Abstract
Recent studies demonstrated that G-protein-coupled receptor 30 (GPR30) on the plasma membrane of gonadotroph cells mediates picomolar, but not nanomolar, levels of estradiol (E2) to rapidly suppress gonadotropin-releasing hormone (GnRH)-induced luteinizing hormone (LH) secretion in the anterior pituitary (AP). While estrone (E1) and estriol (E3) are considered “weak” estrogens that exert suppressive effects through estrogen receptors α and β, it is conceivable that they also strongly suppress GnRH-induced LH secretion via GPR30. Both E1 and E3 are likely present within the blood at picomolar or nanomolar concentrations, indicating that such concentrations are sufficient to suppress GnRH-induced LH secretion. To evaluate this possibility, bovine AP cells were cultured under steroid-free conditions and then incubated with various concentrations (0.01 pM to 10 nM) of E2, E1, or E3, prior to stimulation with GnRH. Notably, GnRH-induced LH secretion from AP cells was inhibited by 1–100 pM E2, 1–10 pM E1, and 1–100 pM E3. GnRH-induced LH secretion from AP cells was not inhibited by lower (0.01–0.1 pM) or higher (1–10 nM) concentrations of E2, E1, and E3. These suppressive effects were inhibited by pre-treatment of AP cells with the GPR30 antagonist G36, but not with the estrogen receptor alpha antagonist. Treatment with E1 or E3 also yielded decreased cytoplasmic cAMP levels in cultured AP cells pre-treated with dopamine and phosphodiesterase inhibitors. Therefore, these results suggest that GPR30 mediates the suppressive effects of E1, E3, and E2 on GnRH-induced LH secretion from bovine AP.
Keywords: Anterior pituitary, Gonadotrope, G protein-coupled estrogen receptor-1, GPR30, Ruminant
Estradiol (E2) modulates the transcriptional expression of the genes that produce gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) within the hypothalamus and the anterior pituitary (AP), respectively, via binding to nuclear-localized estrogen receptors α or β (ERα or ERβ). Moreover, E2 was found to suppress LH, but not follicle stimulating hormone (FSH), production in a rapid manner within the AP via interaction with an as yet uncharacterized membrane receptor [1, 2]. Candidate membrane receptors for this rapid regulatory pathway include membrane ERα, membrane ERβ, STX receptor, and G protein-coupled receptor 30 (GPR30; or G protein-coupled estrogen receptor 1); however, multiple studies indicate that GPR30 is the primary receptor governing this process within the AP [1, 3,4,5].
We previously reported that the nonsteroidal mycoestrogen zearalenone (ZEN), as well as the five known ZEN metabolites, rapidly suppressed GnRH-induced LH secretion [5, 6]. Moreover, some of these compounds, even those that exhibit weak suppression via ERα and ERβ signaling, can yield rapid and strong suppression of GnRH-induced LH secretion via GPR30.
The endogenous estrogens, estrone (E1) and estriol (E3), are weaker agonists for ERα and ERβ than E2, and have therefore been designated “weak” estrogens [7]. However, Watson et al. [7] reported that both E1 and E3 are strong, rapid regulators in rat lactotroph-like tumor cells. Moreover, Selles et al. [8] reported that physiological concentrations of E1 (0.1–10 nM) non-genomically increase aortic nitric oxide synthase and cyclooxygenase activities in female rats. As such, it is possible that E1 and E3 also modulate GnRH-induced LH secretion from the bovine AP via GPR30. Indeed, intramuscular injection of E3 was found to induce an LH surge earlier than an E2 injection in cows and ewes [9, 10].
Notably, previous studies demonstrated that GPR30 mediates rapidly (within a few minutes) picomolar, but not nanomolar, levels of E2, as detected in the blood of luteal phase cows [11, 12], to suppress GnRH-induced LH secretion from the bovine AP [3,4,5]. During pregnancy, cows were found to exhibit increases in the blood concentration of E1, from picomolar levels during the first trimester to a maximum of 7.4 nM [13]. To the best of our knowledge, however, the levels of E3 in the blood of cows are yet to be evaluated. In animals, E3 is synthesized from E1 and E2 [14], and the blood concentration of E3 in non-pregnant women is always significantly higher (3–19-fold) than that of E1 and E2 combined [15]. Thus, the physiological levels of E3 within the blood of cows are also likely within the picomolar or nanomolar range.
Cyclic AMP (cAMP) plays a key role in the cytoplasmic pathway that increases LH secretion by ovine gonadotrophs by modulating Ca2+-activated K+ channels [16, 17]. In the previous study, we described the suppressive effects of E2 pre-treatment on cAMP levels in cultured AP cells; the pre-treatment caused rapid suppression of LH secretion [5].
In this study, we evaluated whether GPR30 mediates picomolar and nanomolar levels of E1 and E3 to suppress GnRH-induced LH secretion from bovine AP cells. We also evaluated the levels of cAMP in cultured AP cells in the presence and absence of pre-treatment with E2, E1, and E3.
Materials and Methods
All experiments were performed according to the Guiding Principles for the Care and Use of Experimental Animals in the Field of Physiological Sciences (Physiological Society of Japan) and approved by the Committee on Animal Experiments of the School of Veterinary Medicine, Yamaguchi University.
Effects of E2, E1, and E3 on GnRH-induced LH secretion from bovine AP cells
G*Power 3 for windows [18] was used to estimate the required number of APs with an α-error probability of 0.05 and a statistical power of 0.95. APs were obtained from post-pubertal Japanese Black heifers (n = 11, 26 months of age). The experiment was repeated eleven times with each of the eleven different pituitary glands, using four wells per treatment. Each experiment began with enzymatic dispersal of AP cells via a previously described method [19] and confirmation of cell viability of greater than 90% by Trypan blue exclusion. Total cell yield was 20.1 × 106 ± 0.9 × 106 cells per pituitary gland. The dispersed cells were then suspended in phenol red-free Dulbecco’s modified Eagle’s medium (DMEM; 21063-029, Gibco, Grand Island, NY, USA) containing 1% nonessential amino acids (100 ×; Gibco), 100 IU/ml penicillin, 50 µg/ml streptomycin, 10% horse serum (Gibco), and 2.5% fetal bovine serum (FBS; Gibco). The horse serum and the FBS had previously been treated with dextran-coated charcoal for removal of steroid hormones. After the cells (2.5 × 105 cells/ml, total 0.5 ml) had been plated in 24-well culture plates (MS-80240, Sumitomo Bakelite, Tokyo, Japan), the cells were maintained at 37°C in a humidified atmosphere of 5% CO2 for 82 h. The wells were washed twice with PBS and then incubated with 490 µl of DMEM containing 0.1% BSA for 2 h. Cells were pre-treated with 5 µl of DMEM alone or with 5 µl of DMEM containing either 0.001 nM to 1,000 nM E2, E1, or E3 (all were from Wako Pure Chemicals, Osaka, Japan). The cells were incubated while gently shaking for 5 min. Cells were then treated for 2 h with 5 µl of 100 nM GnRH (Peptide Institute, Osaka, Japan) dissolved in DMEM to stimulate LH secretion. Pre-treatment with 0.001 nM, 0.01 nM, 0.1 nM, 1 nM, 10 nM, 100 nM, or 1,000 nM E2, E1, or E3 resulted in final concentrations of 0.00001 nM, 0.0001 nM, 0.001 nM, 0.01 nM, 0.1 nM, 1 nM, or 10 nM after GnRH treatment, respectively. The final concentration of GnRH was 1 nM in all treatments [20], except the “control”. Control wells were treated with 5 µl of DMEM, but were not incubated with GnRH. “GnRH” wells were pre-treated with 5 µl of DMEM for 5 min and were then incubated with GnRH for 2 h. After incubation for 2 h, the medium from each well was collected for radioimmunoassay (RIA) analysis of LH levels.
Analysis of the effects of the GPR30 antagonist on E2-, E1-, or E3-induced suppression of GnRH-induced LH secretion from bovine AP cells
APs were obtained from post-pubertal Japanese Black heifers (n = 11, 26 months of age). The experiment was repeated 11 times with each of the 11 different pituitary glands, using four wells per treatment. After the enzymatic dispersal of AP cells, the cells were cultured as described previously at 37°C in 5% CO2 for 82 h. After washing with PBS, the cells were incubated with 485 µl of DMEM containing 0.1% BSA for 2 h. Cells were pre-treated with 5 µL of DMEM alone or with 5 µl of DMEM containing 1 nM of the GPR30 antagonist G36 (Azano Biotech, Albuquerque, NM, USA). After 5 min of gentle shaking, either 5 µl of DMEM alone or 5 µl of DMEM containing 1 nM E2, E1, or E3 was added to each well (final concentration, 0.01 nM). The cells were then incubated with gentle shaking for 5 min, after which they were incubated for 2 h with 5 µl of 100 nM GnRH dissolved in DMEM to stimulate LH secretion. After 2 h of incubation, the medium was collected from each well for RIA analysis of LH levels.
Analysis of the effects of ERα antagonist on E2-, E1-, or E3-induced suppression of GnRH-induced LH secretion from bovine AP cells
We repeated the same design described in the previous section to evaluate the effect of ERα-specific antagonist, 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole (MPP) (Sigma-Aldrich, Saint Louis, MI, USA), on E2-, E1-, and E3-induced suppression of GnRH-induced LH secretion from cultured AP cells obtained from post-pubertal Japanese Black heifers (n = 11; 26 months of age). The final concentration of MPP, E2, E1, and E3 were 0.01 nM.
RIA to measure LH concentrations in culture media
LH concentrations in culture media were assayed in duplicate using double antibody RIA with bovine LH and anti-oLH-antiserum (AFP11743B and AFP192279, National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, CA, USA). This assay has been previously described in detail [5] and has been used to measure changes in blood LH concentrations in Holstein heifers before and after GnRH treatment [21]. The limit of detection was 0.40 ng/ml. At 2.04 ng/ml, the intra- and inter-assay coefficients of variation (CV) were 3.5% and 6.1%, respectively.
Effects of E2, E1, and E3 on the levels of cAMP in cultured AP cells
APs were obtained from post-pubertal Japanese Black heifers (n = 6, 26 months of age). The experiment was repeated six times with each of the six different pituitary glands, using two wells per treatment. After enzymatic dispersal, AP cells were cultured as described previously at 37°C in 5% CO2 for 82 h. After washing with PBS, the cells were incubated with 490 µl of DMEM containing 0.1% BSA for 2 h. Cells were pre-treated with 5 µl of DMEM alone or with 5 µl of DMEM containing 1 nM of E2, E1, or E3 (final concentration, 0.01 nM). After gently shaking for 5 min, cells were treated for 2 h with 5 µl of 100 nM GnRH (final concentration, 1 nM, except the “control”) dissolved in DMEM containing dopamine (0.5 µM, Nacalai Tesque, Kyoto, Japan) and phosphodiesterase inhibitor [0.5 mM 3-isobutyl-1-methyl-xanthine (MIX; Sigma-Aldrich)]. Dopamine and MIX are required to measure cAMP in cultured gonadotroph cells from heterogeneous AP cells because the amount of cAMP in lactotrophs fluctuates; moreover, phosphodiesterase decreases the amount of cAMP to below the detection limits [16]. The concentrations of dopamine and MIX used in the present study were identical to those used in a previous investigation of cAMP in cultured ovine AP cells [16]. There were five treatment conditions: (1) Control, cells treated with 5 µl of DMEM, but not GnRH; (2) GnRH, cells treated with 5 µl of DMEM and GnRH; (3) E2, cells pre-treated with E2 and then treated with GnRH; (4) E1, cells pre-treated with E1 and then treated with GnRH; and (5) E3, cells pre-treated with E3 and then treated with GnRH.
After the 2 h of treatment, the wells were washed twice with PBS and were then used for cAMP extraction with a cAMP Select Enzyme Immunoassay (EIA) Kit (Cayman Chemical, Ann Arbor, MI, USA), as described in detail previously [5]. The limit of detection was 0.09 pmol/ml. At 22.2 pmol/ml, the intra- and inter-assay CV was 5.9 and 7.4%, respectively. The specificities of the cAMP EIA kit evaluated by the company were 100% for cAMP and < 0.01% for cGMP, AMP, ATP, adenosine, and dibutyryl cAMP.
Statistical analysis
The concentrations of LH and cAMP within the control samples from each pituitary gland were averaged, and the mean values were set at 100%. Meanwhile, the concentrations of LH and cAMP in the treated samples from each pituitary gland were averaged, and the mean values were expressed as percentages of the control value. Differences in LH or cAMP concentration were analyzed by one-factor analysis of variance (ANOVA), with concentrations of LH or cAMP as the dependent variable and treatment as the independent variable, followed by post-hoc comparisons using Fisher’s protected least significant difference test (StatView version 5.0 for Windows, SAS Institute, Cary, NC, USA). The level of significance was set at P < 0.05. Data were expressed as mean ± standard error of the mean (SEM).
Results
Effects of E2, E1, or E3 on GnRH-induced LH secretion
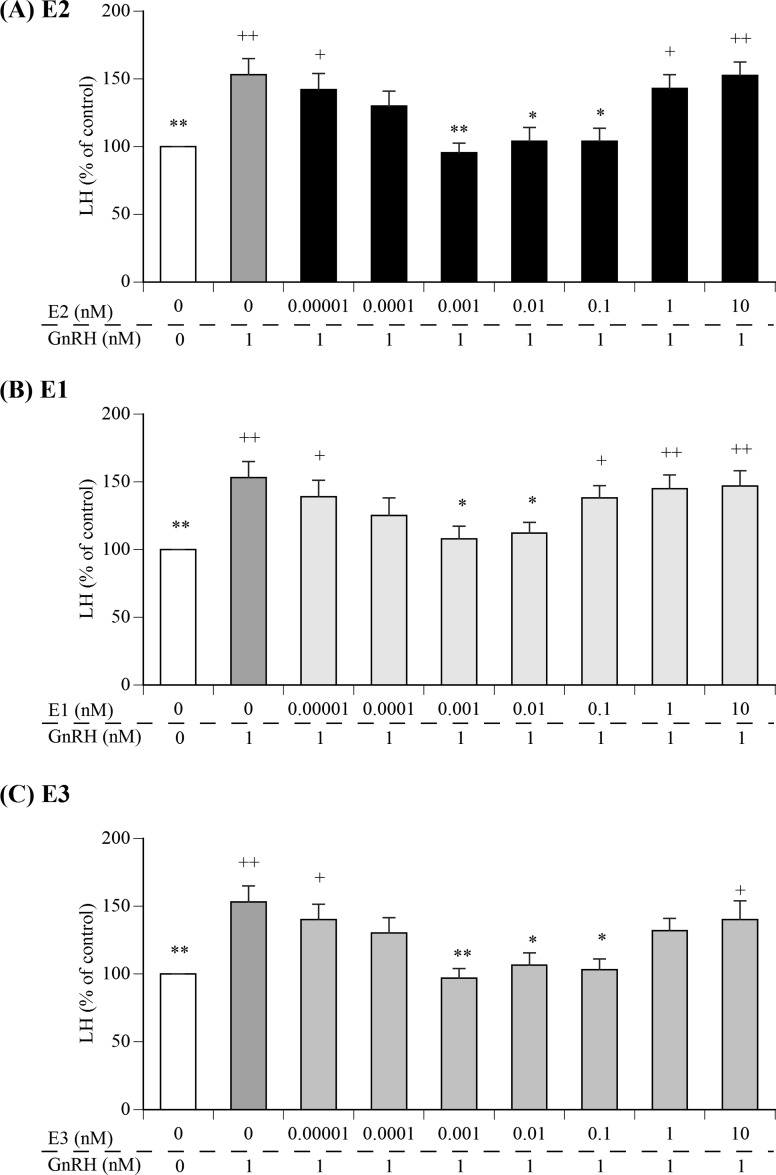
Fig. 1 depicts the effects of various concentrations of E2, E1, or E3 on GnRH-induced LH secretion from cultured AP cells. The media from cells treated with 1 nM GnRH exhibited significantly greater concentrations of LH than that of the control cells. Meanwhile, pre-treatment with 0.001–0.1 nM E2, 0.001–0.01 nM E1, or 0.001–0.1 nM E3, but not with 1–10 nM E2, 0.1–10 nM E1, or 1–10 nM E3,-resulted in marked suppression of GnRH-induced LH secretion from AP cells. In contrast, pre-treatment with low (0.00001–0.0001 nM) or high (1–10 nM) concentrations of E1, E2, and E3 did not decrease the LH concentration.
Fig. 1.
Comparison of the effects of various concentrations of estradiol (E2), estrone (E1), or estriol (E3) in media containing 1 nM gonadotropin-releasing hormone (GnRH) on luteinizing hormone (LH) secretion from cultured bovine anterior pituitary (AP) cells. The concentrations of LH in the control cells (cultured in medium alone; shown as white bars) were averaged and set at 100%, and the mean LH concentration for each treatment group is expressed as a percentage of the control value. + P < 0.05 and ++ P < 0.01 compared to the control; * P < 0.05 and ** P < 0.01 compared to GnRH alone.
Effects of G36 on E2, E1, and E3-mediated suppression of GnRH-induced LH secretion
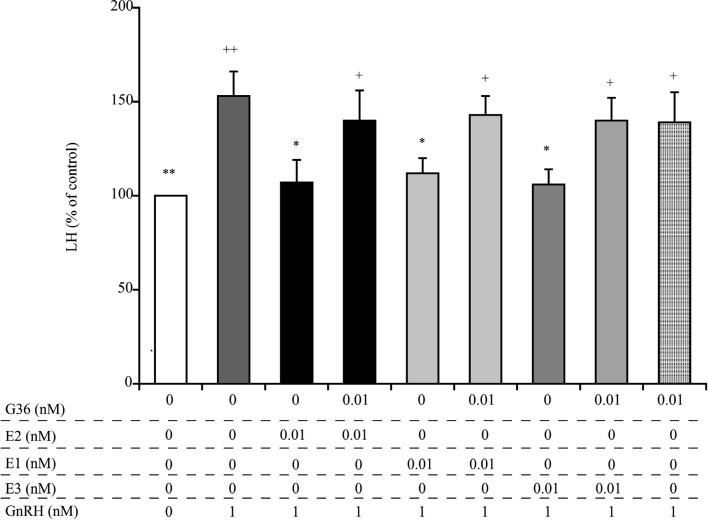
As summarized in Fig. 2, treatment with 1 nM GnRH resulted in a significant increase in LH secretion from cultured AP cells, compared to the untreated control cells, and treatment with the GPR30 antagonist G36 alone had no effect on this process. Notably, however, the observed decreases in LH secretion, mediated by pre-treatment with 0.01 nM E2, E1, and E3, were abrogated by pre-treatment of cells with G36.
Fig. 2.
Comparison of the effects of the GPR30 antagonist G36 on E2, E1, and E3-mediated suppression of GnRH-induced secretion of LH from cultured bovine AP cells. The final concentrations of G36, E2, E1, E3, and GnRH were 0.01 nM, 0.01 nM, 0.01 nM, 0.01 nM, and 1 nM, respectively. The concentrations of LH in the control cells (cultured in medium alone; shown as white bars) were averaged and set at 100%, and the mean LH concentration for each treatment group is expressed as a percentage of the control value. + P < 0.05 and ++ P < 0.01 compared to the control; * P < 0.05 and ** P < 0.01 compared to GnRH alone.
Effects of MPP on E2-, E1-, and E3-mediated suppression of GnRH-induced LH secretion
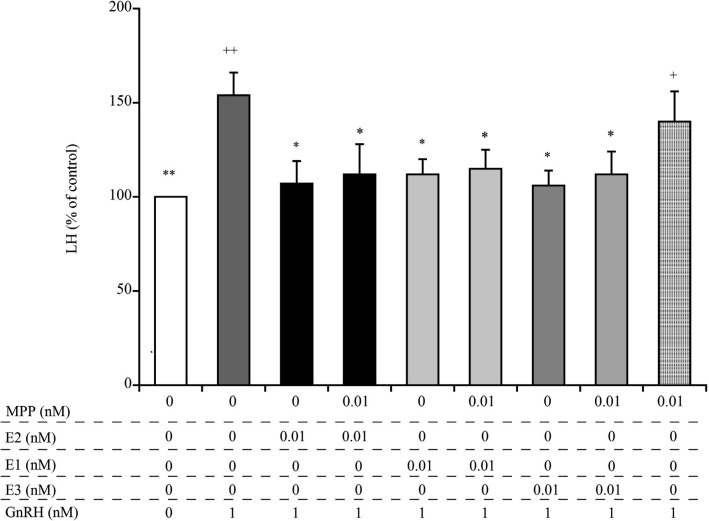
Treatment with the ERα antagonist, MPP, alone had no effect on the GnRH-induced LH secretion (Fig. 3). The observed decrease in LH secretion caused by pre-treatment with 0.01 nM E2, E1, and E3 was not abrogated by pre-treatment with MPP.
Fig. 3.
Comparison of the effects of the estrogen receptor α antagonist, MPP, on E2-, E1-, and E3-mediated suppression of GnRH-induced secretion of LH from cultured bovine AP cells. The final concentrations of MPP, E2, E1, E3, and GnRH were 0.01 nM, 0.01 nM, 0.01 nM, 0.01 nM, and 1 nM, respectively. The concentration of LH in the control cells (cultured in medium alone; shown as white bars) was averaged and set at 100%, and the mean LH concentration for each treatment group was expressed as a percentage of the control value. + P < 0.05 and ++ P < 0.01 compared to the control; * P < 0.05 and ** P < 0.01 compared to GnRH alone.
Effects of E2, E1, or E3 on the levels of cAMP in AP cells
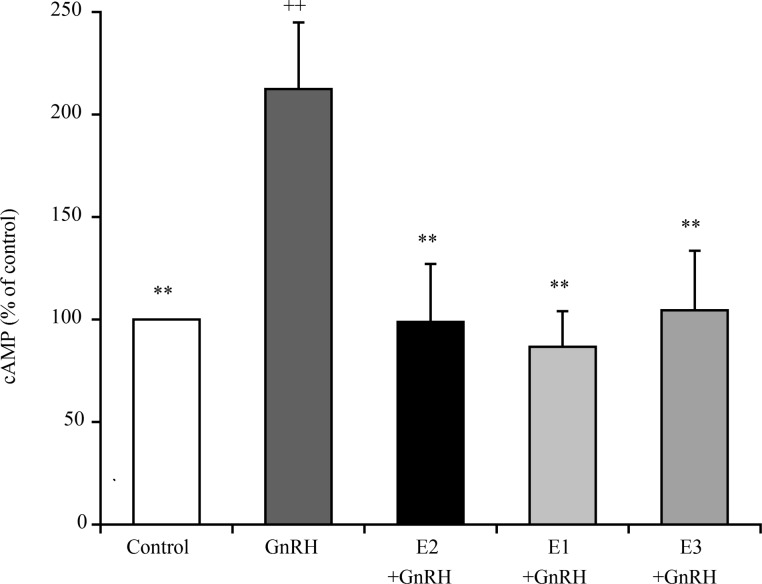
While AP cells treated with 1 nM GnRH exhibited significantly higher levels of cAMP production than the control cells, this effect was abrogated by pre-treatment with 0.01 nM E2, E1, or E3 (Fig. 4).
Fig. 4.
Comparison of the effects of 0.01 nM of E2, E1, and E3 on the levels of cyclic AMP (cAMP) in cultured bovine AP cells treated with 1 nM GnRH. The concentrations of cAMP in the control cells (cultured in medium alone; shown as white bars) were averaged and set at 100%, and the mean cAMP concentration for each treatment group is expressed as a percentage of the control value. ++ P < 0.01 compared to the control; ** P < 0.01 compared to GnRH alone.
Discussion
In the present study, we demonstrated that both E1 and E3, in addition to E2, rapidly suppressed the GnRH-induced LH secretion from cultured bovine AP cells, and that this effect was inhibited by pre-treatment of cells with a GPR30 antagonist. The results of the present study suggested that femtomolar levels of E2, E1, and E3 could not suppress the GnRH-induced LH secretion from the cultured bovine AP cells. ERα is another candidate membrane receptor for the rapid regulatory pathway. However, the present study did not show any significant effect of ERα antagonists on the E2-, E1-, and E3-mediated inhibition of GnRH-induced LH secretion. In addition, multiple studies have shown that GPR30 is the primary receptor governing this process within the AP [1, 3,4,5]. Together, these findings imply that GPR30 mediates the E1-, E2-, and E3-dependent suppression of GnRH-induced LH secretion from AP cells. However, the physiological significance of these results must be carefully considered; in particular, (1) whether the suppressive effects of E1 and E3 are important negative feedback from the ovary or other organs to the AP, and (2) the reason behind the absence of any significant effect of 1–10 nM E1, E2, and E3 on the GnRH-induced LH secretion.
To date, very few reports have been published on the effect of E1 and E3 on LH secretion in vivo. Forrest et al. [9] had reported that intramuscular injection of E3 or E2 could induce an LH surge; interestingly, E3 exerts this effect earlier than E2 in cows and ewes [9]. In addition, Schoenemann et al. [10] reported that an E3 injection could induce an earlier LH surge in ovariectomized cows. Plasma concentration of E1 was found to be positively correlated (r = 0.71) with the number of ovulations in superovulated heifers [22]. Therefore, both E1 and E3 could affect the GnRH-induced LH secretion in vivo. Unlike E2, ZEN, and environmental estrogens [23], the affinity of E1 and E3 for GPR30 has yet to be clarified. However, previous studies have reported that E1, E2, and E3 could non-genetically activate the extracellular signal-regulated kinase (ERK) pathway in rat pituitary cell lines [7, 24]. The ERK pathway is an important pathway downstream of GPR30 that suppresses GnRH-induced LH secretion in bovine AP cells [25]. Therefore, GPR30 might bind with E1 and E3 to activate the ERK pathway to suppress GnRH-induced LH secretion in bovine AP cells.
While little is known regarding the physiological blood concentrations of E1 in ruminants, a previous study indicated that blood E1 concentrations in cows ranged from picomolar to nanomolar levels [13]. Meanwhile, a separate study demonstrated that E3 is a much weaker hormone than E2 and E1 in women [15]. Notably, however, the serum levels of E3 in both pregnant and non-pregnant animals, including cows, have yet to be compared. Although the current opinion held by the medical community is that E3 plays no significant role in non-pregnant animals relative to the other estrogens, Wright et al. [15] found that the blood concentration of E3 (0.6–8.4 nM) in non-pregnant premenopausal women was consistently higher (3–19-fold) than the sum of the concentrations of E1 and E2. Moreover, they found that the blood concentration of E3 fluctuated less in this group of women than that of E1 or E2 [15]. Given that pregnant women exhibit blood E3 concentrations within the picomolar or nanomolar range [26], this might also be the physiological range for E3 in cows. Therefore, the suppressive effect of E1 and E3 on GnRH-induced LH secretion may have important roles at different life stages, for e.g., reproductive cycle, pregnancy, and menopause.
The present study showed that 1–10 nM of E1, E2, and E3 could not suppress GnRH-induced LH secretion. Iqbal et al. [27] had suggested that E2 might have a biphasic effect on LH secretion by gonadotropes, with a rapid initial suppression of LH release (negative feedback) followed by a positive feedback event many hours later. Iqbal et al. [27] had also reported that E2 might activate the MAPK pathway for exerting its time-delayed positive feedback effect. Therefore, the superpharmacological concentration of E1, E2, and E3 may have activated the MAPK pathway earlier. However, the term “positive feedback” must be used with caution, because the GnRH-induced LH secretion from the AP cells pre-treated with 1–10 nM of E1, E2, and E3 was not higher than that from AP cells treated with GnRH only. A more appropriate phrase would be “no suppressing effect,” for 1–10 nM E1, E2, and E3.
The weak suppressive effect of 0.01–0.1 nM E2, compared to 0.001 nM E2, is reasonable, as gonadotropes are surrounded by a high concentration of estradiol and must respond to the increased GnRH in the hypophyseal portal blood to release LH in a state of positive estradiol feedback. E2 rapidly stimulates GnRH secretion from cultured GnRH neurons derived from the embryonic olfactory placode, and the rapid estradiol action is mediated by GPR30 [28]. Therefore, further studies are required to understand the importance of gonadotrope GPR30 in controlling sensitivity to GnRH.
cAMP promotes LH secretion from ovine gonadotroph cells by acting as an intracellular second messenger that rapidly modulates Ca2+-activated K+ channels [16, 17]. Meanwhile, GPR30 forms a plasma membrane complex with membrane-associated guanylate kinase, discs, large homolog 4 (DLG4; or postsynaptic density protein 95), and protein kinase A-anchoring protein 5 (AKAP5), and this complex was shown to inhibit cAMP production in HEK293 cells and CHO cells ectopically expressing GPR30 [29]. In the present study, pre-treatment of AP cells with small amounts of E1, E2, or E3 resulted in decreased cAMP production, suggesting that the GPR30-mediated decreases in cAMP production play an important role in the inhibition of GnRH-induced LH secretion.
However, we must ensure that the cultured bovine AP cells contain not only gonadotrophs, but also lactotrophs. Both E1 and E3 are strong, rapid regulators found in rat lactotroph-like tumor cells [7]. cAMP promotes prolactin synthesis and secretion in lactotrophs [30]. GnRH may indirectly stimulate prolactin secretion in cultured AP cells of ewes in the breeding season [31]. Therefore, some of the observed cAMP maybe derived from lactotrophs. However, this study used DMEM containing dopamine at the same concentration as used in previous studies [5, 16]. Dopamine inhibits GnRH-induced prolactin secretion from cultured AP cells of ewes in breeding season [31]. Dopamine also decreases intracellular cAMP concentrations and inhibits prolactin synthesis and secretion in lactotrophs [32, 33]. Therefore, changes in measured cAMP likely reflect the intracellular cAMP concentration in cultured bovine gonadotrophs.
In conclusion, the results presented in this study suggest that GPR30 mediates the suppressive effects of E1 and E3 as well as E2 on GnRH-induced LH secretion from bovine AP cells.
Acknowledgments
This research was supported by a Grant-in Aid for Scientific Research from Yamaguchi University Foundation (Yamaguchi, Japan) to HK.
References
- 1.Arreguin-Arevalo JA, Nett TM. A nongenomic action of 17beta-estradiol as the mechanism underlying the acute suppression of secretion of luteinizing hormone. Biol Reprod 2005; 73: 115–122. [DOI] [PubMed] [Google Scholar]
- 2.Iqbal J, Latchoumanin O, Clarke IJ. Rapid in vivo effects of estradiol-17beta in ovine pituitary gonadotropes are displayed by phosphorylation of extracellularly regulated kinase, serine/threonine kinase, and 3,5-cyclic adenosine 5-monophosphate-responsive element-binding protein. Endocrinology 2007; 148: 5794–5802. [DOI] [PubMed] [Google Scholar]
- 3.Rudolf FO, Kadokawa H. Expression of estradiol receptor, GPR30, in bovine anterior pituitary and effects of GPR30 agonist on GnRH-induced LH secretion. Anim Reprod Sci 2013; 139: 9–17. [DOI] [PubMed] [Google Scholar]
- 4.Rudolf FO, Kadokawa H. Effects of STX, a novel estrogen membrane receptor agonist, on GnRH-induced luteinizing hormone secretion from cultured bovine anterior pituitary cells. J Vet Med Sci 2014; 76: 1623–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura U, Rudolf FO, Pandey K, Kadokawa H. The non-steroidal mycoestrogen zeranol suppresses luteinizing hormone secretion from the anterior pituitary of cattle via the estradiol receptor GPR30 in a rapid, non-genomic manner. Anim Reprod Sci 2015; 156: 118–127. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura U, Kadokawa H. The nonsteroidal mycoestrogen zearalenone and its five metabolites suppress LH secretion from the bovine anterior pituitary cells via the estradiol receptor GPR30 in vitro. Theriogenology 2015; 84: 1342–1349. [DOI] [PubMed] [Google Scholar]
- 7.Watson CS, Jeng YJ, Kochukov MY. Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation. FASEB J 2008; 22: 3328–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selles J, Polini N, Alvarez C, Massheimer V. Novel action of estrone on vascular tissue: regulation of NOS and COX activity. Steroids 2005; 70: 251–256. [DOI] [PubMed] [Google Scholar]
- 9.Forrest DW, Kaltenbach CC, Dunn TG. Estriol- and estradiol-17 beta-induced luteinizing hormone release in ovariectomized cows and ewes. J Anim Sci 1981; 52: 1106–1113. [DOI] [PubMed] [Google Scholar]
- 10.Schoenemann HM, Humphrey WD, Crowder ME, Nett TM, Reeves JJ. Pituitary luteinizing hormone-releasing hormone receptors in ovariectomized cows after challenge with ovarian steroids. Biol Reprod 1985; 32: 574–583. [DOI] [PubMed] [Google Scholar]
- 11.Endo N, Nagai K, Tanaka T, Kamomae H. Comparison between lactating and non-lactating dairy cows on follicular growth and corpus luteum development, and endocrine patterns of ovarian steroids and luteinizing hormone in the estrous cycles. Anim Reprod Sci 2012; 134: 112–118. [DOI] [PubMed] [Google Scholar]
- 12.Spicer LJ, Echternkamp SE. Ovarian follicular growth, function and turnover in cattle: a review. J Anim Sci 1986; 62: 428–451. [DOI] [PubMed] [Google Scholar]
- 13.Patel OV, Takenouchi N, Takahashi T, Hirako M, Sasaki N, Domeki I. Plasma oestrone and oestradiol concentrations throughout gestation in cattle: relationship to stage of gestation and fetal number. Res Vet Sci 1999; 66: 129–133. [DOI] [PubMed] [Google Scholar]
- 14.Thomas MP, Potter BV. The structural biology of oestrogen metabolism. J Steroid Biochem Mol Biol 2013; 137: 27–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright JV, Schliesman B, Robinson L. Comparative measurements of serum estriol, estradiol, and estrone in non-pregnant, premenopausal women; a preliminary investigation. Altern Med Rev 1999; 4: 266–270. [PubMed] [Google Scholar]
- 16.Adams TE, Wagner TO, Sawyer HR, Nett TM. GnRH interaction with anterior pituitary. II. Cyclic AMP as an intracellular mediator in the GnRH activated gonadotroph. Biol Reprod 1979; 21: 735–747. [DOI] [PubMed] [Google Scholar]
- 17.Sikdar SK, McIntosh RP, Mason WT. Differential modulation of Ca2+-activated K+ channels in ovine pituitary gonadotrophs by GnRH, Ca2+ and cyclic AMP. Brain Res 1989; 496: 113–123. [DOI] [PubMed] [Google Scholar]
- 18.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki S, Kadokawa H, Hashizume T. Direct kisspeptin-10 stimulation on luteinizing hormone secretion from bovine and porcine anterior pituitary cells. Anim Reprod Sci 2008; 103: 360–365. [DOI] [PubMed] [Google Scholar]
- 20.Kadokawa H, Pandey K, Nahar A, Nakamura U, Rudolf FO. Gonadotropin-releasing hormone (GnRH) receptors of cattle aggregate on the surface of gonadotrophs and are increased by elevated GnRH concentrations. Anim Reprod Sci 2014; 150: 84–95. [DOI] [PubMed] [Google Scholar]
- 21.Kadokawa H. Seasonal differences in the parameters of luteinizing hormone release to exogenous gonadotropin releasing hormone in prepubertal Holstein heifers in Sapporo. J Reprod Dev 2007; 53: 121–125. [DOI] [PubMed] [Google Scholar]
- 22.Saumande J, Lopez-Sebastian A. Changes in the plasma concentrations of free and conjugated oestrogens in heifers after treatment to induce superovulation and the relationship with number of ovulations. J Reprod Fertil 1982; 66: 411–416. [DOI] [PubMed] [Google Scholar]
- 23.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol 2006; 102: 175–179. [DOI] [PubMed] [Google Scholar]
- 24.Jeng YJ, Watson CS. Combinations of physiologic estrogens with xenoestrogens alter ERK phosphorylation profiles in rat pituitary cells. Environ Health Perspect 2011; 119: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudolf FO, Kadokawa H. Cytoplasmic kinases downstream of GPR30 suppress gonadotropin-releasing hormone (GnRH)-induced luteinizing hormone secretion from bovine anterior pituitary cells. J Reprod Dev 2016; 62: 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peter M, Dörr HG, Sippell WG. Changes in the concentrations of dehydroepiandrosterone sulfate and estriol in maternal plasma during pregnancy: a longitudinal study in healthy women throughout gestation and at term. Horm Res 1994; 42: 278–281. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal J, Latchoumanin O, Sari IP, Lang RJ, Coleman HA, Parkington HC, Clarke IJ. Estradiol-17beta inhibits gonadotropin-releasing hormone-induced Ca2+ in gonadotropes to regulate negative feedback on luteinizing hormone release. Endocrinology 2009; 150: 4213–4220. [DOI] [PubMed] [Google Scholar]
- 28.Terasawa E, Noel SD, Keen KL. Rapid action of oestrogen in luteinising hormone-releasing hormone neurones: the role of GPR30. J Neuroendocrinol 2009; 21: 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broselid S, Berg KA, Chavera TA, Kahn R, Clarke WP, Olde B, Leeb-Lundberg LM. G protein-coupled receptor 30 (GPR30) forms a plasma membrane complex with membrane-associated guanylate kinases (MAGUKs) and protein kinase A-anchoring protein 5 (AKAP5) that constitutively inhibits cAMP production. J Biol Chem 2014; 289: 22117–22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurer RA. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature 1981; 294: 94–97. [DOI] [PubMed] [Google Scholar]
- 31.Henderson HL, Hodson DJ, Gregory SJ, Townsend J, Tortonese DJ. Gonadotropin-releasing hormone stimulates prolactin release from lactotrophs in photoperiodic species through a gonadotropin-independent mechanism. Biol Reprod 2008; 78: 370–377. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki S, Yamamoto I, Arita J. Mitogen-activated protein kinase-dependent stimulation of proliferation of rat lactotrophs in culture by 3,5-cyclic adenosine monophosphate. Endocrinology 1999; 140: 2850–2858. [DOI] [PubMed] [Google Scholar]
- 33.Ishida M, Mitsui T, Yamakawa K, Sugiyama N, Takahashi W, Shimura H, Endo T, Kobayashi T, Arita J. Involvement of cAMP response element-binding protein in the regulation of cell proliferation and the prolactin promoter of lactotrophs in primary culture. Am J Physiol Endocrinol Metab 2007; 293: E1529–E1537. [DOI] [PubMed] [Google Scholar]