Abstract
The role of IL-17A is important in protection against lung infection with Chlamydiae, an obligate intracellular bacterial pathogen. In this study, we explored the producers of IL-17A in chlamydial lung infection and specifically tested the role of major IL-17A producers in protective immunity. We found that γδT cells and Th17 cells are the major producers of IL-17A at the early and later stages of chlamydial infection, respectively. Depletion of γδT cells in vivo at the early postinfection (p.i.) stage, when most γδT cells produce IL-17A, failed to alter Th1 responses and bacterial clearance. In contrast, the blockade of IL-17A at the time when IL-17A was mainly produced by Th17 (day 7 p.i.) markedly reduced the Th1 response and increased chlamydial growth. The data suggest that the γδ T cell is the highest producer of IL-17A in the very early stages of infection, but the protection conferred by IL-17A is mainly mediated by Th17 cells. In addition, we found that depletion of γδ T cells reduced IL-1α production by dendritic cells, which was associated with a reduced Th17 response. This finding is helpful to understand the variable role of IL-17A in different infections and to develop preventive and therapeutic approaches against infectious diseases by targeting IL-17A.
Keywords: chlamydia, IL-17A, γδ T cell, infection, Th17
Introduction
IL-17A is a T-cell-derived pro-inflammatory cytokine that induces the generation, migration and accumulation of neutrophils. It is generally accepted that IL-17 signaling plays an important role in controlling extracellular infections by enhancing chemokine responses, inducing antimicrobial proteins and recruiting inflammatory cells.1, 2, 3, 4 Although IL-17A is considered less critical for protection against intracellular infections,5 recent data suggest a significant contribution of IL-17A in the protective immunity against some intracellular bacterial infections, such as Francisella tularensis LVS6 and Chlamydiae.7, 8, 9, 10, 11, 12
γδ T cells are functionally differentiated within the fetal thymus before being exposed to foreign antigens, and thus are known as naturally occurring effectors.13, 14 γδ T cells constitute a small percentage of the circulating T cells in humans and in mice, but are predominantly distributed in mucosal tissues such as the peritoneal cavity, gut and lung.14 γδ T cells are the first to develop and are recruited and expanded in response to various mucosal infections.14 Although the study of IL-17A production has largely focused on CD4+ αβ T cells, termed T helper 17 (Th17) cells, a number of recent studies using various experimental mouse models have shown that γδ T cells are also a major producer of IL-17 following intracellular bacterial infections, including Mycobacterium tuberculosis,15 Listeria monocytogenes16 and Salmonella enterica enteritidis.17 Similarly, γδ T cells are also found to be a major source of IL-17A in human tuberculosis patients.18 In addition, other cell types such as CD8+ T cells,19 NK cells20 and neutrophils21 are also reported to be IL-17A producers. Therefore, IL-17A production by the innate immune cells may bridge the innate and adaptive immunity, contributing to protection against intracellular infections.
Chlamydia is an intracellular bacterium that mainly infects mucosal epithelial cells and macrophages, causing different types of human and farm animal diseases. Using a respiratory tract infection model of Chlamydia muridarum (Cm), we and others have shown that chlamydial lung infection can induce IL-17A production and Th17 cell expansion, which plays an important role in the host defense against the infection.7, 11 We also found that IL-17A can promote type-1 T-cell immunity through modulating dendritic cell (DC) function,7 and that IL-17A can synergize with Th1 cytokines to control Chlamydia infections.7, 8, 9 However, the sources of IL-17A production in lung chlamydial infection and, more importantly, the contribution of various IL-17A producers in the host defense against chlamydial infection remain unclear. Therefore, we performed this study to specifically address these questions.
In this study, we demonstrate that γδ T cells and CD4+ T (Th17) cells are the two major producers of IL-17A in the lung at the early and later stages of chlamydial infection, respectively. More importantly, our results indicate that Th17 is the dominant effecter of IL-17A-mediated protection against Cm lung infection, although γδ T cells are the major producer of IL-17A in the very early stages of infection.
Materials and methods
Mice and microorganisms
Female BALB/c mice of 6–8 weeks of age were purchased from Charles River Laboratories (St Constant, Canada) and housed in the animal care facility at the University of Manitoba under pathogen-free conditions. All mice used in experiments were between 6 and 8 weeks old, and matched for sex and age. The research protocol was approved by the institutional ethical committees, and all animal experiments were conducted according to the guidelines of the Canadian Council of Animal Care. Cm, a mouse chlamydial strain, was used for airway infection of the mice. The reproduction and purification of Cm elementary bodies (EBs) were performed as previously described22, 23 and stored at −80 °C until further use. UV-inactivated EBs were used for antigen stimulation in cell culture.
Infection of mice and γδ T cells depletion
For airway infection, a dose of 1 × 103 inclusion-forming units (IFU) of Cm were given intranasally (i.n.) to the mice under appropriate anesthesia in a 40 μl volume of PBS as described.7 For the depletion of airway γδ T cells, mice were treated i.n. with 10 μg anti-TCR γδ monoclonal antibody (mAb; clone GL3, BD Pharmingen, San Diego, CA, USA) at 1 day before infection in 40 μl of PBS. Sham-treated control mice were administered i.n. with isotype-matched anti-Hamster IgG mAb (BD Pharmingen) on the same schedule as anti-TCR γδ mAb delivery. For IL-17A neutralization, γδ T-cell-depleted mice or isotype control mice were further administered i.n. with 10 μg anti-mouse IL-17A mAb (R&D Systems, Minneapolis, MN, USA) at day 7 postinfection (p.i.). The mice were killed at designated time points p.i. and the immune responses and infection were analyzed.
Determination of pulmonary chlamydial loads and histopathological analysis
The mice were intranasally infected with Cm and were killed at the indicated time points. The lungs were homogenized in sucrose phosphate glutamic acid buffer (SPG). To determine chlamydial growth in vivo, lung homogenates were titrated by infection of HeLa cell monolayers as described previously.7 For histopathological analysis, the lungs were fixed in 10% formalin. The lung sections were stained with H&E, and the histological changes of the lung were analyzed by light microscopy.
Cell preparation
Lung mononuclear cells of mice were prepared and analyzed as described previously.24 Briefly, lung tissues were digested in RPMI 1640 containing 2 mg/ml collagenase type XI (Sigma-Aldrich, St Louis, MO, USA) and 100 g/ml DNase I (Sigma-Aldrich) for 60 min at 37 °C and 2 mM EDTA was added during the last 5 min of incubation. After enzymatic digestion, lung leukocytes were further enriched by 35% Percoll (Sigma-Aldrich) centrifugation and followed by lysis of erythrocytes using ACK lysing buffer. Spleen cells were prepared by 2 mg/ml collagenase D (Sigma-Aldrich) digestion. CD11c+ DCs were isolated and purified from splenic single-cell suspensions by using the MACS (Miltenyi Biotec, Auburn, CA, USA) system as previously described.24
Cytokine production assay
Spleen and lung mononuclear cells from Cm-infected-γδ T-cell-depleted mice or isotype control antibody-treated mice were cultured with UV-sterilized Cm (UV-Cm) to test Cm-driven cytokine production, as described.24 Briefly, spleen and lung single-cell suspensions were cultured at a concentration of 7.5 × 106 cells/well and 5 × 106 cells/well, respectively, in the presence or absence of UV-Cm (1 × 105 IFU/ml) stimulation. For the detection of cytokine production by DCs, the isolated DCs were cultured with UV-Cm in 96-well plates at a density of 5 × 105 cells/well. After 72 h culture, the supernatants were collected for cytokine detection by enzyme-linked immunosorbent assay (ELISA; eBioscience, San Diego, CA, USA) as described.24
FACS analysis
For cell-surface staining, designated cells were incubated with fluorochrome-conjugated mAb for 20 min on ice followed by washing with PBS containing 2% bovine serum albumin (BSA). Conjugated antibodies specific for cell surface markers, CD3, CD4, CD8 and DX5 as well as isotype controls were purchased from eBioscience or Biolegend (San Diego, CA, USA). To determine intracellular cytokine expression, cells were cultured with 50 ng/ml PMA (eBioscience), 1 μg/ml ionomycine (eBioscience) and 5 μg/ml brefeldin A (eBioscience) for 5 h at 37 °C. The cells were stained first for surface antigens and then fixed with 4% w/v paraformaldehyde in PBS. The cells were washed with permeabilization buffer which contained 0.1% saponin (Sigma), 2% heat-inactivated FCS and 0.1% NaN3 in PBS followed by incubation with saponin and allophycocyanin-conjugated anti-IFNγ (eBioscience) or anti-IL-17A mAb for 30 min at room temperature. The cells were analyzed by using an LSR II flow cytometer (BD). The absolute number of γδ T cells and IL-17A-producing γδ T, CD4 T, CD8 T and NK cells in the lung was calculated using the total lung mononuclear cell number and the ratio of these cells determined by flow cytometry.
Statistical analysis
Unpaired Student’s t-test was used to compare the significance of differences between groups, and one-way ANOVA was used for analyzing the significance among multiple groups.
Results
γδ T and Th17 are the predominant cells producing IL-17A following Cm lung infection
We first identified the major IL-17A-producing cells in the lung following Cm infection, focusing on CD3+γδ+ T cells (γδ T cells), CD3+CD4+ T cells (CD4 T cells), CD3+CD8+ T cells (CD8 T cells) and CD3-DX5+ cells (NK cells), which were reportedly IL-17A producers. Lung mononuclear cells obtained from infected mice at different time points after infection were examined for cell surface markers and intracellular IL-17A by flow cytometry. We analyzed the percentage of IL-17A producers of particular cell populations (Figures 1a and b) and also the percentage and total number of the particular type of cells (Figures 1c and d) in the lung. We believe the total number is a better reflection of the particular cell population in the infection. As shown in Figure 1b, γδ T cells had the highest level (16%) of background IL-17A production among the cells being tested before infection (day 0). More markedly, following Cm lung infection, the number of IL-17A-producing γδ T cells quickly reached a peak at 24 h (day 1) with 57% of this cell population producing IL-17A (Figures 1b and d), representing 70% of the IL-17A-producing cells in the lung at this time point (Figure 1c). Similar to its quick increase, the IL-17A production by γδ T cells also dropped quickly, reaching close to the background level at day 4 p.i. (Figure 1d). In contrast, the percentage of IL-17A-producing CD3+CD4+ T cells (Th17) increased slowly after infection, reaching its peak at day 10 p.i.. At day 1 p.i., only 2% of the CD4+ T cells were Th17 cells (Figure 1b), representing ~30% of the total IL-17A-producing cells in the lung (Figure 1c) while at day 10, 6% of the CD4+ T cells were Th17 (Figure 1b), representing 85% of the total IL-17A-producing cells in the lung (Figure 1c). Although IL-17A-producing CD8 T cells also increased slightly following Cm lung infection with a peak at day 10 p.i., the level was very low (<1% of CD8 T cells; Figure 1a). IL-17A-producing NK cells are virtually negligible. These data demonstrate that the γδ T cell is the major IL-17A producer at the very early stages of infection (day 1), while at the peak of infection (day 10), Th17 is the major producer of IL-17A. Although the percentage of IL-17A producers within CD4+ T cells was never as high as the IL-17A producers within γδ T cells, the absolute number of Th17 at the later stages of infection was more than three-fold higher than that of the IL-17A producing γδ T cells. The fold increase in Th17 cells represents the increased IL-17A producers in CD4+ T cells and the increased total number of CD4 T cells in the lung at the later stages of infection. Collectively, the data suggest that γδ T cell is the major IL-17A producer in the beginning of the infection, while Th17 is the major IL-17A producer in the later stage of infection when the adaptive immunity plays its role in host defense.
Figure 1.
IL-17A producing cells in the lung following Cm infection. BALB/c mice were intranasally infected with Cm (1 × 103 IFUs). Lung mononuclear cells were isolated and intracellular IL-17A, as well as surface markers, were analyzed at various time points by flow cytometry. (a) Percentage of IL-17A-producing-CD3+γδ+ T, CD3+CD4+ T, CD3+CD8+ T cells and CD3− NK cells in gated lymphocytes. (b) Percentage of IL-17A-producing cells in gated γδ+CD3+T cells, CD4+CD3+ T cells, CD8+CD3+ T cells and DX5+CD3− cells. (c) Percentage of IL17A producing CD3+γδ+ T, CD3+CD4+ T, CD3+CD8+ T cells and CD3− NK cells in total IL-17A-producing cells. (d) Absolute number of IL-17A-producing γδT, CD4+ T, CD8+ T and NK cells. Three independent experiments with four mice in each group were tested, and a representative experiment is shown.
Early IL-17A-producing γδ T cells largely exist in the lung following infection with Cm
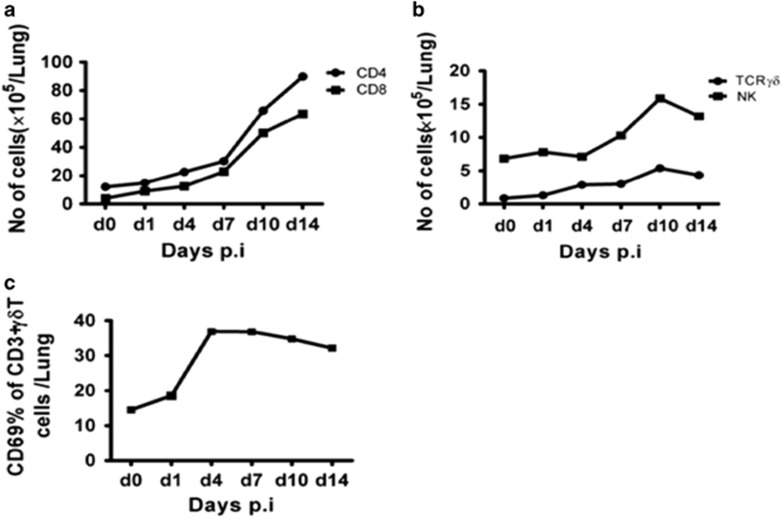
The quick IL-17A production (peak at day 1 p.i.) caused us to wonder about the correlation between γδ T cell activation/expansion and IL-17A production by these cells. We therefore measured IL-17A-producing cell populations and γδ T-cell activation following Cm infection. Interestingly, at day 1 p.i., the total number of γδ T cells did not significantly change compared with CD4 and CD8 T cells (Figures 2a and b), and the expression of CD69, an activation marker, was also minimal (Figure 2c), although it exhibited an upward trend. The absolute number of γδ T cells in the lung increased, steadily peaking at day 10 p.i. while the expression of CD69 peaked at day 3 and remained high during the experimental period (up to day 14). As the peak of IL-17A production by γδ T cells occurred at day 1 p.i. (Figure 1) when the γδ T-cell increase/expansion and activation is minimal (Figure 2), the data suggest that the quick IL-17A-producing γδ T cells likely exist in the lung that can produce IL-17A without full activation (high CD69 expression) following Cm lung infection. Indeed, although the total number of γδ T cells more than doubled at the peak of infection (day 10), the percentage of IL-17A-producers was much lower compared with day 1 p.i. (9% versus 59%), thus the total number of IL-17A-producing γδ T cells was even lower than its baseline. The data also suggest that the newly expanded or recruited γδ T cells following Cm infection are largely not IL-17A producers.
Figure 2.
Increase of particular cell populations and activation of γδ+ T cells in the lung following Cm infection. BALB/c mice were intranasally infected with Cm (1 × 103 IFUs) and killed at the indicated time points. The lung mononuclear cells were prepared and stained with PE-Cy7-anti-CD3, FITC-anti-CD4, PE-anti-CD8, PE-anti-TCRγδ and FITC-anti-DX5 mAbs. The lymphocyte population was analyzed by flow cytometry. The absolute number of CD4+ (CD4+CD3+) T cells and CD8+ (CD8+CD3+) T cells (a), γδ+ (CD3+γδ+) T cells and NK (CD3-DX5+) cells (b) were calculated using total lung mononuclear cell number, and the ratio of the particular cells were determined by flow cytometry. Percentage of CD69 expression was evaluated on CD3+γδ T cells of the lung during Cm infection (c). Three independent experiments with four mice in each group were tested, and a representative experiment is shown.
Depletion of γδ T cells led to reduced IL-17A production but had no significant effect on in vivo chlamydial growth
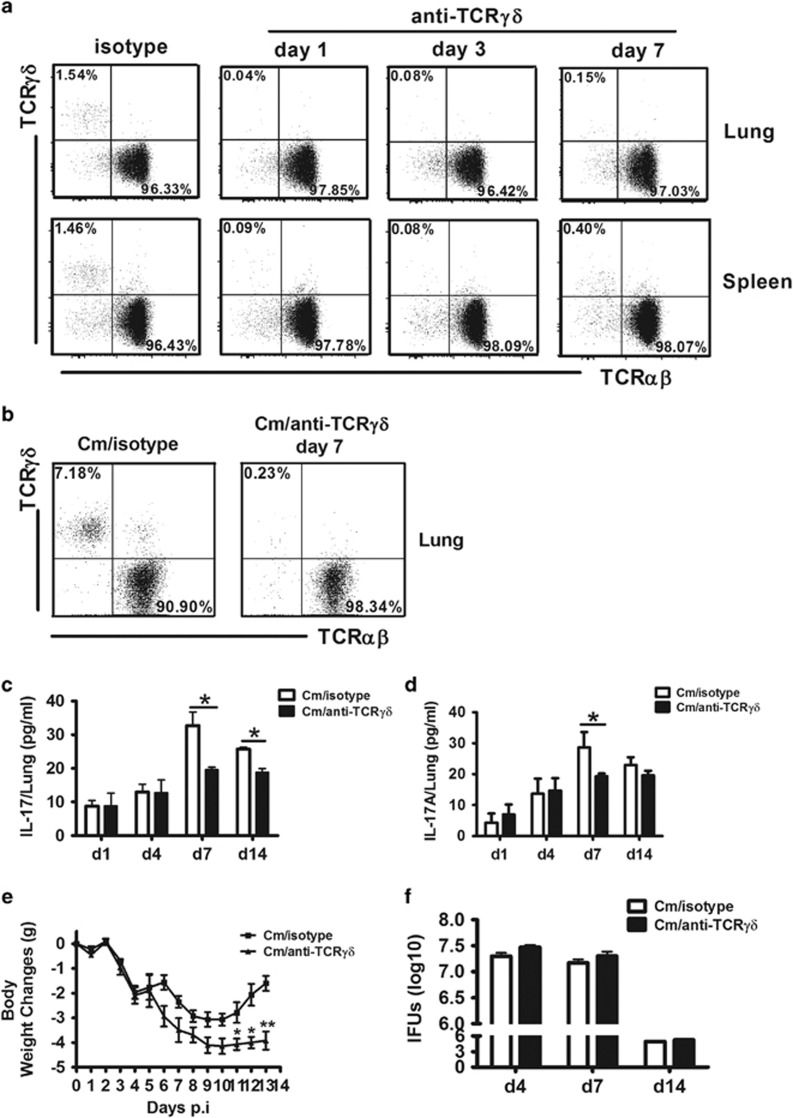
To investigate the influence of γδ T cells on general IL-17A production and disease processes, we depleted γδ T cells from mice by i.n. administration of mAb (GL3) against TCRγδ before Cm infection. This mAb targets the γδ TCR and has been successfully used for in vivo γδ T cell depletion in various infection models.25, 26, 27 We found that more than 95% of γδ T cells were depleted in the lung in naive (Figure 3a) and Cm-infected (Figure 3b) mice when the antibody was delivered intranasally, without a significant effect on αβ T-cell population. We then compared the IL-17A production between γδ T-cell depleted and isotype control antibody-treated mice. We found a significant reduction in IL-17A production by lung mononuclear cells isolated at day 7 and day 14 with UV-Cm stimulation following Cm infection (Figure 3c) and in lung tissue homogenates at day 7 (Figure 3d) in the γδ T-cell-depleted mice compared with the control mice. The data confirm that γδ T cells contribute to IL-17A production following Cm infection.
Figure 3.
Depletion of γδ T cells significantly reduced IL-17A production. BALB/c mice were intranasally treated with 10 μg either anti-TCR γδ mAb (clone GL3) or isotype-matched anti-Hamster IgG mAb (isotype) 1 day before 1 × 103 IFUs Cm airway infection. (a) Successful depletion of γδT cells by the anti-TCR γδ mAb treatment in naive mice at day 1, day 3 and day 7 after Ab administration. Percentage of TCR γδ and TCR αβ in the lung and spleen were analyzed by flow cytometry, gating on CD3+ cells. (b) Percentage of TCR γδ and TCR αβ in the lung following isotype control or anti-TCR γδ mAb administration at day 7 of Cm infection. (c, d) IL-17A production was measured in cell culture supernatants of the lung mononuclear cells (c) and in lung tissue homogenates by ELISA. (d, e) The body weight change in anti-TCR γδ mAb and isotype control mAb-treated-mice following Cm infection. The original body weights of the two groups of mice were similar. (f) Mice were killed on days 4, 7 and 14 p.i. and the lungs were collected and measured for chlamydial growth in vivo as described in the Materials and Methods section. Three independent experiments with four mice in each group were tested. The results are shown as the mean±s.d. *P<0.05, **P<0.01. Note, pooled data of body weight change (e) of 12 mice in each group from three independent experiments are shown.
As we and others have previously shown that IL-17A contributes to the host defense against chlamydial lung infection,7, 8, 9, 10, 11, 12, 13, 14 we then examined the effect of the depletion of γδ T cells, which significantly contributed to IL-17A production in Cm infection, on the disease process. Body weight monitoring showed a larger scale of body weight loss in the γδ T-cell-depleted mice (Figure 3e), especially at the later days post infection, but the lung bacterial loads (IFUs) were not significantly different between the γδ T-cell-depleted and isotype control-treated mice at early (day 4), peak (day 7) and later (day 14) stages of infection (Figure 3f). These results suggest that γδ T cell contributes to IL-17A production and reduces morbidity (measured by less body weight loss), and its role in chlamydial clearance is rather limited.
Depletion of γδ T cells significantly decreased Th17 but not Th1 following Cm infection
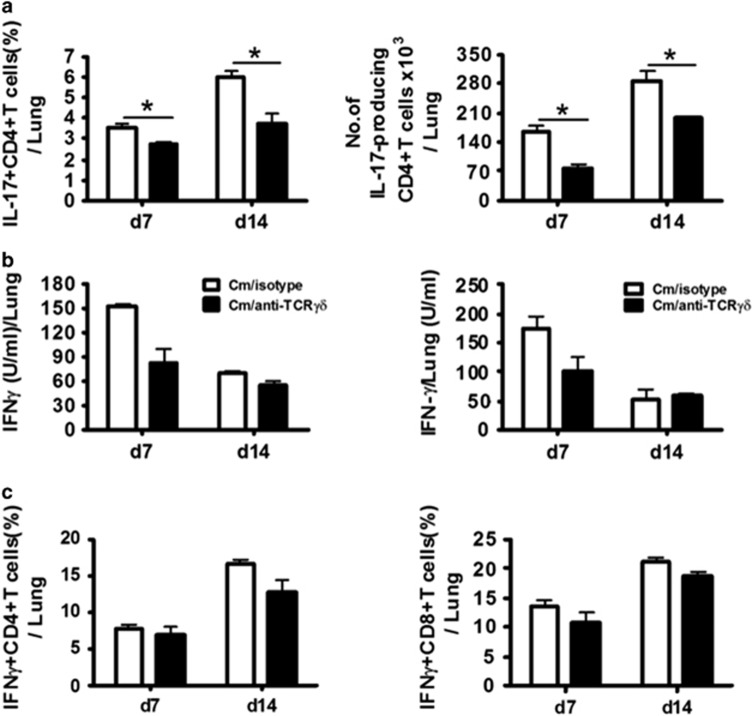
Previous work has shown synergy between the Th17 and Th1 responses in the host defense against chlamydial lung infection, and an enhancing effect of IL-17A on the Th1 response.7, 8, 9 Having shown the ample IL-17A production by γδ T cells and the reduction of general IL-17A production by depletion of this cell type, we then tested if γδ T cells could influence Th17 cells, that is, IL-17A producing CD3+CD4+ T cells. The results showed that the Th17 response was significantly reduced in the γδ T-cell-depleted mice, both in percentage and absolute number at day 7 and day 14 p.i. (Figure 4a). Therefore, γδ T cells do have a promoting effect on the Th17 responses in chlamydial lung infection.
Figure 4.
Depletion of γδT cells significantly affected Th17 but not Th1 responses during Cm infection. Mice were depleted of γδT cells and intranasally infected with Cm, as described in the legend to Figure 3. (a) Percentage of IL-17A-produced CD4+ T cells in the lung tissues were determined by intracellular cytokine staining (left panel) and the absolute number of IL-17A-produced CD4+ T cells in the lung tissues was calculated using total lung mononuclear cell number and the ratio of the IL-17A+CD4 cells determined by flow cytometry (right panel). (b) IFNγ production was measured in the culture supernatants of the lung mononuclear cells (left panel) and lung tissue homogenates (right panel) by ELISA at day 7 and day 14 p.i. (c) Percentage of IFNγ-producing CD4+ T cells (left panel) and CD8+ T cells (right panel) in the lung tissue was determined by intracellular cytokine staining. Three independent experiments with four mice in each group were tested, and a representative experiment is shown. The results are shown as the mean±s.d. *P<0.05.
We then further examined the effect of γδ T-cell depletion on Th1 responses in Cm lung infection. The results showed that there was no significant difference in IFNγ production by cultured lung mononuclear cells (left panel) or lung tissue suspension (right panel) between the γδ T-cell-depleted and isotype control antibody-treated mice (Figure 4b). Consistently, at the single-cell level, there was no significant difference in IFN-γ-producing CD4+ T and CD8+ T cells in the lung between the two groups (Figure 4c). The results suggest that γδ T cells, although producing a large amount of IL-17A at the early stage of infection, have no significant impact on Th1 and Tc1 responses.
Th17, rather than γδ T cell, is critical for enhancing Th1 response and protective immunity against lung infection
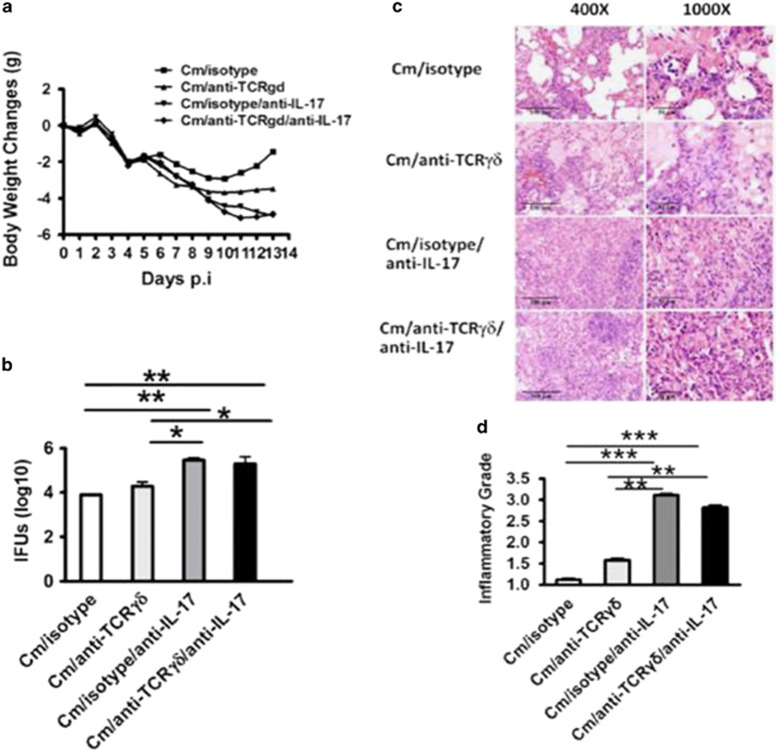
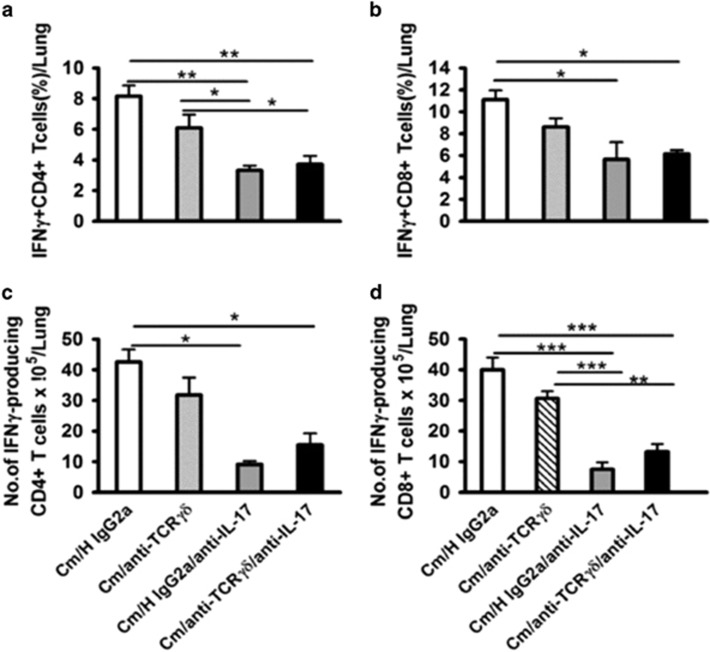
Considering the facts that neutralization of IL-17A has a significant detrimental effect on the Th1 response and chlamydial clearance,7 while depletion of γδ T cells, the major IL-17A producer in early the stage, had no significant impact on chlamydial clearance (Figure 4), we hypothesized that IL-17A-producing γδ T cells and Th17 had different effects on the Th1 response and host defense. To test this hypothesis, we tried to separately test the role of the γδ T cell and Th17 by in vivo antibody depletion/neutralization. On the basis of the kinetics of IL-17A production by γδ T cells and Th17 cells observed in Figure 1 (γδT cell dominated during the first 3 days while Th17 became dominant from day 7), we reasoned that the early depletion of γδ T cells (one day before infection) and later neutralization (day 7) of IL-17A, could, respectively examine the function of IL-17A-producing γδ T cell and Th17 cell. Using this approach, we found that compared with early depletion of γδ T cells, the late neutralization of IL-17A (day 7) thus blocking Th17 had a much more profound impact on the disease process, demonstrated by more serious body weight loss (Figure 5a), bacterial burden (IFU; Figure 5b) and histopathological changes (Figure 5c), semi-quantified by pathological score (Figure 5d). More importantly, the combination of early γδ T-cell depletion and late IL-17A neutralization did not show an added effect on infection and pathology compared with late neutralization of IL-17A alone. The data suggest that IL-17A production by Th17 cells rather than γδ T cells is critical for host defense against Cm lung infection. In addition, unlike the minimal changes of the Th1 response by γδ T depletion (Figure 4), the late neutralization of IL-17A alone significantly reduced the Th1 and Tc1 responses (Figure 6). Again, additional early depletion of γδ T cells in the mice with late IL-17A neutralization had no effect on the percentage (Figures 6a and b) and absolute number (Figures 6c and d) of Th1 and Tc1 cells. The data suggest that the later developed Th17 response, rather than the earlier IL-17A-producing γδ T-cell response, significantly contributes to the development of protective Th1 immunity.
Figure 5.
Later neutralization of IL-17A significantly increased infection compared with the early depletion of γδ T cells. Mice were intranasally infected with 10 μg anti-mouse γδ T cell mAb and 10 μg of H IgG2a isotype control antibody. One day later, two groups of mice were intranasally infected with 1 × 103 IFUs of Cm. At day 7 p.i., two groups of mice were intranasally infected with 10 μg of anti-mouse IL-17A mAb for the neutralization of airway IL-17A. (a) The mice were monitored daily for body weight change. The original body weights of the four groups of mice were similar. (b) The mice were euthanized on day 13 p.i, and the lungs were collected and measured for chlamydial loads in vivo as described in the Materials and Methods section. (c) Histological analysis was performed by staining lung sections with H&E (× 400 and × 1000 magnification under light microscopy). (d) Semi-quantitative analysis of lung inflammation and damage (pathological score). Slides were examined by a blinded pathologist and the inflammatory grades were analyzed as described in the Materials and Methods section. The results are shown as the mean±s.d. of four mice for each group in three independent experiments; *P<0.05; **P<0.01; ***P<0.001.
Figure 6.
Later-neutralized IL-17A significantly reduced IFN-γ production. Mice were neutralized of IL-17A production with or without depletion of γδ T cells in Cm-infected mice as described in the legend to Figure 5. Percentage of IFN-γ-producing CD4+ (a) and CD8+ (b) T cells in the lung tissues at day 13 p.i. were determined by intracellular cytokine staining as described in the Materials and Methods section. The absolute number of IFN-γ-producing CD4+ (c) and CD8+ (d) T cells in the lung tissues was calculated as described in the legend to Figure 2. Three independent experiments with four mice in each group were tested. The results are shown as the mean±s.d.; *P<0.05; **P<0.01; ***P<0.001.
Depletion of γδ T cells had significant effect on IL-1α but not IL-12p40 production by DCs
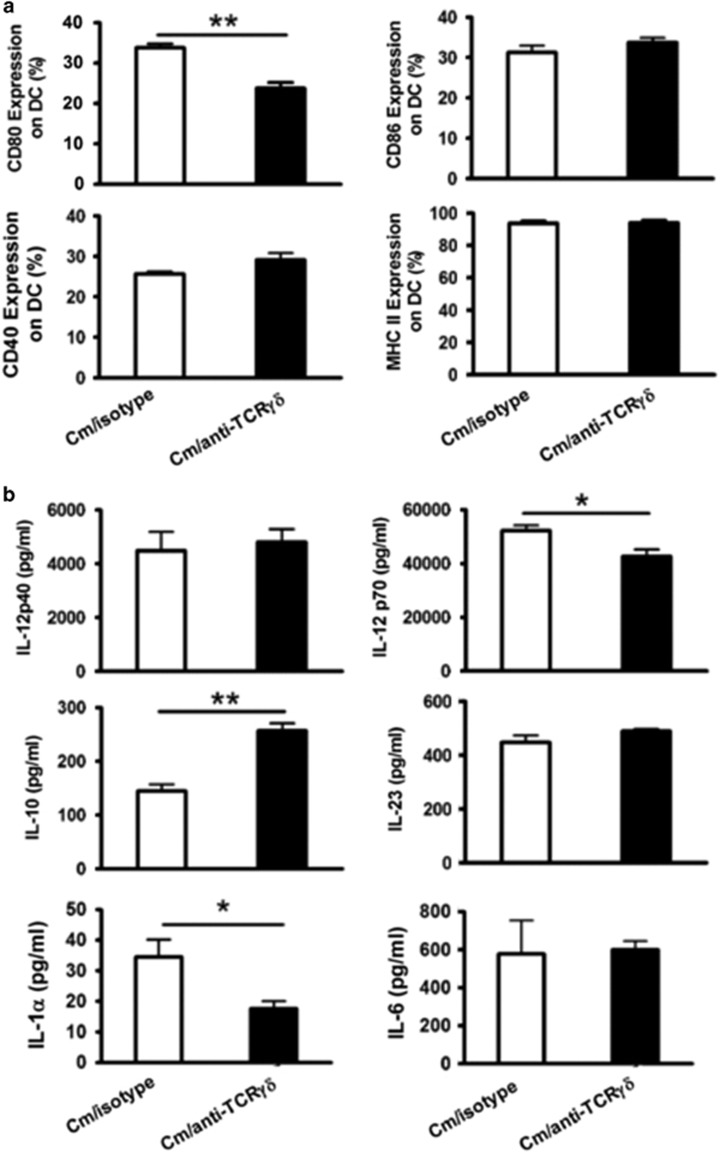
Our previous studies have demonstrated that IL-17A/Th17 can influence co-stimulatory molecule expression and cytokine production by DC, which may be one of the mechanisms by which IL-17A enhances the Th1 response during Cm lung infection.7 To find a clue for the failure of γδ T depletion in altering Th1 and Tc1 responses, we further tested the effect γδ T-cell depletion on DC surface markers and cytokines. We analyzed the DCs isolated from Cm-infected, γδ T-cell-depleted mice and isotype control antibody-treated mice for their surface expression of co-stimulatory molecules, such as CD40, CD80, CD86 and MHC II, and the production of cytokines, such as Th1-promoting cytokines IL-12p40 and IL-12p70, anti-inflammatory cytokines IL-10 and Th17-promoting cytokines IL-23, IL-1α and IL-6. As shown in Figure 7a, Cm-infected, γδ T-cell-depleted mice exhibited significantly reduced CD80 expression but a comparable level of CD86, CD40 and MHC II expression compared with isotype-treated control mice. Interestingly, cytokine analysis showed that depletion of γδ T cells slightly reduced IL-12p70 production but had no significant effect on IL-12p40 production (Figure 7b). The results also showed that depletion of γδ T cells had no significant effect on IL-23 and IL-6 production. Interestingly, the depletion of γδ T cells significantly decreased IL-1α production but increased IL-10 production by DCs. As IL-1α signaling is an important pathway in Th17 development, the data fit the findings shown above that depletion of γδ T cells had a significant effect on Th17 responses.
Figure 7.
Effect of γδ T cell depletion on DC surface markers and cytokine production. Mice were treated as described in the legend to Figure 3, and killed on day 7 p.i. Splenic DCs were isolated using MACS CD11c microbeads as described in the Materials and Methods section. Isolated DCs were double stained with allophycocyanin-conjugated anti-CD11c and PE-labeled antibodies specific to surface markers. (a) The gated CD11c+ DC were analyzed for CD80, CD86, CD40 and MHC II percentages by flow cytometry. (b) Freshly purified CD11c+ DC were cultured at 5 × 105 cells/well in 96-well plates for 72 h, and IL-12p40, IL-12p70, IL-10, IL-23, IL-1α and IL-6 levels in the culture supernatants were measured by ELISA. Three independent experiments with four mice in each group were tested, and a representative experiment is shown. The results are shown as the mean±s.d.; *P<0.05; **P<0.01.
Discussion
We demonstrate in the present study that γδ T and CD4+ T (Th17) cells are mainly responsible for the production of IL-17A in chlamydial lung infection. The dynamics of the two types of cells in producing IL-17A are markedly different, in that γδ T cells are the major producer of IL-17A in the very early stages of infection, while Th17 cells are the major producer of IL-17A in the later stages. More importantly, based on the kinetics of IL-17A production by the two types of cells, we tested their function in modulating Th1 responses and in the clearance of bacteria. For the functional analysis, we tested the effect of two antibody treatment approaches on the host defense. One is to deplete γδ T cells immediately before infection and the other is to block IL-17A at the time point when Th17 becomes the predominant producer of IL-17A while IL-17A producing γδ T cells have reduced below baseline (day 7). We reason that the former reflects the function of γδ T cells while the latter approach reflects the function of Th17. The results showed that depletion of γδ T cells reduced the Th17 response at later time points but had no significant effect on the Th1 response and chlamydial clearance from the lung. In sharp contrast, the blockade of Th17 led to a marked decrease of Th1 and an increase of bacterial growth. Collectively, the data indicate that although γδ T cells are the major IL-17A producer at the early stage of chlamydial lung infection, Th17 cells are the predominant effector for IL-17A-mediated protection in chlamydial lung infection.
Previous studies have shown that γδ T cells play complex roles, protective or detrimental, in various infection modalities in experimental animal models. There are at least three possible mechanisms by which γδ T cells are involved in the immune responses to infection: (1) by regulating innate immune cells by the release of cytokines and chemokines, (2) by lysing infected targets by the release of cytotoxic molecules contained in granules and (3) by inducing conventional CD4 and CD8 T cells. Depletion of γδ T cells by using a genetic knockout approach or by treatment with a specific mAb increased infection with a variety of microorganisms such as Klebsiella, Escherichia coli, Candidiasis and Leishmania major infections.28, 29, 30, 31, 32, 33, 34, 35, 36, 37 However, similar depletion of γδ T cells reduces the infections and diseases caused by Listeria monocytogenes, Candida albicans, Eimeria vermiformis and Salmonella choleraesuis.38, 39, 40, 41 An earlier study by Williams et al. reported that γδ T-cell knockout mice had significantly higher levels of chlamydial growth in the lungs at day 3 and day 7 p.i., but not at the later phase of the infection (day 20).42 Perry et al.43 reported in a Cm genital tract infection model that γδ T-cell-deficient mice had similar levels of chlamydial clearance with wild-type mice. Our data in the present study is largely consistent with the results in the previous studies, showing that γδ T cells can play a supplementary but not essential role in the host defense again chlamydial lung infection. Indeed, although the depletion of γδ T cells led to decreased IL-17A levels and more serious body weight loss following infection, the bacterial load was not significantly different between the γδ T-cell-depleted and intact mice. The data further support the critical importance of Th1 in protection because the present study showed that depletion of γδ T cells had no significant impact on the Th1 response, while blockade of IL-17A significantly reduced the Th1 responses and increased infection. Then, the question is why γδ T cells can promote Th17 responses but have no significant effect on Th1 cell and protection. The reasons are not clear, but it is possible that only a certain level of Th17 is required for enhancing the production of Th1 cytokines and for proper protection. Although the level of IL-17A production by Th17 is reduced in γδ T-cell-depleted mice, the amount is still sufficient for enhancing Th1 responses and for synergizing with Th1 for enhanced protection as we previously reported.8
The lack of any effect on type-1 T-cell responses following the depletion of γδ T cells is rather unexpected. The dominant role of Th1 cells in the host defense against chlamydial infections has been well documented.44, 45 Many previous studies have shown that γδ T cells could promote effector T-cell response, such as Th1 and DTH responses. For example, it was reported that the changes of γδ T cells in M. tuberculosis infection significantly changed the function of protective CD8 cytotoxic T cells. It was likely that the cytokines produced by γδ T cells promoted CD8 T-cell induction in BCG infection.46, 47 The discrepancy between our data and others might be related to the type, route and degree of infections. Notably, the increase of IL-17A production by γδ T cells in chlamydial lung infection is very transient, returning to baseline at 3–4 days following infection. It is interesting that although the γδ T cell response (absolute number) remained increased up to 2 weeks following infection, very few of the later developed γδ T cells produced IL-17A, in sharp contrast with the existing γδ T cells that are largely IL-17A producers. The transient nature of IL-17A production by γδ T cells may partially explain the difference of this cell type compared with Th17 cells in modulating DC function. Our data showed that although γδ T-cell depletion led to reduced CD80 expression and increased IL-10 production by DCs, the impact on IL-12 production is minimal, with only a slight reduction of IL-12p70, while no change was observed in IL-12p40 production. Although IL-12p70 is important for the Th1 response, this minor degree of decrease may not translate into a significant reduction of Th1 responses. In contrast, the reduction of IL-1α is more apparent. As IL-1α signaling is an important pathway for Th17 development, the finding is consistent with the reduction of Th17 following γδ T-cell depletion. It should also be mentioned that although the antibody depletion method is efficient in the depletion of γδ T cells, a small population of residual cells still exists. The residual cell population may still play a role in influencing Th1 cells and other cells.
The assumption that the blockade of IL-17A on day 7 reflects the function of Th17 cells but not γδ T cells is not without controversy. It is true that, at this time point, some γδ T cells may still produce IL-17A. However, because the IL-17A production by γδ T cells returns to baseline or even much below baseline, the effect observed should largely reflect the function of Th17. Due to the lack of Th17 KO mice, this appears the most feasible way at the current stage to address this question.
In conclusion, our data show respective IL-17A production by γδ T cells and Th17 cells at the early stage and later stage of chlamydial lung infection. The IL-17A production by γδ T cells increased very quickly but was very transient in nature, while its production by Th17 increases steadily and maintains a high level. More importantly, our data show that Th17 cells are the major IL-17A producers that promote the Th1 responses and mediate protection in chlamydial infection. The findings in the present study provide new insights into the mechanisms bridging innate and adaptive immunity during lung chlamydial infections, which may have implications in developing effective chlamydial vaccines and in the understanding of host defense mechanisms in other lung infections.
Acknowledgments
This work was supported by grants (to XY) from the Canadian Institutes of Health Research (CIHR), the Manitoba Health Research Council (MHRC) and the Manitoba Institute of Child Health (MICH) and grants (to HB) from the National Natural Science Foundation of China (31070797), the Key Program: 15JCZDJC34900 and 11JCZDJC16200 from Tianjin Municipal Science and Technology Commission (TSTC). XG was a trainee in CIHR National Training Program in Allergy/asthma and a holder of an MICH Studentship. AGJ was a trainee in the CIHR/International Centre for Infectious Diseases (ICID) National Training Program in Infectious Diseases and a holder of an MHRC postdoctoral fellowship. XY was the Canada Research Chair in Infection and Immunity.
Footnotes
The authors declare no conflict of interest.
References
- Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med 2005; 202: 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 2009; 30: 108–119. [DOI] [PubMed] [Google Scholar]
- Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol 2007; 292: L519–L528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, He X, Li X, Qian Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol Immunol 2016; 13: 418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol 2005; 175: 788–795. [DOI] [PubMed] [Google Scholar]
- Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 2009; 31: 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S et al. IL-17/Th17 promotes type 1T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol 2009; 183: 5886–5895. [DOI] [PubMed] [Google Scholar]
- Gao X, Gigoux M, Yang J, Leconte J, Yang X, Suh WK. Anti-chlamydial Th17 responses are controlled by the inducible costimulator partially through phosphoinositide 3-kinase signaling. PloS One 2012; 7: e52657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Ren J, Tang X, Jing Y, Xing D et al. IL-17A synergizes with IFN-gamma to upregulate iNOS and NO production and inhibit chlamydial growth. PloS One 2012; 7: e39214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Gopal R. IL-17 in protective immunity to intracellular pathogens. Virulence 2010; 1: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gao L, Lei L, Zhong Y, Dube P, Berton MT et al. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J Immunol 2009; 183: 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Jiang X, Shen C, Karunakaran KP, Jiang J, Rosin NL et al. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infect Immun 2010; 78: 2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejima T, Shibata K, Yamada H, Hara H, Iwakura Y, Naito S et al. Protective role of naturally occurring interleukin-17A-producing gammadelta T cells in the lung at the early stage of systemic candidiasis in mice. Infect Immun 2011; 79: 4503–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges JF, Graff JC, Jutila MA. Transcriptional profiling of gamma delta T cells. J Immunol 2003; 171: 4959–4964. [DOI] [PubMed] [Google Scholar]
- Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 2006; 177: 4662–4669. [DOI] [PubMed] [Google Scholar]
- Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol 2008; 181: 3456–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz SM, Kohler G, Holscher C, Iwakura Y, Alber G. IL-17A is produced by Th17, gammadelta T cells and other CD4- lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int Immunol 2008; 20: 1129–1138. [DOI] [PubMed] [Google Scholar]
- Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, Wang J et al. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol 2008; 5: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MM, Way SS, Wilson CB. IL-23 promotes the production of IL-17 by antigen-specific CD8 T cells in the absence of IL-12 and type-I interferons. J Immunol 2009; 183: 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos ST, Silver JS, O'Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol 1841776-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten KR, Prakash PS, Unsinger J, Goetzman HS, England LG, Cave CM et al. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infect Immun 78: 4714–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Fan Y, Joyee AG, Wang S, Han X, Bai H et al. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages. J Immunol 2008; 181: 2092–2102. [DOI] [PubMed] [Google Scholar]
- Han X, Wang S, Fan Y, Yang J, Jiao L, Qiu H et al. Chlamydia infection induces ICOS ligand-expressing and IL-10-producing dendritic cells that can inhibit airway inflammation and mucus overproduction elicited by allergen challenge in BALB/c mice. J Immunol 2006; 176: 5232–5239. [DOI] [PubMed] [Google Scholar]
- Joyee AG, Qiu H, Wang S, Fan Y, Bilenki L, Yang X. Distinct NKT cell subsets are induced by different Chlamydia species leading to differential adaptive immunity and host resistance to the infections. J Immunol 2007; 178: 1048–1058. [DOI] [PubMed] [Google Scholar]
- Nian H, Shao H, Zhang G, Born WK, O'Brien RL, Kaplan HJ et al. Regulatory effect of gammadelta T cells on IL-17+ uveitogenic T cells. Invest Ophthalmol Vis Sci 2010; 51: 4661–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezu K, Kawakami K, Miyagi K, Kinjo Y, Kinjo T, Ishikawa H et al. Accumulation of gammadelta T cells in the lungs and their regulatory roles in Th1 response and host defense against pulmonary infection with Cryptococcus neoformans. J Immunol 2004; 172: 7629–7634. [DOI] [PubMed] [Google Scholar]
- Koenecke C, Chennupati V, Schmitz S, Malissen B, Forster R, Prinz I. in vivo application of mAb directed against the gammadelta TCR does not deplete but generates ‘invisible’ gammadelta T cells. Eur J Immunol 2009; 39: 372–379. [DOI] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009; 31: 331–341. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol 2010; 10: 479–489. [DOI] [PubMed] [Google Scholar]
- Moore TA, Moore BB, Newstead MW, Standiford TJ. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol 2000; 165: 2643–2650. [DOI] [PubMed] [Google Scholar]
- Takano M, Nishimura H, Kimura Y, Mokuno Y, Washizu J, Itohara S et al. Protective roles of gamma delta T cells and interleukin-15 in Escherichia coli infection in mice. Infect Immun 1998; 66: 3270–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHiromatsu K, Yoshikai Y, Matsuzaki G, Ohga S, Muramori K, Matsumoto K et al. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med 1992; 175: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladel CH, Blum C, Dreher A, Reifenberg K, Kaufmann SH. Protective role of gamma/delta T cells and alpha/beta T cells in tuberculosis. Eur J Immunol 1995; 25: 2877–2881. [DOI] [PubMed] [Google Scholar]
- King DP, Hyde DM, Jackson KA, Novosad DM, Ellis TN, Putney L et al. Cutting edge: protective response to pulmonary injury requires gamma delta T lymphocytes. J Immunol 1999; 162: 5033–5036. [PubMed] [Google Scholar]
- Jones-Carson J, Vazquez-Torres A, van der Heyde HC, Warner T, Wagner RD, Balish E. Gamma delta T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat Med 1995; 1: 552–557. [DOI] [PubMed] [Google Scholar]
- Rosat JP, MacDonald HR, Louis JA. A role for gamma delta+T cells during experimental infection of mice with Leishmania major. J Immunol 1993; 150: 550–555. [PubMed] [Google Scholar]
- Hisaeda H, Nagasawa H, Maeda K, Maekawa Y, Ishikawa H, Ito Y et al. Gamma delta T cells play an important role in hsp65 expression and in acquiring protective immune responses against infection with Toxoplasma gondii. J Iimmunol 1995; 155: 244–251. [PubMed] [Google Scholar]
- O'Brien RL, Yin X, Huber SA, Ikuta K, Born WK. Depletion of a gamma delta T cell subset can increase host resistance to a bacterial infection. J Immunol 2000; 165: 6472–6479. [DOI] [PubMed] [Google Scholar]
- Emoto M, Nishimura H, Sakai T, Hiromatsu K, Gomi H, Itohara S et al. Mice deficient in gamma delta T cells are resistant to lethal infection with Salmonella choleraesuis. Infect Immun 1995; 63: 3736–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormley FL Jr., Steele C, Wozniak K, Fujihashi K, McGhee JR, Fidel PL Jr. Resistance of T-cell receptor delta-chain-deficient mice to experimental Candida albicans vaginitis. Infect Immun 2001; 69: 7162–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ et al. T-cell alpha beta+and gamma delta+deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA 1996; 93: 11774–11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Grubbs BG, Kelly K, Pack E, Rank RG. Role of gamma-delta T cells in murine Chlamydia trachomatis infection. Infect Immun 1996; 64: 3916–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol 1997; 158: 3344–3352. [PubMed] [Google Scholar]
- Yang X. Role of cytokines in Chlamydia trachomatis protective immunity and immunopathology. Curr Pharm Des 2003; 9: 67–73. [DOI] [PubMed] [Google Scholar]
- Yang X, Brunham R. T lymphocyte immunity in host defence against Chlamydia trachomatis and its implication for vaccine development. Can J Infect Dis 1998; 9: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo N, Sireci G, Meraviglia S, Dieli F, Ivanyi J, Salerno A. gammadelta T cells condition dendritic cells in vivo for priming pulmonary CD8 T cell responses against Mycobacterium tuberculosis. Eur J Immunol 2006; 36: 2681–2690. [DOI] [PubMed] [Google Scholar]
- Dieli F, Caccamo N, Meraviglia S, Ivanyi J, Sireci G, Bonanno CT et al. Reciprocal stimulation of gammadelta T cells and dendritic cells during the anti-mycobacterial immune response. Eur J Immunol 2004; 34: 3227–3235. [DOI] [PubMed] [Google Scholar]