Abstract
Aberrant expression of CXCR4 has been indicated to play a role in the pathogenesis of systemic lupus erythematosus (SLE), but the mechanism of CXCR4 dysregulation in SLE is unclear. This study is aimed to explore the clinical significance and possible mechanisms of abnormal CXCR4 expression on B cells from patients with untreated SLE. Expression of CXCR4 on peripheral B cells was determined by flow cytometry and western blotting. Freshly isolated B cells were cultured with exogenous interleukin 21(IL-21) in the presence or absence of CD40 ligand (CD40L) plus anti-IgM antibody (aIgM), and changes in CXCR4 expression were detected. Involvement of phosphatidylinositol 3 kinase (PI3K)/Akt and Janus kinase/Signal transducer and activator of transcription (JAK/STAT) signaling pathways was assessed by adding blocking agents Ly294002 and AG490. Since CD63 is reported to mediate endosomal recruitment of CXCR4 and BCL6 is capable of silencing CD63 gene transcription, we also measured BCL6 and CD63 gene transcription with real-time PCR. It was shown that CXCR4 expression on B cells was significantly upregulated in SLE patients, especially in those with lupus nephritis, and was positively correlated with SLE Disease Activity Index scores and negatively with the serum complement 3 levels (P<0.05). Downregulation of CXCR4 by IL-21 was intact. In contrast, a similar effect of aIgM plus CD40L in downregulating CXCR4 expression was defective in SLE patients but was restored by co-stimulation with IL-21 in vitro. Both Ly294002 and AG490 promoted downregulation of surface CXCR4 expression on B cells from SLE patients (P=0.078 and P=0.064). Furthermore, B cells from SLE patients exhibited diminished CD63 mRNA and enhanced BCL6 mRNA expression (both P<0.05). To sum up, CXCR4 was overexpressed on SLE B cells, positively correlating with disease activity and kidney involvement. Overactivation of the PI3K/Akt and JAK/STAT pathways as well as defective CD63 synthesis may contribute to CXCR4 dysregulation in SLE.
Keywords: CXCR4, IL-21, signaling pathway, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with heterogeneous clinical manifestations and various immune dysfunctions. It is characterized by disturbances in B-cell homeostasis and the development of a variety of autoantibodies against intra- and extracellular autoantigens.1, 2 Lymphocyte trafficking and positioning within lymphoid organs are controlled by chemokines and their receptors. One important chemokine receptor, CXCR4, is expressed by multiple types of leukocytes3, 4, 5 and is specifically recognized by CXC chemokine ligand 12 (CXCL12).6, 7 CXCR4 is involved in many immune system functions, such as cell chemotaxis, proliferation, apoptosis, survival and differentiation.8 Differential expression of CXCR4 on centroblasts and centrocytes in the germinal center (GC) is critical for segregating the dark zone and the light zone and assists in the maturation and selection of B cells.9, 10 Furthermore, targeted interference of the CXCR4 gene in mice has revealed its critical role in lymphopoiesis, myelopoiesis, GC organization and maintenance of bone marrow stem cell pools.9, 11, 12, 13, 14 Although overexpression of CXCR4 on B cells has been reported both in a lupus mouse model and in SLE patients,15, 16 contradictory results have also been reported.17 Recently, it has been demonstrated that CXCR4 is crucial for the pathogenesis of murine lupus.8 Heightened CXCR4 levels in lupus mice were found to prolong B cell survival and promote migration of these cells to end-organs via CXCL12 gradients.8 In addition, CXCL12 has been shown to be selectively upregulated in the glomeruli and tubules of kidneys from several murine lupus strains with nephritis (for example, NZB/W, BXSB and MRL/lpr) and in patients with lupus nephritis.15, 16, 18, 19 In situ CXCL12 attracts lymphocytes expressing CXCR4, leading to sequestration of these lymphocytes in target organs. Importantly, blocking the interaction between CXCR4 and CXCL12 with an antagonistic peptide early in life or after disease onset was found to inhibit autoantibody production, ameliorate renal disease, reduce disease activity and prolong survival in NZB/W and B6.Sle1Yaa lupus-prone mice.8, 18 Therefore, the CXCL12/CXCR4 axis plays a critical role in recruiting B cells to the kidneys and contributes to nephritis both in mice and in humans.8 Moreover, recent research on the mechanism of lupus protection by malaria infection reported that lupus mice infected with the parasite exhibited attenuated B-cell hyperactivity and restoration of aberrantly elevated pro-inflammatory cytokine profiles via modulation of CXCR4 expression and inhibition of the phosphorylation of downstream signaling molecules, including Akt, NFkappaB and ERK.20 All the above findings indicate that targeting CXCR4 might be promising for lupus therapy.
CXCR4/CXCL12, together with other chemokines and their cognate receptors, contribute to plasmablast migration into bone marrow and inflamed tissues where these cells differentiate into short-lived or long-lived plasma cells and continuously produce autoantibodies such as anti-double-stranded DNA antibody (anti-dsDNA). Although the increased expression of CXCR4 on SLE B cells has been demonstrated, the mechanism of defective CXCR4 regulation in lupus has not yet been explored.
It has been reported that interleukin 21 (IL-21) and B-cell receptor (BCR) crosslinking induce CXCR4 endocytosis and that CD63 mediates endosomal recruitment of CXCR4 in C57BL/6 mice in vitro.21, 22 In addition, overproduction of IL-21 and dysregulation of IL-21 R in certain B-cell subpopulations have been demonstrated in several murine lupus models as well as in SLE patients.23, 24 IL-21, produced mainly by follicular helper T cells (TFH), which have a central role in GC B-cell selection, is able to directly induce B-cell activation, mediate B-cell proliferation and promote plasma cell development, most likely through the downstream pathway of the Janus kinase/Signal transducer and activator of transcription (JAK/STAT) signaling.25 Furthermore, a recent study indicated that phosphatidylinositol 3 kinase (PI3K)/Akt may be involved in CXCR4 regulation.26 Thus, the JAK/STAT and PI3K/Akt pathways are involved in many physical and pathological activities. In this study, we examined the influence of IL-21 on the regulation of CXCR4 in SLE B cells and explored whether the PI3K/Akt and JAK/STAT signaling pathways are involved.
Materials and methods
Patients and controls
Peripheral anticoagulant blood samples were collected from 18 treatment-naive SLE patients who visited Peking Union Medical College Hospital (PUMCH) (17 female, one male; mean age 31±9 years, range 15–45 years). Before visiting PUMCH, these patients had not been diagnosed with SLE, nor had they received any lupus-related therapy. Although a detailed history revealed that some of the patients were likely to have exhibited relevant symptoms or signs of lupus for a long time, they were also defined as treatment-naive SLE patients. All patients fulfilled the revised American College of Rheumatology (ACR) 1997 classification criteria for SLE. Disease activity was evaluated by SLEDAI-2000 criteria (mean 11.7, range 5–19). Peripheral blood samples from 18 age and gender-matched healthy volunteers (17 female, one male; mean age 27±3 years, range 23–32 years) were collected as controls. Approval of this study was obtained from the ethics committees of Peking Union Medical College Hospital Review Board.
Cell preparation
Peripheral blood mononuclear cells (PBMCs) from healthy controls (HC) and SLE patients were prepared by Ficoll–Hypaque density gradient centrifugation. Peripheral B cells were isolated using a negative isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s recommendations. The purity and survival rate of the CD19+ population was typically>95%.
Activation of peripheral B cells in vitro and flow cytometry analysis
Freshly isolated PBMCs or peripheral B cells were incubated with 50 ng/ml IL-21 (PeproTech, Rocky Hill, NJ, USA) for 72 h in the absence or presence of 20 ng/ml CD40L (PeproTech) plus 10 μg/ml anti-human IgM (aIgM) (Sigma-Aldrich, St Louis, MO, USA). The cells were harvested and stained with pre-established combinations of fluorescent mAbs for 30 min on ice in staining buffer (BD Biosciences, Franklin Lakes, NJ, USA). For signaling molecular blockade experiments, isolated B cells were pretreated with 20 μg/ml AG490 (JAK/STAT inhibitor) (Sigma-Aldrich) or 50 μmol/l Ly294002 (PI3K/Akt inhibitor) (Sigma-Aldrich) for 1 h before stimulation with IL-21 and/or CD40L plus aIgM. The following fluorescence-conjugated mAbs and isotype controls from eBioscience (San Diego, CA, USA), BD Biosciences and Biolegend (San Diego, CA, USA) were used: anti-CD19-FITC, anti-CD38-PECY7, anti-IgD-PE, anti-CXCR4-PE, anti-CXCR5-Alexa Fluor 647 and anti-IL-21R-APC.
Western blotting
Isolated B cells were lysed using RIPA buffer (Sigma-Aldrich), and 60–80 μg total cellular protein was electrophoresed on a 10% SDS-polyacrylamide gel electrophoresis gel. The proteins were then transferred onto polyvinylidene-difluoride membranes (Millipore, Darmstadt, Germany) and blocked with 5% non-fat milk for 90 min at room temperature. The membrane was then incubated with antibodies against CXCR4 (Abcam, Cambridge, UK) at 4 °C overnight, with GADPH used as the loading control.
Real-time quantitative PCR
Purified B cells were resuspended in TRIzol reagent (Invitrogen Life Technologies, Waltham, MA, USA), and total RNA was extracted using the RNeasy mini kit (Qiagen, Duesseldorf, Germany). RNA purity was measured by spectrophotometry. Reverse transcription reactions were prepared using the SYBR Premix Ex Taq System (Takara Bio, Mountain View, CA, USA). Real-time PCR was performed using IQ5 System (Bio-Rad, Hercules, CA, USA), and the cycle conditions and relative quantification were completed as described in the manufacturer’s instructions (Bio-Rad). Expression of CD63 and B-lymphoma 6 protein (BCL6) was calculated using the comparative computerized tomography method with efficiency calculations; all mRNA levels were normalized to GAPDH mRNA. The primers used were as follows:
CD63 Forward: 5'-CTGGACAGGATGCAGGCAGA-3';
CD63 Reverse: 5'-CCACAGTAACATTAATGCAGCAGCAGGA-3';
BCL6 Forward: 5'-GGAACCTCCAAATCCGAGAC-3'
BCL6 Reverse: 5'-AGCCCTCAAAGCCACAAGAT-3';
GAPDH Forward: 5'-AAGCCCATCACCATCTTCCA-3';
GAPDH Reverse: 5'-CCTGCCTCACCACCTTCTTG-3'
Statistical analysis
All data were analyzed using SPSS 19.0 software (IBM, Inc., Armonk, NY, USA). The data passing both Kolmogorov–Smirnov and Shapiro–Wilk tests (P>0.05) were considered to have a normal distribution. For data with a normal distribution and homogeneity of variance, one-way analysis of variance with adjusted Bonferroni correction was used to assess differences among groups. The independent sample t-test was used to compare differences between two groups. Correlations were analyzed with Pearson’s correlation. For the data with non-normal distribution, the Mann–Whitney U-test was used to compare differences between two groups. In the co-culture, stimulation experiments and the signaling blockade experiments, repeated measures analysis was applied. For those data not passing Mauchly’s test of sphericity, Greenhouse–Geisser calibration results were chosen. Values of P<0.05 were considered statistically significant.
Results
Abnormal expression of CXCR4 and CXCR5 on SLE B lymphocytes
We first assessed the expression of CXCR4 and CXCR5 on circulating B cells from SLE patients and HC. Flow cytometry analysis revealed upregulation of CXCR4 and downregulation of CXCR5 on peripheral blood CD19+B cells from SLE patients compared with those from HC (CXCR4 mean fluorescence intensity (MFI): 35260±6764 vs 14130±2342, P=0.008; CXCR5 MFI: 5163±766 vs 13660 ±2662, P=0.006; n=11 per group) (Figure 1a). The percentage of CXCR4highCXCR5low/- B cells was significantly increased while the percentage of CXCR4low/-CXCR5high B cells was significantly decreased in SLE patients (39.1± 4.57% and 7.57±1.48%, respectively, n=18) compared with HC (12.11±5.18% and 32.35±6.27%, respectively, n=11) (both P<0.001) (Figure 1b). Western blotting also confirmed remarkable upregulation of total CXCR4 protein in SLE patients (P=0.05, n=3 for each group) (Figure 1c).
Figure 1.
Expression of CXCR4 and CXCR5 on B cells from SLE patients and HC, as detected by flow cytometry. The independent t-test was used for comparisons between the two groups. (a) Expression of CXCR4 and CXCR5 on peripheral CD19+ B cells in SLE patients and in HC. Representative flow cytometry histogram showing the MFI of CXCR4 and CXCR5 on CD19+ B cells in a SLE patient (red line) and in a HC (black line). Bars represent the mean±s.e.m. (n=11 for each group). (b) Representative flow cytometry zebra plots displaying co-expression of CXCR4 and CXCR5 on peripheral CD19+ B cells and comparison of subgroups (n=11 for HC and n=18 for SLE). (c) Western blots of whole-cell lysates of CD19+ B cells from HC and SLE patients. (Blood samples from three SLE patients and three HCs were used for this experiment; CXCR4 protein levels were standardized by GAPDH and analyzed with the t-test.)
The expression level of CXCR4 on SLE B cells correlated with disease activity
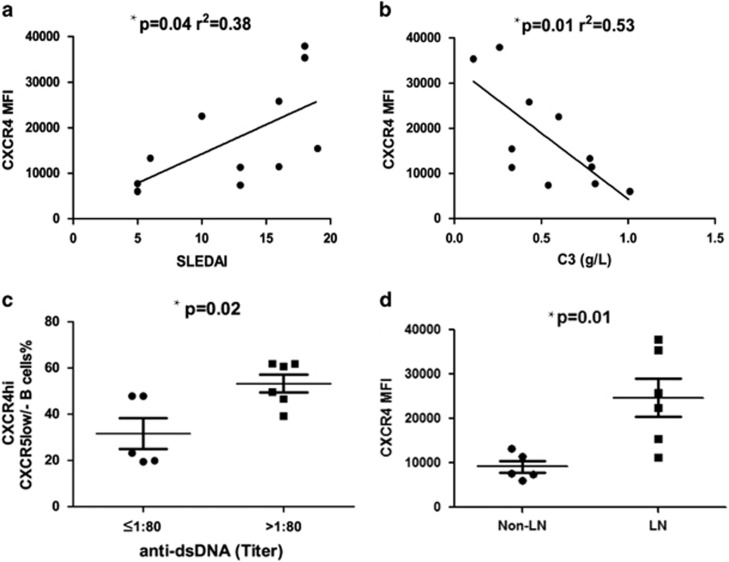
Heightened CXCR4 expression on CD19+ B cells from SLE patients correlated positively with SLEDAI (Systemic Lupus Erythematosus Disease Activity Index) score (r2=0.38; P=0.04; Figure 2a) and negatively with the serum C3 level (r2=0.53; P=0.01; Figure 2b). When patients were stratified by anti-dsDNA titers detected by indirect immunofluorescence, those with higher anti-dsDNA levels (defined as higher than 1:80) had significantly higher percentage of CXCR4highCXCR5low/− B cells (P=0.02; Figure 2c). Notably, CXCR4 levels on CD19+ B cells were more distinctly increased in those SLE patients with nephritis (P=0.01; Figure 2d).
Figure 2.
Correlations between CXCR4 expression and clinical features including the SLEDAI. (a) serum C3 levels, (b) anti-double-strand DNA (c) and lupus nephritis (d). Samples from 11 SLE patients were used for this experiment. Pearson’s correlation and the independent t-test were used for the analysis. Bars show the mean±s.e.m.
Defective downregulation of CXCR4 by BCR crosslinking in SLE B cells
Because IL-21 and IL-21R polymorphisms have been reported to be associated with susceptibility to SLE27 and CXCR4 could be regulated by IL-21 via activation of endocytosis, IL-21R expression on B cells was assessed, confirming enhanced IL-21R expression on B cells from SLE patients compared with HC in our cohort (MFI: 720±67 vs 476±49, P=0.009) (Figure 3a). Considerable IL-21R expression on subgroups of B cells, especially CD19+IgD−CD38− cells, from SLE patients was found (MFI: 401±47 vs 255±34, P=0.02) (Figure 3b). To examine the regulative effect of IL-21 on CXCR4 expression, SLE B cells were stimulated with IL-21 alone or in combination with CD40L plus aIgM. A significant reduction in the percentage of CXCR4+ B cells induced by IL-21 or CD40L plus aIgM was observed in HC (both P<0.05). In SLE patients, the effect of IL-21 appeared to be intact (P<0.05), but CD40L plus aIgM was defective in downregulating the percentage of CXCR4+ B cells (P>0.1 compared with control); however, this could be rescued by co-stimulation with IL-21 (P=0.001, compared with control) (Figure 3c).
Figure 3.
(a and b) Expression of IL-21R on B-cell subpopulations, as detected by flow cytometry. Samples from 18 SLE patients and 14 HC were used for this experiment. The independent t-test was used for comparisons between the two groups. Bars show the mean±s.e.m. (c) Influence of exogenous IL-21 on CXCR4 expression on B cells in the presence or absence of CD40L plus aIgM in vitro (ns: P>0.05). Samples from 11 SLE patients and 10 HCs were used for this experiment. Repeated measures analysis was used for comparison of the different stimulation conditions. Bars show the mean±s.e.m.
The PI3K/Akt and JAK/STAT signaling pathways were involved in IL-21-mediated CXCR4 expression
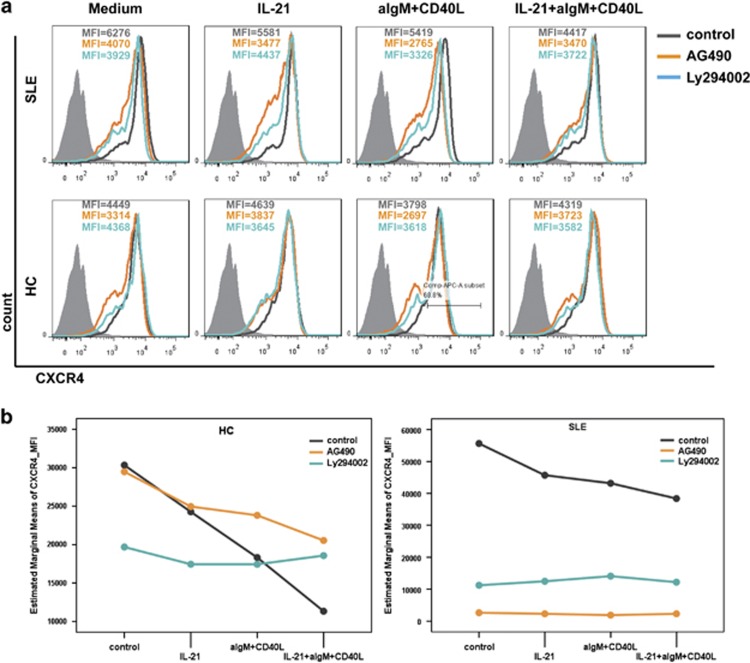
In our previous study, phosphorylation of Akt was found to be overactivated in SLE patients.28 In addition, the association of CXCR4 activation with JAK2 and JAK3 kinases has been reported.29 In the present study, blockade experiments revealed that both Ly294002 (blocking PI3K/Akt) and AG490 (blocking JAK/STAT) strongly inhibit in vitro CXCR4 expression on B cells from SLE patients, independent of the IL-21 stimulation with or without CD40L plus aIgM (P=0.078 and P=0.064). This tendency was not observed in HC (Figure 4).
Figure 4.
Influence of Ly294002 (PI3K/Akt inhibitor) and AG490 (JAK/STAT inhibitor) on CXCR4 expression is independent of IL-21 stimulation in SLE patients. (At least three independent experiments each for HC and SLE were performed.) Isolated peripheral blood B cells were pretreated with AG490 (20 μg/ml) or Ly294002 (50 μmol/l) 1 h before stimulation with CD40L (20 ng/ml) plus anti-IgM (10 μg/ml) with or without IL-21 (50 μg/ml) for 3 days. Surface CXCR4 levels were assessed by flow cytometry. Repeated measures analysis was used for comparison of the different treatment conditions.
Decreased CD63 mRNA and increased BCL6 mRNA in SLE B cells
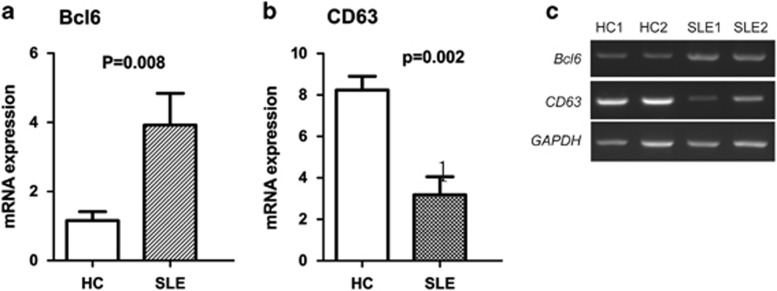
It has been reported that BCL6 upregulates CXCR4 expression on activated B cells by silencing CD63 gene transcription.21 Therefore, we next analyzed expression of BCL6 mRNA and CD63 mRNA in isolated peripheral B cells from HC and SLE patients by real-time quantitative PCR (RT-PCR). We found BCL6 mRNA was upregulated (3.92±0.92 vs 1.16±0.26, P=0.02) and CD63 mRNA downregulated (3.18±0.87 vs 8.23±0.67, P=0.001) in SLE B cells compared with HC B cells (Figure 5).
Figure 5.
(a and b) Expression of BCL6 and CD63 mRNA in purified B cells from HC or SLE patients, as detected by real-time RT-PCR (n=10 for each group). The Mann–Whitney U-test was used for comparison analysis. (c) Representative electrophoresis results are shown.
Discussion
CXCL12-CXCR4 is critically involved in the homing of B cells to lymphoid follicles and inflammatory tissues. Indeed, tight control of the expression of CXCR4 and CXCR5 on immature B cells is crucial for B-cell translocation and migration from the dark zone to the light zone in the GC, which is imperative for the selection and elimination of autoimmune B cells.9 Short-lived plasma cells (PCs) at extra-follicular foci are the main producers of anti-dsDNA, and migration of B cells to extra-follicular sites and their differentiation into PCs are driven by CXCR4-CXCL12.10, 30 In several lupus murine strains, B cells and PCs exhibit elevated CXCR4 expression.8 Increased expression of CXCR4 on B cells and T cells in human lupus has also been reported.16, 31 Consistent with these findings, our study demonstrated that CXCR4 was upregulated in SLE patients and correlated with disease activity and kidney involvement. These results also corroborate the findings of Hanaoka H et al,31 whereby upregulation of CXCR4 expression on circulating B cells with enhanced chemotactic response to CXCL12 in active SLE promoted inflammatory cell infiltration into renal tissue. In our cohort, SLE patients with higher anti-dsDNA titers exhibited a higher proportion of CXCR4highCXCR5low/- B cells. It has been suggested that dysregulated CXCR4/CXCR5 expression on SLE B cells may lead to the mislocalization of autoimmune B cells to extra-follicular sites, may allow them to escape negative selection and apoptosis, and may contribute to autoantibody production and active disease.
A previous study revealed that IL-21 accelerated CXCR4 internalization in mice by inducing endocytosis-related GRK6 expression.21 However, the role of IL-21 in regulating human CXCR4 expression in autoimmunity has not been examined to date. IL-21, which is mainly produced by activated CD4+ T and TFH cells, is essential in regulating B cell responsiveness,25, 32, 33, 34 and has been suggested that IL-21 might serve as a therapeutic target in lupus.35 As demonstrated in our study, SLE B cells expressed higher levels of IL-21R than HC. Accordingly, we examined whether IL-21 has a role in abnormally increased CXCR4 expression in lupus. However, we did find that the downregulation of CXCR4 expression by IL-21 in vitro was intact in B cells from SLE patients. In contrast, BCR crosslinking by CD40L plus aIgM to induce CXCR4 downregulation was defective in SLE, most likely due to the pre-existing over-activation of BCR, although co-stimulation with IL-21 restored CXCR4 downregulation. Because the results of western blotting showed that total CXCR4 protein was increased in SLE patients, mechanisms other than disturbed CXCR4 internalization should exist in SLE. Given the increase in IL-21R expression and intact ability of IL-21 to downregulate CXCR4, IL-21 is probably not responsible for enhanced CXCR4 in SLE. Therefore, we further explored possible signaling pathways.
In our previous study and in the literature, excessive Akt phosphorylation has been confirmed in SLE patients and lupus-prone mice.28, 36, 37 In addition, the association of JAK kinase activation with CXCR4-CXCL2 conjugation has been reported.38 By selectively blocking the PI3K/Akt pathway with Ly294002 or blocking the JAK/STAT pathway with AG490, we found CXCR4 expression was markedly downregulated in SLE B cells, independent of IL-21 and/or BCR stimulation. These results suggest that the abnormally increased CXCR4 levels in SLE may be at least partly due to aberrant over-activation of the PI3K/Akt pathway and the JAK2/STAT3 pathway.
CD63 is a ubiquitously expressed tetraspanin that has been identified as a negative regulator of CXCR4 exocytosis in murine CD4 T cells and B cells.21, 39, 40 CD63 interacts with CXCR4, and the CD63-CXCR4 complex induces CXCR4 trafficking toward endosomes instead of the plasma membrane. BCL6 negatively regulates CD63 mRNA expression in activated B cells, and the level of BCL6 mRNA is inversely correlated with that of CD63 mRNA.21 In this study, we found that B cells from SLE patients expressed higher levels of BCL6 mRNA while producing less CD63 mRNA, which may in turn have led to the dysregulation of CXCR4 on the SLE B cells. Notably, the PI3K/Akt pathway had been reported to participate in BCL6 regulation41, 42 and to contribute to CD63 downregulation.16 Our previous study demonstrated decreased expression of PTEN (a negative regulator of the PI3K/Akt pathway) in SLE, which may contribute to the overactivation of the PI3K/Akt pathway in lupus B cells.28 In the present study, blocking the PI3K/Akt pathway with Ly294002 significantly directly reduced CXCR4 expression as well as indirectly via CD63 upregulation.
In conclusion, CXCR4 was overexpressed on SLE B cells, correlating positively with disease activity and kidney involvement. Overactivation of the PI3K/Akt and JAK/STAT pathways may at least partially contribute to CXCR4 dysregulation in SLE patients. Imbalanced synthesis of CD63 and BCL6 might contribute as well. All these events in turn promote the differentiation of short-lived PCs and the production of anti-dsDNA and are thus involved in the pathogenesis of lupus.
Acknowledgments
We thank the health professional staff from Department of Rheumatology & Clinical Immunology, Peking Union Medical College Hospital and the patients for their participation in this study. This study was supported by grants from the National Natural Science Foundation of China (81325019, 81630044, 81273312, 81601432, 81550023), the National Science Fund for Distinguished Young Scholars of China (813250046), Beijing Municipal Natural Science Foundation (7141008), the Research Special Fund for Public Welfare Industry of Health (20120217), and the Capital Health Research and Development of Special Fund (2011-4001-02).
Author contributions
All authors made substantial contributions. L-dZ and XZ conceived and directed the project and wrote the manuscript. DL and X-nW performed data input and statistical analysis. DL and YL performed flow cytometry experiments. The cell culture experiments were performed by J-wN. Western blotting was performed by CZ. RT-PCR was performed by HC. W-jZ and YL offered important suggestions. LW and Y-yF helped with clinical evaluations of the patients. F-lT and F-cZ collected samples. WH and X-tC provided the concept.
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol 2000; 165: 5970–5979. [DOI] [PubMed] [Google Scholar]
- Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med 2008; 358: 929–939. [DOI] [PubMed] [Google Scholar]
- Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood 2000; 95: 3289–3296. [PubMed] [Google Scholar]
- Nanki T, Lipsky PE. Cutting edge: stromal cell-derived factor-1 is a costimulator for CD4+ T cell activation. J Immunol 2000; 164: 5010–5014. [DOI] [PubMed] [Google Scholar]
- Honczarenko M, Douglas RS, Mathias C, Lee B, Ratajczak MZ, Silberstein LE. SDF-1 responsiveness does not correlate with CXCR4 expression levels of developing human bone marrow B cells. Blood 1999; 94: 2990–2998. [PubMed] [Google Scholar]
- Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J et al. CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev 2010; 29: 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruz A, Samsom M, Alonso JM, Alcami J, Baleux F, Virelizier JL et al. Genomic organization and promoter characterization of human CXCR4 gene. FEBS Lett 1998; 426: 271–278. [DOI] [PubMed] [Google Scholar]
- Wang A, Fairhurst AM, Tus K, Subramanian S, Liu Y, Lin F et al. CXCR4/CXCL12 hyperexpression plays a pivotal role in the pathogenesis of lupus. J Immunol 2009; 182: 4448–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol 2004; 5: 943–952. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med 2001; 194: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 1998; 393: 591–594. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 1998; 393: 595–599. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006; 25: 977–988. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996; 382: 635–638. [DOI] [PubMed] [Google Scholar]
- Chong BF, Mohan C. Targeting the CXCR4/CXCL12 axis in systemic lupus erythematosus. Expert Opin Ther Targets 2009; 13: 1147–1153. [DOI] [PubMed] [Google Scholar]
- Wang A, Guilpain P, Chong BF, Chouzenoux S, Guillevin L, Du Y et al. Dysregulated expression of CXCR4/CXCL12 in subsets of patients with systemic lupus erythematosus. Arthritis Rheum 2010; 62: 3436–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biajoux V, Bignon A, Freitas C, Martinez V, Thelen M, Lima G et al. Expression of CXCL12 receptors in B cells from Mexican Mestizos patients with systemic Lupus erythematosus. J Transl Med 2012; 10: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanian K, Couderc J, Bouchet-Delbos L, Amara A, Berrebi D, Foussat A et al. Role of the chemokine stromal cell-derived factor 1 in autoantibody production and nephritis in murine lupus. J Immunol 2003; 170: 3392–3400. [DOI] [PubMed] [Google Scholar]
- Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 2005; 67: 1772–1784. [DOI] [PubMed] [Google Scholar]
- Badr G, Sayed A, Abdel-Maksoud MA, Mohamed AO, El-Amir A, Abdel-Ghaffar FA et al. Infection of female BWF1 lupus mice with malaria parasite attenuates B cell autoreactivity by modulating the CXCL12/CXCR4 axis and its downstream signals PI3K/AKT, NFkappaB and ERK. PLoS One 2015; 10: e0125340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Kitayama D, Arima M, Sakamoto A, Inamine A, Watanabe-Takano H et al. CXCR4 expression on activated B cells is downregulated by CD63 and IL-21. J Immunol 2011; 186: 2800–2808. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Signoret N, Ishiai M, Marsh M, Kurosaki T, Ravetch JV. B cell antigen receptor engagement inhibits stromal cell-derived factor (SDF)-1alpha chemotaxis and promotes protein kinase C (PKC)-induced internalization of CXCR4. J Exp Med 1999; 189: 1461–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubier JA, Bennett SM, Sproule TJ, Lyons BL, Olland S, Young DA et al. Treatment of BXSB-Yaa mice with IL-21R-Fc fusion protein minimally attenuates systemic lupus erythematosus. Ann N Y Acad Sci 2007; 1110: 590–601. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol 2004; 173: 5361–5371. [DOI] [PubMed] [Google Scholar]
- Deng XM, Yan SX, Wei W. IL-21 acts as a promising therapeutic target in systemic lupus erythematosus by regulating plasma cell differentiation. Cell Mol Immunol 2015; 12: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing W, Pang X, Qu Q, Bai X, Yang W, Bi Y et al. Simvastatin improves the homing of BMSCs via the PI3K/AKT/miR-9 pathway. J Cell Mol Med 2016; 20: 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Luo B, Wang JL, Jiang YW, Wei YS. The association of interleukin-21 polymorphisms with interleukin-21 serum levels and risk of systemic lupus erythematosus. Gene 2014; 538: 94–98. [DOI] [PubMed] [Google Scholar]
- Wu XN, Ye YX, Niu JW, Li Y, Li X, You X et al. Defective PTEN regulation contributes to B cell hyperresponsiveness in systemic lupus erythematosus. Sci Transl Med 2014; 6: 246ra99. [DOI] [PubMed] [Google Scholar]
- Vila-Coro AJ, Rodriguez-Frade JM, Martin De Ana A, Moreno-Ortiz MC, Martinez AC, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J 1999; 13: 1699–1710. [PubMed] [Google Scholar]
- Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol 2009; 183: 3139–3149. [DOI] [PubMed] [Google Scholar]
- Hanaoka H, Okazaki Y, Hashiguchi A, Yasuoka H, Takeuchi T, Kuwana M. Overexpression of CXCR4 on circulating B cells in patients with active systemic lupus erythematosus. Clin Exp Rheumatol 2015; 33: 863–870. [PubMed] [Google Scholar]
- Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol 2007; 179: 5886–5896. [DOI] [PubMed] [Google Scholar]
- Konforte D, Paige CJ. Identification of cellular intermediates and molecular pathways induced by IL-21 in human B cells. J Immunol 2006; 177: 8381–8392. [DOI] [PubMed] [Google Scholar]
- Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med 2010; 207: 155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitoma H, Horiuchi T, Kimoto Y, Tsukamoto H, Uchino A, Tamimoto Y et al. Decreased expression of interleukin-21 receptor on peripheral B lymphocytes in systemic lupus erythematosus. Int J Mol Med 2005; 16: 609–615. [PubMed] [Google Scholar]
- Garcia-Rodriguez S, Callejas-Rubio JL, Ortego-Centeno N, Zumaquero E, Ríos-Fernandez R, Arias-Santiago S et al. Altered AKT1 and MAPK1 gene expression on peripheral blood mononuclear cells and correlation with T-helper-transcription factors in systemic lupus erythematosus patients. Mediators Inflamm 2012; 2012: 495934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylianou K, Petrakis I, Mavroeidi V, Stratakis S, Vardaki E, Perakis K et al. The PI3K/Akt/mTOR pathway is activated in murine lupus nephritis and downregulated by rapamycin. Nephrol Dial Transplant 2011; 26: 498–508. [DOI] [PubMed] [Google Scholar]
- Moriguchi M, Hissong BD, Gadina M, Yamaoka K, Tiffany HL, Murphy PM et al. CXCL12 signaling is independent of Jak2 and Jak3. J Biol Chem 2005; 280: 17408–17414. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Kawano Y, Sato K, Ando Y, Aoki J, Miura Y et al. A CD63 mutant inhibits T-cell tropic human immunodeficiency virus type 1 entry by disrupting CXCR4 trafficking to the plasma membrane. Traffic 2008; 9: 540–558. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ebina H, Koyanagi Y. N-linked glycan-dependent interaction of CD63 with CXCR4 at the Golgi apparatus induces downregulation of CXCR4. Microbiol Immunol 2009; 53: 629–635. [DOI] [PubMed] [Google Scholar]
- Zhang TT, Makondo KJ, Marshall AJ. p110delta phosphoinositide 3-kinase represses IgE switch by potentiating BCL6 expression. J Immunol 2012; 188: 3700–3708. [DOI] [PubMed] [Google Scholar]
- Glauser DA, Schlegel W. The FoxO/Bcl-6/cyclin D2 pathway mediates metabolic and growth factor stimulation of proliferation in Min6 pancreatic beta-cells. J Recept Signal Transduct Res 2009; 29: 293–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.