Abstract
Immunotherapy has the potential to support and expand the body's own armamentarium of immune effector functions, which have been circumvented during malignant transformation and establishment of cancer and is presently considered to be the most promising treatment option for cancer patients. Recombinant antibody technologies have led to a multitude of novel antibody formats, which are in clinical development and hold great promise for future therapies. Among these formats, bispecific antibodies are extremely versatile due to their high efficacy to recruit and activate anti-tumoral immune effector cells, their excellent safety profile, and the opportunity for use in combination with cellular therapies. This review article summarizes the latest developments in cancer immunotherapy using immuno-engagers for recruiting T cells and NK cells to the tumor site. In addition to antibody formats, malignant cell targets, and immune cell targets, opportunities for combination therapies, including check point inhibitors, cytokines and adoptive transfer of immune cells, will be summarized and discussed.
Keywords: Recombinant antibodies, Immuno-engager, TandAb, Bispecific antibodies, ADCC, CD16, Cellular therapy, Immuno-oncology
Introduction
To protect against pathological alterations such as infections, parasites and cancer, vertebrates have evolved a complex network of innate and adaptive immune effector mechanisms. These comprise soluble factors such as toxins, antibodies, chemokines, and several types of immune cells with discrete functions such as phagocytosis and targeted cytotoxicity. Due to the body's permanent exposure to potentially harmful environmental substances, pathogens, commensal bacteria and malignantly transformed cells, maintenance of its homeostasis represents a challenge, which requires the concerted action of a large variety of different immune effector functions. Moreover, pathogens and malignantly transformed cells can actively outsmart the immune system and escape from immunological selection pressure by adaptation, even during an ongoing immune response. The dynamic interplay of pathogens and malignantly transformed cells with the immune system is referred to as ‘immuno-editing’. The process of immuno-editing can be divided into three phases: elimination, equilibrium, and escape [1]. According to this model, pathogens and malignantly transformed cells are eradicated instantaneously (elimination), coexist for some time with the body's defense armamentarium (equilibrium), and, if eradication cannot be achieved, evade immuno-surveillance (escape), allowing for persistence and, consequently, establishment of a potentially life-threatening disease condition.
Current approaches to treat persistent infections and cancer aim either at restoration of the equilibrium phase, thus transforming the pathological condition into a chronic but stable disease, or, ideally, at restoration of the elimination phase, thereby curing the patient. Immuno-surveillance of parasites, infected tissue, and malignantly transformed cells crucially depends on NK cells and cytotoxic T cells (CTLs), which specifically kill target cells after the polarized release of cytotoxic granules. Therefore, it is not surprising that both cell types are subject to numerous immune evasion strategies which have evolved over time and result in the disarming or sequestration of immune cells from the pathological lesion. Conversely, targeted therapies aim at improved recruitment and activation of cytotoxic NK cells and CTLs to the site of infection or malignant alteration.
NK Cells in Cancer Immuno-Surveillance
Even though recent reports have attributed adaptive features to NK cells, they are a part of innate immunity due to the expression of germline-encoded receptors [2,3]. NK cells are distributed throughout the body, but are enriched in the bone marrow, liver, blood, spleen, and lymph nodes. Phenotypically, NK cells are defined by the presence of the cellular markers CD56 and NKp46 (NCR1, CD335), and the absence of T-cell-specific (CD3 and TCR) and B-cell-specific markers (CD19). Furthermore, NK cells are discriminated on the basis of two principal subsets: CD56bright CD16- NK cells, which represent the predominant species in lymphoid organs and are generally characterized by high cytokine production, and CD56dim CD16+ NK cells, which are the predominant species in peripheral blood and are regarded as highly cytotoxic [3]. This simplistic categorization was challenged by previous reports suggesting a much broader spectrum of phenotypic and functional diversity due to stochastic distribution of receptors to individual NK cells and additional shaping by epigenetic modification, DNA methylation, and environmental influences [4]. Adding another level of plasticity to the NK cell population, it is currently under debate whether CD56bright cells differentiate into CD56dim cells [5] or whether CD56dim CD16+ NK cells develop from a different progenitor than CD56bright CD16- NK cells, T cells, B cells, or myeloid cells [6].
CD56bright NK cells are characterized by the absence of CD16 and KIR expression and their potency to secrete immunomodulatory cytokines. Even though resting peripheral blood CD56bright cells are poorly cytotoxic, they display a tremendous proliferative capacity in response to cytokines such as IL-2. In contrast, CD3- CD56dim NK cells express high levels of CD16A and KIR, are highly cytotoxic and are capable of rapid and strong production of IFN-γ following activation [7].
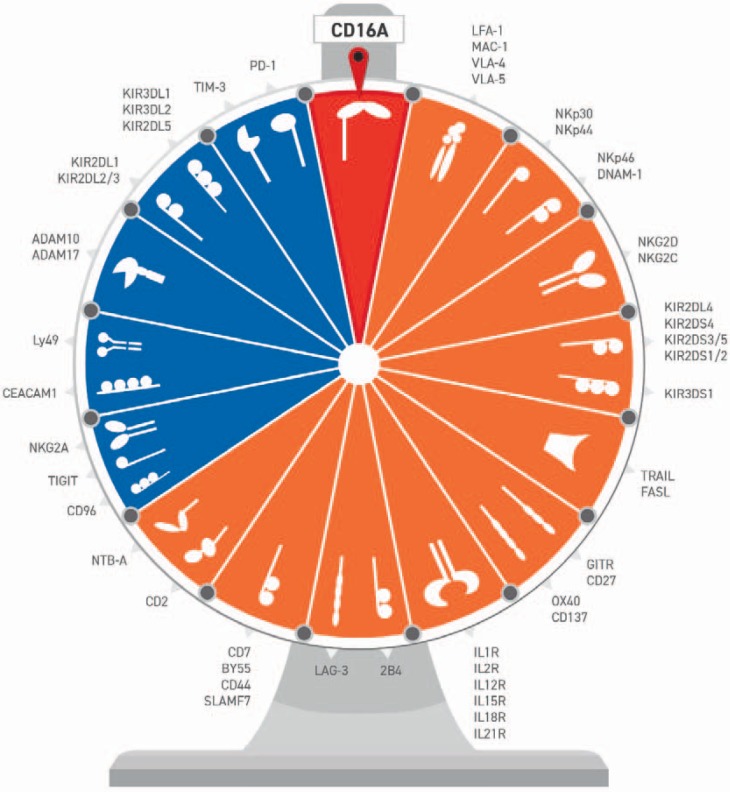
NK cell cytotoxicity is governed by the net result of signaling through inhibitory and activating receptors recognizing self and non-self or altered-self structures on target cells. Among many others (fig. 1), prominent activating NK cell receptors in humans comprise the natural cytotoxicity receptors (NCRs) NKp30, NKp44 and NKp46, NKG2D, DNAM-1 as well as CD16A [8].
Fig. 1.
Major inhibitory and activating NK cell receptors. Inhibitory receptors (blue) are important for self/non-self-discrimination. The net input of individual or several activating receptors (orange/red) triggers cytotoxicity of NK cells towards target cells. NK cell activation can be initiated by loss of inhibitory signaling, e. g. upon downregulation/loss of HLA molecules on the plasma membrane of target cells (‘missing self’), and/or (over)expression of stress-induced ligands on target cells which trigger signaling of activating NK cell receptors (‘induced self’). CD16A = Fc-gamma RIII-alpha; LFA-1 = complement receptor C3 subunit beta; MAC-1 = macrophage integrin VLA-4 = integrin alpha-4; VLA-5 = integrin alpha-5; NKp30 = natural cytotoxicity triggering receptor 3; NKp44 = natural cytotoxicity triggering receptor 2; NKp46 = natural cytotoxicity triggering receptor 1; DNAM-1 = DNAX accessory molecule 1; NKG2D = killer cell lectin-like receptor subfamily K member 1; NKG2C = killer cell lectin-like receptor subfamily C = member 2; KIR2DL = killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail; KIR2DS = killer cell immunoglobulin-like receptor, two domains, short cytoplasmic tail; TRAIL = tumor necrosis factor-related apoptosis-inducing ligand; FASL = Fas ligand; GITR = glucocorticoid-induced TNFR-related protein; CD27 = tumor necrosis factor receptor superfamily member 7; OX40 = tumor necrosis factor receptor superfamily member 4; CD137 = tumor necrosis factor receptor superfamily member 9; ILXR = interleukin X receptor (X indicates number of interleukin); 2B4 = NK cell type I receptor protein 2B4; LAG-3 = lymphocyte-activation gene 3; CD7 = T-cell leukemia antigen; BY55 = natural killer cell receptor BY55; CD44 = GP90 lymphocyte homing/adhesion receptor; SLAMF7 = SLAM family member 7; CD2 = T-cell surface antigen CD2; NTB-A = SLAM family member 6; CD96 = T-cell surface protein tactile; TIGIT = T cell immunoreceptor with Ig and ITIM domains; NKG2A = killer cell lectin-like receptor subfamily C member 1; CEACAM1 = carcinoembryonic antigen-related cell adhesion molecule 1; Ly49 = killer cell lectin-like receptor subfamily A = member 1; ADAM = disintegrin and metalloproteinase domain-containing protein; TIM-3 = T-cell immunoglobulin and mucin domain-containing protein 3; PD-1 = programmed cell death protein 1.
NK cell engagement and activation can be enhanced or triggered by antibodies. Those antibodies can block ligand binding to inhibitory receptors (‘antagonist antibodies’, e.g. KIR) expressed on NK cells or enhance the function of activating receptors (‘agonist antibodies’, e.g. CD137), by mimicking the respective ligand function. This type of engagement does not necessarily lead to a direct activation but can have a supportive effect in a very complex signaling network towards activation and degranulation. Conversely, antibodies directed against CD16A [9] or CD16A and B [10,11] have the potential to directly trigger an activating pathway and degranulation. Another strategy to support and maintain NK cell activity is the inhibition of ADAM17 activity [12,13]. ADAM17 is a metalloproteinase and a potential key player in immuno-oncology [14] that is expressed on NK and cancer cells. Among many other functions, ADAM17 is responsible for the shedding of CD16A. Based on these NK cell characteristics, different NK cell-engaging and/or -activating antibodies are currently in clinical development (table 1). Prominent examples of antagonist antibodies are lirilumab (anti-KIR [15,16]) and monalizumab (anti-NKG2A [17]) and of agonistic antibodies urelumab and utomilumab (anti-CD137 [18,19]).
Table 1.
Overview of antibodies for NK-cell engagement and/or activation
| Targets | Antibody or programs | Format/Isotype | Mode of action | Clinical phase | Company/references |
|---|---|---|---|---|---|
| ADAM-17 | D1 (A12) | hIgG1 | enzyme inhibition | preclinical | [12, 13] |
| CD137 (4-1BB) | Urelumab, Utolimumab | hIgG4, hIgG2 | agonist | I, II | BMS, Pfizer/ [136] |
| CD27 | Varlilumab (CDX-1127) | hIgG1 | agonist | II | Celldex Therapeutics/ [136, 137] |
| CEACAM1 | DIATHIS1 | scFv | antagonist | preclinical | [103, 138, 139] |
| CTLA-4 | Ipilimumab | hIgG1 | agonist | approved | BMS/ [140] |
| GITR | TRX518, INCAGN1876 | hIgG1 | agonist | I–II | Leap Therapeutics, Agenus/ [141, 142] |
| KIR2D-L1,-L2, -L3; | Lirilumab, IPH2101 | hIgG4 | antagonist | I–II | Innate Pharma/ [15] |
| KIR3D-L2 | IPH4102 | hIgG4 | antagonist | I | Innate Pharma/ [143] |
| LAG-3 | TSR-033 | hIgG4 | antagonist | preclinical | Tesaro/ [144, 145] |
| NKG2A | Monalizumab, IPH2201 | hIgG4 | antagonist | I–II | Innate Pharma/ [17] |
| OX40 | MOXR0916, INCAGN1949 | hIgG1 | agonist | II | Genentech, Agenus/ [136, 137, 142] |
| PD-1 | Nivolumab, Prembrolizumab | hIgG4 | antagonist | approved | BMS, Merck & Co./ [146, 147] |
| PD-L1 | Atezolizumab, Avelumab, Durvalumab | hIgG4 | antagonist | approved I–III |
Roche/ [146, 147] Merck KGaA, Pfizer AZ, MedImmune |
| SLAMF7 (CS-1) | Elotuzumab | hIgG1 | agonist | approved | BMS, Abbvie /[148] |
| TIGIT | MTIG7192A, BMS-986207 | hIgG1 | antagonist | I–II | Genentech, BMS/[149, 150]; |
| TIM-3 | TSR-022 | hIgG4 | antagonist | I | Tesaro/ [151] |
Following activation, NK cells release pre-formed granules into the immunological synapse at the NK target interface by exocytosis. These granules contain pore-forming perforin, granzymes, and IFN-γ mRNA, which trigger caspase-dependent and caspase-independent apoptosis of target cells and production of IFN-γ [20,21]. Alternatively, target cell apoptosis can be initiated by Fas ligand or tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in the plasma membrane of NK cells or released from cytotoxic granules which recognize Fas or TRAIL receptors on the target cell. [22]. Importantly, NK cells can be triggered by antibody-dependent cellular cytotoxicity (ADCC) after recognition of antibody-opsonized target cells via CD16A (see below).
Even though NK cells were discovered more than four decades ago, it is only now that their potential in targeted cancer immunotherapy is exploited in clinical settings. Several lines of evidence demonstrate a strong impact of NK cells on cancer immuno-surveillance: i) Based on epidemiological studies, individuals with low cytotoxicity of peripheral NK cells have a higher incidence of cancer [23]. ii) NK cells are subject to numerous tumor immune escape strategies, suggesting that establishment and maintenance of a tumor-promoting microenvironment critically depends on failure of NK cell immuno-surveillance [24,25]. iii) Many tumors depend on blocking tumor infiltration of lymphocytes to limit access for anti-tumor activity of immune cells. Consequently, tumor infiltration of NK cells is a good prognostic marker in several tumor entities including clear renal cell carcinoma, non-small cell lung cancer (NSCLC), and colorectal cancer [26,27,28,29,30]. iv) NK cells mediate cross-talk with the adaptive immune system and therefore have the potential to increase the potency of several immune effector arms [31,32,33,34]. Notably, since NK cell activation depends on the net-activation signal of several activating receptors, NK cell killing is potentially more redundant, making therapy resistance less likely to occur. v) Adoptive transfer of ex vivo expanded NK cells showed high efficacy in early clinical trials [35,36,37]. Furthermore, NK cells display many advantageous features for clinical application. Firstly, NK cells are the first lymphoid cells to repopulate after stem cell transplantation (SCT), reaching normal numbers within 1 month regardless of donor type or patient age [38,39,40]. Therefore, NK cells provide the first opportunity for targeted cellular anti-cancer therapy following SCT. Secondly, NK cells contribute to the graft-versus-tumor/graft-versus-leukemia effect with significantly less or even no graft-versus-host disease (GvHD) compared to allogeneic T cells [36], demonstrating a superior safety profile. Thirdly, NK cells are more easily adaptable to an allogeneic/off-the shelf approach than T cells which require an autologous setting due to otherwise fatal GvHD reactions.
Antibody-Dependent Cellular Cytotoxicity of NK Cells
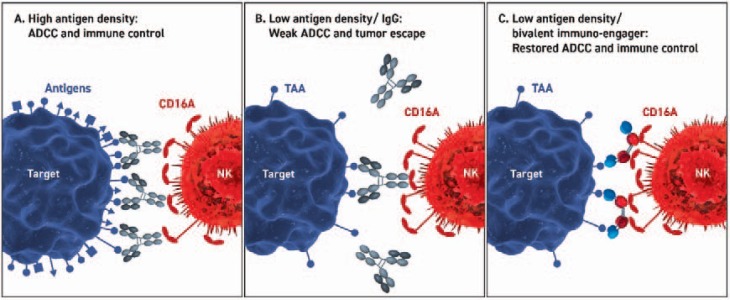
The human body produces antibodies as a defense mechanism against viruses and bacteria. To protect against infectious organisms, a polyclonal IgG response is mounted, whereby multiple antigens and epitopes on infected cells and organisms are recognized. Due to the high density of these antigens, a high degree of opsonization enables multivalent binding of Fc receptors, thus compensating for the low affinities of individual IgGs, and enables strong ADCC and immune control (fig. 2). Conversely, a low density of tumor antigens targeted by monoclonal antibodies, which recognize a single epitope on a single tumor antigen, elicits a low degree of opsonization and, consequently, a limited potential to induce ADCC of immune cells. Interestingly, most monoclonal therapeutic antibodies are blocking antibodies (e.g. cetuximab and panitumumab) and were not developed for immune cell engagement. Therefore, the potential of these molecules to recruit immune cells in a therapeutic setting remains unclear. This limitation could potentially be overcome by bispecific tetravalent immuno-engagers, which mediate robust ADCC and immune control due to multivalent and apparent high affinity binding to CD16A.
Fig. 2.
Scenarios of antibody-mediated immune cell and target cell engagement. Engagement is shown at high antigen density (A) and at low antigen density with IgG-based (B) and recombinant bispecific tetravalent immuno-engagers (C). A At high antigen densities on the target cell (e.g. virus infection, blue) a polyclonal antibody response is initiated leading to saturating opsonization of the target cell and robust ADCC upon Fc binding to CD16A on the NK cell (red). B At limiting antigen densities on the target cell (e.g. malignantly transformed cells), an insufficient degree of opsonization of the target cell by IgG molecules leads to a low level of ADCC and thus tumor immune escape due to few low affinity interactions between the Fc domains and CD16A. C At limiting antigen densities on the target cell (see B) bispecific tetravalent immuno-engagers enable robust ADCC and immune control due to multivalent and apparent high affinity binding to CD16A.
A large variety of mouse and human Fc receptors have been identified which are able to bind the Fc portion of IgG antibodies. The FcγR family comprises four subclasses of receptors - FcγRI, FcγRII, FcγRIII, FcγRIV (only in mouse) - which are expressed on most hematopoietic cells, except for T cells [41,42]. All of these receptors are activating except for the inhibitory FcγRIIb. Most notably, the activating receptor FcγRIIIa (CD16A) is the main receptor facilitating ADCC, whereas all activating FcγRs mediate antibody-dependent cellular phagocytosis (ADCP [43]). Notably, NK cells express only FcγRIIIa but no inhibitory FcγR. For signaling, CD16A associates with homodimeric or heterodimeric ITAM-containing adapter protein complexes of FcεRI-γ chains or CD3ζ chains within the plasma membrane [44]. The affinity of antibodies for CD16A directly correlates with their ability to trigger NK cell activation, thus reducing the antibody dose required for activation [45]. CD16A is the only activating receptor triggering the cytotoxic activity of naïve human NK cells even in the absence of co-stimulatory signals [21,46,47].
The X-ray crystal structure of a human IgG1 Fc fragment-CD16A ectodomain complex has been solved to 3.2 Å resolution [48]. In this complex, the Fc fragment binds asymmetrically to the two Ig domains of CD16A. Residues of the Cγ2 domains and the hinge region of the Fc domain contact with residues in the membrane proximal domain 2 of CD16A and two residues in the linker connecting domain 1 and 2 of CD16A [48].
In addition to NK cells, CD16A has been reported to be expressed on monocytes, macrophages, and γ/δ T cells [49,50]. Two allelic single nucleotide polymorphisms have been identified in human CD16A altering the amino acid in position 158, which is important for interaction with the hinge region of IgGs. The allelic frequencies of the homozygous 158 F/F and the heterozygous 158 V/F alleles are similar within the Caucasian population, ranging between 35 and 52% or 38 and 50%, whereas the homozygous 158 V/V allele is only found in 10–15% [51]. The allelic variant 158V has a higher affinity for IgG than the 158F variant and may therefore confer two advantages in IgG-mediated cancer immunotherapy: i) enhanced potential of NK cells to engage with antibody-opsonized tumor cells and ii) an increased release of granules by NK cells upon encounter with an antibody-opsonized tumor cell [52]. Expression of the allelic variant 158V has been described to correlate with positive clinical response of patients suffering from B-cell lymphoma, leukemia, breast cancer, or colorectal cancer to ADCC-mediating IgG-based therapy [53,54,55,56,57]. Some studies suggest using the performance of patient-derived 158V NK cells in in vitro ADCC assays with antibody-coated tumor cells as a predictive marker for the patients' response to anti-tumor therapy [58]. Notably, IgG antibodies with low or absent fucose side chains are more effective in eliciting ADCC both in vitro and in vivo when compared to conventional IgG antibodies; however, the impact on clinical efficacy has been very limited [59,60].
IgGs can be divided into four subclasses (IgG1, IgG2, IgG3, and IgG4). Among these, IgG1 and IgG3 display the highest affinity to the two known CD16A alleles with affinities in the single digit micromolar range, whereas IgG2 and IgG4 show affinities in the higher micromolar range [61].
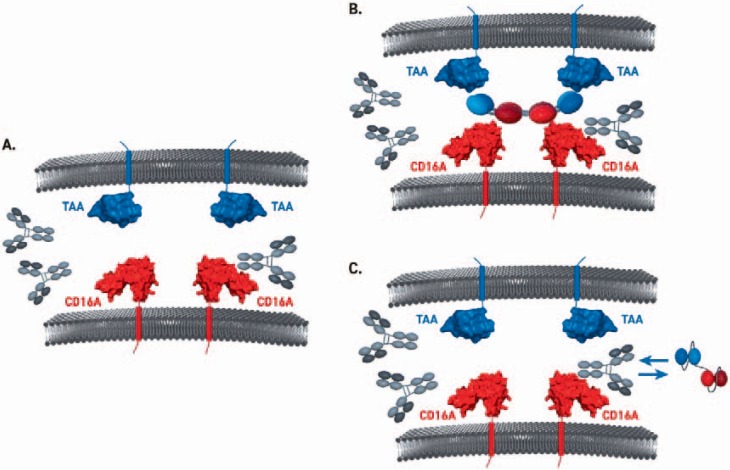
Notably, the binding affinities for IgG1 and IgG3 to CD16A 158V are two-fold higher than for CD16A 158F. Although a number of specific IgGs have shown ADCC-related efficacy in pre-clinical models [62], the observed micromolar affinities of IgGs for CD16A and variations in the binding affinities for different CD16A alleles are unfavorable for therapeutic application of ADCC-inducing antibodies. Moreover, due to high plasma levels of IgG (roughly 10 mg/ml), Fc-based antibody formats face competition for CD16A binding, thereby limiting CD16A occupancy and increasing the required dose of therapeutic antibody. Competition with plasma IgGs might be even more pronounced in disease conditions which are characterized by high levels of plasma IgGs such as multiple myeloma [63]. In the ground state, CD16A on innate immune cells is occupied by polyclonal plasma IgG (fig. 3). This creates a threshold for Fc-based therapeutic antibodies or immuno-engagers which employ the recognition site on CD16A also bound by the Fc proportion of IgG antibodies [64,65], thus limiting therapeutic potential. Importantly, tetravalent bispecific immuno-engagers, which recognize a different epitope on CD16A [9], are virtually unaffected by plasma IgG. This enables high-affinity binding of CD16A and respective tumor antigens leading to strong ADCC and immuno-surveillance.
Fig. 3.
Models for CD16A engagement and IgG competition. A/B In the ground state, CD16A on innate immune cells is occupied by polyclonal plasma IgG. This creates a threshold for Fc-based therapeutic antibodies or immuno-engagers, employing the recognition site on CD16A also bound by the Fc proportion of IgG antibodies, thus limiting therapeutic potential. C Tetravalent bispecific immuno-engagers, which recognize a different epitope on CD16A, are virtually unaffected by plasma IgG. This enables high affinity binding of CD16A and respective tumor antigens leading to strong ADCC and immuno-surveillance.
To increase the binding affinity to CD16A and to allow for CD16A binding independent of patient genotype, a variety of different antibody formats have been developed [66,67,68]. These combine binding domains and, hence, different functionalities in one antibody molecule. These molecules have the potential for greater therapeutic success compared to administration of a mixture of antibodies with the same respective specificities [69,70,71].
Principles of Bispecific Antibody Engineering
Apart from disease settings, PK/PD relationship, patient's treatment status and regimens, the target architecture and biology should ideally be the driver for selection of the appropriate antibody format. However, this information is often unavailable or insufficient for a straightforward, fully rational antibody design approach. Thus, to select the best candidate bearing functional characteristics for optimal therapeutic intervention, extensive molecular engineering of a desired format with multiple iterations combined with high-throughput automated processes for functional screenings are required [72,73]. The simplest bispecific molecules are built from only two antigen binding domains (ABDs) [74] that recognize different antigens. These ABDs are generally composed of Ig heavy and light variable domains of mouse [75] or human origin [76,77,78,79]. The smallest ABD units are single chain antibodies (single chain variable fragment or scFv, [80]) or the larger antigen binding fragments (Fab). Alternatively, heavy chain variable domains of camelids [81] or non-antibody-binding domains [82,83] such as anticalins [84,85] or DARPins [82] serve as building blocks for bispecific molecules. By linking more than two ABDs together, avidity and functionality are substantially improved as demonstrated by the bispecific tetravalent tandem diabodies (TandAb) molecule for CD3- or CD16A-specific immune effector engagement [9,86]. Depending on the linker sequence and linker lengths [87,88,89], the orientation of the VL and VH domain, the number of ABDs and the number of polypeptide chains, the bispecific antibodies can adopt different formats. Prominent examples currently in clinical development include the bispecific tetravalent TandAbs [90], bispecific T-cell engagers (BiTEs [91]), bi- or tri-specific killer cell engagers (BiKEs, TriKEs, [11,92,93], and dual-affinity re-targeting antibodies (DARTs [94]). Adopting a different strategy, two or more ABDs can be genetically fused to a ‘stable’ scaffold allowing further diversity of bispecific molecules. Examples are Fc- or IgG-based bispecific antibody molecules [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97], which have recently entered clinical trials as T-cell recruiters [98].
Bispecific NK- and T-Cell Engagers in Clinical Development
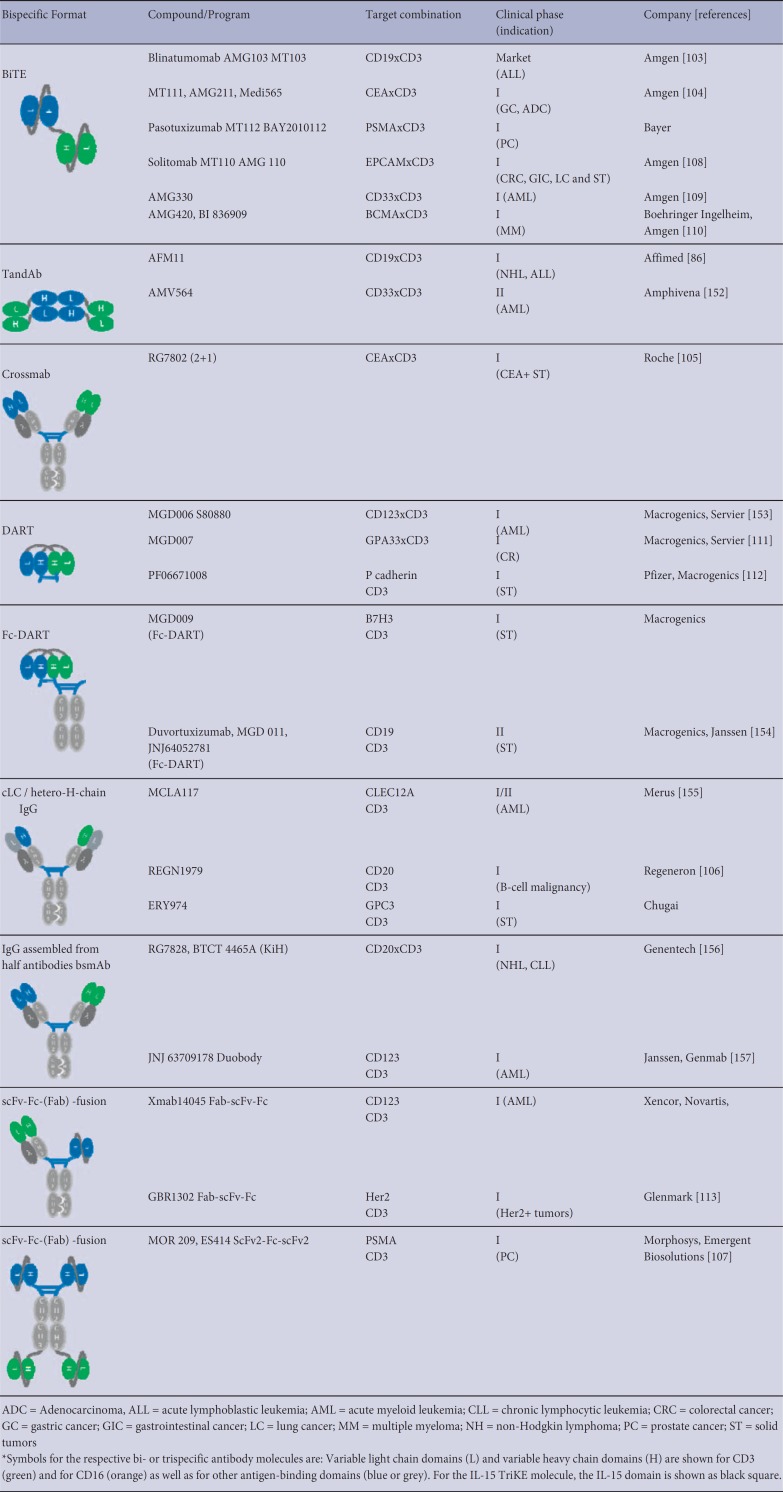
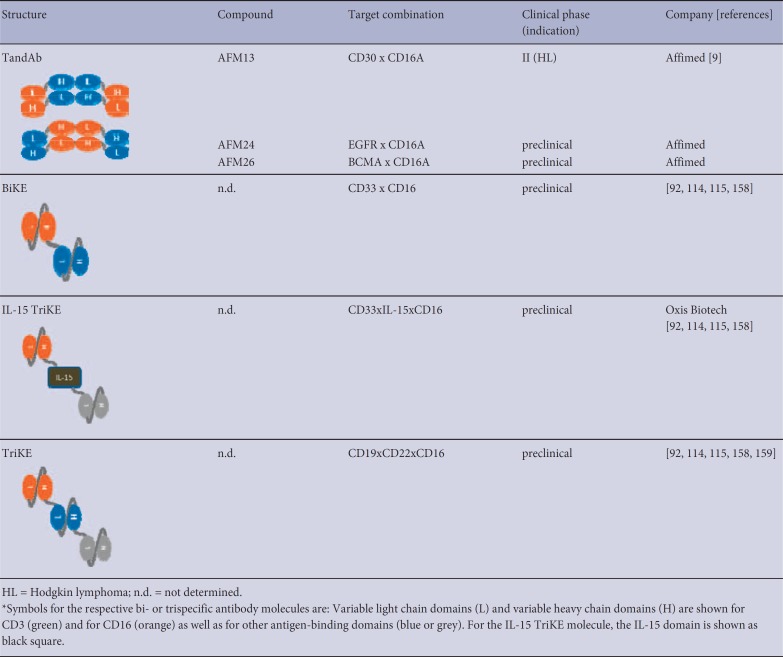
A major subclass of bispecific antibodies recruits cytotoxic lymphocytes such as T and NK cells via ABDs against CD3 and CD16, respectively. For CD3 recruitment, the majority of ABDs derive from the cynomolgus monkey cross-reactive antibody clones SP34 [99] or UCHT1 [100]. In principle, NK cell recruiters are based on two different anti-CD16 domains: clone 3G8 [64,101,102] that is specific for both CD16A and CD16B, and clone LSIV21 [9] that recognizes a CD16A-specific epitope. Examples of bispecific cytotoxic lymphocyte engagers and different designs are the BiTE and TandAb molecules [9,86]. Those bispecific formats are solely composed of ABDs connected to each other via flexible linkers. The formation of the final expression product is largely influenced by its linker lengths. Long linkers between heavy and light variable domains enable intra-molecular pairing of both domains as is the case for both scFv molecules the monovalent BiTE molecule. In contrast, relatively short linkers between heavy and light chain domains allow homo-dimerization of two identical polypeptide chains in a head-to-tail fashion, thereby creating a tetravalent bispecific molecule with two binding sites for each antigen as shown for the TandAb molecule. Other cytotoxic lymphocyte engager molecules that are currently under clinical development are combined with an Fc portion. Table 2 shows the bispecific cytotoxic lymphocyte engagers currently in clinical development. Most formats in development are T-cell engagers comprising an anti-CD3 domain for immune effector cell recruitment in combination with different ABDs directed against tumor-associated antigens such as CD19 [103], CEA [104,105], CD20 [106], PSMA [107], EpCAM [108], CD33 [109], BCMA [110], GPA33 [111], P-cadherin [112], B7-H3, and Her2 [113]. The number of T-cell engagers in clinical development also illustrates the great variety of different bispecific designs, ranging from relatively small molecules to bigger scaffolds employing Fc portions. Even though, to date, T-cell engagers have been the predominant class of bispecific immune cell engagers, they have been challenged by severe side effects in recent clinical trials. Conversely, bispecific NK cell engagers, which only recently have entered the clinic, have shown efficacy and superior safety profiles in clinical [9,86] or late-stage pre-clinical development [10,11,114,115] (table 3).
Table 2.
Overview of bi-specific T-cell engagers in clinical development*
Table 3.
Overview of bi-specific NK-cell engagers in clinical development*
Enhancing NK Cell Efficacy with Cytokines
Cytokines hold great promise to improve the anti-tumor activity of NK cells. Beyond IL-2 and IL-15, which are arguably the best studied cytokines in a therapeutic setting [36], IL-12, IL-18, IL-21, and type I IFNs can be used for in vitro expansion and activation of NK cells before adoptive transfer. The combination of IL-12, IL-15, and IL-18 has recently been reported to induce a population of memory-like NK cells, which showed high efficacy in a phase I clinical trial in acute myeloid leukemia (AML) [35]. Moreover, it has been reported that stimulation of NK cells in vitro with IL-2 or IL-15 for several days resulted in NK cell activation and killing of malignantly transformed cells, which were initially resistant to killing by naïve NK cells [116].
IL-2 and IL-15 bind to the shared dimeric receptor complex comprised of the IL2ββ (15Rβ, CD122) and γc (CD132) chains with only nanomolar affinity thus requiring relatively high concentrations of cytokines for activation. Alternatively, a high-affinity trimeric IL-2 receptor comprised of the IL-2Rαα (CD25) and βγc chain binds to IL-2 with picomolar affinity. CD25 is constitutively expressed by CD56bright NK cells and Tregs; however, its expression requires induction by cytokine stimulation on CD56dim cells [117,118,119,120]. IL-2 has been extensively studied in cancer patients and was demonstrated to be toxic at effective doses. Only few clinical responses were described after monotherapy as high-affinity binding of IL-2 to Tregs results in extensive expansion, sequestration of IL-2, and inhibition of NK cell responses [121]. Interestingly, despite these undesirable side effects, combination therapy of IL-2 with anti-tumor monoclonal antibodies was shown to be safe and demonstrated ADCC-related clinical response in some neuroblastoma and melanoma patients [122].
In contrast, IL-15 does not activate Tregs. IL-15 is trans-presented to the intermediate affinity receptor complex IL-15Rβγc on NK cells by high-affinity receptor chain IL-15α, which is expressed on dendritic cells and macrophages [123]. Ligation induces NK cell differentiation and proliferation as well as cytotoxicity of the NK cells via JAK1, JAK3, and STAT5 signaling [124]. Deletion of IL-15, its receptor, or its downstream signaling proteins results in NK cell lymphopenia [125]. The IL-15α chain can also be secreted and endocytosed, and subsequently trans-present IL-15 to other cells [126,127].
In non-Hodgkin's lymphoma, high concentrations of serum IL-15 following autologous hematopoietic stem cell transplantation are associated with better survival [128]. Moreover, in a phase I clinical trial in patients suffering from metastatic malignancies, daily infusion of recombinant human IL-15 induced NK cell proliferation, albeit, without objective response [129]. Several clinical studies with recombinant human IL-15 are currently ongoing in melanoma, renal cell carcinoma (NCT01021059, NCT01369888), and advanced cancers (NCT01572493, NCT01727076). Another clinical study deals with the support of NK cells after adoptive transfer in leukemia patients (NCT01385423).
Combination of bispecific NK cell-based immuno-engagers with IL-15 stimulation might be a promising therapeutic concept for various malignancies as it combines tumor targeting with improved differentiation, proliferation, and activation of immune cells. Therefore, this combination therapy might be particularly interesting in the context of solid tumors, which inhibit activation and infiltration of NK cells, which is otherwise a positive prognostic marker in renal cell carcinoma, NSCLC, and colorectal cancer [26,27,28,29,30]. Due to the poor bioavailability of IL-15, its short plasma half-life of <40 min, and an unfavorable relation between required dosing and toxicity, the potential of recombinant IL-15 is limited [130]. In contrast, fusion proteins combining IL-15 and its high-affinity receptor subunit IL-15α display reduced renal clearance, increased potency, increased plasma half-life, and increased retention times in lymphoid tissues when compared to IL-15 alone.
Two molecules employing IL-15 and IL-15Rα combinations are currently being tested in in phase I/II mono and combination trials in multiple myeloma, non-Hodgkin's lymphoma, and solid tumors (NCT02452268, NCT02099539, NCT03003728, NCT02523469, NCT03022825, NCT02138734, NCT01946789, NCT02559674, NCT02384954) [119,131].
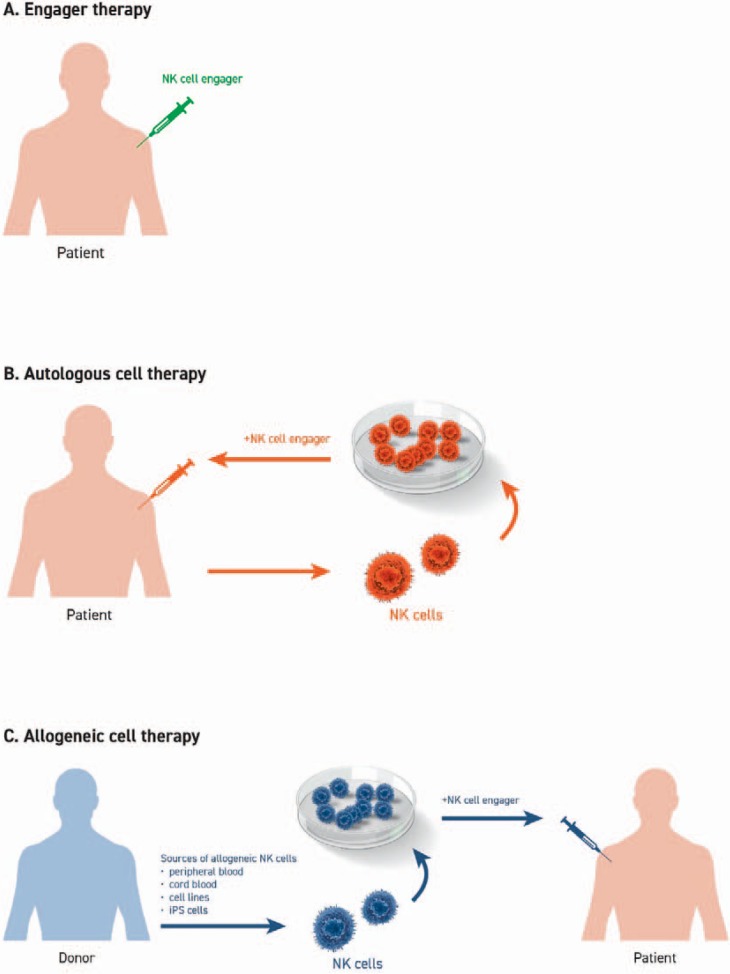
To improve NK cell numbers and anti-tumor activity, a variety of protocols to expand cells ex vivo have been established using NK cells derived from either the patient (autologous setting) or from a healthy donor (allogeneic setting) (fig. 4). The most established source for NK cells is peripheral blood, with current efforts exploring the suitability of NK cells derived from bone marrow, umbilical cord blood, human embryonic stem cells, or induced pluripotent stem cells [132]. Enrichment of NK cells is usually achieved by magnetic depletion of T and B cells with or without additional selection based on CD56.
Fig. 4.
Adoptive NK cells in cancer therapy. A A patient's own NK cells can be stimulated by monotherapy using NK cell engagers to overcome tumor immune evasion and immunosuppression. B Ex vivo expansion and stimulation of autologous NK cells followed by re-infusion in combination with NK cell engagers is a viable therapeutic approach providing increased numbers of activated NK cells. C Alternatively, NK cells can be derived from peripheral blood, cord blood or iPS cells of healthy donors (allogeneic setting) or from immortalized cell lines. After ex vivo stimulation and expansion, NK cells are infused into the patient in combination with NK cell engagers.
Preferably, autologous NK cells are used for cell transfer as these are less likely to promote autoimmune reactions. Indeed, ex vivo cytokine activated and expanded autologous NK cells were shown to be safe; however, no clinical responses in cancer patients with metastatic melanoma, renal cell carcinoma or advance gastrointestinal cancer were seen [133,134,135]. These limitations could potentially be overcome by combination of autologous NK cells (either the patients' endogenous NK cells or reinfused autologous cells after ex vivo stimulation/expansion) in combination with a bispecific immuno-engager and optional IL-15.
Conclusion and Outlook
Immunotherapy is a highly promising approach to cancer therapy. However, efficacy and safety of cellular immunotherapy based on activated or genetically engineered donor-derived immune effector cells need to be improved. Immuno-engagers enable tumor targeting and anti-tumoral cytotoxicity of transferred immune effector cells and therefore represent promising therapeutic avenues. Solid tumors including EGFR+ cancers such as breast cancer, NSCLC and head and neck squamous cell carcinoma, where immunosuppressive tumor microenvironments prevail, could particularly benefit from these therapies. Different variations and combinations of immuno-engager formats, targets on tumor cells, and targets on immune effector cells are currently being investigated. Each of these has unique properties in terms of molecular weight, number of binding sites, specificity, serum half-life, and the potential to mediate cross-talk between different immune cells and must therefore be evaluated for its respective potency. Future and ongoing clinical studies will serve to identify which immuno-engager and which combination product (including cytokines, check point inhibitors and adoptively transferred cells) is best suited for the treatment of a particular cancer and addresses the need for personalized therapy.
Disclosure Statement
Michael Tesar and Joachim Koch are employees of Affimed GmbH.
Acknowledgments
We thank Drs. Adi Hoess, Martin Treder, Erich Rajkovic, Uwe Reusch, Anca Alexandru, and Leonie Kohlhammer for critical reading of the manuscript and helpful discussions. Moreover, we gratefully acknowledge Dr. Anca Alexandru and Leonie Kohlhammer for help in preparing the figures of this article.
References
- 1.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. Of snowflakes and natural killer cell subsets. Nat Biotechnol. 2014;32:140–142. doi: 10.1038/nbt.2810. [DOI] [PubMed] [Google Scholar]
- 5.Chan A, Hong DL, Atzberger A, Kollnberger S, Filer AD, Buckley CD, McMichael A, Enver T, Bowness P. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 6.Wu C, Li B, Lu R, Koelle SJ, Yang Y, Jares A, Krouse AE, Metzger M, Liang F, Lore K, Wu C, Donahue RE, Chen IS, Weissman I, Dunbar CE. Clonal tracking of rhesus macaque hematopoiesis highlights a distinct lineage origin for natural killer cells. Cell Stem Cell. 2014;14:486–499. doi: 10.1016/j.stem.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci U S A. 2011;108:728–732. doi: 10.1073/pnas.1012356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013;34:182–191. doi: 10.1016/j.it.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Reusch U, Burkhardt C, Fucek I, Le Gall F, Le Gall M, Hoffmann K, Knackmuss SH, Kiprijanov S, Little M, Zhukovsky EA. A novel tetravalent bispecific TandAb (CD30/CD16A) efficiently recruits NK cells for the lysis of CD30+ tumor cells. MAbs. 2014;6:728–739. doi: 10.4161/mabs.28591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, Panoskaltsis-Mortari A, Weiner LM, Vallera DA, Miller JS. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012;11:2674–2684. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, Spellman S, Haagenson MD, Lenvik AJ, Litzow MR, Epling-Burnette PK, Blazar BR, Weiner LM, Weisdorf DJ, Vallera DA, Miller JS. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123:3016–3026. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caiazza F, McGowan PM, Mullooly M, Murray A, Synnott N, O'Donovan N, Flanagan L, Tape CJ, Murphy G, Crown J, Duffy MJ. Targeting ADAM-17 with an inhibitory monoclonal antibody has antitumour effects in triple-negative breast cancer cells. Br J Cancer. 2015;112:1895–1903. doi: 10.1038/bjc.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK, Ross JA, Luo X, Weisdorf DJ, Walcheck B, Vallera DA, Miller JS. Targeting Natural killer cells to acute myeloid leukemia in vitro with a CD16 × 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res. 2013;19:3844–3855. doi: 10.1158/1078-0432.CCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowe PR, Corvaia N. ADAM17: a gatekeeper in immune-oncology? Int J Cancer Clin Res. 2016;3:058. [Google Scholar]
- 15.Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, Fuseri N, Bonnafous C, Czerwinski D, Rajapaksa A, Waller E, Ugolini S, Vivier E, Romagne F, Levy R, Blery M, Andre P. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123:678–686. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benson DM, Jr, Cohen AD, Jagannath S, Munshi NC, Spitzer G, Hofmeister CC, Efebera YA, Andre P, Zerbib R, Caligiuri MA. A phase I trial of the anti-KIR antibody IPH2101 and lenalidomide in patients with relapsed/refractory multiple myeloma. Clin Cancer Res. 2015;21:4055–4061. doi: 10.1158/1078-0432.CCR-15-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McWilliams EM, Mele JM, Cheney C, Timmerman EA, Fiazuddin F, Strattan EJ, Mo X, Byrd JC, Muthusamy N, Awan FT. Therapeutic CD94/NKG2A blockade improves natural killer cell dysfunction in chronic lymphocytic leukemia. Oncoimmunology. 2016;5:e1226720. doi: 10.1080/2162402X.2016.1226720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Lee LF, Fisher TS, Jessen B, Elliott M, Evering W, Logronio K, Tu GH, Tsaparikos K, Li X, Wang H, Ying C, Xiong M, VanArsdale T, Lin JC. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res. 2015;3:149–160. doi: 10.1158/2326-6066.CIR-14-0118. [DOI] [PubMed] [Google Scholar]
- 19.Chester C, Ambulkar S, Kohrt HE. 4-1BB agonism: adding the accelerator to cancer immunotherapy. Cancer Immunol Immunother. 2016;65:1243–1248. doi: 10.1007/s00262-016-1829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chua HL, Serov Y, Brahmi Z. Regulation of FasL expression in natural killer cells. Hum Immunol. 2004;65:317–327. doi: 10.1016/j.humimm.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 24.Groth A, Kloss S, von Strandmann EP, Koehl U, Koch J. Mechanisms of tumor and viral immune escape from natural killer cell-mediated surveillance. J Innate Immun. 2011;3:344–354. doi: 10.1159/000327014. [DOI] [PubMed] [Google Scholar]
- 25.Kloess S, Huenecke S, Piechulek D, Esser R, Koch J, Brehm C, Soerensen J, Gardlowski T, Brinkmann A, Bader P, Passweg J, Klingebiel T, Schwabe D, Koehl U. IL-2-activated haploidentical NK cells restore NKG2D-mediated NK-cell cytotoxicity in neuroblastoma patients by scavenging of plasma MICA. Eur J Immunol. 2010;40:3255–3267. doi: 10.1002/eji.201040568. [DOI] [PubMed] [Google Scholar]
- 26.Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, Patsouris ES, Peros G, Horcic M, Tornillo L, Zuber M, Droeser R, Muraro MG, Mengus C, Oertli D, Ferrone S, Terracciano L, Spagnoli GC. Tumor infiltration by FcgammaRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer. 2011;128:2663–2672. doi: 10.1002/ijc.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, Koch M, Weitz J, Kloor M, Zoernig I, Schirmacher P, Brand K, Grabe N, Falk CS. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. 2011;17:678–689. doi: 10.1158/1078-0432.CCR-10-2173. [DOI] [PubMed] [Google Scholar]
- 28.Esendagli G, Bruderek K, Goldmann T, Busche A, Branscheid D, Vollmer E, Brandau S. Malignant and non-malignant lung tissue areas are differentially populated by natural killer cells and regulatory T cells in non-small cell lung cancer. Lung cancer. 2008;59:32–40. doi: 10.1016/j.lungcan.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 29.Del Mar Valenzuela-Membrives M, Perea-Garcia F, Sanchez-Palencia A, Ruiz-Cabello F, Gomez-Morales M, Miranda-Leon MT, Galindo-Angel I, Farez-Vidal ME. Progressive changes in composition of lymphocytes in lung tissues from patients with non-small-cell lung cancer. Oncotarget. 2016;7:71608–71619. doi: 10.18632/oncotarget.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sconocchia G, Spagnoli GC, Del Principe D, Ferrone S, Anselmi M, Wongsena W, Cervelli V, Schultz-Thater E, Wyler S, Carafa V, Moch H, Terracciano L, Tornillo L. Defective infiltration of natural killer cells in MICA/B-positive renal cell carcinoma involves beta(2)-integrin-mediated interaction. Neoplasia. 2009;11:662–671. doi: 10.1593/neo.09296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SC, Srivastava RM, Lopez-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res. 2011;50:248–254. doi: 10.1007/s12026-011-8231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simhadri VL, Hansen HP, Simhadri VR, Reiners KS, Bessler M, Engert A, von Strandmann EP. A novel role for reciprocal CD30-CD30L signaling in the cross-talk between natural killer and dendritic cells. Biol Chem. 2012;393:101–106. doi: 10.1515/BC-2011-213. [DOI] [PubMed] [Google Scholar]
- 33.Ferlazzo G, Morandi B. Cross-talks between natural killer cells and distinct subsets of dendritic cells. Front Immunol. 2014;5:159. doi: 10.3389/fimmu.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sconocchia G, Eppenberger S, Spagnoli GC, Tornillo L, Droeser R, Caratelli S, Ferrelli F, Coppola A, Arriga R, Lauro D, Iezzi G, Terracciano L, Ferrone S. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology. 2014;3:e952197. doi: 10.4161/21624011.2014.952197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S, Neal CC, Yu L, Oh ST, Lee YS, Mulder A, Claas F, Cooper MA, Fehniger TA. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8:357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koehl U, Kalberer C, Spanholtz J, Lee DA, Miller JS, Cooley S, Lowdell M, Uharek L, Klingemann H, Curti A, Leung W, Alici E. Advances in clinical NK cell studies: donor selection, manufacturing and quality control. Oncoimmunology. 2016;5:e1115178. doi: 10.1080/2162402X.2015.1115178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brehm C, Huenecke S, Quaiser A, Esser R, Bremm M, Kloess S, Soerensen J, Kreyenberg H, Seidl C, Becker PS, Muhl H, Klingebiel T, Bader P, Passweg JR, Schwabe D, Koehl U. IL-2 stimulated but not unstimulated NK cells induce selective disappearance of peripheral blood cells: concomitant results to a phase I/II study. PLoS One. 2011;6:e27351. doi: 10.1371/journal.pone.0027351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH, Collins N, Gillio A, George D, Jakubowski A, Heller G, Fazzari M, Kernan N, MacKinnon S, Szabolcs P, Young JW, O'Reilly RJ. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 39.Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, Felicini R, Falcinelli F, Velardi A, Ruggeri L, Aloisi T, Saab JP, Santucci A, Perruccio K, Martelli MP, Mecucci C, Reisner Y, Martelli MF. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 40.Kook H, Goldman F, Padley D, Giller R, Rumelhart S, Holida M, Lee N, Peters C, Comito M, Huling D, Trigg M. Reconstruction of the immune system after unrelated or partially matched T-cell-depleted bone marrow transplantation in children: immunophenotypic analysis and factors affecting the speed of recovery. Blood. 1996;88:1089–1097. [PubMed] [Google Scholar]
- 41.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 42.Kudo K, Imai C, Lorenzini P, Kamiya T, Kono K, Davidoff AM, Chng WJ, Campana D. T lymphocytes expressing a CD16 signaling receptor exert antibody-dependent cancer cell killing. Cancer Res. 2014;74:93–103. doi: 10.1158/0008-5472.CAN-13-1365. [DOI] [PubMed] [Google Scholar]
- 43.Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcγRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Therap. 2008;7:2517–2527. doi: 10.1158/1535-7163.MCT-08-0201. [DOI] [PubMed] [Google Scholar]
- 44.Sun PD. Structure and function of natural-killer-cell receptors. Immunol Res. 2003;27:539–548. doi: 10.1385/IR:27:2-3:539. [DOI] [PubMed] [Google Scholar]
- 45.Bowles JA, Wang SY, Link BK, Allan B, Beuerlein G, Campbell MA, Marquis D, Ondek B, Wooldridge JE, Smith BJ, Breitmeyer JB, Weiner GJ. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108:2648–2654. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114:2657–2666. doi: 10.1182/blood-2009-01-201632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406:267–273. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 49.van Sorge NM, van der Pol WL, van de Winkel JG. FcgammaR polymorphisms: implications for function, disease susceptibility and immunotherapy. Tissue Antigens. 2003;61:189–202. doi: 10.1034/j.1399-0039.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 50.Lanier LL, Ruitenberg JJ, Phillips JH. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988;141:3478–3485. [PubMed] [Google Scholar]
- 51.Lopez-Escamez JA, Saenz-Lopez P, Gazquez I, Moreno A, Gonzalez-Oller C, Soto-Varela A, Santos S, Aran I, Perez-Garrigues H, Ibanez A, Lopez-Nevot MA. Polymorphisms of CD16A and CD32 Fcgamma receptors and circulating immune complexes in Meniere's disease: a case-control study. BMC Med Genet. 2011;12:2. doi: 10.1186/1471-2350-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alderson KL, Sondel PM. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J Biomed Biotechnol. 2011;2011:379123. doi: 10.1155/2011/379123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 54.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, Neri TM, Ardizzoni A. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, Chang HM, Borucka E, Lurje G, Sherrod AE, Iqbal S, Groshen S, Lenz HJ. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 56.Liu D, Tian Y, Sun D, Sun H, Jin Y, Dong M. The FCGR3A polymorphism predicts the response to rituximab-based therapy in patients with non-Hodgkin lymphoma: a meta-analysis. Ann Hematol. 2016;95:1483–1490. doi: 10.1007/s00277-016-2723-x. [DOI] [PubMed] [Google Scholar]
- 57.Angsirisak N, Wittayalertpanya S, Limpanasithikul W, Bunworasate U, Owattanapanich D. Correlation of FcgammaRIIIa polymorphisms to the response of rituximab in Thai patients with diffuse large B-cell lymphoma. Chot Mai Het Thang Phaet. 2015;98:1215–1221. [PubMed] [Google Scholar]
- 58.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 59.Iida S, Kuni-Kamochi R, Mori K, Misaka H, Inoue M, Okazaki A, Shitara K, Satoh M. Two mechanisms of the enhanced antibody-dependent cellular cytotoxicity (ADCC) efficacy of non-fucosylated therapeutic antibodies in human blood. BMC Cancer. 2009;9:58. doi: 10.1186/1471-2407-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 61.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michallet M, Chapuis-Cellier C, Dejoie T, Lombard C, Caillon H, Sobh M, Moreau P, Attal M, Avet-Loiseau H. Heavy+light chain monitoring correlates with clinical outcome in multiple myeloma patients. Leukemia. 2017 doi: 10.1038/leu.2017.209. doi:10.1038/leu.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Palazzo I, Gercel-Taylor C, Kitson J, Weiner LM. Potentiation of tumor lysis by a bispecific antibody that binds to CA19-9 antigen and the Fc gamma receptor expressed by human large granular lymphocytes. Cancer Res. 1990;50:7123–7128. [PubMed] [Google Scholar]
- 65.Garcia de Palazzo I, Holmes M, Gercel-Taylor C, Weiner LM. Antitumor effects of a bispecific antibody targeting CA19-9 antigen and CD16. Cancer Res. 1992;52:5713–5719. [PubMed] [Google Scholar]
- 66.Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017;9:182–212. doi: 10.1080/19420862.2016.1268307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015;67:95–106. doi: 10.1016/j.molimm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Liu H, Saxena A, Sidhu SS, Wu D. Fc Engineering for Developing therapeutic bispecific antibodies and novel scaffolds. Front Immunol. 2017;8:38. doi: 10.3389/fimmu.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robak T, Windyga J, Trelinski J, von Depka Prondzinski M, Giagounidis A, Doyen C, Janssens A, Alvarez-Roman MT, Jarque I, Loscertales J, Rus GP, Hellmann A, Jedrzejczak WW, Kuliczkowski K, Golubovic LM, Celeketic D, Cucuianu A, Gheorghita E, Lazaroiu M, Shpilberg O, Attias D, Karyagina E, Svetlana K, Vilchevska K, Cooper N, Talks K, Prabhu M, Sripada P, Bharadwaj TP, Naested H, Skartved NJ, Frandsen TP, Flensburg MF, Andersen PS, Petersen J. Rozrolimupab, a mixture of 25 recombinant human monoclonal RhD antibodies, in the treatment of primary immune thrombocytopenia. Blood. 2012;120:3670–3676. doi: 10.1182/blood-2012-06-438804. [DOI] [PubMed] [Google Scholar]
- 70.Wilmes GM, Carey KL, Hicks SW, Russell HH, Stevenson JA, Kocjan P, Lutz SR, Quesenberry RS, Shulga-Morskoy SV, Lewis ME, Clark E, Medik V, Cooper AB, Reczek EE. Non-viral adeno-associated virus-based platform for stable expression of antibody combination therapeutics. MAbs. 2014;6:957–967. doi: 10.4161/mabs.28917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobsen HJ, Poulsen TT, Dahlman A, Kjaer I, Koefoed K, Sen JW, Weilguny D, Bjerregaard B, Andersen CR, Horak ID, Pedersen MW, Kragh M, Lantto J. Pan-HER, an antibody mixture simultaneously targeting EGFR, HER2, and HER3, effectively overcomes tumor heterogeneity and plasticity. Clin Cancer Res. 2015;21:4110–4122. doi: 10.1158/1078-0432.CCR-14-3312. [DOI] [PubMed] [Google Scholar]
- 72.Bujak E, Matasci M, Neri D, Wulhfard S. Reformatting of scFv antibodies into the scFv-Fc format and their downstream purification. Methods Mol Biol. 2014;1131:315–334. doi: 10.1007/978-1-62703-992-5_20. [DOI] [PubMed] [Google Scholar]
- 73.Xiao X, Douthwaite JA, Chen Y, Kemp B, Kidd S, Percival-Alwyn J, Smith A, Goode K, Swerdlow B, Lowe D, Wu H, Dall'Acqua WF, Chowdhury PS. A high-throughput platform for population reformatting and mammalian expression of phage display libraries to enable functional screening as full-length IgG. MAbs. 2017;9:996–1006. doi: 10.1080/19420862.2017.1337617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Todorovska A, Roovers RC, Dolezal O, Kortt AA, Hoogenboom HR, Hudson PJ. Design and application of diabodies, triabodies and tetrabodies for cancer targeting. J Immunol Methods. 2001;248:47–66. doi: 10.1016/s0022-1759(00)00342-2. [DOI] [PubMed] [Google Scholar]
- 75.Gram H, Marconi LA, Barbas CF, 3rd, Collet TA, Lerner RA, Kang AS. In vitro selection and affinity maturation of antibodies from a naive combinatorial immunoglobulin library. Proc Natl Acad Sci U S A. 1992;89:3576–3580. doi: 10.1073/pnas.89.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nissim A, Hoogenboom HR, Tomlinson IM, Flynn G, Midgley C, Lane D, Winter G. Antibody fragments from a ‘single pot’ phage display library as immunochemical reagents. EMBO J. 1994;13:692. doi: 10.1002/j.1460-2075.1994.tb06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheets MD, Amersdorfer P, Finnern R, Sargent P, Lindquist E, Schier R, Hemingsen G, Wong C, Gerhart JC, Marks JD. Efficient construction of a large nonimmune phage antibody library: the production of high-affinity human single-chain antibodies to protein antigens. Proc Natl Acad Sci U S A. 1998;95:6157–6162. doi: 10.1073/pnas.95.11.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Knappik A, Ge L, Honegger A, Pack P, Fischer M, Wellnhofer G, Hoess A, Wolle J, Pluckthun A, Virnekas B. Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol. 2000;296:57–86. doi: 10.1006/jmbi.1999.3444. [DOI] [PubMed] [Google Scholar]
- 79.Tiller T, Schuster I, Deppe D, Siegers K, Strohner R, Herrmann T, Berenguer M, Poujol D, Stehle J, Stark Y, Hessling M, Daubert D, Felderer K, Kaden S, Kolln J, Enzelberger M, Urlinger S. A fully synthetic human Fab antibody library based on fixed VH/VL framework pairings with favorable biophysical properties. MAbs. 2013;5:445–470. doi: 10.4161/mabs.24218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ward ES, Gussow D, Griffiths AD, Jones PT, Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989;341:544–546. doi: 10.1038/341544a0. [DOI] [PubMed] [Google Scholar]
- 81.Muyldermans S. Single domain camel antibodies: current status. J Biotechnol. 2001;74:277–302. doi: 10.1016/s1389-0352(01)00021-6. [DOI] [PubMed] [Google Scholar]
- 82.Binz HK, Amstutz P, Pluckthun A. Engineering novel binding proteins from nonimmunoglobulin domains. Nat Biotechnol. 2005;23:1257–1268. doi: 10.1038/nbt1127. [DOI] [PubMed] [Google Scholar]
- 83.Vazquez-Lombardi R, Phan TG, Zimmermann C, Lowe D, Jermutus L, Christ D. Challenges and opportunities for non-antibody scaffold drugs. Drug Discov Today. 2015;20:1271–1283. doi: 10.1016/j.drudis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 84.Hohlbaum AM, Skerra A. Anticalins: the lipocalin family as a novel protein scaffold for the development of next-generation immunotherapies. Expert Rev Clin Immunol. 2007;3:491–501. doi: 10.1586/1744666X.3.4.491. [DOI] [PubMed] [Google Scholar]
- 85.Skerra A. Alternative binding proteins: anticalins - harnessing the structural plasticity of the lipocalin ligand pocket to engineer novel binding activities. FEBS J. 2008;275:2677–2683. doi: 10.1111/j.1742-4658.2008.06439.x. [DOI] [PubMed] [Google Scholar]
- 86.Reusch U, Duell J, Ellwanger K, Herbrecht C, Knackmuss SH, Fucek I, Eser M, McAleese F, Molkenthin V, Gall FL, Topp M, Little M, Zhukovsky EA. A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells. MAbs. 2015;7:584–604. doi: 10.1080/19420862.2015.1029216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hudson PJ, Kortt AA. High avidity scFv multimers; diabodies and triabodies. J Immunol Methods. 1999;231:177–189. doi: 10.1016/s0022-1759(99)00157-x. [DOI] [PubMed] [Google Scholar]
- 88.Le Gall F, Reusch U, Little M, Kipriyanov SM. Effect of linker sequences between the antibody variable domains on the formation, stability and biological activity of a bispecific tandem diabody. Protein Eng Des Sel. 2004;17:357–366. doi: 10.1093/protein/gzh039. [DOI] [PubMed] [Google Scholar]
- 89.Yang F, Wen W, Qin W. Bispecific Antibodies as a development platform for new concepts and treatment strategies. Int J Mol Sci. 2016;18:48. doi: 10.3390/ijms18010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rothe A, Sasse S, Topp MS, Eichenauer DA, Hummel H, Reiners KS, Dietlein M, Kuhnert G, Kessler J, Buerkle C, Ravic M, Knackmuss S, Marschner JP, Pogge von Strandmann E, Borchmann P, Engert A. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:4024–4031. doi: 10.1182/blood-2014-12-614636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective. Pharmacol Ther. 2012;136:334–342. doi: 10.1016/j.pharmthera.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 92.Vallera DA, Felices M, McElmurry RT, McCullar V, Zhou X, Schmohl J, Zhang B, Lenvik A, Panoskaltsis-Mortari A, Verneris MR, Tolar J, Cooley S, Weisdorf D, Blazar BR, Miller JS. IL-15 Trispecific killer engagers (TriKEs) make natural killer cells specific to CD33+ targets while also inducing in vivo expansion, and enhanced function. Clin Cancer Res. 2016;22:3440–3450. doi: 10.1158/1078-0432.CCR-15-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schmohl JU, Gleason MK, Dougherty PR, Miller JS, Vallera DA. Heterodimeric bispecific single chain variable fragments (scFv) killer engagers (BiKEs) enhance NK-cell activity against CD133+ colorectal cancer cells. Target Oncol. 2016;11:353–361. doi: 10.1007/s11523-015-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moore PA, Zhang W, Rainey GJ, Burke S, Li H, Huang L, Gorlatov S, Veri MC, Aggarwal S, Yang Y, Shah K, Jin L, Zhang S, He L, Zhang T, Ciccarone V, Koenig S, Bonvini E, Johnson S. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood. 2011;117:4542–4551. doi: 10.1182/blood-2010-09-306449. [DOI] [PubMed] [Google Scholar]
- 95.Orcutt KD, Ackerman ME, Cieslewicz M, Quiroz E, Slusarczyk AL, Frangioni JV, Wittrup KD. A modular IgG-scFv bispecific antibody topology. Protein Eng Des Sel. 2010;23:221–228. doi: 10.1093/protein/gzp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang JC, Poovassery JS, Bansal P, You S, Manjarres IM, Ober RJ, Ward ES. Engineering multivalent antibodies to target heregulin-induced HER3 signaling in breast cancer cells. MAbs. 2014;6:340–353. doi: 10.4161/mabs.27658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Golay J, Choblet S, Iwaszkiewicz J, Cerutti P, Ozil A, Loisel S, Pugniere M, Ubiali G, Zoete V, Michielin O, Berthou C, Kadouche J, Mach JP, Duonor-Cerutti M. Design and validation of a novel generic platform for the production of tetravalent IgG1-like bispecific antibodies. J Immunol. 2016;196:3199–3211. doi: 10.4049/jimmunol.1501592. [DOI] [PubMed] [Google Scholar]
- 98.Fitzgerald J, Lugovskoy A. Rational engineering of antibody therapeutics targeting multiple oncogene pathways. MAbs. 2011;3:299–309. doi: 10.4161/mabs.3.3.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salmeron A, Sanchez-Madrid F, Ursa MA, Fresno M, Alarcon B. A conformational epitope expressed upon association of CD3-epsilon with either CD3-delta or CD3-gamma is the main target for recognition by anti-CD3 monoclonal antibodies. J Immunol. 1991;147:3047–3052. [PubMed] [Google Scholar]
- 100.Beverley PC, Callard RE. Distinctive functional characteristics of human ‘T’ lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981;11:329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- 101.Arndt MA, Krauss J, Kipriyanov SM, Pfreundschuh M, Little M. A bispecific diabody that mediates natural killer cell cytotoxicity against xenotransplantated human Hodgkin's tumors. Blood. 1999;94:2562–2568. [PubMed] [Google Scholar]
- 102.Li W, Yang H, Dimitrov DS. Identification of high-affinity anti-CD16A allotype-independent human antibody domains. Exp Mol Pathol. 2016;101:281–289. doi: 10.1016/j.yexmp.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, Noppeney R, Hess G, Kallert S, Mackensen A, Rupertus K, Kanz L, Libicher M, Nagorsen D, Zugmaier G, Klinger M, Wolf A, Dorsch B, Quednau BD, Schmidt M, Scheele J, Baeuerle PA, Leo E, Bargou RC. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016;34:1104–1111. doi: 10.1200/JCO.2014.59.1586. [DOI] [PubMed] [Google Scholar]
- 104.Oberst MD, Fuhrmann S, Mulgrew K, Amann M, Cheng L, Lutterbuese P, Richman L, Coats S, Baeuerle PA, Hammond SA. CEA/CD3 bispecific antibody MEDI-565/AMG 211 activation of T cells and subsequent killing of human tumors is independent of mutations commonly found in colorectal adenocarcinomas. MAbs. 2014;6:1571–1584. doi: 10.4161/19420862.2014.975660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bacac M, Klein C, Umana P. CEA TCB: A novel head-to-tail 2:1 T cell bispecific antibody for treatment of CEA-positive solid tumors. Oncoimmunology. 2016;5:e1203498. doi: 10.1080/2162402X.2016.1203498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith EJ, Olson K, Haber LJ, Varghese B, Duramad P, Tustian AD, Oyejide A, Kirshner JR, Canova L, Menon J, Principio J, MacDonald D, Kantrowitz J, Papadopoulos N, Stahl N, Yancopoulos GD, Thurston G, Davis S. A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci Rep. 2015;5:17943. doi: 10.1038/srep17943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hernandez-Hoyos G, Sewell T, Bader R, Bannink J, Chenault RA, Daugherty M, Dasovich M, Fang H, Gottschalk R, Kumer J, Miller RE, Ravikumar P, Wiens J, Algate PA, Bienvenue D, McMahan CJ, Natarajan SK, Gross JA, Blankenship JW. MOR209/ES414, a novel bispecific antibody targeting PSMA for the Treatment of metastatic castration-resistant prostate cancer. Mol Cancer Ther. 2016;15:2155–2165. doi: 10.1158/1535-7163.MCT-15-0242. [DOI] [PubMed] [Google Scholar]
- 108.Ferrari F, Bellone S, Black J, Schwab CL, Lopez S, Cocco E, Bonazzoli E, Predolini F, Menderes G, Litkouhi B, Ratner E, Silasi DA, Azodi M, Schwartz PE, Santin AD. Solitomab, an EpCAM/CD3 bispecific antibody construct (BiTE(R)), is highly active against primary uterine and ovarian carcinosarcoma cell lines in vitro. J Exp Clin Cancer Res. 2015;34:123. doi: 10.1186/s13046-015-0241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krupka C, Kufer P, Kischel R, Zugmaier G, Lichtenegger FS, Kohnke T, Vick B, Jeremias I, Metzeler KH, Altmann T, Schneider S, Fiegl M, Spiekermann K, Bauerle PA, Hiddemann W, Riethmuller G, Subklewe M. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330:reversing a T-cell-induced immune escape mechanism. Leukemia. 2016;30:484–491. doi: 10.1038/leu.2015.214. [DOI] [PubMed] [Google Scholar]
- 110.Hipp S, Tai YT, Blanset D, Deegen P, Wahl J, Thomas O, Rattel B, Adam PJ, Anderson KC, Friedrich M. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia. 2017 doi: 10.1038/leu.2016.388. doi: 10.1038/leu.2016.388. [DOI] [PubMed] [Google Scholar]
- 111.Hurwitz H, Crocenzi T, Lohr J, Bonvini E, Johnson S, Moore P, Wigginton J. A Phase I, first-in-human, open label, dose escalation study of MGD007, a humanized gpA33 × CD3 dual-affinity re-targeting (DART(®)) protein in patients with relapsed/refractory metastatic colorectal carcinoma. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P86. [Google Scholar]
- 112.Root RA, Cao W, Li B, LaPan P, Meade C, Sanford J, Jin M, O'Sullivan C, Cummins E, Lambert M, Sheehan DA, Ma W, Gatto S, Kerns K, Lam K, D'Antona MA, Zhu L, Brady AW, Benard S, King A, He T, Racie L, Arai M, Barrett D, Stochaj W, LaVallie RE, Apgar RJ, Svenson K, Mosyak L, Yang Y, Chichili RG, Liu L, Li H, Burke S, Johnson S, Alderson R, Finlay JW, Lin L, Olland S, Somers W, Bonvini E, Gerber H-P, May C, Moore AP, Tchistiakova L, Bloom L. Development of PF-06671008, a highly potent anti-P-cadherin/anti-CD3 bispecific DART molecule with extended half-life for the treatment of cancer. Antibodies. 2016;5:6. doi: 10.3390/antib5010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moretti P, Skegro D, Ollier R, Wassmann P, Aebischer C, Laurent T, Schmid-Printz M, Giovannini R, Blein S, Bertschinger M. BEAT® the bispecific challenge: a novel and efficient platform for the expression of bispecific IgGs. BMC Proc. 2013;7:O9. [Google Scholar]
- 114.Tay SS, Carol H, Biro M. TriKEs and BiKEs join CARs on the cancer immunotherapy highway. Hum Vaccin Immunother. 2016;12:2790–2796. doi: 10.1080/21645515.2016.1198455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Felices M, Lenvik TR, Davis ZB, Miller JS, Vallera DA. Generation of BiKEs and TriKEs to improve NK cell-mediated targeting of tumor cells. Methods Mol Biol. 2016;1441:333–346. doi: 10.1007/978-1-4939-3684-7_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–2438. [PubMed] [Google Scholar]
- 117.Pillet AH, Bugault F, Theze J, Chakrabarti LA, Rose T. A programmed switch from IL-15- to IL-2-dependent activation in human NK cells. J Immunol. 2009;182:6267–6277. doi: 10.4049/jimmunol.0801933. [DOI] [PubMed] [Google Scholar]
- 118.Pillet AH, Theze J, Rose T. Interleukin (IL)-2 and IL-15 have different effects on human natural killer lymphocytes. Hum Immunol. 2011;72:1013–1017. doi: 10.1016/j.humimm.2011.07.311. [DOI] [PubMed] [Google Scholar]
- 119.Romee R, Leong JW, Fehniger TA. Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. Scientifica. 2014;2014:205796. doi: 10.1155/2014/205796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leong J, Chase J, Romee R, Schneider S, Sullivan R, Fehniger T. Cytokine activation induces CD25 expression and a functional high-affinity IL-2 receptor on CD56dim human NK Cells (P4349) J Immunol. 2013;190((suppl 1)) 183.110. [Google Scholar]
- 121.Waldmann TA. The biology of interleukin-2 and interleukin-15:implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 122.Sondel PM, Hank JA. Combination therapy with interleukin-2 and antitumor monoclonal antibodies. Cancer J Sci Am. 1997;3((suppl 1)):S121–127. [PubMed] [Google Scholar]
- 123.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett. 2010;127:85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chenoweth MJ, Mian MF, Barra NG, Alain T, Sonenberg N, Bramson J, Lichty BD, Richards CD, Ma A, Ashkar AA. IL-15 can signal via IL-15Ralpha, JNK, and NF-kappaB to drive RANTES production by myeloid cells. J Immunol. 2012;188:4149–4157. doi: 10.4049/jimmunol.1101883. [DOI] [PubMed] [Google Scholar]
- 125.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 126.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Rα recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 127.Patidar M, Yadav N, Dalai SK. Interleukin 15:A key cytokine for immunotherapy. Cytokine Growth Factor Rev. 2016;31:49–59. doi: 10.1016/j.cytogfr.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 128.Porrata LF, Inwards DJ, Micallef IN, Johnston PB, Ansell SM, Hogan WJ, Markovic SN. Interleukin-15 affects patient survival through natural killer cell recovery after autologous hematopoietic stem cell transplantation for non-Hodgkin lymphomas. Clin Dev Immunol. 2010;2010:914945. doi: 10.1155/2010/914945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM, Goldman CK, Bryant BR, Decker JM, Chen J, Worthy TYA, Sr WDF, Peer CJ, Sneller MC, Lane HC, Yovandich JL, Creekmore SP, Roederer M, Waldmann TA. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33:74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rhode PR, Egan JO, Xu W, Hong H, Webb GM, Chen X, Liu B, Zhu X, Wen J, You L, Kong L, Edwards AC, Han K, Shi S, Alter S, Sacha JB, Jeng EK, Cai W, Wong HC. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol Res. 2016;4:49–60. doi: 10.1158/2326-6066.CIR-15-0093-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chertova E, Bergamaschi C, Chertov O, Sowder R, Bear J, Roser JD, Beach RK, Lifson JD, Felber BK, Pavlakis GN. Characterization and favorable in vivo properties of heterodimeric soluble IL-15.IL-15Ralpha cytokine compared to IL-15 monomer. J Biol Chem. 2013;288:18093–18103. doi: 10.1074/jbc.M113.461756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Becker PS, Suck G, Nowakowska P, Ullrich E, Seifried E, Bader P, Tonn T, Seidl C. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol Immunother. 2016;65:477–484. doi: 10.1007/s00262-016-1792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Burns LJ, Weisdorf DJ, DeFor TE, Vesole DH, Repka TL, Blazar BR, Burger SR, Panoskaltsis-Mortari A, Keever-Taylor CA, Zhang MJ, Miller JS. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32:177–186. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 134.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17:6287–6297. doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sakamoto N, Ishikawa T, Kokura S, Okayama T, Oka K, Ideno M, Sakai F, Kato A, Tanabe M, Enoki T, Mineno J, Naito Y, Itoh Y, Yoshikawa T. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13:277. doi: 10.1186/s12967-015-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19:1044–1053. doi: 10.1158/1078-0432.CCR-12-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19:997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 138.Hosomi S, Chen Z, Baker K, Chen L, Huang YH, Olszak T, Zeissig S, Wang JH, Mandelboim O, Beauchemin N, Lanier LL, Blumberg RS. CEACAM1 on activated NK cells inhibits NKG2D-mediated cytolytic function and signaling. Eur J Immunol. 2013;43:2473–2483. doi: 10.1002/eji.201242676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dupuis ML, Fiori V, Soriani A, Ricci B, Dominici S, Moricoli D, Ascione A, Santoni A, Magnani M, Cianfriglia M. The human antibody fragment DIATHIS1 specific for CEACAM1 enhances natural killer cell cytotoxicity against melanoma cell lines in vitro. J Immunother. 2015;38:357–370. doi: 10.1097/CJI.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stojanovic A, Fiegler N, Brunner-Weinzierl M, Cerwenka A. CTLA-4 is expressed by activated mouse NK cells and inhibits NK Cell IFN-gamma production in response to mature dendritic cells. J Immunol. 2014;192:4184–4191. doi: 10.4049/jimmunol.1302091. [DOI] [PubMed] [Google Scholar]
- 141.Placke T, Salih HR, Kopp HG. GITR ligand provided by thrombopoietic cells inhibits NK cell antitumor activity. J Immunol. 2012;189:154–160. doi: 10.4049/jimmunol.1103194. [DOI] [PubMed] [Google Scholar]
- 142.Linch SN, McNamara MJ, Redmond WL. OX40 Agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. 2015;5:34. doi: 10.3389/fonc.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Battistella M, Janin A, Jean-Louis F, Collomb C, Leboeuf C, Sicard H, Bonnafous C, Dujardin A, Ram-Wolff C, Kadin ME, Bensussan A, Bagot M, Michel L. KIR3DL2 (CD158k) is a potential therapeutic target in primary cutaneous anaplastic large-cell lymphoma. Br J Dermatol. 2016;175:325–333. doi: 10.1111/bjd.14626. [DOI] [PubMed] [Google Scholar]
- 144.Juno JA, Stalker AT, Waruk JL, Oyugi J, Kimani M, Plummer FA, Kimani J, Fowke KR. Elevated expression of LAG-3, but not PD-1, is associated with impaired iNKT cytokine production during chronic HIV-1 infection and treatment. Retrovirology. 2015;12:17. doi: 10.1186/s12977-015-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Berrien-Elliott MM, Romee R, Fehniger TA. Improving natural killer cell cancer immunotherapy. Curr Opin Organ Transplant. 2015;20:671–680. doi: 10.1097/MOT.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Iraolagoitia XL, Spallanzani RG, Torres NI, Araya RE, Ziblat A, Domaica CI, Sierra JM, Nunez SY, Secchiari F, Gajewski TF, Zwirner NW, Fuertes MB. NK cells restrain spontaneous antitumor CD8+ T cell priming through PD-1/PD-L1 interactions with dendritic cells. J Immunol. 2016;197:953–61. doi: 10.4049/jimmunol.1502291. [DOI] [PubMed] [Google Scholar]
- 147.Giuliani M, Janji B, Berchem G. Activation of NK cells and disruption of PD-L1/PD-1 axis: two different ways for lenalidomide to block myeloma progression. Oncotarget. 2017;8:24031–24044. doi: 10.18632/oncotarget.15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Magen H, Muchtar E. Elotuzumab: the first approved monoclonal antibody for multiple myeloma treatment. Ther Adv Hematol. 2016;7:187–195. doi: 10.1177/2040620716652862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blake SJ, Stannard K, Liu J, Allen S, Yong MC, Mittal D, Aguilera AR, Miles JJ, Lutzky VP, de Andrade LF, Martinet L, Colonna M, Takeda K, Kuhnel F, Gurlevik E, Bernhardt G, Teng MW, Smyth MJ. Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov. 2016;6:446–459. doi: 10.1158/2159-8290.CD-15-0944. [DOI] [PubMed] [Google Scholar]
- 151.da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res. 2014;2:410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Reusch U, Harrington K, Gudgeon C, Fucek I, Ellwanger K, Weichel M, Knackmuss S, Zhukovsky E, Fox JA, Kunkel LA, Guenot J, Walter RB. Characterization of CD33/CD3 tetravalent bispecific tandem diabodies (TandAbs) for the treatment of acute myeloid leukemia. Clin Cancer Res. 2016;22:5829–5838. doi: 10.1158/1078-0432.CCR-16-0350. [DOI] [PubMed] [Google Scholar]
- 153.Al-Hussaini M, Rettig MP, Ritchey JK, Karpova D, Uy GL, Eissenberg LG, Gao F, Eades WC, Bonvini E, Chichili GR, Moore PA, Johnson S, Collins L, DiPersio JF. Targeting CD123 in acute myeloid leukemia using a T-cell-directed dual-affinity retargeting platform. Blood. 2016;127:122–131. doi: 10.1182/blood-2014-05-575704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Walseng E, Nelson CG, Qi J, Nanna AR, Roush WR, Goswami RK, Sinha SC, Burke TR, Jr, Rader C. Chemically programmed bispecific antibodies in diabody format. J Biol Chem. 2016;291:19661–19673. doi: 10.1074/jbc.M116.745588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Van Loo PF, Doornbos R, Dolstra H, Shamsili S, Bakker L. Preclinical evaluation of MCLA117, a CLEC12AxCD3 bispecific antibody efficiently targeting a novel leukemic stem cell associated antigen in AML. Blood. 2015;126:325–325. [Google Scholar]
- 156.Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, Yan X, Clark R, Reyes A, Stefanich E, Mai E, Young J, Johnson C, Huseni M, Wang X, Chen Y, Wang P, Wang H, Dybdal N, Chu YW, Chiorazzi N, Scheer JM, Junttila T, Totpal K, Dennis MS, Ebens AJ. Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med. 2015;7:287ra270. doi: 10.1126/scitranslmed.aaa4802. [DOI] [PubMed] [Google Scholar]
- 157.Gaudet F, Nemeth JF, McDaid R, Li Y, Harman B, Millar H, Teplyakov A, Wheeler J, Luo J, Tam S, Wu S-J, Chen E, Babich A, Elsayed Y, Attar R. Development of a CD123xCD3 bispecific antibody to treat acute myeloid leukemia (AML). In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2016 Apr 16–20; New Orleans, LA. Philadelphia (PA): AACR; Cancer Res. 2016;76((14 suppl)) Abstract 1492. [Google Scholar]
- 158.Miller JS, Felice M, McElmurry R, McCullar V, Zhou X, Tolar J, Schmohl JU, Panoskaltsis-Mortari A, Zhang B, Taras E, Verneris MR, Cooley S, Weisdorf DJ, Blazar BR, Vallera D. Trispecific killer engagers (TriKEs) that contain IL-15 to make NK cells antigen specific and to sustain their persistence and expansion. Blood. 2015;126:232–232. [Google Scholar]
- 159.Schmohl JU, Felices M, Taras E, Miller JS, Vallera DA. Enhanced ADCC and NK cell activation of an anticarcinoma bispecific antibody by genetic insertion of a modified IL-15 cross-linker. Mol Ther. 2016;24:1312–1322. doi: 10.1038/mt.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]