Abstract
Tertiary lymphoid structures (TLS) often develop at sites of persistent inflammation, including cancers and autoimmune diseases. In most cases, the presence of TLS correlates with active immune responses. Because of their proximity to pathological loci, TLS are an intriguing target for the manipulation of immune responses. For several years, it has become clear that lymphotoxin (LT) signalling plays critical roles in lymphoid tissue organogenesis and maintenance. In the current review, we will discuss the role of LT signalling in the development of TLS. With a focus on cancers and autoimmune diseases, we will highlight the correlations between TLS and disease progression. We will also discuss the current efforts and potential directions for manipulating TLS for immunotherapies.
Keywords: autoimmune disease, cancer, immunotherapy, lymphotoxin signalling, tertiary lymphoid structure
Introduction
Inflammation is the immune response to various pathological conditions, such as cancer, autoimmune disease and infection.1 Inflammatory mediators, especially cytokines, chemokines and adhesion molecules, recruit and activate various types of innate and adaptive immune cells to the site of inflammation, including macrophages, DCs, mast cells as well as T and B cells.2 In the case of persistent inflammation, the migration and positioning of immune cells usually follow certain patterns, which are similar to those observed in the secondary lymphoid organs (SLO). These structures have been named tertiary lymphoid structure (TLS; also called tertiary lymphoid organs (TLO)).3, 4 Several cytokines/chemokines and their corresponding receptors have been shown to be involved in the formation of TLS, including lymphotoxins (LTs), tumour necrosis factor (TNF), CCL21, CCL19, CXCL13 and others. Among them, the LT signalling pathway plays key roles.5, 6 In this review, we will highlight the role of LT signalling in the formation of TLS and its application for drug design targeting cancers and autoimmune diseases.
Lymphotoxin signaling: an overview
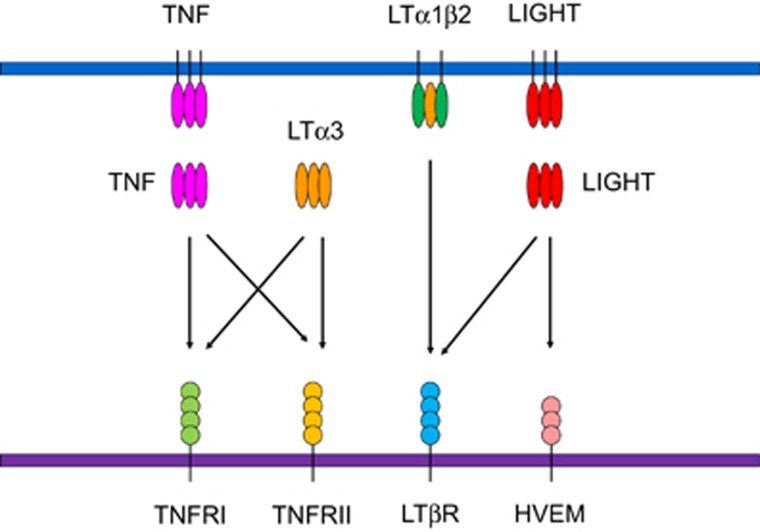
LTs belong to the TNF superfamily. Two distinct forms of LTs, LTα and LTβ, have been identified. They show different expression patterns and cellular distributions. LTα was originally identified because of its ability to induce tumour cell death in vitro; thus, it was named TNFβ.7, 8 Similar to TNF, LTα itself can form a homotrimer that binds to TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2; Figure 1).9 LTα can also form heterodimers together with LTβ as either LTα2β1 or LTα1β2. Among them, LTα1β2 is the predominant form. LTα1β2 binds to lymphotoxin beta receptor (LTβR).10 LTβR is broadly expressed by stromal cells in lymphoid tissues, epithelial cells, monocytes, DCs and other cell types (Table 1).11, 12 LTβR can bind to another TNF superfamily member, LIGHT, which is homologous to lymphotoxins. It has inducible expression and competes with herpes simplex virus glycoprotein D for herpesvirus entry mediator (HVEM), a receptor expressed on T lymphocytes. LIGHT can also provide co-stimulatory signals to T cells by interacting with HVEM.13 Together, these cytokines and receptors form a complex signalling network that plays important roles in several immunological processes.
Figure 1.
The lymphotoxin signalling network. TNF and LTα3 can bind to TNFRI and TNFRII. Both LTα1β2 and LIGHT bind to LTβR. LIGHT can also interact with HVEM and deliver co-stimulatory signals to T cells. TNF and LIGHT can be either membrane-bound or soluble. TNF, tumour necrosis factor; LT, lymphotoxin; TNFRI, TNF receptor I; TNFRII, TNF receptor II; LTβR, lymphotoxin beta receptor; HVEM, herpesvirus entry mediator; LIGHT, homologous to lymphotoxins, inducible expression, competes with herpes simplex virus glycoprotein for HVEM, a receptor expressed on T lymphocytes.
Table 1. Expression profile and phenotype of mice deficient in lymphotoxin-related genes.
| Molecule | Cellular expression | Deficiency in lymphoid neogenesis |
|---|---|---|
| LTα | Primarily on T-, B-, NK and LTi cells | Lack of lymph nodes and Payer’s patches, absence of T–B segregation in spleen |
| LTβ | Primarily on T-, B-, NK and LTi cells | Lack of lymph nodes and Payer's patches, absence of T–B segregation in spleen |
| LTβR | Broadly expressed, except in mature lymphocytes | Lack of lymph nodes and Payer's patches, absence of T–B segregation in spleen |
| LIGHT | Activated T- and NK cells, immature DCs, platelet | Normal |
| TNF | Broadly expressed | Lack Payer’s patches, absence of T–B segregation in spleen |
| TNFRI | Broadly expressed on most nucleated cells | Lack Payer’s patches, absence of T–B segregation in spleen |
| TNFRII | Hematopoietic cells | Normal |
Abbreviations: NK cells, natural killer cells; TNF, tumor necrosis factor.
From slo to tls: the role of lymphotoxin signalling in lymphoid tissue development
Although originally identified as cytotoxins, it later became clear that LTs play a more important role in lymphoid tissue organogenesis and maintenance. The direct evidence came from genetically manipulated mice.14, 15, 16, 17 Mice deficient in LTα show significant defects in lymphoid organ development. Specifically, these mice are born without secondary lymphoid organs, including lymph nodes (LNs) and Payer’s Patches (PPs; Table 1). LTβ-deficient mice phenocopy what has been observed in LTα mice.18, 19 Interestingly, although TNF- and TNFR-deficient mice lack PPs, they still have normal LN.17, 20, 21, 22 These data indicate a more profound role of LTβR signalling in lymphoid tissue organogenesis and maintenance.
LTβR regulates mesenchymal stromal cells for immune cell clustering and patterning
A well-defined role of LTβR signalling during SLO development is its ability to induce the expression of lymphoid chemokines and adhesion molecules for initial immune cell clustering. For lymph node/PP development, LTβR signalling triggered by LT from lymphoid tissue inducer (LTi) cells induces the expression of many chemokines and adhesion molecules, including CCL19, CCL21, CXCL12, CXCL13, ICAM1, VCAM1 and MAdCAM1.23 These molecules coordinate a positive feedback loop by recruiting more LTi cells. They also recruit other immune cells such as B and T cells for further lymphoid tissue maturation and organization. This function of LTβR is true not only in LN/PP development but also in non-lymphoid tissues. In fact, the exogenous expression of LT or LIGHT, which activates LTβR signalling, is able to promote functional lymphoid neogenesis in non-lymphoid tissues.24, 25, 26 The essential role of LTβR signalling in mesenchymal cells for TLS has also been reported.27, 28, 29 In these studies, an arterial TLS model in apoE−/− mice was examined. When LTβR was conditionally deleted from vascular smooth muscle cells, although aorta tertiary lymphoid organ (ATLO) neogenesis was not affected, aberrant ATLO structures, as indicated by reduced size, loose T- and B-cell infiltrates and segregation, were found.
LTβR regulates mesenchymal stromal cell differentiation
Fibroblastic reticular cells (FRCs) and follicular dendritic cells (FDCs) are important organizer stromal cells in T and B zones, respectively. During SLO development, FDCs and FRCs are differentiated from mesenchymal precursors. LTβR signalling was recently found to be important for their differentiation. LN FRCs are mainly derived from adipocyte precursors, and their differentiation is critically dependent on the LTβR-NFκB2-RelB signalling pathway.30 Furthermore, in vivo organogenesis assays showed that embryonic and adult adipocyte precursor cells can migrate into newborn lymph nodes and differentiate into a variety of lymph node stromal cells. FDCs can also arise from ubiquitous perivascular precursors (preFDCs) expressing platelet-derived growth factor receptor β (PDGFRβ). The expansion of preFDCs requires both LTi cells and lymphotoxin.31 The ubiquity of preFDCs and their strategic location at blood vessels may explain the de novo generation of organized TLS at sites of lymphocytic inflammation. Whether a similar LTβR-dependent mechanism controls TLS development remains to be determined.
LTβR regulates HEV for organized lymphocyte recruitment
The LTβR signalling pathway plays critical roles for high endothelial venule (HEV) differentiation and function.32, 33 HEVs are specialized blood vessels that are essential for the migration of lymphocytes into SLO.34 Compared to capillaries, HEVs are distinct in their expression of peripheral node addressins (PNADs). PNADs are a group of highly glycosylated and sulphated forms of sialomucins, such as glycosylation-dependent cell-adhesion molecule-1 (GlyCAM-1), CD34, podocalyxin, endoglycan and endomucin.35, 36 Some sialomucins are also expressed in capillaries, though with lower levels of post-translational modifications. This may be due to the preferential expression of genes involved in glycoconjugate formation in HEVs.36 Besides PNADs, HEVs also express high levels of lymphoid chemokines, such as CCL21.37 DCs promote LT signalling through LTβR for HEV differentiation and function.38 In addition, LTβR signalling is also involved in HEV network growth via regulating VEGF expression. FRCs are the major source of VEGF in lymph nodes.39 LTβR signalling in FRCs is important for VEGF production.40 Alternatively, DCs have also been reported to express VEGF directly for initial HEV formation.40, 41
HEV-like structures are also found in TLS. Because of the similarity between SLO and TLS, it is speculated that LT signalling is also involved in HEV differentiation and function in TLSs. In line with this notion, HEV-like structures are associated with LT-expressing DCs in human breast cancer.42 However, the exact roles of DC and LT signalling on HEV differentiation, growth and function in TLSs remain to be determined.
Different lymphoid tissue inducers for SLO and TLS
In addition to stromal cells and endothelial cells, LTi cells are also critical for LN development, as they deliver the initial triggering of stromal cells. For SLO development, the crucial role of CD4+CD3−RORγt+ LTi cells has been well documented.43, 44, 45 Whether LTi cells play a role in TLS development remains controversial. In a model where TLS was induced by ectopic expression of CCL21 in the thyroid gland, the formation of TLS was independent of LTi cells.46 However, in another study, IL-7 overexpression led to the formation of multiple organized ectopic lymph nodes and caecal patches because of the increased survival of LTi cells.47 Mice overexpressing IL-7 but lacking either RORγ, a factor required for LTi cell generation, or LT had no ectopic lymph nodes. Even so, the data do not exclude the role of Th17 in TLS development, since RORγt is also required for Th17 differentiation. In fact, several studies have linked IL-17 and Th17 to the development of TLSs in various models.48, 49, 50 In addition, B cells have also been reported to be involved in TLS formation in RA patients.51 Recently, an intriguing study showed that B cells can serve as LTi cells by providing LT signalling for microbiota-induced tertiary lymphoid tissue formation, even in RORγt-deficient mice, which lack both LTi cells and Th17 cells.52 Therefore, different LT-producing cells may work as LTi cells depending on the scenarios. For TLS development in cancers, the LT inducer cells have not been reported. Since LT-expressing DCs have been shown to be associated with TLS, it may be an interesting candidate for testing.
Tls in cancer immunotherapy
Correlation between TLS and cancer progression: Clinical study
TLSs form and attract lymphocytes during chronic inflammation. In the case of tumours, TLSs are formed adjacent to malignant cells. Compared to draining lymph nodes (dLNs), these structures are closer to tumour cells, which makes them better places for T cells to meet tumour antigen-carrying DCs and become primed. Clinically, the presence and abundance of TLSs in tumour patients usually correlate with active immune responses and better prognosis.2, 53, 54
One piece of evidence comes from studies in melanoma. The distribution of DCs follows a certain pattern in melanoma tumour tissues.55 Specifically, DC-Lamp+ mature DCs are mostly confined to peritumoural areas, and a higher frequency of DC-Lamp+ mature DCs correlates with better prognosis in patients. The presence of TLS in metastatic melanoma was further identified as lymphoid follicles containing HEVs and clusters of B cells, T cells, FDCs and mature DCs.56 B cells inside these TLSs show signs of clonal amplification, somatic mutation and isotype switching, indicating active immune responses. Similar structures have also been confirmed by another group.57 Furthermore, a 12-chemokine gene expression signature (GES) has been identified and can be used to predict the presence of TLSs. This 12-chemokine GES includes CCL2, CCL3, CCL4, CCL5, CCL8, CCL18, CCL19, CCL21, CXCL9, CXCL10, CXCL11 and CXCL13. Most of these cytokines have been shown to be critical for the formation of SLO and TLS. Interestingly, many of them can be directly upregulated by LT signalling.58
In non-small cell lung cancer (NSCLC) patients, the existence of TLSs has been shown in tumours but not normal tissues.59, 60, 61 These TLSs contained mature DC and T-cell clusters adjacent to B-cell follicles. The density of mature DCs is associated with favourable clinical outcomes. Intriguingly, the density of mature DCs also correlates with tumour infiltrating lymphocytes (TILs), which have been shown to correlate with better prognosis in many different cancers.62 A follow-up study identified that tumour infiltrating T cells are predominantly of the effector-memory phenotype, which is consistent with the important role of TLSs as a site for T-cell priming.63
Mature DCs are not the only cell type that correlates with the presence of TLS and better prognosis. In colorectal carcinoma patients, a higher number of CD3+ T-cell infiltration into tumour-associated lymphoid nodules predicts improved survival.64, 65, 66 In a mouse model of colorectal cancer, the adoptive transfer of splenocytes results in the accumulation of lymphocytes at the TLS, suggesting an active role of TLSs in recruiting lymphocytes to tumour tissues. Higher TILs and the presence of TLSs also correlate with disease-free survival in patients with breast cancer42, 67, 68, 69, 70, 71 and melanoma.55, 57, 64, 72 Taken together, these studies suggest a strong correlation between the presence of TLS and a better prognosis in cancer patients.
On the other hand, however, although the presence of TLS usually correlates with positive clinical outcome, it could also indicate an adverse prognosis in some scenarios. In breast cancer patients, a high number of tumour-infiltrating regulatory T (Ti-Treg) cells was found to be present in the peritumoural areas.73 These Ti-Treg cells are associated with relapse and death in patients. It has been suggested that Treg cells are activated by mature DCs through tumour-associated antigen presentation. Activated Treg cells inhibit the functions of effector T cells, leading to immune escape and tumour progression. Interestingly, the specific recruitment of Treg to TLSs is not through CCL19 or CCL21 because the receptor of both chemokines, CCR7, is equally expressed in effector T cells (Teff) and Treg. Instead, the authors suggested that Treg cells are more likely to be recruited by CCL22, as Treg has a much higher level of CCR4, a receptor for CCL22.73
Targeting lymphotoxin signalling for cancer immunotherapy
Tumour microenvironments are usually inhibitory and prevent effective lymphocyte priming.74, 75 As TLSs are located adjacent to tumour cells, there could be several advantages to priming T cells inside TLSs. First, it is less difficult for antigen-presenting cells (APCs) to take up tumour antigens and migrate to TLSs. Second, the tumour antigen load in TLSs could be higher than tumour dLNs.76 Third, activated effector cells can target tumour cells and stromal cells with less migration.76, 77 In fact, bystander elimination of stromal cells is essential to control tumour growth.78 Considering the significant correlation between the presence of TLSs and better prognosis, it has become an intriguing hypothesis that being able to induce TLS formation might also be a potent strategy to induce anti-tumour immunity (Figure 2).79 Several studies aiming at developing strategies to induce TLSs in order to enhance anti-tumour immune responses have been reported (Table 2).
Figure 2.
Immune responses mediated by tumour-related tertiary lymphoid structures (TLSs). Lymphocytes are recruited from HEV to TLSs, where they form T- and B-cell-rich zones (1). Dendritic cells (DCs) take up antigens and present them to T cells (2). After activation and proliferation, T cells can migrate to tumour cells for destruction (3). Antigens can also be transferred to B cells to induce antibody production (4). Lymphotoxin signalling plays important roles in lymphocyte recruitment, stromal and LTi cell differentiation, and HEV organization. HEV, high endothelial venules. LTβR, lymphotoxin beta receptor.
Table 2. Biologics targeting lymphotoxin signalling for the treatment of cancers and autoimmune diseases.
| Target molecule | Species | Type of agent | Disease/model | Trade name | Stage of clinical development | Reference |
|---|---|---|---|---|---|---|
| Cancer | ||||||
| LTα | Mouse | Antibody-LTα fusion protein | Melanoma | — | — | 24, 80 |
| LTβR | Mouse | α-LTβR agonist antibody | Colon carcinoma | — | — | 81, 82 |
| α-LTβR neutralizing antibody | Sarcoma, Breast cancer | — | — | 83 | ||
| Adenovirus expressing LIGHT | Fibrosarcoma, Colon carcinoma, Breast cancer, Prostate cancer | — | — | 77, 84, 85, 86 | ||
| LIGHT | Mouse | Salmonella expressing LIGHT | Lymphoma | — | — | 87 |
| Antibody-LIGHT fusion protein | Fibrosarcoma, Colon carcinoma | — | — | 103 | ||
| Autoimmune disease | ||||||
| LTα/LTβ | Mouse | α-LTα antibody | RA, EAE | — | — | 88 |
| α-LTβ antibody | EAE | — | — | 89 | ||
| Human | α-LTα antibody | RA | Pateclizumab | Phase 2 (discontinued) | 90 | |
| LTβR | Mouse | LTβR-Ig fusion protein | RA, IBD, EAE, T1D, SS | — | — | 89, 91, 92, 93, 94, 95, 96 |
| Human | LTβR-Ig fusion protein | RA | Baminercept | Phase 2 (discontinued) | 97, 98 | |
| SS | Baminercept | Phase 2 | 99 | |||
| LIGHT | Human | α-LIGHT antibody | Crohn's disease, Ulcerative colitis | SAR252067 | Phase 1 (discontinued) | Sanofi 1Q 2015 report |
| IBD | SAR252067 | Phase 1 | 100 | |||
Abbreviations: EAE, Experimental autoimmune encephalomyelitis; IBD, Inflammatory bowel disease; RA, Rheumatoid arthritis; SS, Sjogren’s syndrome; T1D, Type 1 diabetes.
Because of its central role during lymphoid tissue neogenesis, LTβR has attracted the most attention. Early studies have shown that exogenous expression of LTβR ligands, either LT or LIGHT, is able to promote functional lymphoid neogenesis in non-lymphoid tissues.24, 25, 26 In a proof-of-concept study, the expression of LTβR was evaluated using an immunohistologic survey.81 Most samples (87% to 96%) showed at least 1+ staining for LTβR in human tumours. The wide expression of LTβR in tumour tissues lays the foundation for activating LTβR signalling for TLS induction. An agonistic antibody against LTβR has been developed. In both a human xenograft model and a mouse syngeneic model, the agonist anti-LTβR antibody is able to induce anti-tumour immune responses and increase lymphocyte infiltration into tumour tissues.81 However, the anti-tumour effects were less impressive, possibly because of the wide expression of LTβR, which makes it hard to specifically activate LTβR signalling in tumour tissues. The lack of tumour-specific targeting also raised concerns regarding side effects. In fact, the systemic activation of LT signalling results in serous toxicity.101
The other approach to activate LTβR signalling is through engagement with LIGHT. The expression of LIGHT in non-lymphoid tissues, such as islets, is able to induce development of TLS.25 The ectopic activation of LTβR signalling induces diabetes as a result of autoimmune disease. This process can be blocked by a neutralizing recombinant LTβR fusion protein. Interestingly, forced expression of LIGHT in tumour cells promotes the formation of lymphoid-like structures for direct T-cell sequestration and activation, leading to tumour regression.77, 84 Alternatively, mesenchymal stem cells engineered to express LIGHT can home into tumour tissues and promote anti-tumour immunity.102 However, because of the wide expression of LIGHT receptors, a major challenge in the delivery of LT signalling for cancer immunotherapy is how to specifically target to the tumour (Table 1). To overcome such a limitation, tumour-specific antibody targeting has been utilized to deliver LIGHT specifically to tumour tissues.103 Further study suggested that LTβR signalling activated by LIGHT is able to attract lymphocytes to the tumour tissues, resulting in tumour destruction.
As a ligand of LTβR, LTα is another promising molecule for the activation of LTβR signalling. Several studies have been carried out with recombinant LTα that is fused to an anti-GD2 antibody for specific tumour targeting.24, 80 This fusion protein is able to inhibit tumour growth and prolong survival in a syngeneic mouse melanoma model. Intriguingly, seven days after seven continually daily treatments with this fusion protein, HEV-like structures could already be observed inside tumours, supporting lymphocyte migration.24 These studies suggest that the tumour-specific targeting of LT is able to induce the formation of TLSs in a short period of time and exert anti-tumour effects.24
The strategies discussed above aim to activate LTβR signalling using either an agonist antibody or its ligands. On the other hand, another strategy is to deliver major effector molecules of LT signalling to tumour tissues, such as CCL21 or CCL19. CCL21 is a ligand of CCR7. It is an important effector chemokine that can mediate the homing of DCs and T cells to both lymphoid and non-lymphoid tissues.104, 105 CCL21 overexpression in B16 melanoma results in tumour rejection.106 Therapeutic application of CCL21 in tumour immunotherapy has also been reported.107 Recombinant CCL21 is able to induce tumour regression. The anti-tumour effects were completely abolished in immunodeficient mice or in the absence of T cells, suggesting an essential role of adaptive immunity. Alternatively, cells transduced with CCL21 cDNA can be used instead of recombinant CCL21 protein.108 Interestingly, intratumoral injection of DCs expressing CCL21 showed robust anti-tumour activity and induced regression in all tumours tested. In significant contrast, fibroblasts expressing the same chemokine can only induce regression in 25% of the mice. These results are consistent with the notion that DC is the major target of CCL21.104 In another study, CCL21 was knocked down in a mouse melanoma model and an opposite result was observed.109 Surprisingly, tumour cells expressing higher levels of CCL21 established an immune-tolerant tumour microenvironment, which facilitates tumour progression. The tumour microenvironment in tumours expressing high levels of CCL21 was characterized by a ;higher number of Treg cells. This phenomenon is similar to what has been observed in some breast cancer patient samples, which also show a higher number of Treg in TLSs.73 Considering the profound role of Treg cells during the progression of mouse melanoma, it is interesting to speculate that instead of promoting effector T-cell priming, TLS might facilitate the activation of Treg cells and inhibit effector T cells in tumours or tissues where Treg play a dominant role.
CCL19 and CCL21 share the same receptor, CCR7. Although they have a similar affinity to CCR7, the signalling induced by CCL19 and CCL21 is different.110 Specifically, the binding of CCL19 to CCR7 induces internalization of the receptor, leading to desensitization.111 Furthermore, binding CCL19 desensitized the receptor to subsequent responses to CCL21. Because of these reasons, it is likely that the anti-tumour effects of CCL19 in mouse tumour models are not as impressive as CCL21.112, 113, 114, 115 Overall, CCL19 represents a more moderate, self-limited, and tuneable stimulator for CCR7 activation.
After induction, TLSs in tumour tissues are able to provoke anti-tumour immunity through several mechanisms that are quite unique compared to other therapeutic approaches. First, most current immunotherapies aim to activate/reactivate T cells inside tumours or dLNs. However, T cells inside established tumours are usually exhausted or anergic because of the inhibitory or prolonged activating microenvironment and are difficult to be activated.116 In significant contrast, HEVs in TLSs support the recruitment of naive T cells from the periphery.77, 117, 118 Naive T cells or newly attracted T cells are less exposed to the inhibitory microenvironment and have a lower threshold for activation. Second, the microenvironment in TLSs, including cytokines/chemokines and co-stimulatory signalling, promotes better priming of T cells and prevents them from reaching exhaustion or anergy. Third, as mentioned above, because of higher antigen loads and proximity to tumour cells, TLSs provide a better place for T cells to be primed and to infiltrate inside tumour tissues. Fourth, in situ T-cell priming can also lead to a broader and more comprehensive response, especially for antigens that are hard or unable to be cross-presented by APCs. In summary, the successful induction of TLSs in tumour tissues, either alone or combination with other therapies, may be a potent strategy for tumour immunotherapy.
Tls in autoimmune disease
Role of TLS in autoimmune diseases
TLS can be found in many autoimmune diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and type 1 diabetes (T1D). However, unlike in tumours, the function and prognostic value of TLS in some autoimmune diseases are still controversial, depending on the disease and the loci of TLS formation.
In RA, it has long been observed that tissue-infiltrating lymphocytes can form ectopic follicles in some patients.4 These follicles are organized in a way that is similar to SLO.119, 120 LTβ and CXCL13 are characteristic cytokines/chemokines that can independently predict the presence of TLS in patients, which indicates a critical role of LT signalling during the formation of these follicles.51 There is also a correlation between TLS and disease progression in RA. Specifically, FDCs in TLS express activation-induced cytidine deaminase (AID), an enzyme that is required for somatic hypermutation and the class-switch recombination of Ig genes.121 B cells producing a specific marker of RA, anti-citrullinated protein/peptide antibodies, are found to be in close contact with FDCs. These data provide evidence that TLS in RA may support autoantibody production and contribute to disease progression. However, although the presence of TLS is associated with a higher level of lymphocyte infiltration and systemic inflammation, it does not seem to correlate with the severity of the disease.122 A possible explanation is that clinical disease severity may be influenced by factors other than synovial inflammation.
Nephritis is the major cause of death for SLE. In the analysis of lupus nephritis biopsies, ectopic lymphoid structures were found to be associated with nephritis.123 In those structures, T and B cells were well-organized together with FDCs. These samples were positive for many lymphoid chemokines that are negative in normal renal tissues, including CXCL12, BAFF, CXCL13, CCL21 and CXCL10. The presence of lymphoid structures is associated with in situ B-cell clonal expansion and somatic hypermutation.123 These data suggested a correlation between TLS and disease progression. Similar patterns of TLS formation and chemokine expression were observed in a mouse model.124 Furthermore, DCs in TLSs were shown to express a high level of type 1 interferon (IFN), which might be responsible for the pathogenesis of SLE.
Lymphotoxin signalling as a therapeutic target in autoimmune diseases
In many cases of autoimmune diseases, it is believed that TLSs play a positive role in disease progression, which is consistent with the notion that TLSs are a site of immune activation. As LT signalling is critical for the development of TLS, many efforts have been focused on blocking this signalling as therapy for autoimmune diseases.100, 125
An interesting example is the development of the anti-LTα antibody for the treatment of arthritis. Collagen-induced arthritis (CIA) is a commonly used murine autoimmune disease model in which the disease development depends on T helper (TH) cells.126 In a survey of the gene expression profile in immune cells in CIA, the expression of LTα was found to be specifically in TH cells, especially TH1 and TH17 cells.88 Treating CIA mice with an anti-LTα antibody significantly inhibited disease development. A mechanistic study showed that the administration of anti-LTα antibody depletes TH1 and TH17 cells, resulting in decreased IL-17, IFN-γ, and TNFα production. These data led to the development of the drug Pateclizumab, a humanized anti-LTα antibody. In clinical trials, Pateclizumab was used to treat RA and graft-versus-host disease (GVHD).127, 128 Unfortunately, although proven to be safe in a phase 1 trial, it failed the phase 2 trial for RA because there were no significant benefits over placebo.90 A possible explanation might be the tight regulation during TLS formation.129
Although not successful in LTα blocking, probably because of less efficient blockade, blocking LT signalling with an effective LTβR-Ig130 has been shown to reduce the formation of TLS and inhibit autoimmune disease progression in many different disease models, including T1D, Hashimoto’s thyroiditis, and experimental autoimmune encephalomyelitis (EAE).131, 132, 133, 134 The potent effects of LTβR-Ig in mouse models have pushed a humanized version, Baminercept, into clinical trials.97 Its first clinical trial in RA was discontinued because no significant effect was observed.135 However, further analysis showed that there were significant changes in some biomarkers and with clinical improvements in a subset of patients.98 A second clinical trial against Sjogren’s syndrome (SS) has also reached phase 2. Although there were no benefits for increasing salivary flow or reducing ocular dryness, Baminercept significantly improved the EULAR Sjogren’s syndrome Disease Activity Index (ESSDAI).99
Concluding remarks
Recent breakthroughs in immunotherapy have opened a new era of disease treatment.136 Clinical trials with checkpoint blockade and chimeric antigen receptor-T-cell therapies have shown remarkable long-term efficacy in patients with a variety of cancers.137, 138, 139 Although only a minority of the total treated patients respond to the current immunotherapy treatment, the unprecedented durable responses in some patients have shown that potent effects could be achieved with immunotherapies. Because of its close and active interactions with pathological tissues, TLS has become an intriguing target not only for studying disease progression but also for manipulating immune responses during immunotherapy. Studies during the past years have revealed a central role of LT signalling in the regulation of lymphoid neogenesis. With the manipulation of TLS development by increasing chemokines that attract more lymphocytes, the current durable effects with immunotherapy are expected to reach a broader range of patients soon.
Acknowledgments
YXF holds the Mary Nell and Ralph B. Rogers Professorship in Immunology. This work was supported in part by the US National Institutes of Health through National Cancer Institute grants CA141975 and CA97296, CPRIT grant RR150072, grants from the Chinese Academy of Sciences (XDA09030303), and grants from the Chinese Ministry of Science and Technology (2012ZX10002006, 2011DFA31250 and 2012AA020701) to YXF and a Cancer Resarch Institute Irvington Fellowship to HT.
Footnotes
The authors declare no conflict of interest.
References
- Buckley CD, Barone F, Nayar S, Bénézech C, Caamaño J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol 2015; 33: 715–745. [DOI] [PubMed] [Google Scholar]
- Weinstein AM, Storkus WJChapter six—therapeutic lymphoid organogenesis in the tumor microenvironment. In: Xiang-Yang W, Paul BF(eds). Advances in Cancer Research Volume 128. Academic Press: Cambridge, MA, USA,. 2015, pp 197–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel KM, Cyster JG. Chemokines in lymphopoiesis and lymphoid organ development. Curr Opin Immunol 2001; 13: 172–179. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 2006; 6: 205–217. [DOI] [PubMed] [Google Scholar]
- Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol 2005; 23: 787–819. [DOI] [PubMed] [Google Scholar]
- Zhu M, Fu YX. The role of core TNF/LIGHT family members in lymph node homeostasis and remodeling. Immunol Rev 2011; 244: 75–84. [DOI] [PubMed] [Google Scholar]
- Old L. Tumor necrosis factor (TNF). Science 1985; 230: 630–632. [DOI] [PubMed] [Google Scholar]
- Beutler B, Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature 1986; 320: 584–588. [DOI] [PubMed] [Google Scholar]
- Hohmann H-P, Remy R, Pöschl B, Van Loon A. Tumor necrosis factors-alpha and-beta bind to the same two types of tumor necrosis factor receptors and maximally activate the transcription factor NF-kappa B at low receptor occupancy and within minutes after receptor binding. J Biol Chem 1990; 265: 15183–15188. [PubMed] [Google Scholar]
- Wang Y, Zhu M, Miller M, Fu YX. Immunoregulation by tumor necrosis factor superfamily member LIGHT. Immunol Rev 2009; 229: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JL, French LE. Visualization of lymphotoxin-β and lymphotoxin-β receptor expression in mouse embryos. J Immunol 2002; 168: 5079–5087. [DOI] [PubMed] [Google Scholar]
- Murphy M, Walter BN, Pike-Nobile L, Fanger NA, Guyre PM, Browning JL et al. Expression of the lymphotoxin receptor on follicular stromal cells in human lymphoid tissues. Cell Death Differ 1998; 5: 497–505. [DOI] [PubMed] [Google Scholar]
- Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu G-L et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity 1998; 8: 21–30. [DOI] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 1994; 264: 703–707. [DOI] [PubMed] [Google Scholar]
- Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA et al. Lymphotoxin-α-deficient mice effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol 1995; 155: 1685–1693. [PubMed] [Google Scholar]
- Ryffel B, Di Padova F, Schreier MH, Le Hir M, Eugster HP, Quesniaux VFJ. Lack of type 2T cell-independent b cell responses and defect in isotype switching in TNF-lymphotoxin α-deficient mice. J Immunol 1997; 158: 2126–2133. [PubMed] [Google Scholar]
- Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science 1996; 271: 1289–1291. [DOI] [PubMed] [Google Scholar]
- Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A et al. Abnormal development of secondary lymphoid tissues in lymphotoxin β- deficient mice. Proc Natl Acad Sci USA 1997; 94: 9302–9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin β-deficient mice. Immunity 1997; 6: 491–500. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNFα-deficient mice: A critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med 1996; 184: 1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MW, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA 1997; 94: 8093–8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann B, Luz A, Pfeffer K, Holzmann B. Defective Peyer's patch organogenesis in mice lacking the 55-kD receptor for tumor necrosis factor. J Exp Med 1996; 184: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science 1999; 286: 2098–2102. [DOI] [PubMed] [Google Scholar]
- Schrama D, thor Straten P, Fischer WH, McLellan AD, Bröcker E-B, Reisfeld RA et al. Targeting of lymphotoxin-α to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity 2001; 14: 111–121. [DOI] [PubMed] [Google Scholar]
- Lee Y, Chin RK, Christiansen P, Sun Y, Tumanov AV, Wang J et al. Recruitment and activation of naive T cells in the islets by lymphotoxin β receptor-dependent tertiary lymphoid structure. Immunity 2006; 25: 499–509. [DOI] [PubMed] [Google Scholar]
- Suematsu S, Watanabe T. Generation of a synthetic lymphoid tissue-like organoid in mice. Nat Biotechnol 2004; 22: 1539–1545. [DOI] [PubMed] [Google Scholar]
- Gräbner R, Lötzer K, Döpping S, Hildner M, Radke D, Beer M et al. Lymphotoxin β receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med 2009; 206: 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P et al. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin β receptors. Immunity 2015; 42: 1100–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikakulapu P, Hu D, Yin C, Mohanta SK, Bontha SV, Peng L et al. Artery tertiary lymphoid organs control multilayered territorialized atherosclerosis b-cell responses in aged ApoE−/− Mice. Arterioscler Thrombos Vasc Biol 2016; 36: 1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénézech C, Mader E, Desanti G, Khan M, Nakamura K, White A et al. Lymphotoxin-β receptor signaling through NF-κB2-RelB pathway reprograms adipocyte precursors as lymph node stromal cells. Immunity 2012; 37: 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautler NJ, Kana V, Kranich J, Tian Y, Perera D, Lemm D et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell 2012; 150: 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JL, Allaire N, Ngam-ek A, Notidis E, Hunt J, Perrin S et al. Lymphotoxin-β receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity 2005; 23: 539–550. [DOI] [PubMed] [Google Scholar]
- Onder L, Danuser R, Scandella E, Firner S, Chai Q, Hehlgans T et al. Endothelial cell-specific lymphotoxin-β receptor signaling is critical for lymph node and high endothelial venule formation. J Exp Med 2013; 210: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J-P, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today 1995; 16: 449–457. [DOI] [PubMed] [Google Scholar]
- Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 2004; 4: 360–370. [DOI] [PubMed] [Google Scholar]
- Lee M, Kiefel H, LaJevic MD, Macauley MS, Kawashima H, O'Hara E et al. Transcriptional programs of lymphoid tissue capillary and high endothelium reveal control mechanisms for lymphocyte homing. Nat Immunol 2014; 15: 982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone F, Bombardieri M, Manzo A, Blades M, Morgan P, Challacombe S et al. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid‐like structures in Sjögren's syndrome. Arthritis Rheum 2005; 52: 1773–1784. [DOI] [PubMed] [Google Scholar]
- Moussion C, Girard J-P. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 2011; 479: 542–546. [DOI] [PubMed] [Google Scholar]
- Chyou S, Ekland EH, Carpenter AC, Tzeng T-CJ, Tian S, Michaud M et al. Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J Immunol 2008; 181: 3887–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyou S, Benahmed F, Chen J, Kumar V, Tian S, Lipp M et al. Coordinated regulation of lymph node vascular-stromal growth first by cd11c+ cells and then by T and B cells. J Immunol 2011; 187: 5558–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M, Willenzon S, Kocks J, Davalos-Misslitz AC, Hammerschmidt SI, Schumann K et al. Lymph node T cell homeostasis relies on steady state homing of dendritic cells. Immunity 2011; 35: 945–957. [DOI] [PubMed] [Google Scholar]
- Martinet L, Filleron T, Le Guellec S, Rochaix P, Garrido I, Girard J-P. High endothelial venule blood vessels for tumor-infiltrating lymphocytes are associated with Lymphotoxin β-producing dendritic cells in human breast cancer. J Immunol 2013; 191: 2001–2008. [DOI] [PubMed] [Google Scholar]
- Kim M-Y, Toellner K-M, White A, McConnell FM, Gaspal FM, Parnell SM et al. Neonatal and adult CD4+ CD3− cells share similar gene expression profile, and neonatal cells up-regulate OX40 ligand in response to TL1A (TNFSF15). J Immunol 2006; 177: 3074–3081. [DOI] [PubMed] [Google Scholar]
- Kim M-Y, McConnell FM, Gaspal FM, White A, Glanville SH, Bekiaris V et al. Function of CD4+ CD3− cells in relation to B-and T-zone stroma in spleen. Blood 2007; 109: 1602–1610. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Diehl G, Littman DLymphoid tissue inducer cells in intestinal immunityGut-associated Lymphoid Tissues. Springer: New York, NY, USA,. 2006, pp 59–82. [DOI] [PubMed] [Google Scholar]
- Lira SA, Martin AP, Marinkovic T, Furtado GC. Mechanisms regulating lymphocytic infiltration of the thyroid in murine models of thyroiditis. Crit Rev Immunol 2005; 25: 251–262. [DOI] [PubMed] [Google Scholar]
- Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R et al. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity 2007; 26: 643–654. [DOI] [PubMed] [Google Scholar]
- Deteix C, Attuil-Audenis V, Duthey A, Patey N, McGregor B, Dubois V et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol 2010; 184: 5344–5351. [DOI] [PubMed] [Google Scholar]
- Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 2011; 35: 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol 2011; 12: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol 2001; 167: 1072–1080. [DOI] [PubMed] [Google Scholar]
- Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORγt and LTi cells. J Exp Med 2011; 208: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu-Nosjean M-C, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol 2014; 35: 571–580. [DOI] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Giraldo NA, Kaplon H, Germain C, Fridman WH, Sautès‐Fridman C. Tertiary lymphoid structures, drivers of the anti‐tumor responses in human cancers. Immunol Rev 2016; 271: 260–275. [DOI] [PubMed] [Google Scholar]
- Ladányi A, Kiss J, Somlai B, Gilde K, Fejős Z, Mohos A et al. Density of DC-LAMP+ mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother 2007; 56: 1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipponi A, Mercier M, Seremet T, Baurain J-F, Théate I, van den Oord J et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res 2012; 72: 3997–4007. [DOI] [PubMed] [Google Scholar]
- Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep 2012; 2: 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev 2004; 202: 49–66. [DOI] [PubMed] [Google Scholar]
- Dieu-Nosjean M-C, Antoine M, Danel C, Heudes D, Wislez M, Poulot V et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008; 26: 4410–4417. [DOI] [PubMed] [Google Scholar]
- Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med 2014; 189: 832–844. [DOI] [PubMed] [Google Scholar]
- Germain C, Gnjatic S, Dieu-Nosjean M-C. Tertiary lymphoid structure-associated B cells are key players in anti-tumor immunity. Front Immunol 2015; 6: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S-R, Corrales L, Gajewski TF. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol 2015; 36: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goc J, Germain C, Vo-Bourgais TKD, Lupo A, Klein C, Knockaert S et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014; 74: 705–715. [DOI] [PubMed] [Google Scholar]
- McMullen T, Lai R, Dabbagh L, Wallace T, De Gara C. Survival in rectal cancer is predicted by T cell infiltration of tumour‐associated lymphoid nodules. Clin Exp Immunol 2010; 161: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res 2014; 20: 2147–2158. [DOI] [PubMed] [Google Scholar]
- Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol 2011; 179: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim JY, Park IA, Song IH, Yu JH, Ahn J-H et al. Prognostic significance of tumor-infiltrating lymphocytes and the tertiary lymphoid structures in HER2-positive breast cancer treated with adjuvant trastuzumab. Am J Clin Pathol 2015; 144: 278–288. [DOI] [PubMed] [Google Scholar]
- Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie J-J et al. Human solid tumors contain high endothelial venules: association with T-and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011; 71: 5678–5687. [DOI] [PubMed] [Google Scholar]
- Coronella JA, Spier C, Welch M, Trevor KT, Stopeck AT, Villar H et al. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J Immunol 2002; 169: 1829–1836. [DOI] [PubMed] [Google Scholar]
- Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, De Wind A et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013; 123: 2873–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Park IA, Song IH, Shin S-J, Kim JY, Yu JH et al. Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol 2016; 69: 422–430. [DOI] [PubMed] [Google Scholar]
- Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P et al. High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 2012; 1: 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res 2009; 69: 2000–2009. [DOI] [PubMed] [Google Scholar]
- Tang H, Qiao J, Fu Y-X. Immunotherapy and tumor microenvironment. Cancer Lett 2016; 370: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol 2015; 25: 198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF et al. Increasing tumor antigen expression overcomes ‘ignorance’ to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity 2002; 17: 737–747. [DOI] [PubMed] [Google Scholar]
- Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol 2004; 5: 141–149. [DOI] [PubMed] [Google Scholar]
- Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med 2004; 10: 294–298. [DOI] [PubMed] [Google Scholar]
- Sautès-Fridman C, Fridman WH. TLS in tumors: what lies within. Trends Immunol 2016; 37: 1–2. [DOI] [PubMed] [Google Scholar]
- Reisfeld RA, Gillies SD, Mendelsohn J, Varki NM, Becker JC. Involvement of B lymphocytes in the growth inhibition of human pulmonary melanoma metastases in athymic nu/nu mice by an antibody-lymphotoxin fusion protein. Cancer Res 1996; 56: 1707–1712. [PubMed] [Google Scholar]
- Lukashev M, LePage D, Wilson C, Bailly V, Garber E, Lukashin A et al. Targeting the lymphotoxin-β receptor with agonist antibodies as a potential cancer therapy. Cancer Res 2006; 66: 9617–9624. [DOI] [PubMed] [Google Scholar]
- Hu X, Zimmerman MA, Bardhan K, Yang D, Waller JL, Liles GB et al. Lymphotoxin β receptor mediates caspase-dependent tumor cell apoptosis in vitro and tumor suppression in vivo despite induction of NF-κB activation. Carcinogenesis 2013; 34: 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Ud Din N, Browning DD, Abrams SI, Liu K. Targeting lymphotoxin β receptor with tumor-specific T lymphocytes for tumor regression. Clin Cancer Res 2007; 13: 5202–5210. [DOI] [PubMed] [Google Scholar]
- Yu P, Lee Y, Wang Y, Liu X, Auh S, Gajewski TF et al. Targeting the primary tumor to generate CTL for the effective eradication of spontaneous metastases. J Immunol. 2007; 179: 1960–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Liu Y, Li H, Zhao D, Yang L, Shen J et al. Adenovirus-mediated LIGHT gene modification in murine B-cell lymphoma elicits a potent antitumor effect. Cell Mol Immunol 2010; 7: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Silva DMD, Verma B, Gray A, Brand HE, Skeate JG et al. Forced LIGHT expression in prostate tumors overcomes Treg mediated immunosuppression and synergizes with a prostate tumor therapeutic vaccine by recruiting effector T lymphocytes. Prostate 2015; 75: 280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler M, Le'Negrate G, Krajewska M, Reed JC. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci USA 2007; 104: 12879–12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang EY, Kolumam GA, Yu X, Francesco M, Ivelja S, Peng I et al. Targeted depletion of lymphotoxin-α-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat Med 2009; 15: 766–773. [DOI] [PubMed] [Google Scholar]
- Gommerman JL, Giza K, Perper S, Sizing I, Ngam-ek A, Nickerson-Nutter C et al. A role for surface lymphotoxin in experimental autoimmune encephalomyelitis independent of LIGHT. J Clin Invest 2003; 112: 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy WP, Simon JA, Offutt C, Horn P, Herman A, Townsend MJ et al. Efficacy and safety of pateclizumab (anti-lymphotoxin-α) compared to adalimumab in rheumatoid arthritis: a head-to-head phase 2 randomized controlled study (The ALTARA Study). Arthritis Res Ther 2014; 16: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava RA, Notidis E, Hunt J, Szanya V, Ratcliffe N, Ngam-ek A et al. A role for the lymphotoxin/LIGHT axis in the pathogenesis of murine collagen-induced arthritis. J Immunol 2003; 171: 115–126. [DOI] [PubMed] [Google Scholar]
- Mackay F, Browning JL, Lawton P, Shah SA, Comiskey M, Bhan AK et al. Both the lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology 1998; 115: 1464–1475. [DOI] [PubMed] [Google Scholar]
- Dohi T, Rennert PD, Fujihashi K, Kiyono H, Shirai Y, Kawamura YI et al. Elimination of colonic patches with lymphotoxin β receptor-Ig prevents Th2 cell-type colitis. J Immunol 2001; 167: 2781–2790. [DOI] [PubMed] [Google Scholar]
- Ettinger R, Munson SH, Chao C-C, Vadeboncoeur M, Toma J, McDevitt HO. A critical role for lymphotoxin-β receptor in the development of diabetes in nonobese diabetic mice. J Exp Med 2001; 193: 1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Salomon B, Chen M, Wang Y, Hoffman LM, Bluestone JA et al. Reversal of spontaneous autoimmune insulitis in nonobese diabetic mice by soluble lymphotoxin receptor. J Exp Med 2001; 193: 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatumu MK, Skarstein K, Papandile A, Browning JL, Fava RA, Bolstad AI. Blockade of lymphotoxin-beta receptor signaling reduces aspects of Sjogren’s syndrome in salivary glands of non-obese diabetic mice. Arthritis Res Ther 2009; 11: R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassare A, Fiechtner J, Filipowicz-Sosnowska A, Jeka S, O'Gorman J, Weaver M et al. Preliminary safety and efficacy of baminercept (LTBR-Ig, BG9924) in the treatment of rheumatoid arthritis. Arthritis Rheum 2007; 56: S394. [Google Scholar]
- Bienkowska J, Allaire N, Thai A, Goyal J, Plavina T, Nirula A et al. Lymphotoxin-LIGHT pathway regulates the interferon signature in rheumatoid arthritis. PLoS One 2014; 9: e112545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair WE, Baer AN, Noaiseh G, Parke A, Coca A, Utset T et al. The clinical efficacy and safety of Baminercept, a lymphotoxin-beta receptor fusion protein, in primary Sjögren’s syndrome: results from a randomized, double-blind, placebo-controlled phase II trial. Arthritis Rheumatol 2015; 67: 3844–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov 2013; 12: 147–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune F, Lienard D, Leyvraz S, Mirimanoff R. Regional therapy of melanoma. Eur J Cancer 1993; 29: 606–612. [DOI] [PubMed] [Google Scholar]
- Zou W, Zheng H, He T-C, Chang J, Fu Y-X, Fan W. LIGHT delivery to tumors by mesenchymal stem cells mobilizes an effective antitumor immune response. Cancer Res 2012; 72: 2980–2989. [DOI] [PubMed] [Google Scholar]
- Tang H, Wang Y, Chlewicki LK, Zhang Y, Guo J, Liang W et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer cell 2016; 29: 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JC, Chin RK, Lee Y, Kang H-S, Wang Y, Weinstock JV et al. Differential regulation of CCL21 in lymphoid/nonlymphoid tissues for effectively attracting T cells to peripheral tissues. J Clin Invest 2003; 112: 1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupovic J, Onder L, Gil-Cruz C, Weiler E, Caviezel-Firner S, Perez-Shibayama C et al. Central nervous system stromal cells control local CD8+ T cell responses during virus-induced neuroinflammation. Immunity 2016; 44: 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak L, Igoucheva O, Cho S, Alexeev V. Characterization of the CCL21-mediated melanoma-specific immune responses and in situ melanoma eradication. Mol Cancer Ther 2007; 6: 1755–1764. [DOI] [PubMed] [Google Scholar]
- Sharma S, Stolina M, Luo J, Strieter RM, Burdick M, Zhu LX et al. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol 2000; 164: 4558–4563. [DOI] [PubMed] [Google Scholar]
- Yang S-C, Hillinger S, Riedl K, Zhang L, Zhu L, Huang M et al. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin Cancer Res 2004; 10: 2891–2901. [DOI] [PubMed] [Google Scholar]
- Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 2010; 328: 749–752. [DOI] [PubMed] [Google Scholar]
- Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 2008; 8: 362–371. [DOI] [PubMed] [Google Scholar]
- Bardi G, Lipp M, Baggiolini M, Loetscher P. The T cell chemokine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur J Immunol 2001; 31: 3291–3297. [DOI] [PubMed] [Google Scholar]
- Gao J-Q, Sugita T, Kanagawa N, Iida K, Okada N, Mizuguchi H et al. Anti-tumor responses induced by chemokine CCL19 transfected into an ovarian carcinoma model via fiber-mutant adenovirus vector. Biol Pharm Bull 2005; 28: 1066–1070. [DOI] [PubMed] [Google Scholar]
- Hillinger S, Yang S, Batra R, Strieter R, Weder W, Dubinett S et al. CCL19 reduces tumour burden in a model of advanced lung cancer. Br J Cancer 2006; 94: 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillinger S, Yang S-C, Zhu L, Huang M, Duckett R, Atianzar K et al. EBV-induced molecule 1 ligand chemokine (ELC/CCL19) promotes IFN-γ-dependent antitumor responses in a lung cancer model. J Immunol 2003; 171: 6457–6465. [DOI] [PubMed] [Google Scholar]
- Braun SE, Chen K, Foster RG, Kim CH, Hromas R, Kaplan MH et al. The CC chemokine CKβ-11/MIP-3β/ELC/Exodus 3 mediates tumor rejection of murine breast cancer cells through NK cells. J Immunol 2000; 164: 4025–4031. [DOI] [PubMed] [Google Scholar]
- Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol 2013; 25: 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med 1999; 189: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Hara K, Willis MS, Malin MA, Höpken UE, Gray DH et al. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity 2002; 16: 205–218. [DOI] [PubMed] [Google Scholar]
- Young CL, Adamson TC, Vaughan JH, Fox RI. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthritis Rheum 1984; 27: 32–39. [DOI] [PubMed] [Google Scholar]
- Schröder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci USA 1996; 93: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009; 6: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlings RM, Wijbrandts CA, Mebius RE, Cantaert T, Dinant HJ, van der Pouw‐Kraan TC et al. Synovial lymphoid neogenesis does not define a specific clinical rheumatoid arthritis phenotype. Arthritis Rheum 2008; 58: 1582–1589. [DOI] [PubMed] [Google Scholar]
- Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 2011; 186: 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacionales DC, Kelly KM, Lee PY, Zhuang H, Li Y, Weinstein JS et al. Type I interferon production by tertiary lymphoid tissue developing in response to 2,6,10,14-tetramethyl-pentadecane (Pristane). Am J Pathol 2006; 168: 1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JL. Inhibition of the lymphotoxin pathway as a therapy for autoimmune disease. Immunol Rev 2008; 223: 202–220. [DOI] [PubMed] [Google Scholar]
- Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol 2003; 171: 6173–6177. [DOI] [PubMed] [Google Scholar]
- Chiang EY, Kolumam G, McCutcheon KM, Young J, Lin Z, Balazs M et al. In vivo depletion of lymphotoxin-alpha expressing lymphocytes inhibits xenogeneic graft-versus-host-disease. PLoS One 2012; 7: e33106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emu B, Luca D, Offutt C, Grogan JL, Rojkovich B, Williams MB et al. Safety, pharmacokinetics, and biologic activity of pateclizumab, a novel monoclonal antibody targeting lymphotoxin a: results of a phase I randomized, placebo-controlled trial. Arthritis Res Ther 2012; 144: R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayar S, Campos J, Chung MM, Navarro-Núñez L, Chachlani M, Steinthal N et al. Bimodal expansion of the lymphatic vessels is regulated by the sequential expression of IL-7 and lymphotoxin α1β2 in newly formed tertiary lymphoid structures. J Immunol 2016; 197: 1957–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force WR, Walter BN, Hession C, Tizard R, Kozak CA, Browning JL et al. Mouse lymphotoxin-beta receptor. Molecular genetics, ligand binding, and expression. J Immunol 1995; 155: 5280–5288. [PubMed] [Google Scholar]
- Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity 2000; 12: 471–481. [DOI] [PubMed] [Google Scholar]
- Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol 2002; 169: 424–433. [DOI] [PubMed] [Google Scholar]
- Marinkovic T, Garin A, Yokota Y, Fu Y-X, Ruddle NH, Furtado GC et al. Interaction of mature CD3+ CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest 2006; 116: 2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columba-Cabezas S, Griguoli M, Rosicarelli B, Magliozzi R, Ria F, Serafini B et al. Suppression of established experimental autoimmune encephalomyelitis and formation of meningeal lymphoid follicles by lymphotoxin β receptor-Ig fusion protein. J Neuroimmunol 2006; 179: 76–86. [DOI] [PubMed] [Google Scholar]
- Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 2008; 118: 3537–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin-Frankel J. Cancer immunotherapy. Science 2013; 342: 1432–1433. [DOI] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu W-J, Topalian SL, Hwu P et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]