10, 24]. ROCK1 and cryopreservation could also synergistically activate caspase-dependent apoptotic pathways and cell death following freezing and thawing.
Treatment with an inhibitor of ROCK (ROCKi; Y-27632) has been shown to improve the viability of frozen–thawed human embryonic and induced pluripotent stem cells [25,26,27]. Although the protective mechanism of this small molecule is not entirely clear, it has also been reported to be beneficial for bovine oocyte and blastocyst cryopreservation [28].
This study aimed to determine the effects of vitrification and a ROCKi (Y-27632) on the meiotic and developmental competence of feline oocytes. We examined the protective effects of this small molecule on oocytes against cryoinjury during vitrification and warming.
Materials and Methods
Experiment 1: Identification of a LIMK-associated ROCK pathway in feline COCs
COCs were collected from feline ovaries. The oocytes (n = 75, 3 replicates) were completely separated from the cumulus cells. The presence of LIMK1 and LIMK2 mRNA was investigated using RT-PCR. In addition, oocytes matured in vitro with (n = 90) and without ROCKi (10 µM ROCKi, n = 75) for 12 h were used to quantitatively demonstrate the effects of ROCKi on LIMK1 and LIMK2 mRNA levels.
Experiment 2: Determination of optimal concentrations of ROCKi to promote oocyte meiotic and embryo competence
Experiment 2.1: The COCs were randomly subjected to IVM with different concentrations of ROCKi (10, 20, and 40 µM; n = 90 per group). Oocytes subjected to IVM without ROCKi (0 µM) served as the control group. After 24 h of IVM, the oocytes were assessed for their stage of nuclear maturation. The stages of nuclear maturation of the feline oocytes were classified as germinal vesicle stage (GV), metaphase I (MI), metaphase II (MII), and degenerate oocytes.
Experiment 2.2: This experiment was performed as Experiment 2.1, except that the IVM oocytes (n = 90 per group) were further fertilized in vitro. After IVF, embryo development was classified as cleaved, morula, and blastocyst stages on days 2, 5, and 7 of development, respectively.
Experiment 3: Effects of different concentrations of ROCKi on oocyte meiotic and developmental competence following vitrification and warming
Vitrified–warmed oocytes were cultured in maturation medium containing different concentrations of ROCKi (0, 10, 20, and 40 µM; n = 90 per group). The meiotic competence was assessed after 24 h of IVM. The fertilization competence of the oocytes was assessed at 18 h post-IVF, in terms of the percentages of sperm penetration and pronucleus formation. The developmental competence was determined based on the cleavage, and morula and blastocyst formation rates. Blastocyst quality was examined by cell count.
Reagents
All chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified.
Source of ovaries
Ovaries from domestic cats (Felis catus) were collected after routine ovariohysterectomy and generously provided by the Veterinary Public Health Division of the Bangkok Metropolitan Administration, according to a protocol approved by the Chulalongkorn University of Animal Care and Use. The ovaries were collected in 0.9% (w/v) normal saline solution with 100 IU/ml penicillin and 100 IU/ml streptomycin. Then, the ovaries were transported at approximately 26°C to the laboratory within 2 h.
Oocyte isolation
The ovaries were maintained in a holding medium (HM) (HEPES-buffered M199 supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 100 IU/ml penicillin, and 4 mg/ml bovine serum albumin (BSA; embryo-tested)). The tissues were minced in HM to release cumulus oocyte complexes (COCs). The COCs were morphologically classified under a 40 × magnified stereomicroscope (SMZ645; Nikon, Tokyo, Japan). Only grade A and B oocytes were used in this study. Grade A and B oocytes were fully surrounded by more than 5 layers of compacted cumulus cells and had homogenously darkened cytoplasm.
In vitro maturation and fertilization
In vitro oocyte maturation (IVM) and fertilization (IVF) were performed as previously described [29, 30] with some modifications. For IVM, a group of COCs (approximately 30–40 COCs) was cultured in 500 µl of IVM medium (NaHCO3-buffered M199 with 1 mM sodium pyruvate, 2 mM L-glutamine, 100 IU/ml penicillin, 50 µg/ml gentamicin, 4 mg/ml BSA, 0.05 IU/ml recombinant human follicle-stimulating hormone (rhFSH; Organon, Bangkok, Thailand), and 25 ng/ml epidermal growth factor). After 24 h of IVM, COCs were transferred to 50-µl droplets of IVF medium comprising synthetic oviductal fluid (SOF) with 6 mg/ml BSA. The frozen–thawed semen used in this study was obtained from a fertility-proven male cat. Semen was collected via electroejaculation, then equilibrated at 4°C with an egg yolk TRIS buffer containing 5% (v/v) glycerol for 1 h. The equilibrated sperm were then loaded into freezing straws and layered onto liquid nitrogen vapors for 10 min. The straws were plunged and kept in liquid nitrogen until use. The frozen semen was thawed at 37°C for 30 sec and evaluated subjectively. Aliquots of sperm with more than 50% progressive motility were added to each IVF droplet for a final concentration of 5 × 105 sperm/ml. Oocyte culture was performed at 38.5°C in a humidified atmosphere of 5% CO2 in air.
In vitro embryo culture
After 24 h of IVF, the presumptive zygotes were gently denuded and cultured for 24 h in 50-µl droplets of SOF containing 4 mg/ml BSA and antibiotic (100 IU/ml penicillin and 100 IU/ml streptomycin). Cleaved embryos (day 0 = day of IVF) were further cultured in SOF supplemented with 10% (v/v) fetal calf serum. The culture medium was changed every other day. This culture system was maintained at 38.5°C in a humidified atmosphere of 5% CO2 in air.
Assessment of fertilization competence
After 18 h of IVF, the fertilization competence of vitrified–warmed oocytes was assessed by observation of pronucleus formation. The presumptive zygotes were denuded and fixed in 4% (w/v) paraformaldehyde and stained with 4, 6-diamidino-2-phenylindole (DAPI). Pronucleus formation was examined using an epifluorescence microscope. The IVF treated oocytes or presumptive zygotes were classified, and the percentages categorized as total fertilization (sperm-penetrated oocytes), normal fertilization, abnormal fertilization, and degenerate oocytes were calculated in relation to the total oocytes inseminated. The presence of sperm or a male pronucleus within the ooplasm following IVF indicated a fertilized oocyte. The presumptive zygotes with 2 pronuclei (male and female pronuclei) were classified as normal fertilization. Any abnormalities of pronucleus formation, such as single or multiple pronuclei (> 3), indicated abnormal fertilization. Contracted oocytes without chromatin and/or with dispersed chromatin were classified as degenerate oocytes.
Assessment of embryo development
All cleaved embryos were morphologically observed under an inverted microscope (40 × magnification) to evaluate morulae and blastocysts on day 5 and day 7, respectively (day of IVF = day 0). On day 7, all embryos were fixed in 4% (w/v) paraformaldehyde and stained with 0.1 µg/ml DAPI. Total cell counts were performed using an epifluorescence microscope (Olympus, Japan). The percentages of morulae (> 16 cells without blastocoels) and blastocysts (> 50 cells with blastocoel formation) were recorded.
Oocyte cryopreservation
Open pulled straw (OPS) vitrification of COCs was performed as previously described [15]. The 4-step cryoprotective agent (CPA) exposure technique was performed by incubating the COCs in vitrification media containing different concentrations of ethylene glycol. The COCs were finally loaded into the OPS by capillary effect and immediately submerged into liquid nitrogen. Warming of vitrified COCs was carried out at 37°C by immersing the OPS in HM supplemented with gradually decreasing concentrations of sucrose (0.25 M, 0.125 M, and 0.0625 M, 5 min/step). The vitrified–warmed COCs were kept in HM at 37°C until the next procedure.
RNA extraction
Cumulus cells were mechanically denuded by gentle pipetting. The denuded oocytes were washed in phosphate-buffered saline and kept for RNA extraction [30]. Total RNA was extracted using the Absolutely RNA Nanoprep Kit (Stratagene™, Agilent Technologies, Santa Clara, CA, USA), according to the manufacturer’s instructions, at room temperature (25–30°C). The extracted RNA was assessed for quality using a spectrophotometer (NanoDrop™ 2000, Wilmington, DE, USA) and immediately stored at –80°C.
Quantitative reverse transcription PCR
Reverse transcription (RT) was performed using a First Strand cDNA Synthesis Kit (Superscript III Kit, Invitrogen, Carlsbad, California, USA) according to the manufacturer’s instructions. The products were stored at –20°C for quantitative PCR (qPCR). The relative expression levels of LIM kinase 1 (LIMK1) and LIM kinase 2 (LIMK2) were normalized to the endogenous control gene, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (YWHAZ) [31]. The primers used in this study are listed in Table 1. The LIMK1 and LIMK2 primers were designed based on the sequences of Felis catusLIMK1 and LIMK2, using an NCBI primer design tool, Primer3 software v 4.0.0.
Table 1. Primers for qRT-PCR.
| Gene | Primer (5′-3′ orientation) | Amplicon size (kb) | Reference/ |
| Accession No. | |||
| LIMK1 | Forward: CTGGTCCGAGAGAACAAGAA | 227 | XM_003998592.3 |
| Reverse: ATCTCACACAGGACGATTCC | |||
| LIMK2 | Forward: GTTCAAGTACCACCCAGAGT | 108 | XM_003994813.3 |
| Reverse: CACTTCCCACAGTAAAGGGT | |||
| YWHAZ | Forward: GAAGAGTCCTACAAAGACAGCACGC | 115 | [32] |
| Reverse: AATTTTCCCCTCCTTCTCCTGC |
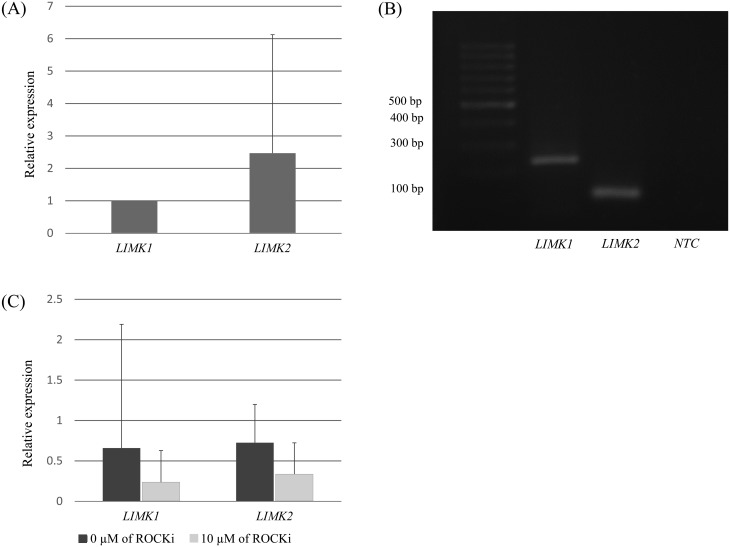
We used an Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with Luminaris Color HiGreen qPCR Master Mix, High ROX (Thermo Scientific, Carlsbad, CA, USA) for qPCR. Each qPCR sample (10 µl) consisted of 2.5 µl of DNA template mixture (2 µl of reverse transcription product or no template as a control, with 0.5 µl of yellow sample buffer) and 7.5 µl of a mixture containing 5 µl of Luminaris Color HiGreen qPCR Master Mix, High ROX, 0.3 µl of each forward and reverse primer, and 1.9 µl of nuclease-free water. The thermal cycling conditions were as follows: pretreatment with uracil-DNA glycolysase (UDG) with 50°C 2 min, activate Tag DNA polymerase with 95°C for 10 min, and 55 cycles of 95°C for 15 sec for denaturation, 60°C for 30 sec for annealing, and 72 for 60 sec for extension. The relative expression levels were analyzed by Sequence Detection System (SDS) Software (Applied Biosystems). LIMK1 expression in fresh oocytes prior to oocyte maturation served as a control, in order to compare its relative expression to LIMK2. The relative expression of these kinases after 12 h of maturation were also compared with control (no ROCKi) and 10 µM ROCKi-treated oocytes (Fig. 1A and 1C).
Fig. 1.
The mRNA expression of LIMK1 and LIMK2 in feline oocytes (A). The PCR products were confirmed by electrophoresis (B). qRT-PCR expression of LIMK1 and LIMK2 in feline oocytes matured for 12 h with or without ROCKi (10 µM) (C).
The PCR products were confirmed by electrophoresis on a 1% (w/v) agarose gel (Bio-Rad, Hercules, CA, USA) containing 5% (v/v) RedSafe Nucleic Acid Staining Solution (iNtRON Biotechnology, Gyeonggi-do, Korea) in TBE (Tris/Borate/EDTA) buffer. The amplified products (Fig. 1B) were examined under UV light using a gel documentation system (Syngene, Cambridge, UK).
Statistical analysis
All experiments were performed in triplicate or quadruplicate. Data were tested for normality and equal variance using the median test, and are expressed as mean ± standard deviation (SD) for each experiment. The fertilization competence of vitrified-warmed oocytes was defined based on the percentages of total fertilization, normal fertilization, abnormal fertilization, and degenerate oocytes. The percentage of cleaved embryos was defined as the number of cleaved embryos relative to the total number of oocytes in each group. The oocytes that were fertilized by sperm (sperm penetration) and the early stage embryos prior to cellular division at 48 h post-fertilization were excluded. The proportions of morulae and blastocysts were relative to the total number of cleaved embryos. Logistic regression was used to test the differences in the maturation stage of the oocytes, fertilization competence, and developmental rate of the embryos (cleavage, and morula and blastocyst formation rates). Multiple analysis of variance (ANOVA) and least significant difference post-hoc tests were used for statistical comparisons of the cell numbers within the blastocysts. Differences in quantitative mRNA expression were assessed by Student’s t-test (relative expression between LIMK1 and LIMK2 in fresh oocytes) and ANOVA (Experiment 1). All statistical analyses were performed with SPSS version 22.0.0 (IBM, Armonk, NY, USA). P values less than 0.05 were considered statistically significant.
Results
Experiment 1: Identification of a LIMK-associated ROCK pathway in feline COCs
Feline oocytes expressed LIMK1 and LIMK2, as shown in Fig. 1A and 1C. qRT-PCR revealed that LIMK2 mRNA was expressed at a greater level than LIMK1 (P > 0.05, Fig. 1A). The sizes of the PCR products were confirmed by electrophoresis (Fig. 1B). ROCKi supplementation during IVM downregulated LIMK1 and LIMK2 in feline oocytes, compared with the levels in the control (0 µM ROCKi). This finding indicated the bioactivity of the ROCKi in feline oocytes.
Experiment 2: Determination of toxicity of ROCKi on meiotic and embryo competence
The effects of the ROCKi on oocyte meiotic and embryo competence are shown in Table 2. The ROCKi affected the meiotic resumption of the feline oocytes during IVM in a dose-dependent manner. ROCKi at 10 µM did not improve MII rates compared to the control (66.3 ± 6.2% vs. 58.0 ± 4.3%). High concentrations of the inhibitor (20 µM and 40 µM) significantly decreased meiotic competence, which appeared to be caused by a high incidence of MI arrest (Table 2). By contrast, treating the oocytes with the ROCKi during IVM did not affect embryo developmental competence, in terms of cleavage, or morula or blastocyst formation rates.
Table 2. The effect of the ROCKi on IVM and embryo development of non-cryopreserved feline COCs.
| ROCKi concentration (µM) |
Stage of nuclear maturation |
Stage of embryo development |
Blastocyst cell number | |||||
| Degenerate (%) | GV (%) | MI (%) | MII (%) | Cleavage * | Morula ** | Blastocyst ** | ||
| 0 | 6.9 ± 0.5 | 12.2 ± 0 | 22.4 ± 0.5 | 58.0 ± 4.3 a) | 55.7 ± 4.4 | 59.3 ± 22.1 | 52.5 ± 12.7 | 147.2 ± 11.0 |
| 10 | 7.7 ± 0.4 | 4.8 ± 3.7 | 21.1 ± 7.6 | 66.3 ± 6.2 a) | 53.3 ± 9.5 | 60.3 ± 7.0 | 40.9 ± 22.8 | 176.3 ± 17.6 |
| 20 | 7.3 ± 0.4 | 4.8 ± 4.0 | 35.8 ± 8.6 | 47.8 ± 6.9 b) | 58.6 ± 14.4 | 53.3 ± 9.5 | 35.1 ± 6.4 | 135.4 ± 12.8 |
| 40 | 7.8 ± 0.4 | 7.8 ± 1.4 | 49.4 ± 10.1 | 35.1 ± 12.7 c) | 48.6 ± 11.6 | 58.7 ± 15.1 | 37.0 ± 1.5 | 143.8 ± 14.8 |
Data represent mean ± SD. a), b), c) within the same column, different superscripts denote values that differ significantly (P < 0.05). *, ** in relation to the number of oocytes and cleaved embryos, respectively.
Experiment 3: Effects of different concentrations of ROCKi on the development of oocytes and embryos following vitrification and warming
Vitrification negatively affected the meiotic resumption and developmental competence of immature feline oocytes. The addition of the ROCKi in the maturation medium did not significantly improve the rate of oocyte entry into MII. ROCKi treatment of vitrified–warmed oocytes significantly induced meiotic arrest at MI, irrespective of the ROCKi concentration (P < 0.05). In addition, the highest concentration of the inhibitor (40 µM) significantly reduced MII rates when compared with the lower concentrations and the control (Table 4). Supplementation with the ROCKi (40 µM) also significantly increased the generation of degenerate oocytes compared with the control and lower ROCKi treatment conditions (P < 0.05).
Table 4. The effect of ROCKi treatment on IVM and embryo development of vitrified–warmed feline COCs.
| ROCKi concentration (µM) |
Stage of nuclear maturation |
Stage of embryo development |
Blastocyst cell number | |||||
| Degenerate (%) | GV (%) | MI (%) | MII (%) | Cleavage * | Morula ** | Blastocyst ** | ||
| 0 | 11.3 ± 2.5 a) | 16.0 ± 7.9 | 20.0 ± 7.3 a) | 53.6 ± 9.9 a) | 27.4 ± 2.1 a) | 16.1 ± 4.3 | 6.4 ± 4.5 | 66.0 ± 18.4 |
| 10 | 13.1 ± 4.0 a) | 10.2 ± 1.2 | 29.3 ± 10.7 b) | 46.6 ± 6.6 a) | 36.1 ± 3.1 b) | 24.2 ± 6.2 | 12.1 ± 6.6 | 68.4 ± 12.2 |
| 20 | 7.4 ± 2.6 a) | 21.9 ± 13.3 | 26.4 ± 6.7 b) | 43.8 ± 13.6 a) | 33.2 ± 7.5 b) | 22.3 ± 3.8 | 16.2 ± 5.2 | 96.3 ± 35.8 |
| 40 | 21.1 ± 3.9 b) | 10.3 ± 5.5 | 31.5 ± 10.8 b) | 36.2 ± 29.8 b) | 25.6 ± 5.1 a) | 12.4 ± 11.3 | 6.4 ± 4.5 | 81.0 ± 36.8 |
Data represent mean ± SD. a), b) within the same column, different superscripts denote values that differ significantly (P < 0.05). *, ** in relation to the number of oocytes and cleaved embryos, respectively.
Following fertilization for 18 h, the percentages of sperm-penetrated oocytes (total fertilization) did not significantly differ among the experimental groups (P > 0.05, Table 3). The ROCKi at concentrations of 10 µM and 20 µM significantly improved the percentage of normal fertilization (2-pronucleus zygotes) compared with the control treatment (P < 0.05). Similar to its effects on oocyte maturation, the highest concentration of ROCKi (40 µM) adversely affected the percentage of normally fertilized oocytes (P < 0.05). Our study revealed that vitrification and warming induced degeneration of the oocytes rather than abnormal fertilization (Table 3). However, the different concentrations of the ROCKi did not statistically affect the percentages of degenerate oocytes.
Table 3. The effect of the ROCKi on the fertilization competence of vitrified-warmed feline oocytes.
| ROCKi concentration (µM) |
Total oocyte | % total fertilization | % normal fertilization | % abnormal fertilization | % degeneration |
| 0 | 55 | 50.5 ± 0.9 | 42.8 ± 8.6 a) | 9.1 ± 1.5 | 48.4 ± 2.8 |
| 10 | 55 | 52.8 ± 0.5 | 49.4 ± 3.4 b) | 3.6 ± 0.6 | 47.2 ± 0.5 |
| 20 | 57 | 50.9 ± 1.5 | 45.8 ± 6.1 c) | 5.3 ± 1.0 | 49.1 ± 1.5 |
| 40 | 46 | 47.6 ± 4.1 | 39.3 ± 3.1 d) | 13.0 ± 1.7 | 48.2 ± 7.7 |
Data represent mean ± SD. a), b) within the same column, different superscripts denote values that differ significantly (P < 0.05).
Similar to its effects on the fertilization competence of the vitrified–warmed oocytes, the ROCKi at 10 µM and 20 µM was found to promote developmental competence, in terms of the cleavage rate (36.1 ± 3.1 and 33.2 ± 7.5%, respectively), compared to the 40 µM dose (25.6 ± 5.1%) and the control treatment (27.4 ± 2.1%) (P < 0.05, Table 4). Although the differences were not statistically significant, the cleaved embryos from the 10 µM and 20 µM ROCKi treatment groups developed into morulae and blastocysts at higher rates than the control. There was no effect of the ROCKi on blastocyst quality as assessed by embryonic cell number.
Discussion
In the present study, we examined the main mediators of the ROCK cascade (LIM kinases) and the effects of a specific ROCKi on feline oocyte meiotic and developmental competence. The ROCK cascade and its roles in somatic cells have been intensively studied [31,32,33]. However, knowledge on the presence and functions of the LIM kinases in oocytes is fairly limited. In experiment 1, we identified LIMK1 and LIMK2 mRNA expression in feline oocytes. This expression indicated that, as observed in other species such as swine and mouse, the ROCK cascade plays an important role in oocyte viability and competence [5, 8, 34], particularly the homeostasis of the oocyte cytoskeleton [5, 8, 21, 35]. In addition, ROCK signaling is involved in the caspase-3-dependent apoptotic cascade [31, 36,37,38]. ROCK1 is phosphorylated by caspase-3, then regulates the phosphorylation of the downstream LIMK proteins, which are associated with membrane stability [31, 33, 39]. LIMK1 and LIMK2 are serine/threonine kinases that work cooperatively to control polymerization of the actin cytoskeleton via phosphorylation and inactivation of cofilin, an actin-depolymerizing factor [31, 33]. This finding is in agreement with previous reports indicating that LIMK1 plays an essential role in controlling microtubule function [25, 32]. In the mouse, LIMK1 was gradually detected after germinal vesicle breakdown and peaked at MI and MII [40]; it localized to the microtubule organizing center of the spindle pole of MII oocytes. Although the activity of the LIM kinases has yet to be examined throughout the time course of feline oocyte maturation, we determined the effect of the ROCKi on the levels of LIM kinase mRNA after 12 h of maturation in this study, since MI stage oocytes were expected to present predominantly at this maturation time [41].
In this study, addition of the ROCKi (10 µM) during oocyte maturation downregulated LIM kinases, but it did not significantly arrest meiotic resumption compared with the control treatment. However, the higher ROCKi concentrations (20 µM and 40 µM) used in this study proportionally increased the meiotic arrest of oocytes. This finding is similar to other studies that indicated that a high concentration (50 µM) of a ROCKi negatively affected oocyte maturation in swine and mice [5, 8, 42]. It is possible that higher concentrations of the ROCKi could further downregulate the LIM kinases, which are essential for meiotic resumption [40] and cytoskeletal reorganization [39, 43]. Interestingly, it appears that a small proportion of the MI-arrested oocytes induced by the ROCKi treatment (20 µM and 40 µM) could resume meiosis, progress to MII, and become fertilized during IVF, as the percentages of MII oocytes were lower than the cleavage rates (Table 2). This result contradicts a previous report that indicated that a ROCKi irreversibly affected porcine oocyte maturation and blocked cumulus expansion [44]. In addition, ROCKi treatment did not significantly improve the cytoplasmic maturation of the non-vitrified oocytes in terms of cleavage and blastocyst formation rates (Table 2).
In experiment 3, the nuclear and cytoplasmic maturation of feline oocytes following vitrification and warming were significantly compromised when compared with the non-frozen control oocytes. Oocytes are the largest cells in the body and contain complex structures that render them susceptible to injuries during the freezing procedure. Cryoinjuries affect meiosis, fertilization, and pregnancy rates [11,12,13, 15, 28, 45, 46]. The poor fertilization competence and early embryo development following vitrification (Table 3 and 4) suggest that the cryoinjuries predominantly induced oocyte degeneration rather than defective fertilization.
Our results revealed that supplementation of ROCKi in the oocyte maturation medium did not significantly improve the nuclear maturation of vitrified–warmed oocytes, although it improved as assessed by the fertilization competence and cleavage rate. Although the mechanism of the ROCKi-mediated reduction in or protection against cryoinjury during vitrification and warming is not clear, it is possible that optimal concentrations of ROCKi (10 µM to 20 µM) may influence the quality of cumulus cells and oocytes by supporting the mitochondrial and cytoskeletal dynamics of vitrified–warmed oocytes [5, 8, 47, 48]. In addition, the positive effects of the ROCKi may also be related to previous findings that a ROCKi suppressed caspase-3, -8, and -10 activity in pluripotent stem cells [36, 49,50,51]. Further study should be performed to clarify these pathways in order to improve the freezing technique and developmental competence of feline oocytes.
This study demonstrated the expression of members of the ROCK cascade (LIM kinases) in feline oocytes. An appropriate dose of ROCKi (10 µM) did not negatively affect oocyte and embryo development. The optimal concentration of the ROCKi promoted cytoplasmic maturation of vitrified–warmed oocytes, which is essential to their fertilization competence.
Acknowledgments
The authors would like to thank the Veterinary Public Health Division of Bangkok Metropolitan Administration for providing ovary samples. This study was financially supported, in part, by the Thailand Research Fund (RSA5680028), the 90th Anniversary Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University, and the Special Task Force for Activating Research (GSTAR 59-007-31-005).
References
- 1.Wood TC, Wildt DE. Effect of the quality of the cumulus-oocyte complex in the domestic cat on the ability of oocytes to mature, fertilize and develop into blastocysts in vitro. J Reprod Fertil 1997; 110: 355–360. [DOI] [PubMed] [Google Scholar]
- 2.Arav A, Natan Y. Vitrification of oocytes: from basic science to clinical application. Adv Exp Med Biol 2013; 761: 69–83. [DOI] [PubMed] [Google Scholar]
- 3.Edgar DH, Gook DA. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum Reprod Update 2012; 18: 536–554. [DOI] [PubMed] [Google Scholar]
- 4.Luvoni GC, Pellizzari P, Battocchio M. Effects of slow and ultrarapid freezing on morphology and resumption of meiosis in immature cat oocytes. J Reprod Fertil Suppl 1997; 51: 93–98. [PubMed] [Google Scholar]
- 5.Duan X, Liu J, Dai XX, Liu HL, Cui XS, Kim NH, Wang ZB, Wang Q, Sun SC. Rho-GTPase effector ROCK phosphorylates cofilin in actin-meditated cytokinesis during mouse oocyte meiosis. Biol Reprod 2014; 90: 37. [DOI] [PubMed] [Google Scholar]
- 6.Luvoni GC. Cryosurvival of ex situ and in situ feline oocytes. Reprod Domest Anim 2012; 47(Suppl 6): 266–268. [DOI] [PubMed] [Google Scholar]
- 7.Turathum B, Saikhun K, Sangsuwan P, Kitiyanant Y. Effects of vitrification on nuclear maturation, ultrastructural changes and gene expression of canine oocytes. Reprod Biol Endocrinol 2010; 8: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Duan X, Xiong B, Cui XS, Kim NH, Rui R, Sun SC. ROCK inhibitor Y-27632 prevents porcine oocyte maturation. Theriogenology 2014; 82: 49–56. [DOI] [PubMed] [Google Scholar]
- 9.Chian RC, Wang Y, Li YR. Oocyte vitrification: advances, progress and future goals. J Assist Reprod Genet 2014; 31: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang Y, Rakwongrit D, Phermthai T, Somfai T, Nagai T, Parnpai R. Cryopreservation of immature buffalo oocytes: effects of cytochalasin B pretreatment on the efficiency of cryotop and solid surface vitrification methods. Anim Sci J 2012; 83: 630–638. [DOI] [PubMed] [Google Scholar]
- 11.Saragusty J, Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 2011; 141: 1–19. [DOI] [PubMed] [Google Scholar]
- 12.Cocchia N, Ciani F, Russo M, El Rass R, Rosapane I, Avallone L, Tortora G, Lorizio R. Immature cat oocyte vitrification in open pulled straws (OPSs) using a cryoprotectant mixture. Cryobiology 2010; 60: 229–234. [DOI] [PubMed] [Google Scholar]
- 13.Comizzoli P, Wildt DE, Pukazhenthi BS. In vitro compaction of germinal vesicle chromatin is beneficial to survival of vitrified cat oocytes. Reprod Domest Anim 2009; 44(Suppl 2): 269–274. [DOI] [PubMed] [Google Scholar]
- 14.Luvoni GC. Current progress on assisted reproduction in dogs and cats: in vitro embryo production. Reprod Nutr Dev 2000; 40: 505–512. [DOI] [PubMed] [Google Scholar]
- 15.Tharasanit T, Manee-In S, Buarpung S, Chatdarong K, Lohachit C, Techakumphu M. Successful pregnancy following transfer of feline embryos derived from vitrified immature cat oocytes using stepwise cryoprotectant exposure technique. Theriogenology 2011; 76: 1442–1449. [DOI] [PubMed] [Google Scholar]
- 16.Baust JG, Gao D, Baust JM. Cryopreservation: An emerging paradigm change. Organogenesis 2009; 5: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boroda AV, Kipryushina YO, Yakovlev KV, Odintsova NA. The contribution of apoptosis and necrosis in freezing injury of sea urchin embryonic cells. Cryobiology 2016; 73: 7–14. [DOI] [PubMed] [Google Scholar]
- 18.Martin G, Sabido O, Durand P, Levy R. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol Reprod 2004; 71: 28–37. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Mende J, Hellström-Lindberg E, Joseph B, Zhivotovsky B. Freezing induces artificial cleavage of apoptosis-related proteins in human bone marrow cells. J Immunol Methods 2000; 245: 91–94. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Cowley S, Flaim CJ, James W, Seymour L, Cui Z. The roles of apoptotic pathways in the low recovery rate after cryopreservation of dissociated human embryonic stem cells. Biotechnol Prog 2010; 26: 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang WL, Addona T, Nair DG, Qi L, Ravikumar TS. Apoptosis induced by cryo-injury in human colorectal cancer cells is associated with mitochondrial dysfunction. Int J Cancer 2003; 103: 360–369. [DOI] [PubMed] [Google Scholar]
- 22.Lee SR, Xu YN, Jo YJ, Namgoong S, Kim NH. The Rho-GTPase effector ROCK regulates meiotic maturation of the bovine oocyte via myosin light chain phosphorylation and cofilin phosphorylation. Mol Reprod Dev 2015; 82: 849–858. [DOI] [PubMed] [Google Scholar]
- 23.Simopoulou M, Asimakopoulos B, Bakas P, Boyadjiev N, Tzanakaki D, Creatsas G. Oocyte and embryo vitrification in the IVF laboratory: a comprehensive review. Folia Med (Plovdiv) 2014; 56: 161–169. [DOI] [PubMed] [Google Scholar]
- 24.MacLean-Fletcher S, Pollard TD. Mechanism of action of cytochalasin B on actin. Cell 1980; 20: 329–341. [DOI] [PubMed] [Google Scholar]
- 25.Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev 2009; 76: 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurosawa H. Application of Rho-associated protein kinase (ROCK) inhibitor to human pluripotent stem cells. J Biosci Bioeng 2012; 114: 577–581. [DOI] [PubMed] [Google Scholar]
- 27.Maldonado M, Luu RJ, Ramos ME, Nam J. ROCK inhibitor primes human induced pluripotent stem cells to selectively differentiate towards mesendodermal lineage via epithelial-mesenchymal transition-like modulation. Stem Cell Res (Amst) 2016; 17: 222–227. [DOI] [PubMed] [Google Scholar]
- 28.Hochi S, Ito K, Hirabayashi M, Ueda M, Kimura K, Hanada A. Effect of nuclear stages during IVM on the survival of vitrified-warmed bovine oocytes. Theriogenology 1998; 49: 787–796. [DOI] [PubMed] [Google Scholar]
- 29.Sananmuang T, Techakumphu M, Tharasanit T. The effects of roscovitine on cumulus cell apoptosis and the developmental competence of domestic cat oocytes. Theriogenology 2010; 73: 199–207. [DOI] [PubMed] [Google Scholar]
- 30.Thongkittidilok C, Tharasanit T, Sananmuang T, Buarpung S, Techakumphu M. Insulin-like growth factor-1 (IGF-1) enhances developmental competence of cat embryos cultured singly by modulating the expression of its receptor (IGF-1R) and reducing developmental block. Growth Horm IGF Res 2014; 24: 76–82. [DOI] [PubMed] [Google Scholar]
- 31.Filliers M, Goossens K, Van Soom A, Merlo B, Pope CE, de Rooster H, Smits K, Vandaele L, Peelman LJ. Gene expression profiling of pluripotency and differentiation-related markers in cat oocytes and preimplantation embryos. Reprod Fertil Dev 2012; 24: 691–703. [DOI] [PubMed] [Google Scholar]
- 32.Thongkittidilok C, Tharasanit T, Songsasen N, Sananmuang T, Buarpung S, Techakumphu M. Epidermal growth factor improves developmental competence and embryonic quality of singly cultured domestic cat embryos. J Reprod Dev 2015; 61: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amano T, Tanabe K, Eto T, Narumiya S, Mizuno K. LIM-kinase 2 induces formation of stress fibres, focal adhesions and membrane blebs, dependent on its activation by Rho-associated kinase-catalysed phosphorylation at threonine-505. Biochem J 2001; 354: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 1998; 393: 805–809. [DOI] [PubMed] [Google Scholar]
- 35.Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem 2001; 276: 670–676. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Liu XB, Cheng C, Xu DL, Lu QH, Ji XP. Rho-kinase inhibition is involved in the activation of PI3-kinase/Akt during ischemic-preconditioning-induced cardiomyocyte apoptosis. Int J Clin Exp Med 2014; 7: 4107–4114. [PMC free article] [PubMed] [Google Scholar]
- 37.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 1999; 285: 895–898. [DOI] [PubMed] [Google Scholar]
- 38.Koyanagi M, Takahashi J, Arakawa Y, Doi D, Fukuda H, Hayashi H, Narumiya S, Hashimoto N. Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J Neurosci Res 2008; 86: 270–280. [DOI] [PubMed] [Google Scholar]
- 39.Leverrier Y, Ridley AJ. Apoptosis: caspases orchestrate the ROCK n bleb. Nat Cell Biol 2001; 3: E91–E93. [DOI] [PubMed] [Google Scholar]
- 40.Street CA, Bryan BA. Rho kinase proteinspleiotropic modulators of cell survival and apoptosis. Anticancer Res 2011; 31: 3645–3657. [PMC free article] [PubMed] [Google Scholar]
- 41.Ortíz-López L, Morales-Mulia S, Ramírez-Rodríguez G, Benítez-King G. ROCK-regulated cytoskeletal dynamics participate in the inhibitory effect of melatonin on cancer cell migration. J Pineal Res 2009; 46: 15–21. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Zhu Y, Cao Y, Wang Q, Du J, Tian J, Liang Y, Ma W. LIM kinase activity is required for microtubule organising centre positioning in mouse oocyte meiosis. Reprod Fertil Dev 2016; 29: 791–804. [DOI] [PubMed] [Google Scholar]
- 43.Tharasanit T, Techakumphu M, Lohachit C. Reorganisation of cell cytoskeleton of cat oocytes matured in vitro. TJVM 2008; 38: 8. [Google Scholar]
- 44.Chan CC, Khodarahmi K, Liu J, Sutherland D, Oschipok LW, Steeves JD, Tetzlaff W. Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp Neurol 2005; 196: 352–364. [DOI] [PubMed] [Google Scholar]
- 45.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 2003; 4: 446–456. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki H, Koyama K, Kabashima K, Fang J, Matsuzaki M. Temporary Inhibition of Germinal Vesicle Breakdown by Rho Kinase Inhibitor Y-27632 is Detrimental to Oocyte Maturation. J Mamm Ova Res 2011; 28: 126. [Google Scholar]
- 47.Barritt LC, Miller JM, Scheetz LR, Gardner K, Pierce ML, Soukup GA, Rocha-Sanchez SM. Conditional deletion of the human ortholog gene Dicer1 in Pax2-Cre expression domain impairs orofacial development. Indian J Hum Genet 2012; 18: 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gook DA, Osborn SM, Johnston WI. Cryopreservation of mouse and human oocytes using 1,2-propanediol and the configuration of the meiotic spindle. Hum Reprod 1993; 8: 1101–1109. [DOI] [PubMed] [Google Scholar]
- 49.Ferreira EM, Vireque AA, Adona PR, Meirelles FV, Ferriani RA, Navarro PA. Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology 2009; 71: 836–848. [DOI] [PubMed] [Google Scholar]
- 50.Martorell-Riera A, Segarra-Mondejar M, Reina M, Martínez-Estrada OM, Soriano FX. Mitochondrial fragmentation in excitotoxicity requires ROCK activation. Cell Cycle 2015; 14: 1365–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ichikawa H, Nakata N, Abo Y, Shirasawa S, Yokoyama T, Yoshie S, Yue F, Tomotsune D, Sasaki K. Gene pathway analysis of the mechanism by which the Rho-associated kinase inhibitor Y-27632 inhibits apoptosis in isolated thawed human embryonic stem cells. Cryobiology 2012; 64: 12–22. [DOI] [PubMed] [Google Scholar]