Abstract
Background
Some evidence suggests that chronic kidney disease is a risk factor for lower-extremity peripheral artery disease. We aimed to quantify the independent and joint associations of two measures of chronic kidney disease (estimated glomerular filtration rate [eGFR] and albuminuria) with the incidence of peripheral artery disease.
Methods
In this collaborative meta-analysis of international cohorts included in the Chronic Kidney Disease Prognosis Consortium (baseline measurements obtained between 1972 and 2014) with baseline measurements of eGFR and albuminuria, at least 1000 participants (this criterion not applied to cohorts exclusively enrolling patients with chronic kidney disease), and at least 50 peripheral artery disease events, we analysed adult participants without peripheral artery disease at baseline at the individual patient level with Cox proportional hazards models to quantify associations of creatinine-based eGFR, urine albumin-to-creatinine ratio (ACR), and dipstick proteinuria with the incidence of peripheral artery disease (including hospitalisation with a diagnosis of peripheral artery disease, intermittent claudication, leg revascularisation, and leg amputation). We assessed discrimination improvement through c-statistics.
Findings
We analysed 817 084 individuals without a history of peripheral artery disease at baseline from 21 cohorts. 18 261 cases of peripheral artery disease were recorded during follow-up across cohorts (median follow-up was 7·4 years [IQR 5·7–8·9], range 2·0–15·8 years across cohorts). Both chronic kidney disease measures were independently associated with the incidence of peripheral artery disease. Compared with an eGFR of 95 mL/min per 1·73 m2, adjusted hazard ratios (HRs) for incident study-specific peripheral artery disease was 1·22 (95% CI 1·14–1·30) at an eGFR of 45 mL/min per 1·73 m2 and 2·06 (1·70–2·48) at an eGFR of 15 mL/min per 1·73 m2. Compared with an ACR of 5 mg/g, the adjusted HR for incident study-specific peripheral artery disease was 1·50 (1·41–1·59) at an ACR of 30 mg/g and 2·28 (2·12–2·44) at an ACR of 300 mg/g. The adjusted HR at an ACR of 300 mg/g versus 5 mg/g was 3·68 (95% CI 3·00–4·52) for leg amputation. eGFR and albuminuria contributed multiplicatively (eg, adjusted HR 5·76 [4·90–6·77] for incident peripheral artery disease and 10·61 [5·70–19·77] for amputation in eGFR <30 mL/min per 1·73 m2 plus ACR ≥300 mg/g or dipstick proteinuria 2+ or higher vs eGFR ≥90 mL/min per 1·73 m2 plus ACR <10 mg/g or dipstick proteinuria negative). Both eGFR and ACR significantly improved peripheral artery disease risk discrimination beyond traditional predictors, with a substantial improvement prediction of amputation with ACR (difference in c-statistic 0·058, 95% CI 0·045–0·070). Patterns were consistent across clinical subgroups.
Interpretation
Even mild-to-moderate chronic kidney disease conferred increased risk of incident peripheral artery disease, with a strong association between albuminuria and amputation. Clinical attention should be paid to the development of peripheral artery disease symptoms and signs in people with any stage of chronic kidney disease.
Funding
American Heart Association, US National Kidney Foundation, and NIDDK
Lower-extremity peripheral artery disease (PAD) affects 8–10 million adults in the US1 and more than 200 million adults around the world.2 Its prevalence increased by 24% globally in the last decade.2 PAD increases the risk of adverse clinical outcomes3,4 and impairs lower-extremity function.5 PAD is particularly important for those on hemodialysis, and indeed its incident rate (~400 per 1,000 patient-years) is much higher than that for coronary heart disease and stroke (~100–150 per 1,000 patient-years) in this clinical population.6 Several previous studies have explored the association of mild and moderate stages of chronic kidney disease (CKD) with PAD.7–14 However, most of them were cross-sectional7–10 and/or investigated either of the two kidney measures to define and stage CKD, estimated glomerular filtration rate (eGFR) or albuminuria,9–12 but not both. This kind of limited evidence may have contributed to the lack of inclusion of CKD amongst the risk factors for PAD in the 2016 guidelines on PAD from the American Heart Association (AHA) and the American College of Cardiology (ACC).15 Therefore, we aimed to quantify the independent and joint associations of eGFR and albuminuria with future risk of PAD using data from 817,084 adults within 21 cohorts in the CKD Prognosis Consortium (CKD-PC).16 These rich data allowed us to also evaluate prediction improvement of PAD with these CKD measures and explore several different types of PAD such as leg amputation and revascularization.
Methods
Study Selection
Details of the CKD-PC are described elsewhere.16,17 Briefly, the CKD-PC is an international consortium aiming to provide evidence that can improve prevention and management of CKD and currently consists of over 70 prospective cohorts including participants from 40 countries/regions with data on eGFR, albuminuria, and clinical outcomes. This current study used data from 9 general population cohorts, 8 cohorts of subjects with at high risk of cardiovascular disease (such as diabetes mellitus), and 4 cohorts exclusively enrolling patients with CKD. These prospective studies had data on incident PAD whereas other cohorts in the CKD-PC did not. This study was approved for use of deidentified data by the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health, and the need for informed consent was waived.
Cohorts with baseline measurements of eGFR and albuminuria, at least 1000 participants (not applied to cohorts preferentially enrolling individuals with CKD), and at least 50 PAD events were eligible for inclusion. Transfer of individual participant data or standardized analysis of outputs for meta-analysis took place between July 2015 and January 2017, with baseline measurements during 1972–2014.
Variables at Baseline
GFR was primarily estimated by the CKD-EPI creatinine-based equation,18 since serum creatinine is the most widely used filtration marker in clinical practice.19 However, as a secondary analysis, we explored eGFR based on the CKD-EPI cystatin-c equation in six studies with relevant data, as this has demonstrated a stronger relationship to clinical outcomes than creatinine-based eGFR.20 For albuminuria, as recommended by CKD guidelines,21 we preferred urine albumin-to-creatinine ratio (ACR), but semi-quantitative assessment of proteinuria using a dipstick test was also accepted.16
We defined the following factors in the AHA/ACC Pooled Cohort Equations22 as traditional atherosclerotic risk factors: age, gender, race (blacks vs. non-blacks), smoking status (current vs. former/never), systolic blood pressure, antihypertensive drug use, and diabetes (defined as fasting glucose ≥7.0 mmol/L, non-fasting glucose ≥11.1 mmol/L, hemoglobin A1c ≥6.5%, use of antidiabetic drugs, or self-reported diabetes) and levels of total and high-density lipoprotein cholesterols. A history of other cardiovascular disease (CVD) (coronary heart disease, stroke, and heart failure) was not an exclusion criterion and was treated as a covariate in our study. We took this approach since risk factor profiles are not necessarily the same between PAD and other CVDs. For example, smoking and diabetes are particularly strong predictors of PAD.1 There are also a few unique aspects for PAD evaluation and monitoring (e.g., ankle brachial index and foot examination).2,23 Indeed, a previous risk prediction tool for new development of intermittent claudication from the Framingham Heart Study incorporates a history of coronary heart disease as a predictor.24
PAD outcomes
Given heterogeneous literature regarding how to define incident PAD,11,12,24–26 we investigated the following definitions of PAD: 1. Study-specific PAD (comprehensively defined in each study based on ICD codes or self-report of PAD diagnosis, leg revascularization, leg amputation, intermittent claudication, or repeated ankle-brachial index as available); 2. PAD-related hospitalizations (ICD-9 codes 440.2 [atherosclerosis of native arteries of the extremities] and 440.4 [chronic total occlusion of artery of the extremities] or equivalents in ICD-10); 3. Leg revascularization (ICD-9 codes 38.18 [endarterectomy, lower limb arteries], 39.25 [aorta-iliac-femoral bypass], 39.29 [other peripheral vascular shunt or bypass], 39.50 [angioplasty of other non-coronary vessel] or self-report); and 4. Leg amputation (ICD codes 84.1x [amputation of lower extremity]). Appendix 1 (appendix pp 3–5) details any deviations in definitions for each cohort.
Statistical analyses
Analyses were restricted to subjects aged 18 years or older without a history of PAD at baseline. We excluded any subject with missing values for eGFR, albuminuria, or traditional risk factors at baseline.18 However, we included a few studies that systematically lacked data on some traditional risk factors (details about one or a few missing variables in some cohorts can be found in appendix pp 6–7). All estimates were obtained within each cohort first and then meta-analyzed by a fixed-effect model, with the number of events in each cohort as weights, to have consistent weights between the analysis of risk relationship and risk prediction.18,27 Meta-analyses were performed for analyses with estimates from ≥3 cohorts.
Using Cox proportional hazards models, we first quantified the associations of eGFR and albuminuria with PAD outcomes in the general population and high-risk cohorts after adjusting for each other and traditional risk factors. eGFR and ACR were modeled by linear splines with knots at 30, 45, 60, 75, and 90 ml/min/1.73m2 and 10, 30, and 300 mg/g, respectively. eGFR 95 ml/min/1.73m2 and ACR 5 mg/g were set as reference.18 ACR was log-transformed, as were all continuous traditional risk factors.22,28 We used Zellner’s seemingly unrelated regression29 to evaluate whether the associations of eGFR and ACR with different definitions of PAD were significantly different or not. We also quantified PAD risk by cross-categories of eGFR and albuminuria in the context of the new international CKD staging system.21 For this analysis of cross-categories of CKD measures, as previously done,28,30 we combined ACR <10, 10–29, 30–299, and ≥300 mg/g and dipstick proteinuria, negative (reference), ± (trace), 1+, and ≥2+, respectively. The same categories of dipstick proteinuria was used when general population and high risk cohorts with data on dipstick were explored in other analyses.
Subsequently, we conducted subgroup analyses by age, sex, race, and history of diabetes, hypertension (defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medications), use of statins, and CVDs. Interaction was tested using meta-regression for average coefficients for spline terms weighted on the number of events in each study (for eGFR, only spine terms <90 ml/min/1.73m2 were taken into account). We also separately analyzed the subpopulation with CKD including participants with low eGFR <60 ml/min/1.73m2 or high albuminuria (ACR ≥30 mg/g or dipstick proteinuria ≥1+)17 from the general population and high-risk cohorts and all participants in the four CKD cohorts. For the analysis of the CKD population, eGFR 50 ml/min/1.73m2 and ACR 100 mg/g were set as reference, and dipstick proteinuria was categorized into negative/trace (reference), 1+, 2+, and ≥3+, as done previously.31
Next, we estimated the difference in Harrell’s c-statistics,32 a parameter of risk discrimination accounting for censoring, between prediction models that included or excluded kidney measures (eGFR, albuminuria, or both). To mitigate the methodological advantage for kidney measures having several spline terms, in these prediction analyses, eGFR was modeled with two linear terms with a knot at 60 ml/min/1.73m2, as previously done.18
All models showed good calibration according to visual evaluation of predicted vs. observed risk in the vast majority of cohorts.33 The assessment of heterogeneity was based on the I2 statistic and the χ2 test. Random-effects meta-regression analysis was performed to explore sources of heterogeneity when heterogeneity was high (I2 statistic >75%34). All analyses were performed with Stata/MP 13 (www.stata.com), and a P-value <0.05 was considered statistically significant.
Role of the funding source
The funders had no role in the study design, data collection, analysis, data interpretation, or writing of the report. KM had full access to all analyses and all authors had final responsibility for the decision to submit for publication, informed by discussions with collaborators.
Results
Study Characteristics
A total of 817,084 individuals free of PAD history, with mean age of 54 (SD 12) years, were followed for a median of 7.4 years (Table 1). Overall, 33% were diabetic and 9% had history of CVDs. The prevalence of eGFR <60 ml/min/1.73m2 and high albuminuria were 17% and 5% in general population cohorts, 14% and 20% in high-risk cohorts, and 84% and 66% in CKD cohorts, respectively. During follow-up, 18,261 incident cases of PAD based on study-specific definitions were reported across all cohorts, 8,014 cases of PAD-related hospitalizations from 8 cohorts, 2,549 cases of leg revascularization from 10 cohorts, and 1,754 cases of leg amputation from 7 cohorts.
Table 1.
Demographic characteristics of included cohorts
| study | N | Age, mean (years) |
Female (%) |
Black (%) |
Smoking (%) |
Hypertension (%) |
Diabetes (%) |
Total chol, mean (SD) (mmol/L) |
HDL chol, mean (SD) (mmol/L) |
History of CVD (%) |
eGFR <60 (%) |
ACR ≥30 (%) |
Study- Specific PAD events (n) |
PAD hospital- ization (n) |
PAD revascularization (n) |
PAD amputation (n) |
follow- up, median (IQR) (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General population | |||||||||||||||||
| ARIC | 10256 | 63 (6) | 5668 (55%) | 2251 (22%) | 1481 (14%) | 4770 (47%) | 1656 (16%) | 5.2 (1.0) | 1.3 (0.4) | 1349 (13%) | 616 (6%) | 796 (8%) | 307 | 214 | 170 | 73 | 15.8 [14.2–16.8] |
| BIS | 1553 | 80 (6) | 842 (54%) | 0 (0%) | 76 (5%) | 1419 (91%) | 362 (23%) | 5.6 (1.2) | 1.5 (0.5) | 367 (24%) | 533 (34%) | 359 (23%) | 101 | NA | NA | NA | 4.0 [3.9–4.1] |
| CHS | 2824 | 78 (5) | 1680 (59%) | 459 (16%) | 209 (7%) | 1448 (51%) | 486 (17%) | 5.3 (1.0) | NA | 843 (30%) | 1162 (41%) | 551 (20%) | 135 | NA | NA | NA | 9.9 [5.6–15.0] |
| ESTHER* | 5467 | 62 (7) | 2958 (54%) | 0 (0%) | 797 (15%) | 3281 (60%) | 993 (18%) | 5.7 (1.3) | 1.4 (0.4) | 967 (18%) | 598 (11%) | 608 (11%) | 67 | NA | 67 | NA | 10.7 [5.3–10.9] |
| KHS* | 244763 | 44 (10) | 163356 (67%) | 0 (0%) | 93459 (38%) | 63408 (26%) | 15021 (6%) | 4.9 (0.9) | 1.3 (0.3) | 2706 (1%) | 50608 (21%) | 8790 (4%) | 1695 | 1695 | NA | NA | 12.4 [10.6–14.2] |
| MESA | 6693 | 62 (10) | 3532 (53%) | 1836 (27%) | 871 (13%) | 3005 (45%) | 839 (13%) | 5.0 (0.9) | 1.3 (0.4) | 0 (0%) | 874 (13%) | 638 (10%) | 72 | NA | NA | NA | 8.5 [7.7–8.6] |
| PREVEND | 6481 | 51 (13) | 3449 (53%) | 63 (1%) | 2173 (34%) | 2313 (36%) | 270 (4%) | 5.7 (1.1) | 1.3 (0.4) | 377 (6%) | 172 (3%) | 742 (11%) | 63 | NA | 63 | NA | 12.5 [12.2–12.8] |
| Rancho Bernardo | 1427 | 70 (12) | 857 (60%) | 1 (0%) | 112 (8%) | 719 (50%) | 196 (14%) | 5.5 (1.0) | 1.4 (0.4) | 181 (13%) | 521 (37%) | 203 (14%) | 157 | NA | NA | NA | 13.7 [6.4–18.2] |
| SCREAM_DIP* | 106300 | 51 (14) | 57102 (54%) | 0 (0%) | NA | 71857 (68%) | 19131 (18%) | 5.4 (1.1) | 1.4 (0.4) | 13471 (13%) | 9842 (9%) | 5227 (5%) | 1263 | 1150 | 396 | 216 | 4.3 [2.8–5.7] |
| Total for GP | 385764 | 48 (11) | 239444 (62%) | 4610 (1%) | 99178 (26%) | 152220 (39%) | 38954 (10%) | 5.1 (1.0) | 1.3 (0.3) | 20261 (5%) | 64926 (17%) | 17914 (5%) | 3860 | 3059 | 696 | 289 | 10.1 [8.4–11.7] |
| High-risk population | |||||||||||||||||
| ADVANCE | 10580 | 66 (6) | 4489 (42%) | 35 (0%) | 1586 (15%) | 8732 (83%) | 10580 (100%) | 5.2 (1.2) | 1.3 (0.4) | 2641 (25%) | 1656 (16%) | 3246 (31%) | 665 | NA | NA | NA | 5.0 [4.5–5.0] |
| Geisinger | 40704 | 61 (14) | 20737 (51%) | 1101 (3%) | 6182 (15%) | 30132 (74%) | 31381 (77%) | 4.8 (1.1) | 1.2 (0.4) | 11882 (29%) | 8191 (20%) | 10839 (27%) | 911 | 667 | 387 | 249 | 3.2 [1.7–5.0] |
| GLOMMS-II | 9752 | 66 (14) | 4896 (50%) | 0 (0%) | 85 (1%) | 442 (5%) | 646 (7%) | NA | NA | 774 (8%) | 3622 (37%) | 2650 (27%) | 271 | NA | 198 | 115 | 4.9 [2.7–7.5] |
| Maccabi | 212198 | 58 (14) | 104245 (49%) | 0 (0%) | 4588 (2%) | 122703 (58%) | 80188 (38%) | 4.9 (1.1) | 1.3 (0.3) | 8080 (4%) | 27717 (13%) | 34820 (16%) | 6669 | NA | NA | NA | 5.0 [2.3–8.1] |
| Mt Sinai BioMe | 4086 | 57 (13) | 2481 (61%) | 1395 (34%) | 719 (18%) | 3510 (86%) | 2358 (58%) | 4.8 (1.1) | 1.4 (0.5) | 693 (17%) | 1127 (28%) | 1249 (31%) | 543 | 156 | 66 | NA | 4.1 [2.6–5.3] |
| NZDCS | 25904 | 61 (14) | 12796 (49%) | 67 (0%) | 3755 (14%) | 19171 (80%) | 25904 (100%) | 5.3 (1.1) | 1.3 (0.4) | 4825 (19%) | 6234 (24%) | 1954 (8%) | 2021 | 1592 | 415 | 468 | 9.3 [7.3–10.6] |
| RCAV | 54114 | 63 (12) | 1761 (3%) | 9405 (17%) | NA | 40839 (75%) | 39926 (74%) | 4.6 (1.1) | NA | 7029 (13%) | NA | 11583 (21%) | 1529 | 1313 | 302 | 334 | 7.4 [6.4–8.3] |
| SCREAM ACR | 61321 | 53 (13) | 26795 (44%) | 0 (0%) | NA | 52313 (85%) | 34173 (56%) | 5.1 (1.1) | 1.3 (0.4) | 11426 (19%) | 8217 (13%) | 16196 (26%) | 1283 | 1148 | 392 | 272 | 3.6 [2.3–5.1] |
| SMART | 3181 | 57 (13) | 921 (29%) | 0 (0%) | 922 (29%) | 2075 (66%) | 801 (25%) | 5.1 (1.4) | 1.2 (0.4) | 1808 (57%) | 602 (19%) | 987 (31%) | 105 | NA | 93 | 27 | 5.8 [2.4–9.6] |
| Total for HR | 421840 | 59 (13) | 179121 (42%) | 12003 (3%) | 17837 (4%) | 279917 (66%) | 225957 (54%) | 4.9 (1.1) | 1.3 (0.3) | 49158 (12%) | 57366 (14%) | 83524 (20%) | 13997 | 4876 | 1853 | 1465 | 4.9 [3.3–6.3] |
| CKD population | |||||||||||||||||
| CanPREDDICT | 1468 | 67 (13) | 570 (39%) | 25 (2%) | NA | 1434 (98%) | 704 (48%) | 4.3 (1.3) | 1.2 (0.4) | 440 (30%) | 1463 (100%) | 1079 (74%) | 74 | NA | NA | NA | 4.8 [2.7–5.0] |
| GCKD | 4502 | 60 (12) | 1845 (41%) | 0 (0%) | 695 (15%) | 4325 (96%) | 1498 (33%) | 5.5 (1.3) | 1.4 (0.5) | 1391 (31%) | 3545 (79%) | 2565 (57%) | 130 | NA | NA | NA | 2.0 [2.0–2.1] |
| SRR-CKD | 2527 | 67 (15) | 848 (34%) | 0 (0%) | NA | 2436 (96%) | 876 (35%) | 5.1 (1.5) | NA | 576 (23%) | 2497 (99%) | 2006 (79%) | 127 | 79 | NA | NA | 2.8 [2.0–4.4] |
| Sunnybrook | 983 | 61 (18) | 447 (45%) | 0 (0%) | 93 (9%) | 841 (86%) | 396 (40%) | 4.9 (1.3) | 1.4 (0.5) | 357 (36%) | 489 (50%) | 629 (64%) | 73 | NA | NA | NA | 2.9 [1.7–4.8] |
| Total for CKD | 9480 | 63 (14) | 3710 (39%) | 25 (0%) | 788 (8%) | 9036 (95%) | 3474 (37%) | 5.1 (1.4) | 1.3 (0.4) | 2764 (29%) | 7994 (84%) | 6279 (66%) | 404 | 79 | 0 | 0 | 2.8 [2.0–3.4] |
| All cohorts | 817084 | 54 (12) | 422275 (52%) | 16638 (2%) | 117803 (14%) | 441173 (54%) | 268385 (33%) | 5.0 (1.0) | 1.3 (0.3) | 72183 (9%) | 130286 (16%) | 107717 (13%) | 18261 | 8014 | 2549 | 1754 | 7.4 [5.7–8.9] |
Data are n, n (%), mean (SD), or median (IQR). For definitions of study acronyms and references, see online appendix 3. HDL: high-density lipoprotein, CVD: cardiovascular disease, eGFR: estimated glomerular filtration rate, ACR: urine albumin-to-creatinine ratio, PAD: peripheral artery disease.
Studies with dipstick proteinuria
Independent Associations of eGFR and Albuminuria with Incident PAD Outcomes
The adjusted risk of incident PAD was largely constant above eGFR 60 ml/min/1.73m2 and steadily increased below eGFR 60 ml/min/1.73m2, with a similar risk gradient across the four definitions of PAD (Figure 1A–1D). Compared to eGFR 95 ml/min/1.73m2, the hazard ratio (HR) of incident study-specific PAD was 1.22 (95% CI, 1.14–1.30) at eGFR 45 ml/min/1.73m2, 1.68 (1.52–1.86) at eGFR 30 ml/min/1.73m2, and 2.06 (1.70–2.48) at eGFR 15 ml/min/1.73m2 (Figure 1A). The risk gradient was slightly steeper for eGFR based on cystatin than when based on serum creatinine below <90 ml/min/1.73m2 (appendix p 11), although we were able to only meta-analyze study-specific PAD due to limited availability of cystatin C.
Figure 1.
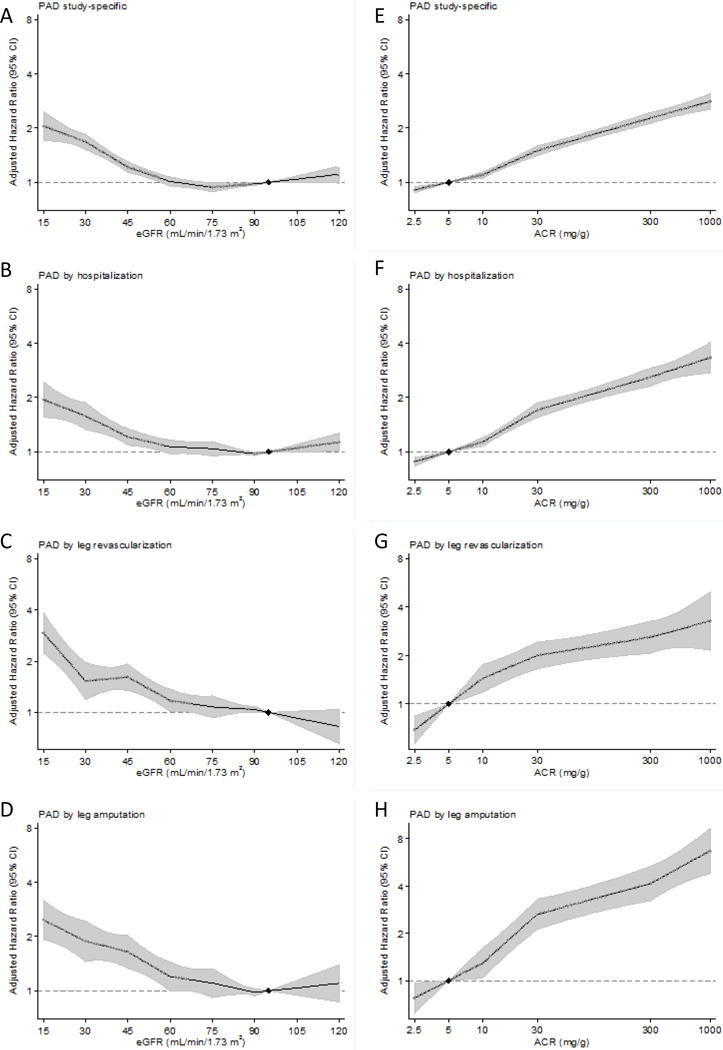
Adjusted hazard ratios and 95% confidence intervals (shaded areas) for each definition of peripheral artery disease according to eGFR (panels A-D) and ACR (panels E-H). The reference value is eGFR 95 mL/min/1.73m2 and ACR 5 mg/g (diamonds). Adjusted for age, sex, race or ethnic origin, smoking, systolic blood pressure, antihypertensive drugs, diabetes, total and HDL cholesterol concentrations, and albuminuria (ACR or dipstick) or eGFR, as appropriate. Panels A-D included cohorts with dipstick proteinuria, and panels E-H were based on cohorts with ACR data.
The associations of ACR with PAD outcomes were generally linear on the log-log scale (Figure 1E–1H), with significantly increased risk even within the range below the current clinical threshold of abnormality (<30 mg/g). Compared to ACR 5 mg/g, the HR of incident study-specific PAD was 1.10 (95% CI, 1.06–1.14) at ACR 10 mg/g, 1.50 (1.41–1.59) at ACR 30 mg/g, and 2.28 (2.12–2.44) at ACR 300 mg/g (Figure 1E). The risk relationship appeared largely similar for study-specific PAD, PAD-related hospitalizations (Figure 1F), and leg revascularization (Figure 1G) but was steepest for leg amputation (Figure 1H). For example, the adjusted HR at ACR 300 mg/g vs. 5 mg/g was 3.68 (95% CI 3.00–4.52) for leg amputation and ~2.5 for the other three outcomes. Moreover, the adjusted HR of leg amputation for log-ACR as a linear term was significantly greater than that of study-specific PAD (p <0.001 by the seemingly unrelated regressions).
Although qualitatively consistent associations were seen in most cohorts, we observed high heterogeneity (I2 statistic >75%) for HR at eGFR 45 vs. 95 ml/min/1.73m2 for study-specific PAD and PAD-related hospitalization (appendix p 12). However, in the meta-regression analyses, none of the covariates appeared to explain the difference in HRs across studies (appendix p 27). HR at ACR 30 vs. 5 mg/g did not demonstrate high heterogeneity in any PAD outcomes (appendix p 13). Regarding subgroups, although statistically significant interactions were observed in some combinations of PAD definitions and subgroups (appendix pp 14–20), CKD measures were generally associated with increased risk of incident PAD in every subgroup tested. Similar patterns were seen when we analyzed CKD population (appendix p 21).
Joint Associations of eGFR and Albuminuria with Incident PAD Outcomes
We confirmed multiplicative contributions of eGFR and albuminuria to increased PAD risk by modeling their cross-categories in the general/high-risk cohorts including ones with dipstick proteinuria (Figure 2). Regardless of PAD definition, the highest risk was observed in the category of severely reduced eGFR <30 ml/min/1.73m2 plus severely elevated ACR ≥300 mg/g, with HRs of ~6 to 11 compared to the reference category of eGFR ≥90 ml/min/1.73m2 plus ACR <10 mg/g or negative dipstick proteinuria. The categories with mild to moderate abnormality of both eGFR (30–59 ml/min/1.73m2) and ACR (30–299 mg/g) showed 2.1–4.4 times higher risk of PAD outcomes. Lower eGFR and higher ACR were associated with increased risk of PAD even when the other CKD measure was normal (e.g., eGFR 30–59 ml/min/1.73m2 showed a HR of 1.2–2.4 even when ACR <10 mg/g; and ACR 30–299 mg/g showed a HR of 1.8–2.2 even when eGFR ≥90 ml/min/1.73m2). Generally similar patterns were found when we analyzed CKD population (appendix p 22).
Figure 2.
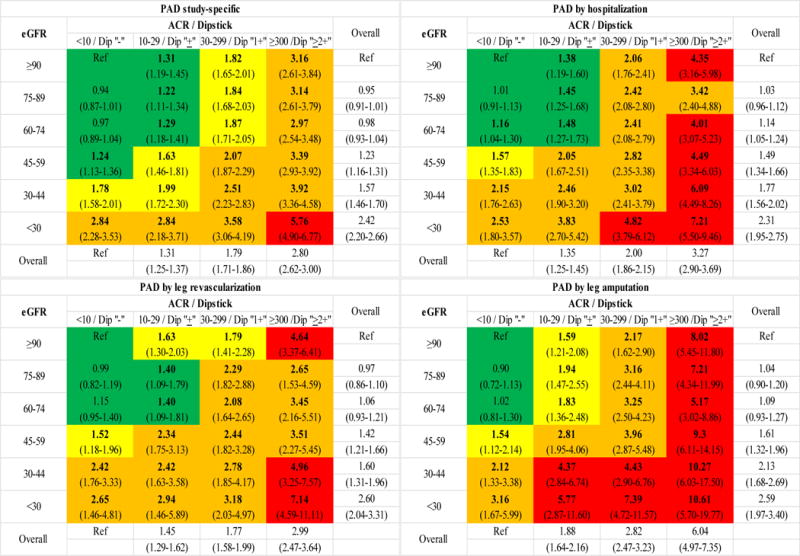
Categorical analysis of peripheral artery disease outcome definitions with eGFR and ACR in the combined general population and high-risk cohorts. Panels show adjusted hazard ratios derived from categorical analysis of the general population and high-risk cohorts. Dipstick −, ±, 1+, and ≥2+ were combined with ACR categories, as appropriate. Color coding is based on following cutoffs: green indicating <1.5; yellow 1.5 − <2; orange 2−<4; and red ≥4. Bold indicates statistical significance.
Improvement in Risk Prediction of PAD with Kidney Measures
C-statistics based on traditional risk factors ranged from 0.750 to 0.772 across the four PAD outcomes in the general and high-risk cohorts with ACR data (Figure 3). The addition of CKD measures significantly improved PAD risk discrimination beyond traditional risk factors. For all PAD outcomes, the improvement in risk was more evident with ACR than with eGFR (e.g., Δc-statistic: 0.018 [95% CI, 0.015–0.020] vs. 0.010 [0.008–0.011] for study-specific PAD). The improvement was particularly evident for leg amputation when adding ACR, with Δc-statistic 0.058 (95% CI 0.045–0.070). We saw some incremental improvements in c-statistics when eGFR and ACR were added simultaneously. The greater risk improvement with ACR over eGFR was also seen when cystatin C was taken as the filtration marker rather than serum creatinine (appendix p 23).
Figure 3.
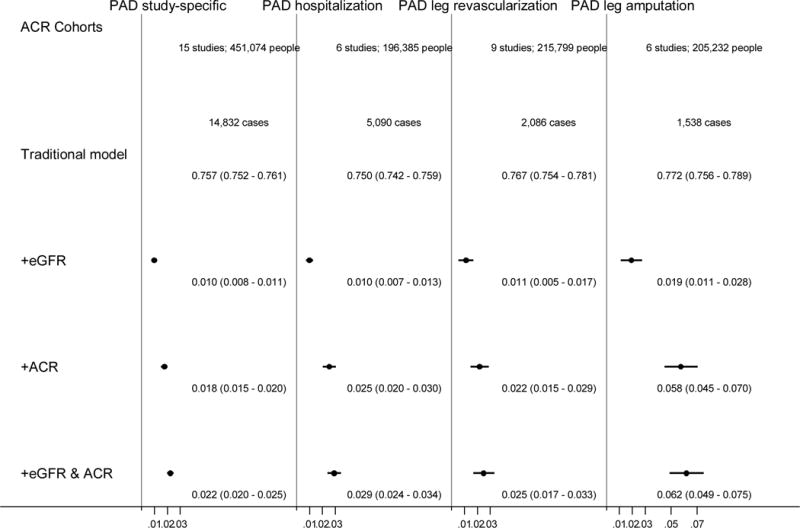
Difference in C statistics and 95% CIs for each definition of peripheral artery disease after addition of kidney measures to traditional models (age, sex, race, smoking status, systolic blood pressure, antihypertensive drug use, and diabetes, levels of total and high-density lipoprotein cholesterols, and history of cardiovascular disease) in the combined general population and high-risk cohorts.
To compare the contributions of the kidney measures and traditional risk factors to PAD risk, we added each of them in turn to demographic predictors (age, gender, and race) (Figure 4). Of the traditional risk factors, diabetes and a history of other CVDs were consistently the strongest predictors. Of note, ACR consistently improved the risk prediction more than these two potent predictors regardless of PAD outcomes. The contribution of eGFR to risk prediction of PAD was similar to or slightly greater than traditional risk factors other than diabetes and history of CVD. The risk discrimination improvement of PAD was confirmed with dipstick but not as much as ACR data (appendix pp 24–25). When we investigated CKD population, the pattern for the contributions of eGFR, ACR, and traditional risk factors to PAD risk prediction was largely similar (appendix p 26), with ACR as one of the most potent predictors.
Figure 4.
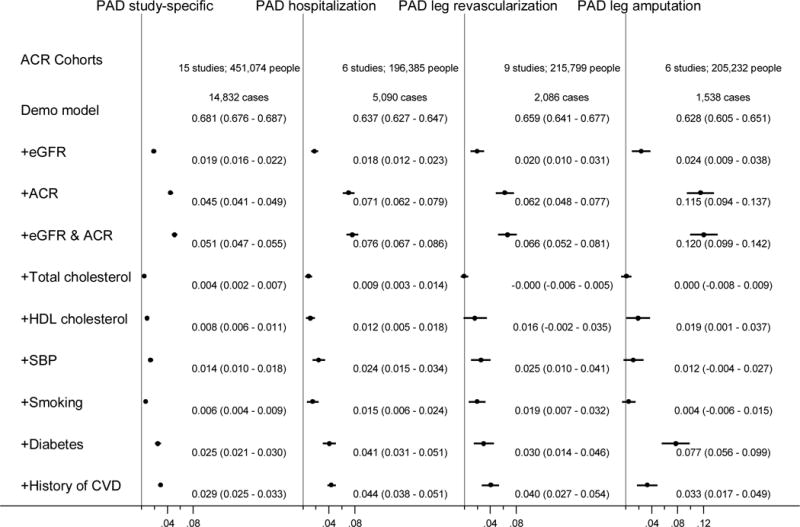
Difference in C statistics and 95% CIs for each definition of peripheral artery disease after addition of kidney measures and traditional risk factors to the demographic model (age, sex, and race) in the combined general population and high-risk cohorts.
Discussion
This international collaborative meta-analysis of individual level data in ~0.8 million individuals free of PAD at baseline demonstrates that both eGFR and ACR were independently associated with future risk of PAD. Even mild to moderate CKD conferred 1.5 to 4 times higher risk of PAD beyond traditional risk factors. For ACR, we observed risk gradient even within the range currently considered normal or mildly elevated (i.e., <30 mg/g).21 The associations were largely consistent across different cohorts as well as key demographic and clinical subgroups such as those with vs. without diabetes or hypertension. Reflecting their strong associations, both kidney measures improved the prediction of PAD risk beyond traditional risk factors, with more evident improvements with ACR than with eGFR. It is noteworthy that the contribution of these kidney measures (particularly ACR) to PAD risk prediction was greater than or similar to any modifiable traditional risk factors including diabetes and history of CVDs. Of interest, ACR substantially improved the prediction of leg amputation.
Although most previous studies have not analyzed the CKD-PAD association longitudinally with both eGFR and albuminuria,7–12 two previous investigations by Bello et al.13 and Garimella et al.14 have done so. However, the former included individuals with a history of PAD at baseline and used a wide definition of PAD including atherosclerotic events beyond lower-extremity such as aortic aneurysm and renal artery stenosis.13 The latter used decline in ABI below ≤0.9 as an outcome variable.14 Therefore, our study expanded to clinical lower-extremity PAD including leg amputation. Other unique aspects of our study include meta-analysis of individual level data (mostly unpublished data), a collaborative investigation of international cohorts, extensive subgroup analyses, and a sophisticated evaluation of c-statistics.
Overall, our results suggest important pathophysiological contributions of CKD to the development of PAD above and beyond traditional risk factors, although the current study is not designed to elucidate mechanisms. Nonetheless, it is worth emphasizing that both CKD measures contributed to PAD risk even among those without diabetes or hypertension, suggesting that eGFR and albuminuria are not merely end-organ damage markers of these traditional atherosclerotic risk factors. Indeed, there are several plausible mechanisms linking CKD to PAD, which include, but are not limited to, activation of renin-angiotensin system, oxidative stress, inflammation, hypercoagulability, abnormal calcium-phosphate metabolism, elevation of lipoprotein(a), and accumulation of uremic toxins.35 In addition, albuminuria is linked to endothelial dysfunction and/or microvascular damage.36 This aspect may explain the particularly strong contribution of albuminuria to the risk of leg amputation. The development of critical limb ischemia as a severe form of PAD has been suggested to be due to a compromised microcirculation resulting in an impaired collateral formation and wound healing.37,38
It is noteworthy that higher ACR was associated with incident PAD even within the range currently considered normal or mildly elevated (i.e., <30 mg/g).21 This pattern was seen also for other cardiovascular outcomes (e.g., cardiovascular mortality, coronary heart disease, and heart failure),18,28 making some experts propose a lower threshold of “elevated” albuminuria.39 Decisions on thresholds for albuminuria should involve comprehensive consideration about the distribution of a relevant biomarker in the target population, the need of age- or sex-specific thresholds, contribution to clinical outcomes, and cost-effectiveness of clinical management triggered by identifying “abnormal” values of that biomarker.28,40–42 In terms of distribution, 19.1% of participants in our general population cohorts had ACR <10 mg/g. Nonetheless, it seems worth paying attention to any future evidence informing this important issue, particularly cost-effectiveness of any interventions targeting mildly elevated ACR below 30 mg/g.
The strong association between CKD and PAD may not be surprising since CKD is sometimes regarded as an equivalent atherosclerotic disease in terms of prognosis,43 but our study has clinical implications since there are unique aspects to the diagnosis and management of PAD. Although the AHA/ACC 2016 Guideline on PAD does not specify CKD as a risk factor of PAD,15 our results indicate that individuals with CKD even at mild to moderate stages may warrant clinical attention to leg signs and symptoms of PAD. Annual foot care is currently recommended in patients with diabetes,23 but adherence to this recommendation is only ~30%.44 Thus, as the first step to improve this low adherence, those with both diabetes and CKD (particularly when albuminuria is present) may be a reasonable target to strongly encourage regular foot care. From the practical point of view, it is important that the assessment of kidney function and albuminuria is already recommended in patients with diabetes as well as in those with hypertension.21,23,45 Thus, in these clinical populations, the CKD measures should be readily available to classify the risk of PAD. Moreover, a few groups have proposed prediction models for PAD risk for the general population,24,33 but none of those take into consideration CKD measures. In this context, it is important that CKD measures improved PAD prediction even among individuals without diabetes or hypertension in our study.
Although this is the most comprehensive study yet conducted for the prospective association of CKD with incident PAD, the results should be interpreted with appropriate caution. As mentioned, the definitions of PAD outcomes varied across cohorts. In addition, some definitions (e.g., clinical diagnosis and hospitalization for PAD included as a part of study-specific PAD in several studies) might be prone to ascertainment bias, particularly among advanced CKD. Nonetheless, it is important that the results were consistent across different PAD outcomes including a harder outcome of leg amputation. Similarly, the methods used to assess creatinine, albuminuria, and traditional risk factors were not necessarily consistent across cohorts, although we standardized their definitions as much as possible (appendix pp 6–7). Our study population predominantly consisted of whites and blacks, and thus confirmatory investigation is needed for other racial/ethnic groups. Also, as any other observational studies, residual confounding due to unevaluated potential confounders (e.g., physical activity) could have occurred.
In conclusion, even mild to moderate CKD conferred ~1.5–4 times higher risk of incident PAD beyond and above traditional atherosclerotic risk factors. The albuminuria-amputation relationship was remarkably strong. Our results suggest that clinical attention should be paid to the development of leg symptoms and clinical signs of PAD in persons with any stages of CKD.
Panel: Research in context
Evidence before this study
Lower-extremity peripheral artery disease (PAD) is an important complication for patients on hemodialysis, and indeed its incident rate is much higher than that for coronary heart disease and stroke in this clinical population. For less severe stages of chronic kidney disease (CKD), several previous studies have explored the risk for PAD, but most of them were cross-sectional and/or investigated either of the two kidney measures to define and stage CKD, estimated glomerular filtration rate (eGFR) or albuminuria, but not both. This kind of limited evidence may have contributed to the lack of inclusion of CKD amongst the risk factors for PAD in the 2016 guidelines on PAD from the American Heart Association and the American College of Cardiology.
Added value of this study
This individual-level data meta-analysis, with 18,261 incident PAD cases from 0.8 million participants from 21 cohorts, examined the prospective and independent associations of eGFR and albuminuria with future risk of peripheral artery disease (PAD). We observed that both reduced eGFR and albuminuria were independently associated with future risk of PAD. Even mild to moderate CKD conferred 1.5 to 4 times higher risk of PAD beyond traditional risk factors. Accordingly, both kidney measures improved the prediction of PAD risk beyond traditional risk factors, with more evident improvements with albuminuria than with eGFR. Of interest, albuminuria was particularly strongly associated with the risk of leg amputation and substantially improved its risk prediction.
Implications of all the available evidence
Our results indicate that individuals with CKD even at mild to moderate stages may warrant clinical attention to leg signs and symptoms of PAD. Annual foot care is currently recommended in patients with diabetes, but adherence to this recommendation is low. Thus, as the first step to improve this low adherence, those with both diabetes and CKD (particularly when albuminuria is present) may be a reasonable target to strongly encourage regular foot care. From the practical point of view, it is important that the assessment of kidney function and albuminuria is already recommended in patients with diabetes as well as in those with hypertension. Thus, in these clinical populations, the CKD measures should be readily available to classify the risk of PAD.
Supplementary Material
Acknowledgments
Funding: The CKD-PC Data Coordinating Center is funded in part by a program grant from the US National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100446-01). This specific study was supported by a grant from the American Heart Association (#14CRP20380886). A variety of sources have supported enrollment and data collection including laboratory measurements, and follow-up in the collaborating cohorts of the CKD-PC. These funding sources include government agencies such as national institutes of health and medical research councils as well as foundations and industry sponsors listed on appendix pp 9–10. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Contributors:
KM, JC, RTG, CPK, VS, and MW conceived of the study concept and design. KM, SHB, JC and the CKD-PC investigators/collaborators listed below acquired the data. KM and the Data Coordinating Center members listed below analyzed the data. All authors took part in the interpretation of the data. KM, SHB, JC, and FK drafted the manuscript, and all authors provided critical revisions of the manuscript for important intellectual content. All collaborators shared data and were given the opportunity to comment on the manuscript. JC obtained funding for CKD-PC and individual cohort and collaborator support is listed on appendix pp 9–10.
CKD-PC investigators/collaborators (study acronyms/abbreviations are listed on appendix p 8): ADVANCE: John Chalmers, Mark Woodward, Hisatomi Arima, Vlado Perkovic; ARIC: Josef Coresh, Kunihiro Matsushita, Morgan Grams, Yingying Sang; BIS: Elke Schaeffner, Natalie Ebert, Peter Martus; CanPREDDICT: Adeera Levin, Ognjenka Djurdjev, Mila Tang; CARE FOR HOMe: Gunnar Heine, Sarah Seiler, Adam Zawada, Insa Emrich; CHS: Michael Shlipak, Mark Sarnak, Ronit Katz, Jade Hiramoto; ESTHER: Hermann Brenner, Ben Schöttker, Dietrich Rothenbacher, Kai-Uwe Saum; GCKD: Anna Köttgen, Florian Kronenberg, Markus Schneider, Kai-Uwe Eckardt; Geisinger: Jamie Green, H Lester Kirchner, Alex R Chang; GLOMMS-II: Corri Black, Angharad Marks, Gordon Prescott, Laura Clark, Nick Fluck; Gubbio: Massimo Cirillo; KHS: Sun Ha Jee, Heejin Kimm, Yejin Mok; Maccabi: Gabriel Chodick, Varda Shalev; MASTERPLAN: Jack F. M. Wetzels, Peter J Blankestijn, Arjan D van Zuilen, M Bots; MESA: Carmen Peralta, Jade Hiromoto, Ronit Katz, Mark Sarnak; Mt Sinai BioMe: Erwin Bottinger, Girish N Nadkarni, Stephen B Ellis, Rajiv Nadukuru; NZDCS: Timothy Kenealy, C Raina Elley, John F Collins, Paul L Drury; PREVEND: Ron T Gansevoort, Stephan JL Bakker, Hiddo J Lambers Heerspink; Rancho Bernardo: Simerjot K Jassal, Jaclyn Bergstrom, Joachim H Ix, Elizabeth Barrett-Connor; RCAV: Csaba Kovesdy, Kamyar Kalantar-Zadeh; SCREAM: Juan J Carrero, Alessandro Gasparini, Abdul Rashid Qureshi, Peter Barany Study; SMART: Frank Visseren, Ale Algra, Yolanda van der Graaf; SRR-CKD: Marie Evans, Mårten Segelmark, Maria Stendahl, Staffan Schön; Sunnybrook: Navdeep Tangri, Maneesh Sud, David Naimark; ULSAM: Johan Ärnlöv, Lars Lannfelt, Anders Larsson;
CKD-PC Steering Committee: Josef Coresh (Chair), Ron T Gansevoort, Morgan E. Grams, Stein Hallan, Csaba P Kovesdy, Andrew S Levey, Kunihiro Matsushita, Varda Shalev, Mark Woodward
CKD-PC Data Coordinating Center: Shoshana H Ballew (Assistant Project Director), Jingsha Chen (Programmer), Josef Coresh (Principal investigator), Morgan E Grams (Director of Nephrology Initiatives), Lucia Kwak (Programmer), Kunihiro Matsushita (Director), Yingying Sang (Lead programmer), Mark Woodward (Senior statistician)
Declarations of Interest: All coauthors have completed ICMJE forms. Dr. Matsushita reports grants from American Heart Association, grants from US National Kidney Foundation, grants from US National Institutes of Health, during the conduct of the study; grants and personal fees from Kyowa Hakko Kirin, grants and personal fees from Fukuda Denshi, outside the submitted work. Dr. Ärnlöv reports personal fees from AstraZeneca, outside the submitted work. Dr. Coresh reports grants from NIH (National Institute for Health), grants from NKF (National Kidney Foundation), during the conduct of the study; grants from NIH (National Institute for Health), grants from NKF (National Kidney Foundation), outside the submitted work; In addition, Dr. Coresh has a patent PCT/US2015/044567 Provisional patent [Coresh, Inker and Levey] filed 8/15/2014 – Precise estimation of glomerular filtration rate from multiple biomarkers issued. Dr. Woodward reports personal fees from Amgen, outside the submitted work. Dr. Shlipak reports other from TAI Diagnostics, personal fees from Cricket Health, Inc., outside the submitted work.
Contributor Information
Kunihiro Matsushita, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Shoshana H. Ballew, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Prof Josef Coresh, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Prof Hisatomi Arima, Department of Preventive Medicine and Public Health, Faculty of Medicine, Fukuoka University, Fukuoka, Japan.
Prof Johan Arnlov, Division of Family Medicine and Primary Care, Department of Neurobiology, Care Science and Society, Karolinska Institutet, Huddinge, Sweden; School of Health and Social Studies, Dalarna University, Falun, Sweden.
Prof Massimo Cirillo, Department “Scuola Medica Salernitana”, University of Salerno, Italy.
Natalie Ebert, Charité University Medicine, Institute of Public Health, Berlin, Germany.
Jade S. Hiramoto, Division of Vascular and Endovascular Surgery, Department of Surgery, University of California San Francisco, San Francisco, CA, USA.
Prof Heejin Kimm, Institute for Health Promotion, Department of Epidemiology and Health Promotion, Graduate School of Public Health, Yonsei University, Seoul, Korea.
Prof Michael G. Shlipak, San Francisco VA Medical Center, San Francisco, USA and University of California, San Francisco, USA.
Prof Frank L.J. Visseren, Department of Vascular Medicine, University Medical Center Utrecht, Utrecht, the Netherlands.
Prof Ron T. Gansevoort, Department of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Prof Csaba P. Kovesdy, Memphis Veterans Affairs Medical Center, Memphis, TN and University of Tennessee Health Science Center, Memphis, TN.
Prof Varda Shalev, Maccabi Institute for Research and Innovation, Maccabi Healthcare Services, and Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Prof Mark Woodward, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD; The George Institute for Global Health, University of Sydney, Sydney, NSW, Australia; The George Institute for Global Health, University of Oxford, Oxford, United Kingdom.
Prof Florian Kronenberg, Division of Genetic Epidemiology, Department of Medical Genetics, Molecular and Clinical Pharmacology, Medical University of Innsbruck, Innsbruck, Austria.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Executive Summary: Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:447–54. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–40. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60:686–95 e2. doi: 10.1016/j.jvs.2014.03.290. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Applegate WB, Bonds DE, et al. Ankle brachial index values, leg symptoms, and functional performance among community-dwelling older men and women in the lifestyle interventions and independence for elders study. J Am Heart Assoc. 2013;2:e000257. doi: 10.1161/JAHA.113.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.2011 USRDS Annual Data Report. Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2011. [Internet]. 2011. Available from: http://www.usrds.org/atlas.aspx. [Google Scholar]
- 7.Baber U, Mann D, Shimbo D, Woodward M, Olin JW, Muntner P. Combined role of reduced estimated glomerular filtration rate and microalbuminuria on the prevalence of peripheral arterial disease. Am J Cardiol. 2009;104:1446–51. doi: 10.1016/j.amjcard.2009.06.068. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Mohler ER, 3rd, Xie D, et al. Risk factors for peripheral arterial disease among patients with chronic kidney disease. Am J Cardiol. 2012;110:136–41. doi: 10.1016/j.amjcard.2012.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvin E, Kottgen A, Coresh J. Kidney function estimated from serum creatinine and cystatin C and peripheral arterial disease in NHANES 1999–2002. Eur Heart J. 2009;30:1918–25. doi: 10.1093/eurheartj/ehp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wattanakit K, Folsom AR, Criqui MH, et al. Albuminuria and peripheral arterial disease: results from the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2008;201:212–6. doi: 10.1016/j.atherosclerosis.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wattanakit K, Folsom AR, Selvin E, Coresh J, Hirsch AT, Weatherley BD. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007;18:629–36. doi: 10.1681/ASN.2005111204. [DOI] [PubMed] [Google Scholar]
- 12.Wattanakit K, Folsom AR, Selvin E, et al. Risk factors for peripheral arterial disease incidence in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2005;180:389–97. doi: 10.1016/j.atherosclerosis.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Bello AK, Hemmelgarn B, Lloyd A, et al. Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin J Am Soc Nephrol. 2011;6:1418–26. doi: 10.2215/CJN.09741110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garimella PS, Ix JH, Katz R, et al. Association of albumin-creatinine ratio and cystatin C with change in ankle-brachial index: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2015;65:33–40. doi: 10.1053/j.ajkd.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary. Circulation. 2016 doi: 10.1161/CIR.0000000000000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushita K, Ballew SH, Astor BC, et al. Cohort Profile: The Chronic Kidney Disease Prognosis Consortium. Int J Epidemiol. 2013;42:1660–8. doi: 10.1093/ije/dys173. [DOI] [PubMed] [Google Scholar]
- 17.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–51. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. The lancet Diabetes & endocrinology. 2015;3:514–25. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–7. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–43. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements. 2013;3:1–150. doi: 10.1016/j.kisu.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 23.Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murabito JM, D’Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation. 1997;96:44–9. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 25.Garg PK, Biggs ML, Carnethon M, et al. Metabolic syndrome and risk of incident peripheral artery disease: the cardiovascular health study. Hypertension. 2014;63:413–9. doi: 10.1161/HYPERTENSIONAHA.113.01925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660–7. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaptoge S, Di Angelantonio E, Pennells L, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–20. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zellner A. An Efficient Method of Estimating Seemingly Unrelated Regressions and Tests for Aggregation Bias. Journal of the American Statistical Association. 1962;57:348. [Google Scholar]
- 30.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–40. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–73. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 33.D’Agostino RB, Vasan RS, Pencina MJ, et al. General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 34.Borenstein M, Hedges L, JPT H, HR R. Introduction to meta-analsys. West Sussex: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 35.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 36.El Nahas M. Cardio-Kidney-Damage: a unifying concept. Kidney Int. 2010;78:14–8. doi: 10.1038/ki.2010.123. [DOI] [PubMed] [Google Scholar]
- 37.Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–41. doi: 10.1016/j.jvs.2009.08.073. [DOI] [PubMed] [Google Scholar]
- 38.Jorneskog G. Why critical limb ischemia criteria are not applicable to diabetic foot and what the consequences are. Scand J Surg. 2012;101:114–8. doi: 10.1177/145749691210100207. [DOI] [PubMed] [Google Scholar]
- 39.Ratto E, Leoncini G, Viazzi F, et al. Microalbuminuria and cardiovascular risk assessment in primary hypertension: should threshold levels be revised? Am J Hypertens. 2006;19:728–34. doi: 10.1016/j.amjhyper.2005.12.018. discussion 35–6. [DOI] [PubMed] [Google Scholar]
- 40.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 41.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–90. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 42.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 43.Tonelli M, Muntner P, Lloyd A, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380:807–14. doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 44.Wesselink SF, Lingsma HF, Robben P, Mackenbach JP. Guideline adherence and health outcomes in diabetes mellitus type 2 patients: a cross-sectional study. BMC Health Serv Res. 2015;15:22. doi: 10.1186/s12913-014-0669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


