Abstract
Background
Neuroendocrine malignancy is indolent, yet relentless in its propensity to metastasize to the liver, where it may cause bizarre paraneoplastic syndromes. The pathophysiologic mechanism behind this predilection for hepatic metastasis is twofold: the portal venous system drains the most likely primary sites for neuroendocrine tumors, and the relatively immunosuppressed environment within the hepatic parenchyma is permissive for tumor growth. The standard of care for patients with metastatic neuroendocrine tumor is surgical resection of at least 90% of the tumor burden.
Methods
This report describes CT-guided percutaneous cryoablation of an inoperable mesenteric carcinoid tumor that had previously demonstrated hepatic metastases utilizing hydrodissection to safely and effectively prevent further metastasis while priming the immune system to eradicate this malignancy systemically.
Results
CT-guided percutaneous cryoablation is minimally invasive, has intrinsic analgesic properties, and may contribute to sensitization of the immune system against tumor antigens.
Conclusion
Percutaneous cryoablation with hydrodissection can be used to target intraabdominal malignancy in poor surgical candidates. This procedure is safe, effective, and minimally invasive.
Keywords: Abscopal effect, Carcinoid tumor, Cryoablation, Mesenteric tumor, Metastasis, Neuroendocrine tumor
Introduction
Small bowel carcinoid malignancy arises from neuroendocrine enterochromaffin cells located throughout the gastrointestinal tract, most frequently in the midgut. Ten percent of these tumors may produce large quantities of metabolically active humoral substances, including serotonin and histamine. This serotonin is secreted into the portal venous drainage where it is metabolized in the liver. The malignancy metastasizes through lymphatic channels, which typically follow venous drainage, and the midgut is no exception. The superior mesenteric lymph nodes drain the jejunum, ileum, cecum, and appendix, while the inferior mesenteric nodes drain the hindgut and the celiac nodes drain the foregut. Metastasis to the liver allows the paraneoplastic carcinoid syndrome to manifest, which is characterized by episodic flushing and diarrhea. This is because the serotonin produced in the metastatic cells directly enters circulation through the hepatic veins, thus avoiding first-pass hepatic inactivation. Chronic cardiac exposure to high levels of humoral substances produced by metastases leads to right heart failure; the left heart is protected by pulmonary inactivation of these substances. In the setting of primary bronchial carcinoid, the carcinoid syndrome can manifest in the absence of hepatic metastasis because the humoral substances avoid the portal venous drainage and subsequent hepatic degradation.
The adaptive immune system provides robust defense against infection as well as malignancy, and any cell or molecule not recognized as self is phagocytosed by antigen-presenting cells (APCs). Once inside the lysosome, the antigen is processed and displayed via major histocompatibility complex (MHC) molecules. MHC class I presents intracellular antigens, while MHC class II presents extracellular antigens. These MHC-antigen complexes are subsequently bound by a T-cell receptor with affinity for that specific antigen. APCs that display MHC class I are compatible with CD8 T cells and cause proliferation of cytotoxic lymphocytes specific for intracellular antigens. MHC class II binds with T-cell receptors on CD4 T cells and leads to B-cell stimulation and specific antibody production against extracellular antigens. After the binding requirement has been satisfied, proinflammatory cytokines (TNF-alpha, IL-2, IL-12) are released, resulting in a proliferation of T cells with affinity for the foreign antigen initially recognized by the APCs.
The characteristics of the APCs are instrumental in modulating the immune response in any given organ, and in the liver, they produce a tolerogenic environment. Kupffer cells and plasmacytoid dendritic cells are two types of APC that are expressed in the liver, preferentially around the portal tract, to detect foreign antigens. Both of these cell lineages have been demonstrated to secrete IL-10, an anti-inflammatory cytokine, which attenuates the immune response [1]. This contributes to production of an intrahepatic environment that is amenable to tumor growth and metastasis.
This report describes an experimental palliative modality using CT-guided cryoablation to target inoperable mesenteric malignancies and decrease morbidity from hepatic metastasis. The patient was a 67-year-old female with a remote history of resection of a small bowel primary malignancy for which pathology showed carcinoid tumor present at the surgical margins. This operation was complicated by development of a large ventral hernia, which contained both small bowel and colon. Surveillance imaging studies demonstrated a persistent mesenteric calcified mass consistent with carcinoid tumor deep to the ventral hernia. The tumor being encased in bowel and in an abdomen which had previously been demonstrated to contain numerous adhesions, achieving 90% tumor cytoreduction presented an interesting challenge. The carcinoid tumor had previously demonstrated hepatic metastasis, requiring frequent invasive procedures, including two chemoembolizations and three cryoablation procedures over the previous 3 years to eradicate hepatic lesions. Three independent surgeons refused to operate due to the entanglement of the lesion within the bowel and its proximity to a mesenteric vessel. Additionally, proton beam therapy was declined for the same reason. Adapting to these circumstances necessitated an alternative treatment modality, and the recent development of smaller-diameter cryoprobes has allowed percutaneous cryoablation to be used widely for hepatic lesions as well as complex cases which would be otherwise untreatable [2]. Image guidance can be obtained with ultrasound, contrast-enhanced ultrasound, CT, PET, virtual navigation systems, and fusion imaging. For this case, CT guidance was determined to be optimal due to the location of the tumor deep within the abdomen as well as the excellent demarcation of the margins of the ice ball shown on CT, which were used to determine adequate ablation intraoperatively [3].
Percutaneous cryoablation has a number of advantages over thermal ablation techniques as well as open and laparoscopic techniques, including intrinsic analgesic properties, minimal invasiveness, and a favorable cellular and molecular immunologic response. Intrinsic tissue architecture is predominantly preserved during cryoablation, which minimizes irreversible damage to nontarget ablated tissue, enhancing the safety profile of this procedure [4]. Preservation of the molecular structure of tumor antigens as well as disruption of the tumor cell plasma membrane results in 13–20% of dendritic cells in the draining lymph nodes presenting tumor antigen and inducing a clonal T-cell proliferation compared to 7% in radiofrequency ablation [5]. Furthermore, cryoablation has been demonstrated to induce a systemic antitumor, proinflammatory cytokine response as well as statistically significant reductions in secondary tumor growth and propensity for pulmonary metastasis in a mouse model [6]. Patients with metastatic pancreatic cancer treated with cryotherapy demonstrated increased overall survival compared to first-line chemotherapy, and a positive correlation between overall survival and pretreatment immune function was demonstrated, providing additional evidence for the existence of an immune-regulated antitumor response following cryoablation [7]. It is hypothesized that cryoablation of this mesenteric carcinoid will induce an immune response abrogating the propensity for hepatic metastasis.
Materials and Methods
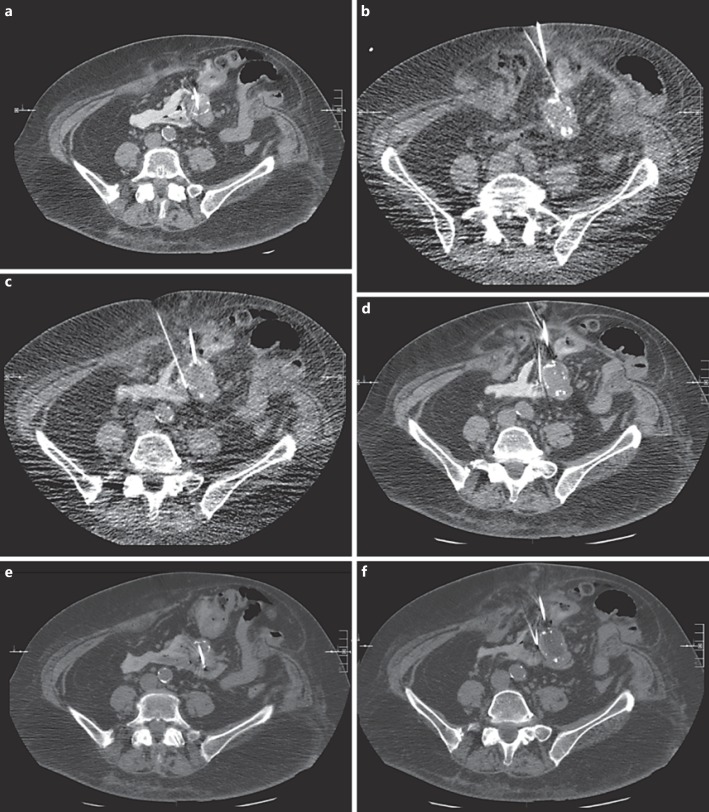
Meticulous planning revealed a small anterior window which was adequate to access the tumor percutaneously. Informed written consent was obtained, and the patient was positioned supine on the CT gantry under general anesthesia, prepped and draped in the usual sterile fashion. Limited CT images were performed with metal guide markers to delineate the lower abdominal midline, partially calcified mesenteric mass and for procedure planning (Fig. 1a). Using CT guidance, a single 17-gauge cryoablation probe was inserted from an anterior percutaneous approach into the mesenteric mass. Subsequently, three 21-gauge needles were inserted under CT/fluoroscopic guidance adjacent to the lateral margins of the mass. Utilizing normal saline diluted with a small amount of contrast (3 mL of Visipaque), approximately 40 mL of solution was injected through each 21-gauge needle for hydrodissection and to displace bowel from the mass to allow cryoablation to be safely performed. Once hydrodissection was complete, an additional 17-gauge probe was inserted from an anterior approach into a lower portion of the mesenteric mass. At this point, cryoablation was performed with limited CT images obtained during cryoablation to evaluate the progression of the ice ball. This demonstrated satisfactory coverage of the mesenteric mass (Fig. 2a, b, c, d, e, f). An additional freeze cycle with intervening thaw cycle was performed. Following removal of the cryoablation probes and 21-gauge needles, limited CT was performed, revealing hypodensity within the region without evidence of nontarget ablation to adjacent bowel. A follow-up CT scan at 1 month showed minimal rim enhancement and no new liver lesions, confirming >90% tumor ablation (Fig. 1b, c, d, e).
Fig. 1.
Mesenteric carcinoid tumor cryoablation. a Preoperative abdominal CT image demonstrating a calcified mesenteric mass surrounded by bowel loops, ventral hernia, and an anterior window to access the mass percutaneously. b Postoperative abdominal CT image demonstrating hypodensity in the region of the mass without evidence of ablation to surrounding bowel and contrast used for hydrodissection. c One-month follow-up CT confirming >90% tumor coverage with minimal rim enhancement. d Preoperative abdominal CT image with measurements of the lesion. e Four-month follow-up CT with measurements revealing a 20–25% decrease in tumor size.
Fig. 2.
Probe placement and hydrodissection. a Initial cryoprobe embedded in the mass. b First 21-gauge needle lateral to the mass hydrodissecting the bowel away from the target zone anterolaterally. c Second 21-gauge needle medially hydrodissecting additional bowel loops. d Third 21-gauge needle hydrodissecting posteromedially. e Second cryoprobe embedded in the inferior portion of the mass. f CT image obtained during the freeze cycle demonstrating expanding ice ball within the mesenteric mass.
This case was reviewed by UF IRB-03 Chairman, and it was determined that as a single case report, it did not require IRB approval.
Results
With surgery and radiation deemed to be unacceptably risky, this mass was targeted for percutaneous cryoablation with intraoperative hydrodissection to mobilize the bowel away from the target zone. Following removal of the cryoablation probes and 21-gauge hydrodissection needles, limited CT was performed, revealing excellent ablation of the tumor without evidence of nontarget ablation to adjacent bowel. The patient was discharged 1 day after the procedure without any abdominal pain and in good condition. CT scan 1 month after the operation demonstrated 90% tumor ablation and no new liver lesions. Four-month follow-up CT revealed a 20–25% decrease in size of the mesenteric carcinoid compared to the preablation size and continued absence of hepatic metastasis. CT every 4 months will be utilized to surveil for detectable hepatic metastases, which were likely to be present as micrometastases at the time of the cryoablation. This is secondary to the patient's presentation with frequent metastasis and the epidemiology of small bowel origin carcinoid tumors.
Discussion
Carcinoid tumors that originate from the small bowel are more likely to metastasize than those from other primary sites; these tumors show tropism for the liver, with 82% of neuroendocrine tumor patients showing liver metastases at autopsy. These metastases are typically multifocal, with a median of 13 metastases, of which less than half are radiologically detectable: therefore, it is likely that hepatic micrometastases are present at the time of cryoablation. The 5-year survival for patients with metastatic carcinoid tumor is 19–38% [8]. The standard of care for these patients is reduction of the tumor burden by 90%, with the intent to eradicate it completely. Recent advances in transvascular modalities to target hepatic metastases have reduced the need to surgically resect these hepatic metastases; however, morbidity and mortality from these procedures remain significant. Treatment-related mortality from transarterial chemoembolization is approximately 2–3%, while numerous complications have been reported, ranging from mild postembolization syndrome (60–80% morbidity) to reversible and irreversible hepatic decompensation (31 and 15% morbidity) [9]. Our patient required multiple procedures over the course of several years due the propensity of her small bowel origin mesenteric primary carcinoid tumor to metastasize hepatically. This calcified mesenteric mass was deemed unresectable surgically by three independent opinions and was not amenable to proton beam therapy due to its location encased in the bowel and its proximity to a mesenteric vessel. Leaving the mass in situ and continuing to treat the hepatic metastases would have been a suboptimal approach and would have exposed the patient to significant procedure-related morbidity and mortality. It is vital that referring physicians recognize the expanding oncologic role of ablative techniques such as cryotherapy for unusual and difficult cases to better serve this patient population.
Our hypothesis is that cryoablation of this mesenteric carcinoid tumor will induce a systemic antitumor response which will inhibit further hepatic metastases of residual intraabdominal carcinoid malignancy, effectively and safely extending this patient's 5-year survival and decreasing morbidity from frequent invasive procedures. Due to the relatively immunosuppressed environment intrahepatically, micrometastases present at the time of the procedure may exhibit differential behavior. Further investigation will reveal whether the clonal, tumor-specific, cytotoxic T-cell response induced by cryoablation is indeed able to penetrate the hepatic parenchyma and suppress hepatic growth of micrometastases.
Conclusion
Percutaneous image-guided cryoablation has been successfully utilized at our institution to achieve treatment and potential cure of a mesenteric carcinoid malignancy through cytoreduction. This minimally invasive technique can be safely applied to treat suboptimal surgical candidates and inoperable mesenteric malignancies.
Disclosure Statement
The authors declare no conflicts of interest.
Appendix
Continuing Medical Education Questions
| 1. Which lymph nodes drain the jejunum? |
| A superior mesenteric |
| B inferior mesenteric |
| C internal iliac |
| D para-aortic |
| E celiac |
| 2. Antigen-presenting cells display intracellular antigens via _________ molecules, stimulating __________ cells specific for intracellular antigen. |
| A MHC class 2: CD8 |
| B MHC class 2: CD4 |
| C MHC class 2: NK |
| D MHC class 1: CD8 |
| E MHC class 1: CD4 |
| 3. Which molecule is secreted by hepatic metastases in the paraneoplastic carcinoid syndrome? |
| A insulin |
| B a specific antibody |
| C serotonin |
| D epinephrine |
| E norepinephrine |
| 4. Plasmacytoid dendritic cells are antigen-presenting cells found in greater proportion in the hepatic parenchyma compared to other lymphoid organs. Which anti-inflammatory cytokine do they secrete that attenuates the immune response in this organ? |
| A TNF-alpha |
| B IL-2 |
| C IL-6 |
| D IL-10 |
| E IL-12 |
| 5. Right heart failure is a consequence of long-standing carcinoid syndrome. Which primary site may manifest as carcinoid syndrome in the absence of hepatic metastases? |
| A small intestine |
| B bronchi |
| C rectum |
| D stomach |
| E appendix |
MHC, major histocompatibility complex.
References
- 1.Chan T, Wiltrout RH, Weiss JM. Immunotherapeutic modulation of the suppressive liver and tumor microenvironments. Int Immunopharmacol. 2011;11:879–889. doi: 10.1016/j.intimp.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shyn PB, Mauri G, Alencar RO, Tatli S, Shah SH, Morrison PR, Catalano PJ, Silverman SG. Percutaneous imaging-guided cryoablation of liver tumors: predicting local progression on 24-hour MRI. AJR Am J Roentgenol. 2014;203:W181–W191. doi: 10.2214/AJR.13.10747. [DOI] [PubMed] [Google Scholar]
- 3.Mauri G, Nicosia L, Varano GM, Shyn P, Sartori S, Tombesi P, Di Vece F, Orsi F, Solbiati L. Unusual tumour ablations: report of difficult and interesting cases. Ecancermedicalscience. 2017;11:733. doi: 10.3332/ecancer.2017.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vikingstad EM, de Ridder GG, Glisson RR, Cardona DM, DiPalma D, Eward WC, Brigman BE, Nelson RC, Kim CY. Comparison of acute histologic and biomechanical effects of radiofrequency ablation and cryoablation on periarticular structures in a swine model. J Vasc Interv Radiol. 2015;26:1221–1228. doi: 10.1016/j.jvir.2015.04.013. e1. [DOI] [PubMed] [Google Scholar]
- 5.Rao P, Escudier B, de Baere T. Spontaneous regression of multiple pulmonary metastases after radiofrequency ablation of a single metastasis. Cardiovasc Intervent Radiol. 2011;34:424–430. doi: 10.1007/s00270-010-9896-9. [DOI] [PubMed] [Google Scholar]
- 6.Joosten JJ, Muijen GN, Wobbes T, Ruers TJ. In vivo destruction of tumor tissue by cryoablation can induce inhibition of secondary tumor growth: an experimental study. Cryobiology. 2001;42:49–58. doi: 10.1006/cryo.2001.2302. [DOI] [PubMed] [Google Scholar]
- 7.Niu L, Chen J, He L, Liao M, Yuan Y, Zeng J, Li J, Zuo J, Xu K. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas. 2013;42:1143–1149. doi: 10.1097/MPA.0b013e3182965dde. [DOI] [PubMed] [Google Scholar]
- 8.Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. The epidemiology of metastases in neuroendocrine tumors. Int J Cancer. 2016;139:2679–2686. doi: 10.1002/ijc.30400. [DOI] [PubMed] [Google Scholar]
- 9.Curley SA, Stuart KE, Schwartz JM, Carithers RL, Hunter KU. Nonsurgical therapies for localized hepatocellular carcinoma: transarterial embolization, radiotherapy, and radioembolization. Waltham, MA: UpToDate; (accessed March 14, 2017). [Google Scholar]