Abstract
In the last two decades, monoclonal antibodies have revolutionized the therapy of cancer patients. Although antibody therapy has continuously been improved, still a significant number of patients do not benefit from antibody therapy. Therefore, rational optimization of the antibody molecule by Fc engineering represents a major area of translational research to further improve this potent therapeutic option. Monoclonal antibodies are able to trigger a variety of effector mechanisms. Especially Fc-mediated effector functions such as antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement- dependent cytotoxicity (CDC) are considered important in antibody therapy of cancer. Novel mechanistic insights into the action of monoclonal antibodies allowed the development of various Fc engineering approaches to modulate antibodies' effector functions. Strategies in modifying the Fc glycosylation profile (Fc glyco-engineering) or approaches in engineering the protein backbone (Fc protein engineering) have been intensively evaluated. In the current review, Fc engineering strategies resulting in improved ADCC, ADCP and CDC activity are summarized and discussed.
Keywords: ADCC, CDC, Antibody engineering
Introduction
Monoclonal antibodies have improved significantly the therapeutic options of patients and are standard in the treatment of cancer today [1,2,3,4]. However, a considerable number of patients still do not benefit from antibody therapy and relapse remains a serious issue. Thus, further optimization of the antibody molecule to achieve a higher therapeutic efficacy represents a major area in current translational research.
New avenues for the generation of rationally designed ‘fit-for-purpose’ antibodies were opened by a better understanding of antibody effector functions and their relative contribution to the antibody's therapeutic efficacy. Effector functions of antibodies are complex, and antibodies are able to kill cancer cells by various mechanisms in vitro (fig. 1). These include direct induction of cell death by receptor cross-linkage or blockade of receptor-ligand interactions, complement-dependent cytotoxicity (CDC), and recruitment of effector cells for antibody-dependent cell-mediated cytotoxicity (ADCC) or antibody-dependent cellular phagocytosis (ADCP) by engagement of activating Fcγ receptors (FcγR). In particular, as revealed in various animal models, Fc-mediated effector cell recruitment and functions such as ADCC or ADCP were suggested to play crucial roles for tumor-targeting antibodies. For example, the antibodies rituximab or trastuzumab lost their therapeutic activity in genetically modified mice either lacking expression of activating FcγR or being defective in FcγR signaling, while their efficacy was enhanced in FcγRIIb knocked-out mice [5,6]. Moreover, analysis of isotype switch variants with different ratios of affinities to activating and inhibitory FcγR (A:I ratio), revealed that antibodies with a higher A:I ratio had superior therapeutic activity [7]. However, the role of distinct FcγR is complex, as FcγRIIb binding promoted the pro-apoptotic activities of agonistic antibodies targeting members of the tumor necrosis factor receptor superfamily [8,9,10,11]. The importance of FcγR engagement was also supported by clinical observations showing that homozygous expression of the FcγRIIIa-V158 allele and/or the FcγRIIa-H131 allele correlated with higher response rates and prolonged overall survival [12,13]. FcγRIIIa-V158 binds the IgG1 Fc domain with higher affinity compared to FcγRIIIa-F158, resulting in stronger ADCC activity [14]. The functional consequences of FcγRIIa-H131 versus FcγRIIa-R131 engagement are not yet understood in detail. In addition, the relative contribution of selected effector cell populations (i.e. NK cells, monocytes, macrophages, granulocytes), has not been completely clarified [15,16,17].
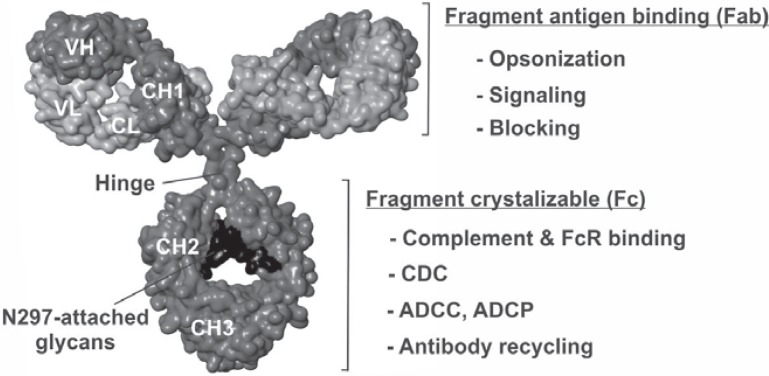
Fig. 1.
Structure and functions of therapeutic IgG1 antibodies. Antibody model of a human IgG1 molecule (kindly provided by Dr. M.R. Clark [81]) with antibody light and heavy chains depicted in light grey and dark grey, respectively, and the glycans attached to amino acid N-297 in black (VL, VH, antibody variable regions of the light and heavy chain; CH1-3, constant heavy chain regions 1–3; CL constant light chain). The effector functions of therapeutic antibodies in cancer therapy include direct mechanisms of action, which are mediated by the Fab domain, and indirect functions, which in addition require the Fc portion. Long plasma and body retention times are due to antibody uptake and recycling by interaction of the Fc domain with the neonatal Fc receptor.
Whereas the observations outlined above indicate that efficient FcγR engagement determines the efficacy of therapeutic antibodies in patients, the role of complement has not strictly been proven. In certain mouse models C1q knockout resulted in loss of therapeutic activity of rituximab [18,19] pointing to an important role of CDC in tumor cell elimination, but this was not observed in other animal models [5,7,16]. In patients, clear evidence is lacking. However, rapid consumption of complement during rituximab infusion and the observation that application of plasma as a source of complement increased the clinical efficacy of rituximab at least in individual patients may suggest a contribution of CDC in antibody therapy [20,21].
Further complexity derives from the fact that complement may also affect effector cell-mediated killing. Thus, both complement deposition and anaphylatoxin formation were shown to enhance effector cell cytotoxicity, indicating interplay between complement and Fc receptor-mediated killing in distinct situations [22,23]. Intriguingly, as demonstrated for rituximab, in other situations complement fixation may impair ADCC activity and therapeutic activity [24,25]. Thus, C3b deposition triggered by target cell-bound antibodies was shown to hinder the interaction of the antibody with FcγRIIIa on NK cells, resulting in diminished ADCC [24]. Therefore, efficient complement fixation is beneficial when induction of CDC, complement-dependent cell-mediated cytotoxicity (CDCC), or complement-dependent cellular phagocytosis (CDCP) is possible [26]. However, when not, it may even impede other effector functions and potentially diminish therapeutic effects. In conclusion, available experimental evidence may suggest that only a selected repertoire of effector functions is available depending on target antigen characteristics, tumor type, tumor burden, tumor location, antibody isotype, and availability of distinct components of the patients' immune system [27,28,29].
Based on these observations, antibody Fc engineering strategies were developed with the aim to further improve the performance of therapeutic antibodies by enhancing the antibody's ability to induce CDC or to trigger effector cell-mediated killing [30,31,32]. In addition, other modifications were established to abolish selected Fc functions, which for example may allow reducing unwanted side effects. Here, different Fc engineering technologies to improve ADCC and CDC activity of human IgG1 antibodies are summarized.
Enhancing ADCC and ADCP Activity
Glyco-Engineering
The presence of the oligosaccharide attached to the asparagine residue at position 297 (N297) in IgG1 antibodies is critical for binding to FcγR and the complement factor C1q (fig. 2). While non-glycosylated antibodies displayed significant structural changes in the CH2 domain [33] leading to a closed conformation hindering binding to FcγR or C1q, antibodies with a monosaccharide attached to the Fc domain still mediated effector functions via FcγR [34]. The impact of Fc glycosylation on antibodies' ADCC activity was supported using CAMPATH-1H expressed in different cell lines resulting in distinct glycosylation profiles [35]. Based on these and other related observations, controlled alteration of the Fc-attached oligosaccharide to manipulate Fc-mediated antibody functions became one major issue in antibody engineering [36]. In particular, a reduced content of fucose or sialic acid was correlated with improved ADCC activity (fig. 2; table 1) [37,38]. Based on these findings, various strategies have been developed allowing rational engineering of the glycosylation profile to boost ADCC.
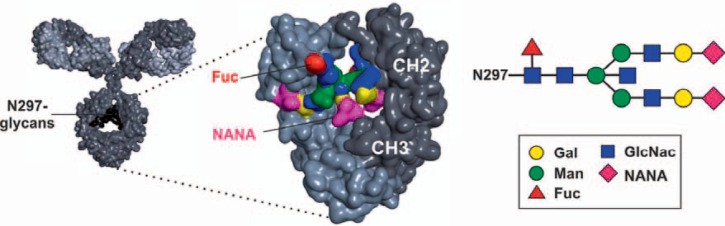
Fig. 2.
N-297 attached glycans in antibody effector functions. IgG antibodies typically contain a glycan (black) attached to amino acid N297 in each heavy chain (dark grey, heavy chain, light grey, light chain). The N-297 attached glycan contains a core structure comprising N-acetylglucosamine (GlcNAc) and mannose (Man) residues, which can be extended by fucose (Fuc), a bisecting GlcNAc and galactose (Gal) and sialic acid (NANA) residues.
Table 1.
Approaches to manipulate antibody fucosylation
| Technique | Fucosylation level | Reference |
|---|---|---|
| Unmodified/mutated cell line (YB02, LEC13) | variable | [35, 37] |
| N-acetylglucosaminyltransferase III (GnT III) overexpression | variable | [43] |
| Co-expression of heterologous GDP-6-deoxy-D-lyxo-4-hexulose reductase | variable | [84] |
| FUT8 gene knockout | afucosylated | [40] |
| FUT8 siRNA knockdown | afucosylated | [85] |
| Antibody expression in the presence of the glycosidase inhibitor kifunensine | variable | [45] |
| Zinc-finger nuclease mediated knockdown of GDP-fucose transporter gene | afucosylated | [86] |
FUT8 = Alpha-(1,6)-fucosyltransferase.
For reduction in core fucosylation different technologies are available. The most straight forward approach is the use of cell lines, such as the Lec13 CHO mutant with reduced capacity to incorporate fucose in the antibody-attached oligosaccharide [39]. In addition, generation of completely non-fucosylated antibodies was achieved by knockout of the FUT8 gene encoding the α1,6-fucosyltransferase (α1,6-FucT) in cell lines such as CHO (Potelligent® technology) [40].
In an alternative approach, overexpression of N-acetylglucosaminyltransferase III (GnT III) led to the attachment of a bisecting GlcNAc residue (GlycoMAb™ technology), directly resulting in improved FcR binding [41]. Importantly, the bisected oligosaccharide is not a suitable substrate for α1,6-FucT, resulting in non-fucosylated antibody species [42]. By applying this strategy, an anti-neuroblastoma antibody was glyco-engineered. The 20-fold improvement in NK cell-mediated ADCC correlated with the relative content of bisected, non-fucosylated oligosaccharides [43]. Similar results were observed for a glyco-engineered rituximab variant [41] and a CD19 antibody [44]. Subsequently, alternative strategies have been developed to reduce core fucosylation in mammalian cells (table 1) [40,41,43,45]. Mainly mammalian cell lines have been used in the production of glyco-engineered antibodies, but also non-mammalian expression hosts such as yeasts and plants genetically modified to allow production of antibodies with defined carbohydrate structures might be an option. Besides engineering the expression host, also chemo-enzymatic modification has been used for rational remodeling of Fc-bound N-glycans. Although antibodies with well-defined carbohydrate structures and optimized effector functions can be realized, a more complex production process may generate additional costs [46].
From a mechanistic point of view, Ferrara and colleagues [47] unraveled that high affinity interaction of human IgG1 with FcγRIIIa is due to reciprocal action between the receptor carbohydrate and areas of the antibody's Fc part that are only accessible when fucose is lacking. Importantly, co-crystal structures of FcγRIIIa and non-fucosylated Fc domains suggested that the N-glycan structure at the asparagine residue at position 162 (N162) of FcγRIIIa interacts with the Fc-bound carbohydrate structure [48,49]. FcγRIIIa and FcγRIIIb are the only human FcγR which are glycosylated at this position, explaining the selective increase in the antibodies' affinity for FcγRIII [47,48,49].
Besides the obvious impact of fucose levels on FcγRIIIa-triggered NK cell-mediated ADCC, also the cytolytic and phagocytic activities of neutrophils expressing the highly homologous FcγRIIIb isoform is modulated by lack of core fucosylation. While phagocytosis of target cells coated with non-fucosylated antibodies was improved [50], in another study impaired neutrophil-mediated ADCC was observed after removal of fucose [51,52].
Besides fucose, also other sugar residues in the Fc-attached oligosaccharide were shown to modulate ADCC activity. Recently, hypergalactosylation of IgG1 was correlated with enhanced ADCC activity [53], although the effects were less pronounced compared to removal of fucose. Also, incorporation of a bisecting sugar residue by enzymatic remodeling resulted in enhanced ADCC activity [54]. Scallon and colleagues [55] demonstrated that for selected antibodies a higher level of sialic acid resulted in reduced ADCC activity, while for other antibody specificities differences in sialic acid levels had no impact on FcγRIIIa binding but unexpectedly on antigen binding capacity. Expression of modified sialidase A in host cell lines resulted in expression of non-sialylated monoclonal antibodies, consistently demonstrating stronger ADCC activity than sialylated counterparts [56].
Protein Engineering
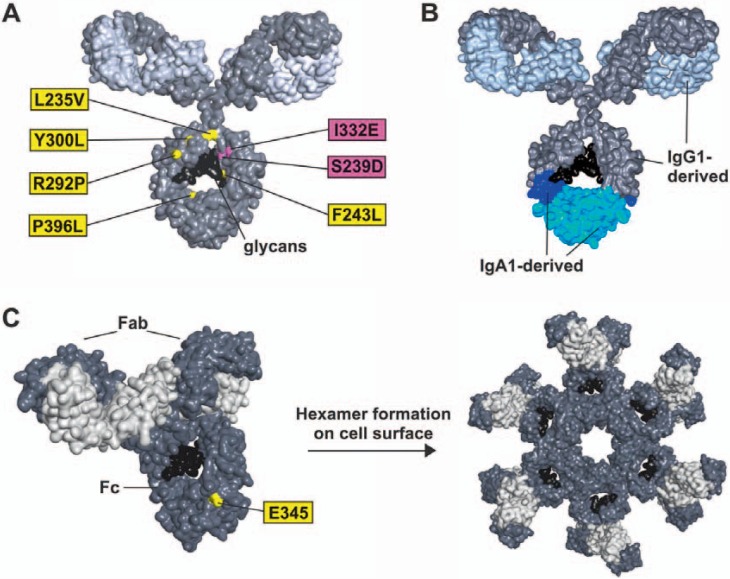
Exchanging amino acids directly in the FcγR binding site within the antibody's Fc domain was a logical strategy to manipulate Fc/FcR interactions (fig. 3; table 2). Shields and colleagues [57] exchanged all solvent exposed amino acids in the Fc domain of human IgG1 to alanine and thereby identified various Fc domain variants with altered FcγR binding profiles. In this pioneering approach a triple variant (S298A-E333A-K334A) was identified, which exerted a higher affinity to FcγRIIIa but showed diminished binding to FcγRIIa and FcγRIIb. This modification resulted in enhanced ADCC activity with NK cells, especially when the low-affinity FcγRIIIa-F158 allele was expressed homozygously. Subsequently, a variety of Fc variants with increased FcγRIIIa binding profile and increased ADCC activity were discovered by following different experimental strategies [58,59,60]. Although many of the described amino acid exchanges enhanced affinity to FcγRIIIa, these variants often differed in their affinities to other FcγR. Lazar and colleagues [59] generated an Fc variant (S239D-I332E) with enhanced FcγRIIIa binding affinity which also displayed a higher affinity to FcγRIIa and FcγRIIb (fig. 3A; table 2). Antibodies harboring the S239D-I332E amino acid modification were more efficient in inducing NK cell-mediated ADCC than native IgG1 molecules. Impressively, this variant even triggered stronger ADCC than the triple Fc variant described by Shields and colleagues [57]. With regard to rituximab, the S239D-I332E variant showed improved ADCC activity with NK cells and a modest enhancement in ADCP by macrophages. In contrast, CDC activity was not affected. In non-human primates this S239D-I332E rituximab variant depleted half of the circulating B cells at an approximately 50-fold lower dose level than the non-engineered IgG1 counterpart [59]. The S239D-I332E modification was also applied to engineer a CD19 antibody [61,62]. While the corresponding native CD19 IgG1 antibody had no B-cell depletion activity in monkeys, the Fc-optimized variant efficiently eliminated peripheral B cells [63]. The S239D-I332E variant was recently proven to enhance ADCC activity also in CD33 or CD133 antibodies targeting AML tumor cells [64].
Fig. 3.
Fc protein-engineering strategies for enhanced ADCC or CDC. A Antibody model of a human IgG1 molecule engineered by exchanging selected amino acid positions. Yellow: amino acid substitutions introduced in Margetuximab (MacroGenics); [82]. Purple: amino acid substitutions introduced in MOR208, Xmab-5574 (Xencor, MorphoSys); [59]. B Antibody model of an antibody molecule engineered by exchanging larger amino acid stretches between the IgG1 and IgA1 isotypes (IgGA cross-isotype; [65]). C IgG1 molecule with marked E345 position. Antibodies harboring amino acid exchanges at this (or other) positions are expressed as monomeric IgG molecules but form hexamers at the cell surface of target cells after antigen binding [73].
Table 2.
Selected engineered IgG1-Fc variants with enhanced ADCC activity
| Variant | FcγRIIIa binding | FcγRIIb binding | ADCC induction | X-fold reduction in EC50 value | Complement activation | Reference |
|---|---|---|---|---|---|---|
| Wild type | ↑ | ↑ | ↑ | – | + | |
| S298A-E333A-K334A | ↑↑ | ↓ | ↑↑↑ | 10–100 | n.d.a. | [57] |
| S239D-I332E | ↑↑↑ | ↑↑ | ↑↑↑ | 10–100 | + | [59] |
| S239D-I332E-A330L | ↑↑↑↑ | ↑↑↑ | ↑↑↑ | 10–100 | – | [59] |
| L235V-F243L-R292P-Y300L-P396L | ↑↑↑ | ↓ | ↑↑↑ | 10–100 | n.d.a. | [60, 83] |
EC50 = Effective concentration 50 %; ↑ = enhanced activity/binding compared to wt; ↓ = reduced activity/binding compared to wt; n.d.a. = no data available.
While many Fc protein engineering approaches are based on substitution of selected individual amino acids, alternative strategies have been developed by exchanging larger amino acid stretches between different isotypes. These so-called cross-isotypes demonstrated interesting profiles of effector functions. For example, Kelton and colleagues [65] reported the engineering of an IgGA ‘cross-isotype’ antibody which combines selected effector functions of both IgG and IgA (fig. 3B). IgGA binds to FcαRI, FcγRI and FcγRIIa, while no binding to FcγRIIIa was observed. A trastuzumab IgGA potently activated both neutrophils and macrophages and mediated higher CDC than IgG1 or IgA antibodies.
Combining Glyco- and Protein Engineering
Most engineering strategies focus on either introducing amino acid exchanges in the protein backbone or in modifying the Fc-bound glycosylation profile. Recently, also combined Fc engineering approaches have been described. The ADCC-optimized S298A-E333A-K334A Fc variant was glyco-engineered by expression in Lec13 cells to reduce fucose content. This double-engineered variant displayed improved NK cell-mediated ADCC activity [66]. Yet, in a similar approach employing S239D-I332E, S239D-I332E-A330L Fc variants, improved ADCC activity was not observed, although the affinity of FcγRIIIa binding was 10-fold increased [67,68]. The gain in FcγRIIIa affinity for selected protein-engineered and glyco-engineered variants may therefore be based on different mechanisms. While protein-protein interactions are improved by amino acid substitutions, stronger interactions between Fc- and FcR-attached carbohydrates may account for improved FcR binding. These data may further suggest that a certain threshold in FcγRIIIa engagement has to be overcome to reach maximal NK cell-mediated ADCC. This threshold is already overcome by selected protein-engineered Fc variants or by non-fucosylated Fc parts. However, these findings need to be confirmed in a more complex in vivo situation.
Enhancing CDC Activity
Protein engineering could also be applied to improve IgG1 antibodies' capacity to trigger CDC (table 3). Several studies investigated the impact of selected amino acid substitutions in the CH2 domain on complement activation [69,70]. Idusogie and colleagues [69] identified amino acids K326 and E333 to be critical in C1q binding. Moore et al. [70] analyzed a variety of Fc variants thereby unraveling S267, H268 and S324 to be important for effective CDC. Variants carrying K326W or E333S substitutions resulted in enhanced CDC activity. However, CDC was not further enhanced by their combination although it resulted in a 5-fold higher C1q binding capacity compared to variants carrying the individual substitutions. Interestingly, whereas variants carrying either K326W or E333S substitutions lacked significant ADCC activity, the double-mutant K326A-E333A demonstrated enhanced CDC activity, while ADCC activity was unaffected. A roughly 7-fold improvement in CDC activity was reached by exchanging three amino acids (S267E-H268F-S324T). This triple-mutant displayed substantially diminished ADCC activity, which could be restored by introducing two additional amino acid exchanges G236A and I332E, resulting in wild type-like ADCC activity and an even further enhanced CDC activity. An alternative approach was developed by Natsume and colleagues [71] by designing mixed IgG1/IgG3 Fc variants with significantly enhanced CDC activity.
Table 3.
Selected engineered IgG1-Fc variants with enhanced CDC activity
| Variant | ID | C1q- fold binding | CDC (fold) potency | ADCC (fold) potency | Reference |
|---|---|---|---|---|---|
| Wild type | 1 | 1 | 1 | ||
| K326W | 3 | 2 | 0 | [69] | |
| K326W-E333S | 5 | 2 | 0 | [69] | |
| S267E-H268F-S324T | EFT | 47 | 6.9 | 0.045 | [70] |
| S267E-H268F-S324T-G236A-I332E | EFT + AE | 23 | 1.2 | [70] | |
| IgG1/IgG3 chimera | 113F | 7.1 | 3.7 | 1 | [70, 71] |
| E345K (hexamer variant) | 1 | >10 | 2–5 | [74] | |
| E430G (hexamer variant) | 1 | >10 | 2–5 | [74] |
n.d.a. = No data available.
Most CDC-optimized Fc variants were designed to more potently trigger CDC while maintaining FcγR-mediated ADCC activity. Recently, Lee and colleagues [26] described novel Fc mutations enabling selective binding to C1q without concomitant engagement of Fcγ receptors. The engineered Fc domains were introduced into the rituximab antibody and potently triggered CDC. Moreover, even without FcγR engagement this antibody was capable of activating NK cells and macrophages in the presence of complement, presumably by interaction of opsonins deposited to target cells and complement receptors on effector cells. Thus, target cells were efficiently killed by CDCC and CDCP. Interestingly, in a preclinical in vivo mouse model the kinetics and efficacy of target cell clearance were comparable to those of molecules triggering FcγR-mediated effector functions.
Even though less well established, also glyco-engineering may be applied to modulate CDC activity of therapeutic antibodies. For example, Quast and colleagues [72] showed that high levels of terminal sialic acid significantly impaired CDC activity mediated by rituximab.
Dual ADCC and CDC Optimization
As outlined above, various engineering strategies aim at individually improving selected effector mechanisms, such as ADCC or CDC. The rational choice of an engineering strategy for clinical application is not trivial, because the relative contribution of individual effector mechanism to the therapeutic activity of monoclonal antibodies in vivo is not fully understood. Therefore, concomitant enhancement of different effector functions might be desirable [30].
Natsume and colleagues [71] addressed this problem by reducing the fucose level in the background of IgG1/IgG3 mixed isotype variants. These variants demonstrated improved CDC activity compared to IgG1 and IgG3 variants, and interestingly enhanced NK cell-mediated ADCC activity. Wirt and colleagues [73] developed a similar approach by generating non-fucosylated variants of the EFTAE-Fc variant described by Moore and colleagues [70]. The EFTAE variant displays significantly improved C1q binding, resulting in stronger CDC-mediated lysis of tumor cells. The derived non-fucosylated variant, in addition to improved CDC activity, also demonstrated enhanced NK-cell mediated ADCC, due to high-affinity FcγRIIIa binding.
Based on the finding that IgG antibodies can organize into hexamers on the cell surface after antigen binding, thereby building optimal docking sites for C1q, de Jong and colleagues [74,75] translated this natural concept to design antibodies with improved effector functions. Mutations that enhanced hexamer formation and complement activation by IgG1 antibodies were identified (fig. 3C). Especially Fc domains with E345K or E430G amino acid exchanges potently enhanced CDC activity and interestingly also improved ADCC in the background of selected antibodies.
Finally, in certain clinical settings, in which interactions with immune cells or complement can lead to adverse events, Fc-mediated functions may be undesirable. Thus, in sharp contrast to typical tumor targeting antibodies, antibodies which exclusively rely on target antigen binding or neutralization of receptors, such as certain immune checkpoint blockers, antibodies harboring immunological silent Fc domains may be advantageous. Non-immune stimulatory Fc domains were generated using different approaches [76]. This type of ‘Fc silencing’ technology has recently been applied to the anti-PD-L1 antibody atezolizumab.
Perspectives of Fc-Engineered Antibodies in Clinical Applications
Until 2017, three Fc engineered antibodies have received approval for clinical use in cancer therapy (table 4). Two of them are typical tumor targeting antibodies that were optimized for enhanced effector cell recruitment by Fc glyco-engineering: mogamulizumab, which is specific for C-C chemokine receptor type 4 (CCR4) and which has been approved for the treatment of adult T-cell leukemia/lymphoma (ATLL) in Japan in 2012, contains a non-fucosylated Fc domain generated by applying the Potelligent® technology [77]. Obinutuzumab, which has received approval by the Food and Drug Administration (FDA) in the treatment of chronic lymphocytic leukemia (CLL) in 2016, is a low-fucose CD20 antibody that was generated by GlycoMAb™ technology [78]. In contrast, the third approved antibody, atezolizumab, which targets PD-L1 and which is employed for immune checkpoint blockade by blocking interaction of PD-L1 with its inhibitory receptor PD-1 on T cells, contains an aglycosylated Fc domain and lacks Fc receptor and complement binding [79]. This was achieved by replacing the N-glycosylation site asparagine 298 by alanine. Atezolizumab has been approved by the FDA for treatment of patients with locally advanced or metastatic urothelial carcinoma in 2016. In the next years, Fc-engineered antibodies are expected to gain increasing importance in particular in cancer therapy, and currently a variety of Fc-engineered antibodies targeting various antigens are in advanced stages of clinical development (table 5). These include glyco-engineered antibodies (e.g. ublituximab) as well as protein-engineered antibodies (e.g. margetuximab, MOR208; fig. 3A) generated by different Fc engineering technologies. Despite this obvious success, it is still not clear whether antibody Fc engineering indeed translates into a higher therapeutic efficacy in patients since comparative studies with corresponding non-engineered antibodies are difficult to realize and have not been performed to date. Whereas atezolizumab and mogamulizumab have not been compared to native antibodies with the same target antigen specificity, obinutuzumab was tested head-to-head against the native IgG1 CD20 antibody rituximab. Intriguingly, in a phase III randomized trial in CLL patients under chlorambucil chemotherapy, obinutuzumab was superior compared to rituximab regarding overall response rates and median progression-free survival [80], which may reflect benefits from Fc optimization. However, improved overall survival was not observed in other clinical trials or indications [81]. In addition, one may consider that the antibodies are not derived from the same clone and differ in more than one property. Thus, obinutuzumab not only is more efficacious in triggering ADCC as a consequence of Fc engineering, but is also more potent in inducing homotypic aggregation and direct target cell death, which may render it difficult to assess the impact of Fc engineering in this clinical setting. Expanding experience with Fc-engineered second-generation antibody variants of clinically approved antibodies such as CetuGEX, a glyco-engineered variant of the epidermal growth factor receptor-targeting antibody cetuximab that is currently tested in a clinical phase II trial, may offer the opportunity to compare efficacies of Fc-engineered antibodies versus native IgG1 counterparts and provide novel insights.
Table 4.
Fc-engineered antibodies approved for clinical use
| Antibody (company) | Trade name | Antigen | Engineering strategy | Indication | Year of approval | Reference |
|---|---|---|---|---|---|---|
| Mogamulizumab (Kyowa Hakko Kirin) | Poteligeo | CCR4 | non-fucosylated (host: FUT8-deficient CHO; Potelligent® technology) | ATLL | 2012# | [77] |
| Obinutuzumab (Roche) | Gazyva | CD20 | low fucose (GNT III over-expression; GlycoMAb™ technology) | CLL | 2013 | [78] |
| Atezolizumab (Roche) | Tecenriq | PD-L1 | non-glycosylated (AA substitution: N298A) | urothelial carcinoma | 2016 | [79] |
CCR4 = C-C chemokine receptor type 4; PD-L1 = programmed cell death 1 ligand 1, FUT8 = alpha-(1,6)-fucosyltransferase; GNT III = N-acetylglucosaminyltransferase III; AA = amino acid; ATLL = adult T-cell leukemia/lymphoma; CLL = chronic lymphocytic leukemia.
Country-specific approval (Japan).
Table 5.
Examples of Fc-engineered antibodies in advanced stages of clinical development
| Antibody (Company) | Antigen | Engineering strategy | Indication | Status | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| Ublituximab (TG Therapeutics) | CD20 | low fucose (host: YB2/0; EMABling® technology) | CLL, DLBCL | phase III | NCT02612311, NCT02793583 |
| Margetuximab (MacroGenics) | HER2 | AA substitutions: L235V/F243L/R292P/Y300L/P396L | breast cancer | phase III | NCT02492711 |
| Talacotuzumab, JNJ-56022473 (CSL, Janssen Biotech) | CD123 | AA substitutions: 239D/I332E (Xmab® technology) | AML | phase II/III | NCT02472145 |
| MOR208, Xmab-5574 (Xencor, MorphoSys) | CD19 | AA substitutions: S239D/I332E (Xmab® technology) | DLBCL, | phase II/III | NCT02763319 |
| Inebilizumab, MEDI-551 (MedImmune LLC) | CD19 | non-fucosylated (host: FUT8-deficient CHO; Potelligent® technology) | DLBCL, CLL | phase II | NCT01453205, NCT01466153 |
| BI 836826 (Boehringer Ingelheim) | CD37 | AA substitutions: S239D/I332E (Xmab® technology) | CLL | phase II | NCT02624492 |
| CetuGEX (Glycotope GmbH) | EGFR | reduced fucosylation, optimized galactosylation and degree of branching; GEX™ platform) | squamous cell carcinoma of the head and neck | phase II | NCT02052960 |
| PankoMab-GEX (Glycotope GmbH) | TA-MUC1 | reduced fucosylation, optimized galactosylation and degree of branching; GEX™ platform) | ovarian cancer | phase II | NCT01899599 |
| Lumretuzumab (Roche) | HER3 | Low fucose (GNT III over-expression; GlycoMab® technology) | NSCLC | phase II | NCT02204345 |
FUT8 = α1,6-fucosyltransferase; GNT III = N-acetylglucosaminyltransferase III; AA = amino acid; CLL = chronic lymphocytic leukemia; DLBCL = diffuse large B-cell lymphoma; AML = acute myeloid leukemia; NSCLC = non-small cell lung cancer. #Country-specific approval (Japan).
Conclusions
In the last two decades, monoclonal antibodies have revolutionized the therapy of cancer, although not all patients benefit. Currently, new generations of engineered antibodies are frequently entering the clinic with rationally designed effector functions. While strategies in improving antibodies' Fc affinity to selected FcγRs to improve immunotherapy of cancer is widely accepted, the concept of enhancing complement activation is still under debate and to our knowledge CDC-optimized antibody variants have not been tested in the clinic to date.
While a variety of engineered Fc variants have been described, detailed comparisons between larger panels of protein-engineered and/or glyco-engineered Fc variants with distinct profiles of effector functions are missing. Especially when analyzed in carefully designed in vivo models, such data may allow to unravel the relative contribution of selected effector cell populations to successful antibody therapy and to identify Fc variants with the highest potential in a given clinical application. Together with a more detailed understanding in the development and progression of diseases, the outlined technologies may allow the design of antibodies with tailor-made effector functions that are optimally suited for application in a given clinical situation.
Disclosure Statement
The authors declare no conflicts of interest
Acknowledgements
Dr. Rob de Jong and Professor Dr. Paul Parren (Genmab B.V.) are kindly acknowledged for providing the pdb coordinate file used to illustrate the IgG hexamer model. M.P. is supported by the Mildred-Scheel professorship program by the Deutsche Krebshilfe e.V. and by a research grant by the Deutsche Forschungsgemeinschaft (Pe1425/5-1). C.K and M.P. are supported by a research grant by the Wilhelm Sander Foundation (2014.134.1). We apologize to all investigators whose important work in the field could not be cited because of space limitations.
References
- 1.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 2.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell. 2012;148:1081–1084. doi: 10.1016/j.cell.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichert JM. Marketed therapeutic antibodies compendium. MAbs. 2012;4:413–415. doi: 10.4161/mabs.19931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 6.de Haij S, Jansen JH, Boross P, Beurskens FJ, Bakema JE, Bos DL, Martens A, Verbeek JS, Parren PW, van de Winkel JG, Leusen JH. In vivo cytotoxicity of type I CD20 antibodies critically depends on Fc receptor ITAM signaling. Cancer Res. 2010;70:3209–3217. doi: 10.1158/0008-5472.CAN-09-4109. [DOI] [PubMed] [Google Scholar]
- 7.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 8.Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White AL, Chan HT, Roghanian A, French RR, Mockridge CI, Tutt AL, Dixon SV, Ajona D, Verbeek JS, Al-Shamkhani A, Cragg MS, Beers SA, Glennie MJ. Interaction with FcgammaRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J Immunol. 2011;187:1754–1763. doi: 10.4049/jimmunol.1101135. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Ravetch JV. Apoptotic and antitumor activity of death receptor antibodies require inhibitory Fcgamma receptor engagement. Proc Natl Acad Sci U S A. 2012;109:10966–10971. doi: 10.1073/pnas.1208698109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, Li Y, Pitti R, Totpal K, Yee S, Ross S, Vernes JM, Lu Y, Adams C, Offringa R, Kelley B, Hymowitz S, Daniel D, Meng G, Ashkenazi A. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 2011;19:101–113. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, Neri TM, Ardizzoni A. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 13.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Dall'Ozzo S, Tartas S, Paintaud G, Cartron G, Colombat P, Bardos P, Watier H, Thibault G. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004;64:4664–4669. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 15.Biburger M, Aschermann S, Schwab I, Lux A, Albert H, Danzer H, Woigk M, Dudziak D, Nimmerjahn F. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity. 2011;35:932–944. doi: 10.1016/j.immuni.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veeramani S, Wang SY, Dahle C, Blackwell S, Jacobus L, Knutson T, Button A, Link BK, Weiner GJ. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118:3347–3349. doi: 10.1182/blood-2011-05-351411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, Botto M, Introna M, Golay J. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–1587. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 19.Golay J, Cittera E, Di Gaetano N, Manganini M, Mosca M, Nebuloni M, van Rooijen N, Vago L, Introna M. The role of complement in the therapeutic activity of rituximab in a murine B lymphoma model homing in lymph nodes. Haematologica. 2006;91:176–183. [PubMed] [Google Scholar]
- 20.Klepfish A, Schattner A, Ghoti H, Rachmilewitz EA. Addition of fresh frozen plasma as a source of complement to rituximab in advanced chronic lymphocytic leukaemia. Lancet Oncol. 2007;8:361–362. doi: 10.1016/S1470-2045(07)70106-7. [DOI] [PubMed] [Google Scholar]
- 21.Xu W, Miao KR, Zhu DX, Fang C, Zhu HY, Dong HJ, Wang DM, Wu YJ, Qiao C, Li JY. Enhancing the action of rituximab by adding fresh frozen plasma for the treatment of fludarabine refractory chronic lymphocytic leukemia. Int J Cancer. 2011;128:2192–2201. doi: 10.1002/ijc.25560. [DOI] [PubMed] [Google Scholar]
- 22.Gelderman KA, Tomlinson S, Ross GD, Gorter A. Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 2004;25:158–164. doi: 10.1016/j.it.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Shushakova N, Skokowa J, Schulman J, Baumann U, Zwirner J, Schmidt RE, Gessner JE. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgammaRs in immune complex-induced lung disease. J Clin Invest. 2002;110:1823–1830. doi: 10.1172/JCI200216577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang SY, Racila E, Taylor RP, Weiner GJ. NK-cell activation and antibody-dependent cellular cytotoxicity induced by rituximab-coated target cells is inhibited by the C3b component of complement. Blood. 2008;111:1456–1463. doi: 10.1182/blood-2007-02-074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang SY, Veeramani S, Racila E, Cagley J, Fritzinger DC, Vogel CW, St John W, Weiner GJ. Depletion of the C3 component of complement enhances the ability of rituximab-coated target cells to activate human NK cells and improves the efficacy of monoclonal antibody therapy in an in vivo model. Blood. 2009;114:5322–5330. doi: 10.1182/blood-2009-01-200469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CH, Romain G, Yan W, Watanabe M, Charab W, Todorova B, Lee J, Triplett K, Donkor M, Lungu OI, Lux A, Marshall N, Lindorfer MA, Goff OR, Balbino B, Kang TH, Tanno H, Delidakis G, Alford C, Taylor RP, Nimmerjahn F, Varadarajan N, Bruhns P, Zhang YJ, Georgiou G. IgG Fc domains that bind C1q but not effector Fcgamma receptors delineate the importance of complement-mediated effector functions. Nat Immunol. 2017;18:889–898. doi: 10.1038/ni.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boross P, Jansen JH, de Haij S, Beurskens FJ, van der Poel CE, Bevaart L, Nederend M, Golay J, van de Winkel JG, Parren PW, Leusen JH. The in vivo mechanism of action of CD20 monoclonal antibodies depends on local tumor burden. Haematologica. 2011;96:1822–1830. doi: 10.3324/haematol.2011.047159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, Wu Y, Meng YG, Gribling P, Lin Z, Nguyen K, Tran T, Zhang Y, Rosen H, Martin F, Chan AC. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 29.Dayde D, Ternant D, Ohresser M, Lerondel S, Pesnel S, Watier H, Le Pape A, Bardos P, Paintaud G, Cartron G. Tumor burden influences exposure and response to rituximab: pharmacokinetic-pharmacodynamic modeling using a syngeneic bioluminescent murine model expressing human CD20. Blood. 2009;113:3765–3772. doi: 10.1182/blood-2008-08-175125. [DOI] [PubMed] [Google Scholar]
- 30.Natsume A, Niwa R, Satoh M. Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug Des Devel Ther. 2009;3:7–16. [PMC free article] [PubMed] [Google Scholar]
- 31.Presta LG. Molecular engineering and design of therapeutic antibodies. Curr Opin Immunol. 2008;20:460–470. doi: 10.1016/j.coi.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Strohl WR. Optimization of Fc-mediated effector functions of monoclonal antibodies. Curr Opin Biotechnol. 2009;20:685–691. doi: 10.1016/j.copbio.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Feige MJ, Nath S, Catharino SR, Weinfurtner D, Steinbacher S, Buchner J. Structure of the murine unglycosylated IgG1 Fc fragment. J Mol Biol. 2009;391:599–608. doi: 10.1016/j.jmb.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 34.Kao D, Danzer H, Collin M, Gross A, Eichler J, Stambuk J, Lauc G, Lux A, Nimmerjahn F. A monsaccharide residue is sufficient to maintain mouse and human IgG subclass activity and directs IgG effector functions to cellular Fc receptors. Cell Rep. 2015;13:2376–2385. doi: 10.1016/j.celrep.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 35.Lifely MR, Hale C, Boyce S, Keen MJ, Phillips J. Glycosylation and biological activity of CAMPATH-1H expressed in different cell lines and grown under different culture conditions. Glycobiology. 1995;5:813–822. doi: 10.1093/glycob/5.8.813. [DOI] [PubMed] [Google Scholar]
- 36.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 37.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 38.Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471–478. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Patnaik SK, Stanley P. Lectin-resistant CHO glycosylation mutants. Methods Enzymol. 2006;416:159–182. doi: 10.1016/S0076-6879(06)16011-5. [DOI] [PubMed] [Google Scholar]
- 40.Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R, Sakurada M, Uchida K, Shitara K, Satoh M. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87:614–622. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- 41.Davies J, Jiang L, Pan LZ, LaBarre MJ, Anderson D, Reff M. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnol Bioeng. 2001;74:288–294. [PubMed] [Google Scholar]
- 42.Schachter H. The joys of HexNAc. The synthesis and function of N- and O-glycan branches. Glycoconjugate J. 2000;17:465–483. doi: 10.1023/a:1011010206774. [DOI] [PubMed] [Google Scholar]
- 43.Umana P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17:176–180. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- 44.Barbin K, Stieglmaier J, Saul D, Stieglmaier K, Stockmeyer B, Pfeiffer M, Lang P, Fey GH. Influence of variable N-glycosylation on the cytolytic potential of chimeric CD19 antibodies. J Immunother. 2006;29:122–133. doi: 10.1097/01.cji.0000175684.28615.7b. [DOI] [PubMed] [Google Scholar]
- 45.van Berkel PH, Gerritsen J, van Voskuilen E, Perdok G, Vink T, van de Winkel JG, Parren PW. Rapid production of recombinant human IgG With improved ADCC effector function in a transient expression system. Biotechnol Bioeng. 2010;105:350–357. doi: 10.1002/bit.22535. [DOI] [PubMed] [Google Scholar]
- 46.Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang LX. Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci U S A. 2017;114:3485–3490. doi: 10.1073/pnas.1702173114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrara C, Stuart F, Sondermann P, Brunker P, Umana P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem. 2006;281:5032–5036. doi: 10.1074/jbc.M510171200. [DOI] [PubMed] [Google Scholar]
- 48.Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, Umana P, Benz J. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizushima T, Yagi H, Takemoto E, Shibata-Koyama M, Isoda Y, Iida S, Masuda K, Satoh M, Kato K. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells. 2011;16:1071–1080. doi: 10.1111/j.1365-2443.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibata-Koyama M, Iida S, Misaka H, Mori K, Yano K, Shitara K, Satoh M. Nonfucosylated rituximab potentiates human neutrophil phagocytosis through its high binding for FcgammaRIIIb and MHC class II expression on the phagocytotic neutrophils. Exp Hematol. 2009;37:309–321. doi: 10.1016/j.exphem.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, Bleeker WW, Dechant M, Beyer T, Repp R, van Berkel PH, Vink T, van de Winkel JG, Parren PW, Valerius T. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood. 2008;112:2390–2399. doi: 10.1182/blood-2008-03-144600. [DOI] [PubMed] [Google Scholar]
- 52.Derer S, Glorius P, Schlaeth M, Lohse S, Klausz K, Muchhal U, Desjarlais JR, Humpe A, Valerius T, Peipp M. Increasing FcgammaRIIa affinity of an FcgammaRIII-optimized anti-EGFR antibody restores neutrophil-mediated cytotoxicity. MAbs. 2014;6:409–421. doi: 10.4161/mabs.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomann M, Reckermann K, Reusch D, Prasser J, Tejada ML. Fc-galactosylation modulates antibody-dependent cellular cytotoxicity of therapeutic antibodies. Mol Immunol. 2016;73:69–75. doi: 10.1016/j.molimm.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog. 2005;21:1644–1652. doi: 10.1021/bp050228w. [DOI] [PubMed] [Google Scholar]
- 55.Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol. 2007;44:1524–1534. doi: 10.1016/j.molimm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Naso MF, Tam SH, Scallon BJ, Raju TS. Engineering host cell lines to reduce terminal sialylation of secreted antibodies. MAbs. 2010;2:519–527. doi: 10.4161/mabs.2.5.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, Fox JA, Presta LG. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 58.Bowles JA, Wang SY, Link BK, Allan B, Beuerlein G, Campbell MA, Marquis D, Ondek B, Wooldridge JE, Smith BJ, Breitmeyer JB, Weiner GJ. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108:2648–2654. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, Vielmetter J, Carmichael DF, Hayes RJ, Dahiyat BI. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, Huang L, Vijh S, Johnson S, Bonvini E, Koenig S. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67:8882–8890. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 61.Horton HM, Bernett MJ, Pong E, Peipp M, Karki S, Chu SY, Richards JO, Vostiar I, Joyce PF, Repp R, Desjarlais JR, Zhukovsky EA. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68:8049–8057. doi: 10.1158/0008-5472.CAN-08-2268. [DOI] [PubMed] [Google Scholar]
- 62.Kellner C, Zhukovsky EA, Potzke A, Bruggemann M, Schrauder A, Schrappe M, Kneba M, Repp R, Humpe A, Gramatzki M, Peipp M. The Fc-engineered CD19 antibody MOR208 (XmAb5574) induces natural killer cell-mediated lysis of acute lymphoblastic leukemia cells from pediatric and adult patients. Leukemia. 2013;27:1595–1598. doi: 10.1038/leu.2012.373. [DOI] [PubMed] [Google Scholar]
- 63.Zalevsky J, Leung IW, Karki S, Chu SY, Zhukovsky EA, Desjarlais JR, Carmichael DF, Lawrence CE. The impact of Fc engineering on an anti-CD19 antibody: increased Fcgamma receptor affinity enhances B-cell clearing in nonhuman primates. Blood. 2009;113:3735–3743. doi: 10.1182/blood-2008-10-182048. [DOI] [PubMed] [Google Scholar]
- 64.Koerner SP, Andre MC, Leibold JS, Kousis PC, Kubler A, Pal M, Haen SP, Buhring HJ, Grosse-Hovest L, Jung G, Salih HR. An Fc-optimized CD133 antibody for induction of NK cell reactivity against myeloid leukemia. Leukemia. 2017;31:459–469. doi: 10.1038/leu.2016.194. [DOI] [PubMed] [Google Scholar]
- 65.Kelton W, Mehta N, Charab W, Lee J, Lee CH, Kojima T, Kang TH, Georgiou G. IgGA: a ‘cross-isotype’ engineered human Fc antibody domain that displays both IgG-like and IgA-like effector functions. Chem Biol. 2014;21:1603–1609. doi: 10.1016/j.chembiol.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 66.Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 67.Repp R, Kellner C, Muskulus A, Staudinger M, Nodehi SM, Glorius P, Akramiene D, Dechant M, Fey GH, van Berkel PH, van de Winkel JG, Parren PW, Valerius T, Gramatzki M, Peipp M. Combined Fc-protein- and Fc-glyco-engineering of scFv-Fc fusion proteins synergistically enhances CD16a binding but does not further enhance NK-cell mediated ADCC. J Immunol Methods. 2011;373:67–78. doi: 10.1016/j.jim.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Masuda K, Kubota T, Kaneko E, Iida S, Wakitani M, Kobayashi-Natsume Y, Kubota A, Shitara K, Nakamura K. Enhanced binding affinity for FcgammaRIIIa of fucose-negative antibody is sufficient to induce maximal antibody-dependent cellular cytotoxicity. Mol Immunol. 2007;44:3122–3131. doi: 10.1016/j.molimm.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Idusogie EE, Wong PY, Presta LG, Gazzano-Santoro H, Totpal K, Ultsch M, Mulkerrin MG. Engineered antibodies with increased activity to recruit complement. J Immunol. 2001;166:2571–2575. doi: 10.4049/jimmunol.166.4.2571. [DOI] [PubMed] [Google Scholar]
- 70.Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs. 2010;2 doi: 10.4161/mabs.2.2.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Natsume A, In M, Takamura H, Nakagawa T, Shimizu Y, Kitajima K, Wakitani M, Ohta S, Satoh M, Shitara K, Niwa R. Engineered antibodies of IgG1/IgG3 mixed isotype with enhanced cytotoxic activities. Cancer Res. 2008;68:3863–3872. doi: 10.1158/0008-5472.CAN-07-6297. [DOI] [PubMed] [Google Scholar]
- 72.Quast I, Keller CW, Maurer MA, Giddens JP, Tackenberg B, Wang LX, Munz C, Nimmerjahn F, Dalakas MC, Lunemann JD. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J Clin Invest. 2015;125:4160–4170. doi: 10.1172/JCI82695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wirt T, Rosskopf S, Rösner T, Eichholz K, Kahrs A, Lutz S, Kretschmer A, Valerius T, Klausz K, Otte A, Gramatzki M, Peipp M, Kellner C. An Fc double-engineered CD20 antibody with enhanced ability to trigger complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity. Transfus Med Hemother. 2017;44 doi: 10.1159/000479978. DOI: 10.1159/000479978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Jong RN, Beurskens FJ, Verploegen S, Strumane K, van Kampen MD, Voorhorst M, Horstman W, Engelberts PJ, Oostindie SC, Wang G, Heck AJ, Schuurman J, Parren PW. A Novel platform for the potentiation of therapeutic antibodies based on antigen-dependent formation of IgG hexamers at the cell surface. PLoS Biol. 2016;14:e1002344. doi: 10.1371/journal.pbio.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ, van de Winkel JG, Wilson IA, Koster AJ, Taylor RP, Saphire EO, Burton DR, Schuurman J, Gros P, Parren PW. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343:1260–1263. doi: 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER, Scallon B, Teplyakov A, Malia TJ, Strohl WR. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods. 2014;65:114–126. doi: 10.1016/j.ymeth.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 77.Beck A, Reichert JM. Marketing approval of mogamulizumab: a triumph for glyco-engineering. MAbs. 2012;4:419–425. doi: 10.4161/mabs.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee HZ, Miller BW, Kwitkowski VE, Ricci S, DelValle P, Saber H, Grillo J, Bullock J, Florian J, Mehrotra N, Ko CW, Nie L, Shapiro M, Tolnay M, Kane RC, Kaminskas E, Justice R, Farrell AT, Pazdur R. U.S. Food and drug administration approval: obinutuzumab in combination with chlorambucil for the treatment of previously untreated chronic lymphocytic leukemia. Clin Cancer Res. 2014;20:3902–3907. doi: 10.1158/1078-0432.CCR-14-0516. [DOI] [PubMed] [Google Scholar]
- 79.Inman BA, Longo TA, Ramalingam S, Harrison MR. Atezolizumab: A PD-L1-blocking antibody for bladder cancer. Clin Cancer Res. 2017;23:1886–1890. doi: 10.1158/1078-0432.CCR-16-1417. [DOI] [PubMed] [Google Scholar]
- 80.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy MS, Illmer T, Opat S, Owen CJ, Samoylova O, Kreuzer KA, Stilgenbauer S, Dohner H, Langerak AW, Ritgen M, Kneba M, Asikanius E, Humphrey K, Wenger M, Hallek M. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 81.Sehn LH, Goy A, Offner FC, Martinelli G, Caballero MD, Gadeberg O, Baetz T, Zelenetz AD, Gaidano G, Fayad LE, Buckstein R, Friedberg JW, Crump M, Jaksic B, Zinzani PL, Padmanabhan Iyer S, Sahin D, Chai A, Fingerle-Rowson G, Press OW. Randomized phase II trial comparing obinutuzumab (GA101) with rituximab in patients with relapsed CD20+ indolent B-cell non-Hodgkin lymphoma: final analysis of the GAUSS study. J Clin Oncol. 2015;33:3467–3474. doi: 10.1200/JCO.2014.59.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clark MR. IgG effector mechanisms. Chem Immunol. 1997;65:88–110. [PubMed] [Google Scholar]
- 83.Nordstrom JL, Gorlatov S, Zhang W, Yang Y, Huang L, Burke S, Li H, Ciccarone V, Zhang T, Stavenhagen J, Koenig S, Stewart SJ, Moore PA, Johnson S, Bonvini E. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res. 2011;13:R123. doi: 10.1186/bcr3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.von Horsten HH, Ogorek C, Blanchard V, Demmler C, Giese C, Winkler K, Kaup M, Berger M, Jordan I, Sandig V. Production of non-fucosylated antibodies by co-expression of heterologous GDP-6-deoxy-D-lyxo-4-hexulose reductase. Glycobiology. 2010;20:1607–1618. doi: 10.1093/glycob/cwq109. [DOI] [PubMed] [Google Scholar]
- 85.Mori K, Kuni-Kamochi R, Yamane-Ohnuki N, Wakitani M, Yamano K, Imai H, Kanda Y, Niwa R, Iida S, Uchida K, Shitara K, Satoh M. Engineering Chinese hamster ovary cells to maximize effector function of produced antibodies using FUT8 siRNA. Biotechnol Bioeng. 2004;88:901–908. doi: 10.1002/bit.20326. [DOI] [PubMed] [Google Scholar]
- 86.Haryadi R, Zhang P, Chan KF, Song Z. CHO-gmt5, a novel CHO glycosylation mutant for producing afucosylated and asialylated recombinant antibodies. Bioengineered. 2013;4:90–94. doi: 10.4161/bioe.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]