Abstract
Purpose
To report a case of severe ocular rosacea following ipilimumab plus nivolumab treatment in a patient with metastatic malignant skin melanoma.
Methods
Case report and review of the literature.
Results
A 68-year-old male with newly diagnosed metastatic malignant cutaneous melanoma was treated with first-line ipilimumab plus nivolumab, which resulted in a partial response. Four months after initiation of treatment, the patient developed red eyelids and conjunctivae, with painful gritty eyes, limiting his capacity to read. Following a diagnosis of severe ocular rosacea and dry eyes, treatment including corticosteroids, antimicrobial agents, and eyelid hygiene was started, and within 3 months, the ocular complaints resolved.
Conclusion
Treatment with checkpoint inhibitor immunotherapy for metastatic melanoma may trigger several ocular immune-related adverse events. This case describes severe ocular rosacea as an adverse event following ipilimumab plus nivolumab treatment.
Keywords: Ocular rosacea, Ipilimumab, Nivolumab, Metastatic melanoma, Toxicity
Established Facts
• Immunotherapy for metastatic melanoma may result in ocular adverse events such as uveitis and orbital inflammation.
Novel Insights
• Ocular rosacea is a rare but potential adverse event of anti-CTLA-4 and anti-PD-1 treatment.
Introduction
The monoclonal antibodies ipilimumab and nivolumab have recently been introduced as therapy for metastatic cutaneous melanoma. Ipilimumab is an antibody directed against inhibitory CTLA-4 proteins on the surface of activated T cells, while nivolumab blocks signalling of the inhibitory PD-1 receptor of tumour-resident T cells, thus enhancing the immune system to attack tumour cells. Treatment with so-called “checkpoint inhibitor immunotherapy” results in a higher survival for patients with metastatic cutaneous melanoma, however, at the cost of sometimes severe immune-related adverse effects (irAEs) [1, 2]. The most common irAEs include dermatologic and gastrointestinal complaints, such as skin rashes and diarrhoea as a result of dermatitis and colitis, respectively, and general fatigue [1, 2], which may be caused by endocrinopathies, including thyroid gland disorders or hypophysitis. Ocular irAEs are rare but have been reported in 1.3% of patients receiving anti-CTLA-4 treatment [3], and 1.6% of patients receiving anti-PD-1 treatment [4]. Typical ocular irAEs are uveitis and orbital inflammation. This report presents a case of severe ocular rosacea following combination treatment with ipilimumab and nivolumab, and reviews the literature regarding ocular surface irAEs.
Case Description
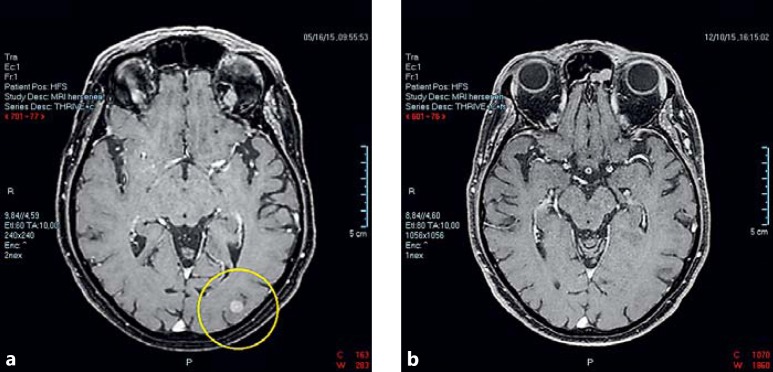
A 68-year-old male was diagnosed in 2015 with metastatic malignant cutaneous melanoma and treated with immunotherapy. He had had a pigmented lesion removed from his back in 2006. The patient was treated in a clinical trial (NCT01621490) and received, starting June 2015, 4 doses of ipilimumab in combination with nivolumab, followed by 5 doses of nivolumab monotherapy. Subsequently, a partial response (RECIST 1.1 criteria) [5] was seen, with regression of cerebral and extracerebral metastases on magnetic resonance imaging and computed tomography (Fig. 1). Adverse effects included diarrhoea, skin rash, and renal insufficiency, for which the immunotherapy was interrupted for 1 month and for which the patient received prednisone in August 2015.
Fig. 1.
Magnetic resonance imaging scans of cerebral metastasis. a Cerebral metastasis of the cutaneous melanoma at baseline. b Seven months later, the lesions have regressed following ipilimumab plus nivolumab treatment.
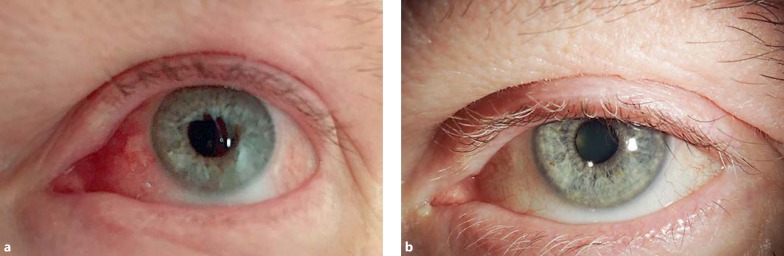
Prior to the immunotherapy, the patient had had some minor complaints of dry eyes, without the need to visit an ophthalmologist. In late October 2015, 4 months after the start of immunotherapy, when prednisone for other adverse events had been tapered, the patient developed new complaints of dry eyes, aggravating in December 2015 to complaints of severe dry eyes, redness of periorbital skin, swelling of his face, and nasal congestion. At that time, he also developed asymptomatic grade 4 lipase and grade 3 amylase elevations, without clinical signs of pancreatitis, and symptomatic adrenal insufficiency, for which hydrocortisone substitution therapy was started. His immunotherapy treatment was discontinued permanently. The patient received artificial tears and in February 2016, prednisone was restarted at 30 mg per day for facial swelling and nasal congestion, which reduced the facial swelling and nasal congestion, but not the ocular problems (Fig. 2a); this led to referral of the patient to the Department of Ophthalmology of the Leiden University Medical Center in March 2016. On examination, the patient had typical facial rosacea, bilateral severe redness of the eyelids with many telangiectasias, congested Meibomian glands with surrounding inflammation, severe nasal and temporal conjunctival injection, diffuse severe punctate keratitis with adherent mucus, and no signs of uveitis or any other intraocular problems. Schirmer's test for tear secretion was less than 5 mm for each eye. Upon a diagnosis of severe ocular rosacea, topical treatment was started with corticosteroids (fluorometholone), a steroidal/antimicrobial ointment (hydrocortisone/oxytetracycline/polymyxine B) for the eyelids, and eyelid scrubbing twice daily. Lubricants were continued. Within 3 months, the ocular complaints had resolved and the corneal epithelium had recovered, showing a smooth and shiny surface without any punctate staining. There were telangiectasias on the lower eyelids, but no Meibomian congestion or inflammation, with excellent oil production. The patient experienced a great relieve and had discontinued the use of corticosteroids (Fig. 2b). Treatment was continued with eyelid hygiene and lubricants.
Fig. 2.
Slit-lamp photography. a Ocular rosacea after ipilimumab and nivolumab treatment. The redness of the eyelids and injection of the conjunctiva are clearly visible. The left eye is shown; both eyes had a similar appearance. b After adequate treatment for the ocular rosacea, the redness and inflammation have disappeared.
Discussion
Several ocular irAEs have been described with ipilimumab treatment, such as uveitis, vitritis, peripheral ulcerative keratitis, choroiditis, serous retinal detachment, and orbitopathy [6]. Only a few ocular irAEs have been described during nivolumab treatment, which include dry eyes, conjunctivitis, blurred vision, and iritis [4, 7, 8]. Our case presents ocular rosacea following treatment with ipilimumab plus nivolumab. A summary of the literature regarding irAEs of the ocular surface is provided (Table 1).
Table 1.
Overview of ocular surface immune-related adverse events for ipilimumab and nivolumab in the literature
| First author | Age/sex | Current immunotherapy | Previous immunotherapies | Ocular surface immune-related adverse events | Treatment for immune-related adverse events | Outcome of ocular complaints |
|---|---|---|---|---|---|---|
| Papavasileiou [6] | 55/F | Ipilimumab, bevacizumab | Not reported | Peripheral ulcerative keratitis | Topical corticosteroids, topical antibiotics, acyclovir | Resolved |
| Voskens [17], 2013 | 57/M | Ipilimumab | DTIC, sorafenib | Conjunctivitis | Lubrication | Resolved |
| Voskens [15], 2012 | 53/F | Ipilimumab | Dacarbazine, sorafenib | Iridocyclitis, marginal keratitis | Systemic corticosteroids | Resolved |
| Henderson [18] | 55/M | Ipilimumab | Not reported | Episcleritis, orbital inflammation | Topical steroids | Improved |
| Zimmer [4] | 78/M | Nivolumab | Interferon-α | Conjunctivitis | Topical corticosteroids | Not resolved |
| 49/F | Nivolumab | Vemurafenib, dabrafenib, ipilimumab | Dry eyes | Topical therapy | Not resolved | |
| Nguyen [7] | 58/M | Nivolumab | Not reported | Dry eyes; corneal perforation | Lubrication, topical cyclosporine, punctal occlusion | Improved |
| 46/F | Nivolumab | Not reported | Dry eyes | Lubrication, topical cyclosporine | Improved | |
| Montaudie [16] | 56/M | Nivolumab | Not reported | Dry eyes, sarcoidosis | Systemic corticosteroids | Resolved |
As checkpoint inhibitors stimulate immune responses rather aspecifically, adverse immunological events can be expected. Immune responses involve the adaptive immune response and can be due to T-cell responses. Our case suggests that adaptive immune responses play an important role in rosacea, as has also been suggested by Nguyen et al. [7] in the occurrence of complaints of dry eyes following nivolumab. The importance of T-cell-mediated autoimmunity in the lacrimal glands, which leads to lack of tear production, has previously been indicated [9]. Although the pathophysiology of ocular rosacea is still not fully understood, a local immune response is considered important [10]. Analysis of the skin in rosacea patients has shown an elevated presence of T cells, with mainly CD4+ expression and CD8+ expression to a lesser extent [11]. Treatment with either ipilimumab or nivolumab has been shown to increase the level of CD4+ and CD8+ cells in tumour tissue, thus supporting the suggestion of T-cell mediation in the pathophysiology of our case [12, 13]. Parallel to the situation in dry eyes, treatment with ipilimumab and nivolumab may probably have triggered a T-cell-mediated autoimmune response in the eyelids, leading to Meibomian gland disease, in combination with tear gland disease, which is responsible for the lack of tear production.
The patient in our case responded initially to systemic corticosteroids and had complete resolution of ocular irAEs after topical treatment with corticosteroids and antimicrobial agents, together with eyelid hygiene. Most ocular irAEs following immunotherapy have been successfully treated with topical corticosteroids, and only rarely systemic therapy has been required [14]. Systemic steroids were required for ocular irAEs after immunotherapy in a patient developing iridocyclitis and marginal keratitis [15] and a patient developing dry eyes and sarcoidosis [16]. In one case, punctal occlusion was used for dry eye treatment [7], but the authors noted the ambiguity of this procedure, which could worsen the complaints of dry eyes when clearance of inflammatory mediators from the ocular surface is delayed.
This report shows ocular rosacea as a rare but potential irAE of the ocular surface after treatment with immunotherapy for metastatic melanoma. As in most ocular irAEs, our patient responded well to corticosteroids. With immunotherapy being approved for an increasing number of indications, clinicians should be aware of the potential adverse events this treatment may elicit, including rare events, such as ocular rosacea.
Statement of Ethics
Written informed consent was obtained from the patient for this report. The institute's medical ethics committee of the Leiden University Medical Center declared that there was no objection to this study.
Disclosure Statement
N.J.B. and M.J.J. declare no conflicts of interest. J.B.A.G.H. reports having received institutional research grants from BMS, MSD, and GSK and having advisory roles for MSD, BMS, Pfizer, Roche, Ibsen, Novartis, and NEON Therapeutics.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr, Lao CD, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 3.Tarhini A. Immune-mediated adverse events associated with ipilimumab CTLA-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica (Cairo) 2013;2013:857519. doi: 10.1155/2013/857519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I, Schmidgen MI, Gutzmer R, Utikal JS, Goppner D, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210–225. doi: 10.1016/j.ejca.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Papavasileiou E, Prasad S, Freitag SK, Sobrin L, Lobo AM. Ipilimumab-induced ocular and orbital inflammation - a case series and review of the literature. Ocul Immunol Inflamm. 2016;24:140–146. doi: 10.3109/09273948.2014.1001858. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen AT, Elia M, Materin MA, Sznol M, Chow J. Cyclosporine for dry eye associated with nivolumab: a case progressing to corneal perforation. Cornea. 2016;35:399–401. doi: 10.1097/ICO.0000000000000724. [DOI] [PubMed] [Google Scholar]
- 8.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClellan AJ, Volpe EA, Zhang X, Darlington GJ, Li DQ, Pflugfelder SC, de Paiva CS. Ocular surface disease and dacryoadenitis in aging C57BL/6 mice. Am J Pathol. 2014;184:631–643. doi: 10.1016/j.ajpath.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes AD, Steinhoff M. Integrative concepts of rosacea pathophysiology, clinical presentation and new therapeutics. Exp Dermatol. 2016 doi: 10.1111/exd.13143. DOI: 10.1111/exd.13143. [DOI] [PubMed] [Google Scholar]
- 11.Buhl T, Sulk M, Nowak P, Buddenkotte J, McDonald I, Aubert J, Carlavan I, Deret S, Reiniche P, Rivier M, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015;135:2198–2208. doi: 10.1038/jid.2015.141. [DOI] [PubMed] [Google Scholar]
- 12.Weber JS, Hamid O, Chasalow SD, Wu DY, Parker SM, Galbraith S, Gnjatic S, Berman D. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother. 2012;35:89–97. doi: 10.1097/CJI.0b013e31823aa41c. [DOI] [PubMed] [Google Scholar]
- 13.Choueiri TK, Fishman MN, Escudier B, McDermott DF, Drake CG, Kluger H, Stadler WM, Perez-Gracia JL, McNeel DG, Curti B, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2839. DOI: 10.1158/1078-0432.CCR-15-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fierz FC, Meier F, Chaloupka K, Boni C. Intraocular inflammation associated with new therapies for cutaneous melanoma - case series and review. Klin Monbl Augenheilkd. 2016;233:540–544. doi: 10.1055/s-0042-102668. [DOI] [PubMed] [Google Scholar]
- 15.Voskens C, Cavallaro A, Erdmann M, Dippel O, Kaempgen E, Schuler G, Schuler-Thurner B, Heinzerling L. Anti-cytotoxic T-cell lymphocyte antigen-4-induced regression of spinal cord metastases in association with renal failure, atypical pneumonia, vision loss, and hearing loss. J Clin Oncol. 2012;30:e356–e357. doi: 10.1200/JCO.2011.41.4359. [DOI] [PubMed] [Google Scholar]
- 16.Montaudie H, Pradelli J, Passeron T, Lacour JP, Leroy S. Pulmonary sarcoid-like granulomatosis induced by nivolumab. Br J Dermatol. 2016 doi: 10.1111/bjd.14808. DOI: 10.1111/bjd.14808. [DOI] [PubMed] [Google Scholar]
- 17.Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, Bergmann T, Bockmeyer CL, Eigentler T, Fluck M, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson AD, Thomas DA. A case report of orbital inflammatory syndrome secondary to ipilimumab. Ophthal Plast Reconstr Surg. 2015;31:e68–e70. doi: 10.1097/IOP.0000000000000081. [DOI] [PubMed] [Google Scholar]