Abstract
Background
Graves disease (GD) is an autoimmune condition characterized by the presence of antibodies against the thyrotropin receptor (TRAB), which stimulate the thyroid gland to produce excess thyroid hormone. Theoretically, TRAB could stimulate highly differentiated thyroid cancer tissue and/or metastases to produce thyroid hormone.
Case
A 68-year-old male, with weight loss and palpitations, was diagnosed with thyrotoxicosis. A later MRI, due to persistent shoulder pain, revealed multiple bone metastases. A biopsy was diagnostic for follicular variant of papillary thyroid carcinoma, and total thyroidectomy was performed. One week after thyroidectomy the patient was admitted with severe hyperthyroidism. TRAB was >40 IU/mL (normal <0.7 IU/mL). High-dose antithyroid drug treatment was followed by high-dose radioactive iodine-131 (RAI) and local radiotherapy covering the right shoulder. Antithyroid drug treatment continued until after the fourth RAI dose. Hypothyroidism did not occur until following the fifth RAI treatment.
Summary and Conclusions
We present a patient initially diagnosed with thyrotoxicosis and subsequently with metastatic follicular variant of papillary thyroid cancer. It is suggested that TRAB stimulated the highly differentiated extrathyroidal metastatic thyroid tissue to produce excessive amounts of thyroid hormone, delayed diagnosis, and potential aggravation of the course of thyroid cancer.
Keywords: Thyroid cancer, Thyrotropin receptor antibodies, Metastases, Post-thyroidectomy thyroid storm, Case report
What Is Known about This Topic?
• The prevalence of thyrotoxicosis in patients with thyroid cancer is unknown. In view of the sparse reports, it is most likely very low. Thyrotropin receptor antibodies are seldom measured or found in patients with thyroid cancer, but the presence of these antibodies could, at least theoretically, influence the course and clinical presentation of well-differentiated thyroid cancer.
What Does This Case Report Add?
• The combination of thyrotoxicosis and thyroid cancer may lead to delayed diagnosis and aggravation of the latter. As indicated by our case report, thyrotropin receptor antibodies can stimulate not only normal thyroid tissue, but most likely also highly differentiated metastatic thyroid cancer tissue to produce thyroid hormones. It may be important to acknowledge this possibility in patients with thyrotoxicosis and thyroid cancer.
Introduction
Graves disease (GD) is an autoimmune condition characterized by the presence of antibodies binding to and stimulating the thyrotropin receptor (TRAB), resulting in hyperthyroidism [1]. GD is common, with a lifetime risk of around 5% [2]. The etiology of GD is inadequately understood, but is generally thought to be the result of interactions between genetic [3] and environmental triggers, of which cigarette smoking [4, 5] and iodine intake [6] have received most attention. Patients with GD have an increased burden of other morbidities [7], including an increased risk of thyroid cancer (TC) [8, 9], although surveillance bias may, at least partly, explain the latter. Whether GD, which is associated with excess mortality [10], affects the prognosis of differentiated TC is still a matter of controversy [11, 12, 13, 14]. Theoretically, TRAB, due to stimulation of thyroid tissue, could not only induce thyroid malignancy but also make a tumor and/or distant metastases develop more aggressively.
Patients diagnosed with GD within a short period of time after thyroidectomy for TC are very rare. In the following we present a case of a 68-year-old male with GD and metastatic TC (papillary adenocarcinoma of follicular type), where the presence of TRAB complicated treatment of the latter. The case report is presented in its current form after written and oral consent from the patient.
Patient
Eighteen months prior to admission to our department of endocrinology, in June 2014, a 68-year-old male contacted his primary care physician (GP) with a history of unintended weight loss (15 kg) and intermittent palpitations. He was diagnosed with thyrotoxicosis and treated with methimazole and propranolol. No further evaluation of the cause of thyrotoxicosis was carried out. Thyrotropin (TSH), triiodothyronine (T3), and thyroxine (T4) were measured regularly from the initiation of antithyroid drugs. Table 1 summarizes the TSH, T3, and T4 levels at key time points throughout the case history. Prior medical history included lower back and right shoulder pain, starting approximately 6 months before the diagnosis of thyrotoxicosis and unsuccessfully treated with physiotherapy and over the counter analgesics. An MRI scan, performed 2 months prior to admission to our department, showed bone metastases to several vertebrae (Th12, L1, L2, and L5) as well as to the right shoulder. A biopsy from the shoulder showed differentiated adenocarcinoma with cells positive for cytokeratin 7, vimentin, thyroid transcription factor-1, thyroglobulin, and thyroid peroxidase (TPO). Whereas cytokeratin 20, caudal type homeobox 2 (CDX2), and prostate specific antigen (PSA) were negative. It was concluded that the biopsy represented metastatic well-differentiated thyroid carcinoma.
Table 1.
Key laboratory results
| TSH (0.3–4.0 U/L) |
T3 (1.3–3.5 nmol/L) |
T4 (60–130 nmol/L) |
TRAB (<0.7 IU/mL) |
TBC (0.6–1.22) |
TG (<50 μg/L) |
A-TGAb (<60×103 IU/L) |
|
|---|---|---|---|---|---|---|---|
| At first contact with GP (18 months PAD) | <0.01 | 7.2 | 188 | NA | NA | NA | NA |
| At the diagnosis of metastasis (2 months PAD) | <0.01 | 3.5 | 65 | NA | 0.54 | NA | NA |
| Pre-thyroidectomy (1 month PAD) | <0.01 | NA | 171 | NA | NA | NA | NA |
| At admission with thyroid storm(few days PAD) | <0.01 | 12.3 | 309 | NA | NA | NA | NA |
| At admission to our department | <0.01 | 2.9 | 181 | >40.0 | NA | NA | NA |
| Prior to the first RAI (1 month AAD) | <0.01 | 2.7 | 74 | 36.0 | 0.65 | 159,936 | 11,470 |
| After the first RAI (2 months AAD) | <0.01 | 4.2 | 127 | 32.3 | 0.61 | 144,905 | 9,459 |
| Prior to second RAI (4 months AAD) | <0.01 | 36 | 1.9 | NA | 0.88 | 114,592 | 4,271 |
| Prior to third RAI (8 months AAD) | <0.01 | 65 | 2.0 | 9.8 | 0.57 | 30,655 | 1,259 |
| Prior to fourth RAI (15 months ADD) | 0.28 | 46 | NA | 6.0 | NA | 12,280 | 709 |
| Prior to fifth RAI (18 months ADD) | 0.79 | 62 | NA | 4.2 | NA | 6,887 | 345 |
| Prior to sixth RAI (27 months ADD) | 1.6 | 6.1 | 1.1 | 3.1 | 0.73 | NA | NA |
| Post sixth RAI (29 months ADD) | 0.21 | 71 | NA | NA | NA | 2,078 | 91 |
| Currently (32 months ADD) | 0.11 | 18a | 1.1a | NA | NA | 1,381 | 32 |
The table shows serum levels of thyrotropin (TSH), triiodothyronine (T3), and thyroxine (T4), thyrotropin receptor antibodies (TRAB), TBC (thyroxin binding capacity), thyroglobulin (TG), and thyroglobulin antibodies (A-TGAb) at key points in the case report. PAD, prior to admission to our department; AAD, after admission to our department; NA, not available.
Free thyroid hormone levels (not protein bound): free-T4 (10–22 pmol/L), free-T3 (3.5–6.5 pmol/L). Normal values are in parentheses.
The patient had a total thyroidectomy, including removal of macro-pathologically suspected lymph nodes. Examination of resected specimens revealed a semi-lobulated mass measuring 40 × 20 × 25 mm (right lobe), 35 × 20 × 20 mm (left lobe), and an isthmus mass measuring 25 × 15 × 5 mm. The histopathological examination demonstrated a classic multinodular goiter with 2 malignant foci, one in the right lobe measuring 13 mm and the other in the left lobe measuring 1 mm. Histology examination of the 2 foci showed growth of 2 histologically identical tumors. Both foci were confirmed as papillary adenocarcinoma of follicular type and were followed up by immunohistochemical staining showing tumor cells positive for cytokeratin 19, HBME-1 (Hector Battifora mesothelial cell) and TPO. There were neither macro-pathological nor histopathological signs of thyroid autoimmunity.
After total thyroidectomy, the patient was started on liothyronine, 40 μg daily, according to an in-house standard treatment protocol. One week postoperatively he was admitted through the emergency department at his local hospital due to nausea, vomiting, and thyrotoxic symptoms including palpitations, weight loss, tremor, emotional lability, confusion, and insomnia. Biochemically, he had a suppressed serum TSH of <0.01 U/L (normal 0.3–4.0 U/L), a T4 of 309 nmol/L (normal 60–130), and a T3 of 12.3 mU/L (normal 1.3–3.5). According to the diagnostic criteria suggested by Akamizu et al. [15], the patient fulfilled the criteria for thyroid storm type 1: thyrotoxicosis (yes) and at least 1 CNS manifestation (yes; delirium), and 1 of the following: fever (yes; temperature 38.2°C), tachycardia (yes; pulse 111/min), congestive heart failure (no; no signs of heart failure), or GI/hepatic manifestations (yes; nausea). Treatment with liothyronine was discontinued and high-dose propylthiouracil (PTU) (400 mg ×3 daily), glucocorticoids (hydrocortisone succinate 50 mg ×4 daily), and propranolol (40 mg ×2 daily) were started according to an in-house protocol.
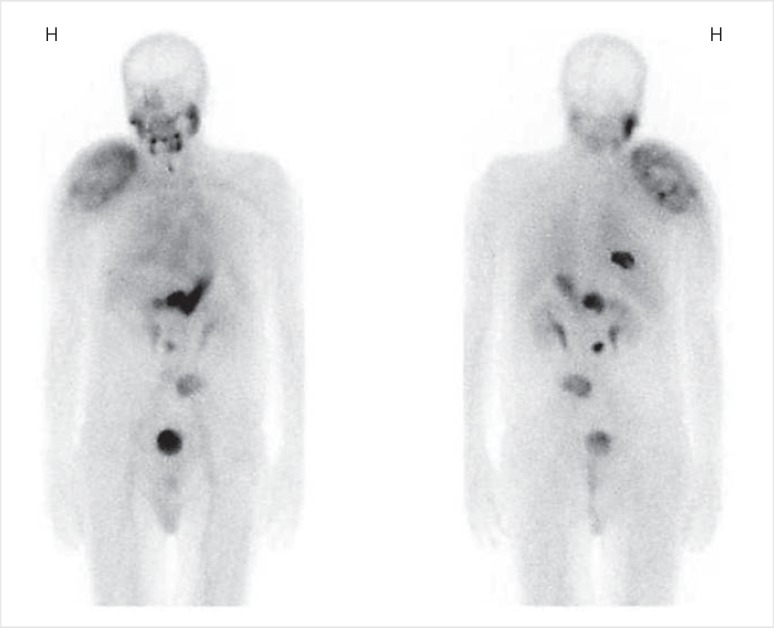
The patient was transferred to our endocrinology department in order to receive high-dose radioactive iodine-131 (RAI) ablation and radiotherapy to his skeletal metastases (30 Gy in 10 daily fractions, 5 fractions per week) via the department of oncology and nuclear medicine. Figure 1 shows a whole-body technetium-99m pertechnetate scintigraphy, performed 3 weeks after thyroid surgery, demonstrating technetium uptake in the metastatic tissue and an empty thyroid bed.
Fig. 1.
Whole-body technetium-99m pertechnetate scintigraphy, performed after thyroidectomy, clearly visualizing the large metastatic mass to the right shoulder, as well as metastases at right costa 9, Th12, L3, and L5 (posterior projection). Physiological uptake can be seen in the salivary glands, the ventricle, and the bladder. H = right side. Left image = anterior projection. Magnification, ×1.2.
Postoperatively, RAI (3.7 GBq per treatment session) was given for 3 sessions over 7 months after admission to our department. In total the patient received (by April 2017) 6 doses, with the last 2 RAI dosages at 7.4 Gbq (totaling to 29.6 Gbq). The fourth RAI was given 7 months after the third treatment session, and the last 2 sessions were given with a 4-month interval. After the first dose of RAI, the T3 and T4 levels decreased to normal, but TSH remaining suppressed. After the fourth dose of RAI, TSH concentration became measurable. During the fall of 2014 the patient was taken off PTU, with TSH, T3, and T4 levels remaining within normal limits. However, after the fourth RAI dose (fall of 2015) the patient became hypothyroid and began treatment with T4. The patient remained hyper- or euthyroid for more than 1.5 years after total thyroidectomy. Currently (March 2017), the patient is still being treated with T4 and remains clinically and biochemically euthyroid (Table 1).
Thyroglobulin level was clearly elevated prior to surgery at 159,936 g/L (normal <50 g/L) even when taking thyroglobulin antibodies into account, indicating a high tumor load. The thyroglobulin, and thyroglobulin antibodies, decreased during the RAI treatments (Table 1). As evident from Table 1, the level of thyroglobulin steadily declined but still (after 6 RAI treatments) remains measurable. No further RAI doses are planned, and there are currently no signs of growth of the metastatic tissue, when evaluated with FDG-PET-CT.
The first recorded measurement of thyroid antibodies was done when the patient was admitted with thyroid storm, 10 days after total thyroidectomy and 18 months after starting antithyroid drugs. TRAB were highly positive at >40.0 IU/L (normal <0.7 IU/L), and TPO antibodies were negative: 6 × 103 IU/L (positive >15 × 103 IU/L). TRAB positivity was confirmed by 3 repeated measurements over a time period of 2 months. All measurements of TRAB were done at our local laboratory using a Brahms TRAK human RIA analysis system (Brahms GmbH, Henningsdorf, Germany). TRAB has remained elevated throughout the observation period, as summarized in Table 1.
Discussion
Within the last 15 years, to our knowledge, only 2 cases have illustrated GD and coexisting metastatic papillary adenocarcinoma [16, 17]. Al-Omari et al. [16] presented a case of a 65-year-old male with diffuse goiter, cold nodules on a thyroid scan, and classical signs of thyrotoxicosis. The patient had lung metastases and continued to be thyrotoxic following total thyroidectomy. Suzuki et al. [17] published a case of a 59-year-old female with metastatic TC, diagnosed after a chest X-ray had revealed metastatic masses. Biopsy showed metastatic TC. She was treated with total thyroidectomy and RAI, was euthyroid at the time of diagnosis of the TC, but had elevated TRAB. After the thyroidectomy, the patient became and continued to be thyrotoxic. The prevalence of thyrotoxicosis in patients with TC is unknown, but, in view of the sparse reports, it is most likely very low.
Our patient, despite total thyroidectomy, remained thyrotoxic. In TC, thyrotoxicosis can occur under several conditions: (1) co-occurrence of GD and TC, (2) de novo production of thyroid hormones from the TC, (3) co-occurrence of autonomic thyroid nodule(s) and TC, and (4) destruction of normal thyroid tissue by TC, RIA, or surgery. In our opinion, options 3 and 4 are unlikely, since the patient remained hyperthyroid long after thyroid surgery. In addition, due to the long duration of thyrotoxicosis and the fact that the patient developed thyrotoxicosis long before treatment with RIA, thyrotoxicosis due to destruction of normal thyroid tissue is also unlikely. Although, we cannot exclude de novo production of thyroid hormones from the metastatic tissue, we suggest that this 68-year-old male, with co-existing GD and metastatic papillary thyroid adenocarcinoma of follicular type, had TRAB stimulation of the metastatic thyroid tissue, causing this highly differentiated tumor tissue to produce uncontrolled amounts of thyroid hormones.
As the first evaluation of TRAB was carried out 18 months after the diagnosis of thyrotoxicosis and 1 month after thyroid surgery, we can only speculate on the exact timing of the development of TRAB in this patient. The development of TRAB has been described after thyroid destruction [18], including RAI [19], and following subacute thyroiditis [20]. In our patient, RAI and subacute thyroiditis can be excluded, as the patient had TRAB before RAI and did not have any history of symptoms related to thyroiditis prior to diagnosis. The development of TRAB and GD shortly after partial thyroidectomy has been described [21]. We cannot exclude that the TRAB in this patient is the result of thyroid surgery, although this has never been described after total thyroidectomy and would be expected to occur with a much longer lag time [20]. On the other hand, the lack of histopathological signs of autoimmunity within the thyroid gland could indicate that TRAB is the result of thyroid surgery.
We cannot clarify whether GD preceded the TC or vice versa in our case. GD has been associated with a 10- to 12-fold increased risk of TC [8, 9]. It is, however, unclarified whether this observation is due to a chance finding, due to more extensive examination of affected individuals, or indicates a direct carcinogenic effect of GD. In our opinion, knowing the time it takes to develop TC and the rapid onset of GD, it is most likely that the patient had TC for a number of years and subsequently developed GD. The clinical presentation, with a debut of lower back and right shoulder pain months before symptoms of thyrotoxicosis, favors this interpretation. In a study by Kikuchi et al. [22], the mean time to develop a follicular carcinoma, after exposure to radiation, was 20.5 years. Since the clinical presentation of the patient was as expected in thyrotoxicosis, further investigation into the etiology of the symptoms was not in focus. It is unlikely that an early standard thyroid scintigraphy, knowing that there were only 2 small cancer foci within the thyroid gland, would have led to a different diagnostic strategy and an earlier diagnosis of malignancy.
The treatment of choice in metastatic TC is thyroidectomy followed by RAI. However, the presence of TRAB and subsequent thyrotoxicosis can hinder treatment efficacy. In patients with thyrotoxicosis the iodine metabolism is increased, thus potentially rendering the RAI treatment less efficient [19]. This could be the case in our patient due to a continuous stimulation of the metastases by TRAB. Unfortunately, it was not possible, immunohistochemically, to demonstrate the presence of TSH receptors or the binding of TRAB in the metastasis due to the lack of available tissue. Although there was a significant drop in thyroglobulin levels after the RAI treatments, iodine uptake in the metastases was visualized even on TxWBS (whole-body scan after therapy dose) after the fifth RAI, and persistently measurable thyroglobulin levels remain to this day. The patient also remained euthyroid without any treatment for 1 year before becoming hypothyroid in the fall of 2015 (after the fourth RAI). This indicates persistent thyroid hormone production by the metastatic tissue. This further underlines our argument that the patient had TRAB stimulation of the metastatic TC tissue to produce thyroid hormones.
The combination of GD and TC may lead to delayed diagnosis and aggravation of TC. As indicated by our case report, TRAB can stimulate not only normal thyroid tissue, but most likely also highly differentiated metastatic TC tissue to produce thyroid hormones.
Disclosure Statement
There are no relevant conflicts of interest pertaining to this manuscript.
References
- 1.Smith TJ, Hegedus L. Graves' Disease. N Engl J Med. 2016;375:1552–1565. doi: 10.1056/NEJMra1510030. [DOI] [PubMed] [Google Scholar]
- 2.Carle A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, Laurberg P. Epidemiology of subtypes of hyperthyroidism in Denmark: a population-based study. Eur J Endocrinol. 2011;164:801–809. doi: 10.1530/EJE-10-1155. [DOI] [PubMed] [Google Scholar]
- 3.Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves' disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001;86:930–934. doi: 10.1210/jcem.86.2.7242. [DOI] [PubMed] [Google Scholar]
- 4.Brix TH, Hansen PS, Kyvik KO, Hegedus L. Cigarette smoking and risk of clinically overt thyroid disease: a population-based twin case-control study. Arch Intern Med. 2000;160:661–666. doi: 10.1001/archinte.160.5.661. [DOI] [PubMed] [Google Scholar]
- 5.Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf) 2013;79:145–151. doi: 10.1111/cen.12222. [DOI] [PubMed] [Google Scholar]
- 6.Laurberg P, Pedersen KM, Vestergaard H, Sigurdsson G. High incidence of multinodular toxic goitre in the elderly population in a low iodine intake area vs. high incidence of Graves' disease in the young in a high iodine intake area: comparative surveys of thyrotoxicosis epidemiology in East-Jutland Denmark and Iceland. J Intern Med. 1991;229:415–420. doi: 10.1111/j.1365-2796.1991.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 7.Brandt F, Thvilum M, Almind D, Christensen K, Green A, Hegedus L, Brix TH. Morbidity before and after the diagnosis of hyperthyroidism: a nationwide register-based study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066711. e66711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YK, Lin CL, Chang YJ, Cheng FT, Peng CL, Sung FC, Cheng YH, Kao CH. Cancer risk in patients with Graves' disease: a nationwide cohort study. Thyroid. 2013;23:879–884. doi: 10.1089/thy.2012.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shu X, Ji J, Li X, Sundquist J, Sundquist K, Hemminki K. Cancer risk in patients hospitalised for Graves' disease: a population-based cohort study in Sweden. Br J Cancer. 2010;102:1397–1399. doi: 10.1038/sj.bjc.6605624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandt F, Almind D, Christensen K, Green A, Brix TH, Hegedus L. Excess mortality in hyperthyroidism: the influence of preexisting comorbidity and genetic confounding: a Danish nationwide register-based cohort study of twins and singletons. J Clin Endocrinol Metab. 2012;97:4123–4129. doi: 10.1210/jc.2012-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yano Y, Shibuya H, Kitagawa W, Nagahama M, Sugino K, Ito K, Ito K. Recent outcome of Graves' disease patients with papillary thyroid cancer. Eur J Endocrinol. 2007;157:325–329. doi: 10.1530/EJE-07-0136. [DOI] [PubMed] [Google Scholar]
- 12.Hales IB, McElduff A, Crummer P, Clifton-Bligh P, Delbridge L, Hoschl R, Poole A, Reeve TS, Wilmshurst E, Wiseman J. Does Graves' disease or thyrotoxicosis affect the prognosis of thyroid cancer. J Clin Endocrinol Metab. 1992;75:886–889. doi: 10.1210/jcem.75.3.1517381. [DOI] [PubMed] [Google Scholar]
- 13.Belfiore A, Garofalo MR, Giuffrida D, Runello F, Filetti S, Fiumara A, Ippolito O, Vigneri R. Increased aggressiveness of thyroid cancer in patients with Graves' disease. J Clin Endocrinol Metab. 1990;70:830–835. doi: 10.1210/jcem-70-4-830. [DOI] [PubMed] [Google Scholar]
- 14.Pellegriti G, Mannarino C, Russo M, Terranova R, Marturano I, Vigneri R, Belfiore A. Increased mortality in patients with differentiated thyroid cancer associated with Graves' disease. J Clin Endocrinol Metab. 2013;98:1014–1021. doi: 10.1210/jc.2012-2843. [DOI] [PubMed] [Google Scholar]
- 15.Akamizu T, Satoh T, Isozaki O, Suzuki A, Wakino S, Iburi T, Tsuboi K, Monden T, Kouki T, Otani H, Teramukai S, Uehara R, Nakamura Y, Nagai M, Mori M, Japan Thyroid Association Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22:661–679. doi: 10.1089/thy.2011.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Omari AA, Haddad FH, Khushman HM, Malkawi OM. Graves disease and papillary thyroid cancer. An association that can be missed. Saudi Med J. 2005;26:1280–1282. [PubMed] [Google Scholar]
- 17.Suzuki K, Nakagawa O, Aizawa Y. A case of pulmonary metastatic thyroid cancer complicated with Graves' disease. Endocr J. 2001;48:175–179. doi: 10.1507/endocrj.48.175. [DOI] [PubMed] [Google Scholar]
- 18.Hegedus L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003;24:102–132. doi: 10.1210/er.2002-0016. [DOI] [PubMed] [Google Scholar]
- 19.Bonnema SJ, Hegedus L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev. 2012;33:920–980. doi: 10.1210/er.2012-1030. [DOI] [PubMed] [Google Scholar]
- 20.Bennedbaek FN, Gram J, Hegedus L. The transition of subacute thyroiditis to Graves' disease as evidenced by diagnostic imaging. Thyroid. 1996;6:457–459. doi: 10.1089/thy.1996.6.457. [DOI] [PubMed] [Google Scholar]
- 21.Yu HM, Park SH, Lee JM, Park KS. Graves' disease that developed shortly after surgery for thyroid cancer. Endocrinol Metab (Seoul) 2013;28:226–230. doi: 10.3803/EnM.2013.28.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikuchi S, Perrier ND, Ituarte P, Siperstein AE, Duh QY, Clark OH. Latency period of thyroid neoplasia after radiation exposure. Ann Surg. 2004;239:536–543. doi: 10.1097/01.sla.0000118752.34052.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]