Transplantation of hematopoietic stem/progenitor cells (HSPC) has become one of the most effective treatments for several hematological malignancies, cancers and genetic immune disorders. However, the lack of histocompatible donor sources and the number of stem cells suitable for engraftment are major constraints in the clinic, and these constraints greatly hamper the use of stem cell therapy. In humans, fetal liver and bone marrow are the two major locations of hematopoiesis before and after birth, respectively. Thus, understanding the specialized stem cell microenvironment supporting the self-renewal and expansion of HSPC in fetal liver and bone marrow is not only essential for extending the current knowledge of stem cell biology but is also beneficial to adult regenerative medicine in the clinic.
Despite intensive research over the past several decades, there has not been a clear overall picture of how the hematopoietic stem cell niche functions in the expansion of primitive and self-renewing HSPCs. Many studies have shown that the expansion of HSPCs depends on some well-known growth factors, such as stem cell factors, flt3 ligands, thrombopoietin, interleukin 6, interleukin-3 and angiopoietin-like 5, which function in different combinations,1, 2, 3 and newly recognized factors such as notch ligand and stemregenin 1.4, 5 However, whether cytokines alone lead to HSPC expansion with self-renewing capacity remains unclear, as previous studies have also indicated that direct interactions between HSPCs and cellular components, such as blood vessel endothelial cells, osteoblasts, mesenchymal stem cells and so on, in the stem cell niche are crucial for the regulation of hematopoiesis.6, 7 Moreover, there are ongoing debates about whether HSPCs lose long-term grafting ability during in vitro expansion as a result of the loss of a proper stem cell-forming niche, which has not yet been accurately formatted in existing stem cell protocols.8 New insights into the hematopoietic stem cell niche are required.
In a previous study, we showed that human hematopoietic stem cells in the fetal liver, cord blood and bone marrow, which carry the actual long-term in vivo repopulating activity, possess a unique phenotype of CD34hiCD133hi, while the CD34hiCD133neg phenotype defines hematopoietic progenitor cells and only contributes to the differentiation of specific lineages.9 According to another study, mesenchymal stem cells engineered to express growth factors can robustly support the expansion of CD34hiCD133hi cells, and both cell-cell contact and soluble factors are essential for this process.10 Yong et al.11 recently identified a novel human liver progenitor cell population in the fetal liver with a unique phenotype of CD34loCD133lo. This pluripotent cell population could give rise to multiple cell lineages, including hepatocytes, endothelial cells, adipocytes and osteocytes; thus, CD34loCD133lo cells contribute an essential component in the fetal liver niche. Furthermore, CD34loCD133lo cells express principal growth factors that are important for stem cell expansion such as stem cell factor, insulin-like growth factor 2, C–X–C motif chemokine 12 (CXCL12) and factors in the angiopoietin-like protein family. The co-culture of fetal liver CD34loCD133lo cells with autologous fetal liver CD34hi HSPCs or allogenic cord blood HSPCs supports the ex vivo expansion of HSPCs from both resources. In addition, this new co-culture-based expansion only relies on the soluble factors secreted from CD34loCD133lo cells and is cell-cell contact independent.
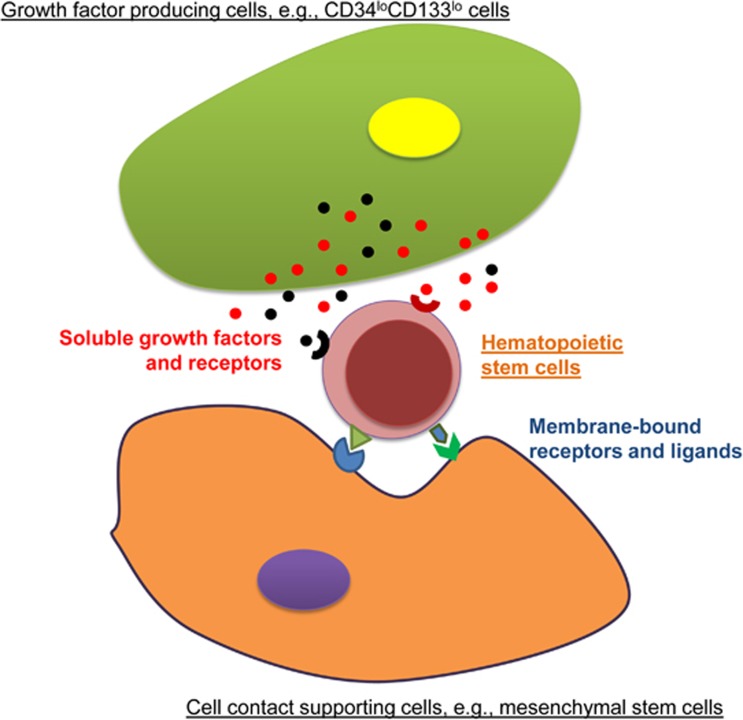
Considering these results, it is thought that there is a network of various cell types that reconstitute the hematopoietic stem cell niche in the fetal liver. Some groups of stromal cells in stem cell niche could be responsible for producing growth factors to support stem cell expansion, for example, CD34loCD133lo cells, while certain cell types provide cell-cell contact with additional stimulating signals, for example, mesenchymal stem cells; other cell types potentially have the two overlapping features (Figure 1). These cells are referred to as stromal cells, which contribute soluble and/or membrane-bound signals to HSPCs as ‘feeder cells’, while those that are not involved in stem cell maintenance act as ‘non-feeder cells’. Considering the complexity of the stem cell niche and the limited time and extent of stem cell expansion that researchers can currently achieve, the existing conditions for ex vivo stem cell expansion remain far from optimal. To achieve long-lasting robust ex vivo stem cell renewal and expansion, new factors should be explored to better mimic the hematopoietic stem cell niche. Theoretically, soluble and fixed components in a co-culture system can be obtained from the growth factors and the membrane-bound proteins that are present on feeder cells isolated from fetal livers, respectively. However, it is likely to be infeasible to continuously culture all potential feeder cell types in a single ex vivo culture system, considering the difficulty in obtaining and maintaining these cells for even a short period of time without changing their original features. Yong demonstrated that over time, primary CD34loCD133lo feeder cells rapidly change in vitro, leading to a dramatic decrease in the HSPC expansion efficiency when cultured for more than 7 days.11 Thus, a feeder-free system in a 2D or a 3D format is likely to be more sustainable. In addition, although Yong detected the expression of a few key growth factors at the mRNA level, their protein translation, which is the real effector, could vary; the few known factors may only represent a small portion of the natural cocktail.
Figure 1.
Two major types of feeder cells for HSPCs in a fetal liver stem cell niche.
Can we overcome these issues and customize a feeder-free system with a combination of optimized soluble and immobilized factors? The following strategy to characterize and simplify the stem cell niche could be explored: first, gently disassociate the cellular network in the fetal liver, followed by an ex vivo 3D co-culture-based screening system and cell ablation to identify the essential cell components under the most robust conditions for the expansion of HSPCs. Proteomics and bioinformatics should subsequently be applied to identify the principal growth factors secreted and to characterize the membrane-bound receptor/ligand patterns formed between feeder cells and HSPCs. Finally, selected combinations should be verified in a novel feeder-free culture system with both soluble and anchored components.
Acknowledgments
QC is supported by the Singapore National Research Foundation Fellowship (NRF-NRFF2017-03).
Footnotes
The author declares no conflict of interest.
References
- Zhang CC, Kaba M, Iizuka S, Huynh H, Lodish HF. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood 2008; 111: 3415–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammaitoni L, Bruno S, Sanavio F, Gunetti M, Kollet O, Cavalloni G et al. Ex vivo expansion of human adult stem cells capable of primary and secondary hemopoietic reconstitution. Exp Hematol 2003; 31: 261–270. [DOI] [PubMed] [Google Scholar]
- Heike T, Nakahata T. Ex vivo expansion of hematopoietic stem cells by cytokines. Biochim Biophys Acta 2002; 1592: 313–321. [DOI] [PubMed] [Google Scholar]
- Dahlberg A, Delaney C, Bernstein ID. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood 2011; 117: 6083–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JE Jr, Brunstein CG, Boitano AE, DeFor TE, McKenna D, Sumstad D et al. Phase I/II trial of stemregenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell 2016; 18: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane GM, Jeffery E, Morrison SJ. Adult haematopoietic stem cell niches. Nat Rev Immunol 2017. e-pub ahead of print 12 June 2017; doi:10.1038/nri.2017.53. [DOI] [PubMed]
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 2006; 6: 93–106. [DOI] [PubMed] [Google Scholar]
- Weidner CI, Walenda T, Lin Q, Wolfler MM, Denecke B, Costa IG et al. Hematopoietic stem and progenitor cells acquire distinct DNA-hypermethylation during in vitro culture. Sci Rep 2013; 3: 3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Khoury M, Limmon G, Choolani M, Chan JK, Chen J. Human fetal hepatic progenitor cells are distinct from, but closely related to, hematopoietic stem/progenitor cells. Stem Cells 2013; 31: 1160–1169. [DOI] [PubMed] [Google Scholar]
- Khoury M, Drake A, Chen Q, Dong D, Leskov I, Fragoso MF et al. Mesenchymal stem cells secreting angiopoietin-like-5 support efficient expansion of human hematopoietic stem cells without compromising their repopulating potential. Stem Cells Dev 2011; 20: 1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong KS, Keng CT, Tan SQ, Loh E, Chang KT, Tan TC et al. Human CD34(lo)CD133(lo) fetal liver cells support the expansion of human CD34(hi)CD133(hi) hematopoietic stem cells. Cell Mol Immunol 2016; 13: 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]