Abstract
Background
The benefit of neoadjuvant therapy (NAC) in patients with estrogen receptor positive (ER+) HER2− breast cancers and in invasive lobular cancer (ILC) is uncertain due to low rates of pathologic complete response (pCR). Our aim was to determine if pathologic features can identify subsets likely to benefit from NAC.
Methods
Patients with stage I–III ER+, HER2− breast cancer receiving NAC were retrospectively reviewed. Endpoints were downstaging to breast-conserving surgery (BCS) and nodal pCR after NAC. Patients were grouped by progesterone receptor (PR) status and grade/differentiation (high grade or poor [HP] vs non-HP).
Results
From 2007–2016, 402 ER+/HER2− cancers in patients receiving NAC were identified. Median age was 50 years, 98% were clinical stage II–III, and 75% cN+. Overall pCR rate was 5%; breast pCR in 7%, nodal pCR in 15% of cN+ patients (p<.0001). Patients with ILC initially ineligible for BCS (n=56) were less likely to downstage than those with ductal (n=183), 16% vs 48% (p≤.0001); with a similar trend in the axilla (p=.086). Rates of BCS eligibility after NAC were highest in PR−/HP (62%) and lowest in PR+/non-HP (29%) patients (p=.005). In the axilla, nodal pCR among cN+ patients (n=301) ranged from 0–35% (p<.0001) within these groups, and was most frequent in PR−/HP patients.
Conclusions
ER+/HER2− patients most likely to benefit from NAC are those with PR− and HP tumors. Patients with ILC are unlikely to downstage in the breast or axilla compared to IDC. Use of these criteria can assist in defining initial treatment approach.
Keywords: pathologic features, breast cancer, neoadjuvant chemotherapy, estrogen receptor, hormone receptor, downstaging, nodal pathologic complete response
INTRODUCTION
Neoadjuvant chemotherapy (NAC) in patients with operable breast cancer offers several potential benefits, including downstaging the primary tumor to allow breast conservation1,2 as well as eradicating axillary nodal disease with a reduction in the need for axillary dissection.1–3 Rates of pathologic complete response (pCR) to NAC vary widely by breast cancer subtype and are consistently lower in hormone receptor positive (HR+)/HER2 negative (HER2−) breast cancers, ranging from 2–11%, than in triple-negative and HER2+ breast cancer where pCR rates range from 18–45%.4–9 Although pCR rates are lowest in HR+/HER2− disease, patients with this subtype have a high partial response rate to NAC4, which may allow breast-conserving surgery (BCS) in patients initially requiring mastectomy, but there are inconsistent data regarding the rates of BCS after NAC for HR+/HER2− patients compared to other subtypes.4,9
Patients with invasive lobular carcinoma (ILC), which is classically estrogen receptor positive (ER+), low grade, and well differentiated, have also been shown to have a poor response to NAC, with significantly lower rates of pCR than seen in patients with infiltrating ductal cancer.10–12 The lower rates of pCR translate into lower rates of BCS after NAC than seen with invasive ductal carcinoma (IDC)9–12, but whether these differences are the result of histology per se or other features, such as grade and receptor status, has been questioned.13,14
The issue of selection of patients likely to benefit from NAC has achieved new importance with the demonstration in prospective trials15–17 that patients presenting with axillary nodal metastases can be accurately staged with sentinel lymph node biopsy after NAC, allowing the avoidance of axillary dissection in those who achieve nodal pCR. Additionally, rates of pCR in the axillary nodes have been shown to be higher than in the breast in HR+/HER2− patients4,18, providing further impetus for the use of NAC in this subgroup of patients. The purpose of this study was to compare rates of breast and nodal pCR in patients with infiltrating ductal and infiltrating lobular carcinoma, and to examine the impact of routinely reported pathologic features on the rate of pCR in HR+/HER2− and downstaging to BCS in patients receiving NAC.
METHODS
Study population
This was an Institutional Review Board-approved, Health Insurance Portability and Accountability Act (HIPAA)-compliant study. A retrospective review of the Memorial Sloan Kettering Cancer Center database was conducted to identify all patients with stage I–III breast cancer treated with NAC between 2007–2016. Patients were eligible for inclusion if they were ER+ and/or progesterone receptor positive (PR+) and HER2− based on their pretreatment core biopsy. Hormone receptor positivity was defined as ≥10% of cells staining positive for ER or PR. This definition was chosen since there is little debate about the use of NAC in patients with lower levels of hormone receptor expression. HER2 receptor was defined as negative with immunohistochemical staining of 0 or 1+, or fluorescent in situ hybridization that was not-amplified. Patients were excluded if they had an excisional biopsy for diagnosis, or if any portion of their surgery, including sentinel lymph node biopsy, was completed prior to receipt of NAC. Patients previously treated with breast-conservation therapy with an ipsilateral breast cancer recurrence were also excluded.
The primary endpoints of the study were breast tumor downstaging to allow BCS in patients initially requiring mastectomy, and nodal pCR. Upfront and post-NAC eligibility for BCS was recorded prospectively by the treating breast surgeon. Patients eligible for BCS at presentation or those requiring mastectomy due to contraindications to BCS, such as inflammatory breast cancer or multicentric disease, were excluded from the breast endpoint calculation, while patients who were clinically node negative (cN0) at presentation were excluded from the nodal pCR endpoint calculation. pCR in this study was defined as the absence of invasive and in situ disease in the breast and the nodes.
Clinicopathologic features
Patients were considered clinically node positive (cN+) if they had suspicious palpable lymph nodes at presentation or if carcinoma was identified in the lymph nodes by needle biopsy. Pathologic features collected from the initial core biopsy pathology reports included ER and PR expression, nuclear grade, differentiation using the Scarff –Bloom-Richardson classification19, and histology. Patients with mixed ductal and lobular histology were classified as having lobular histology. Classic lobular carcinomas where classified as grade 1 and well differentiated when not otherwise specified in the pathology report.
The associations between each of the pathologic features and downstaging to eligibility for BCS or nodal pCR were assessed using the Chi-square test, and multivariate logistic regression analysis. A subsequent analysis was based on grouping of PR status and grade/differentiation, with PR+ vs PR− grouped with high grade or poor differentiation vs non-high grade and non-poor differentiation. These associations were tested using the Chi-square test. The 4 ER−/PR+ patients were excluded. All analyses were conducted in SAS v 9.4. P-values less than 0.05 were considered statistically significant.
RESULTS
From 2007–2016, 391 patients, 11 with bilateral cancer, receiving NAC for HR+/HER2− cancers were identified, yielding a sample of 402 cancers. Clinicopathologic characteristics of the study population are listed in Table 1. Median patient age was 50 years (range 27–79 years), and median clinical tumor size was 5.0 cm. 68% of patients had clinical T2 and T3 tumors, and 301 (75%) were cN+ at presentation. Of the cN+ patients, 45 (15%) had suspicious palpable nodal disease and 256 (85%) had biopsy-confirmed nodal involvement. 310 (77%) cancers were ductal and 91 (23%) were pure or mixed lobular cancers. The clinicopathologic characteristics of the ductal and lobular groups are compared in Table 1. Ductal cancers were more commonly high grade or poorly differentiated than lobular cancers. Doxorubicin, cyclophospahamide, and a taxane was the most common type of chemotherapy, received in 390 (97%) cases.
Table 1.
Clinicopathologic characteristics
| Clinicopathologic Characteristics |
Total (%) n=402 |
Ductal n=310 |
Lobular n=91 |
p-value (IDC vs ILC) |
|---|---|---|---|---|
| Age, years, median (range) | 50 (27, 79) | |||
| Clinical Tumor size (median) | 5.0 cm | |||
| Clinical T | 0.004* | |||
| T1/T2 | 181 (45%) | 152 (49%) | 29 (32%) | |
| T3/T4 | 219 (54%) | 157 (51%) | 62 (68%) | |
| Tx | 2 (0.5%) | 1 (0.3%) | 0 | |
| Clinical N | 0.0002 | |||
| N0 | 101 (25%) | 65 (21%) | 36 (40%) | |
| N1 | 252 (63%) | 211 (68%) | 41 (45%) | |
| N2/N3 | 49 (12%) | 34 (11%) | 14 (15%) | |
| Clinical stage | 0.184 | |||
| 1 | 10 (2%) | 6 (2%) | 4 (4%) | |
| 2 | 181 (45%) | 146 (47%) | 35 (38%) | |
| 3 | 211 (52%) | 158 (51%) | 52 (57%) | |
| Differentiation | <0.0001* | |||
| Well/Moderate | 186 (46%) | 115 (37%) | 71 (78%) | |
| Poor | 198 (49%) | 186 (60%) | 12 (13%) | |
| Unknown | 18 (4%) | 9 (3%) | 8 (9%) | |
| Nuclear grade | <0.0001* | |||
| 1/2 | 188 (47%) | 113 (36%) | 75 (82%) | |
| 3 | 115 (29%) | 109 (35%) | 6 (7%) | |
| Unknown | 99 (25%) | 88 (28%) | 10 (11%) | |
| Histology | NA | |||
| Ductal | 310 (77%) | 310 (100%) | NA | |
| Lobular | 91 (23%) | NA | 91 (100%) | |
| Unknown | 1 (0.2%) |
unknowns excluded from analysis
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma
The overall pCR rate was 5% (n=21). Breast pCR was seen in 7% (n=27) of the 402 cancers and nodal pCR in 15% (n=45) of the 301 cN+ patients (p<.001). Overall pCR was more common in ductal than lobular cancers, with 6% (n=19) of ductal carcinomas and 1% (n=1) of lobular carcinomas having pCR (p=0.055). One occult cancer presenting with nodal metastases with unknown histology had a pCR.
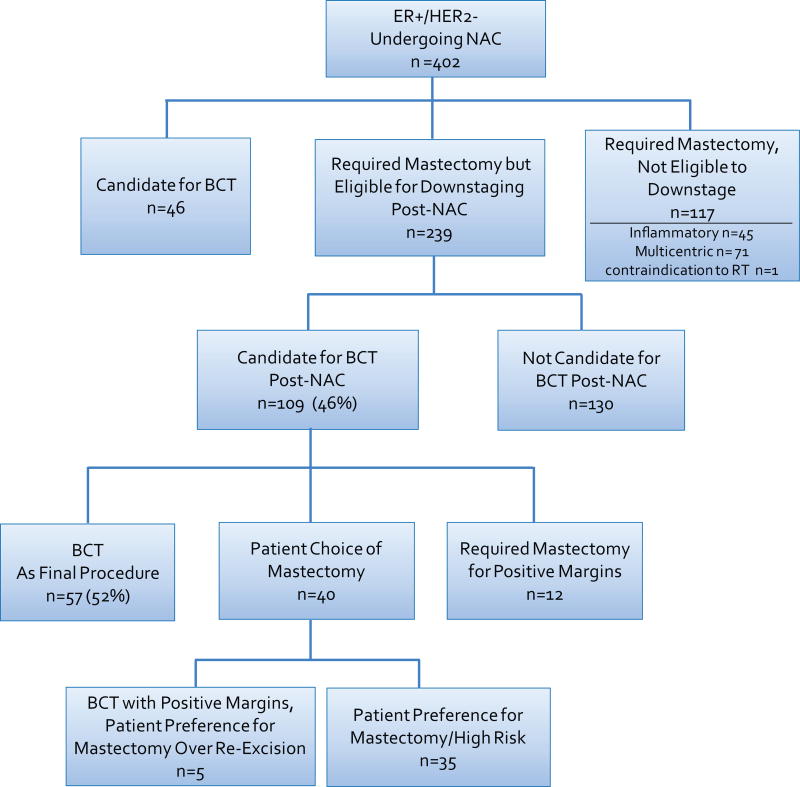
239 of the 402 cancers were potentially eligible for downstaging to BCS with NAC (Fig. 1), and 109 were felt to be candidates for BCS post treatment. 12 of these patients ultimately required mastectomy. This resulted in 97 (41%) patients who had BCS or were eligible to do so but opted for mastectomy. These 97 patients were considered successfully downstaged for BCS after NAC.
Fig. 1.
Breast-conserving surgery cohort
ER, estrogen receptor; NAC, neoadjuvant chemotherapy; BCT, breast-conserving therapy; RT, radiation therapy
Multiple clinicopathologic features were associated with downstaging to BCS and nodal pCR in univariate analysis (Table 2). A significantly higher rate of downstaging to BCS was seen in patients with IDC compared to ILC (48% vs 16%, p<0.0001). Axillary pCR was also more frequent among ductal carcinomas, but this difference did not reach statistical significance (16% vs 7%, p=0.086). Poorly differentiated tumors and those lacking PR expression were significantly associated with downstaging to BCS, while grade, differentiation, and lack of PR expression were all significantly associated with nodal pCR. In a multivariate model (Table 3) including differentiation, PR status, and histology, which was limited to patients with complete data, histology was significantly associated with downstaging to BCS. Patients with lobular carcinomas were 78% less likely to downstage (odds ratio [OR] 0.22, p=0.0007) than those with ductal carcinoma. The only factor that remained significantly associated with nodal pCR was poor differentiation (OR 2.667, p=0.014).
Table 2.
Univariate analysis of rate of breast and nodal downstaging for various pathologic features
| Breast | Axilla | |||
|---|---|---|---|---|
| Tumor Pathologic Characteristic | Downstaged to BCS (n=239) |
p-value | Nodal pCR (n=301) |
p-value |
| Histology* | <0.0001 | 0.086 | ||
| Ductal | 48% (88/183) | 16% (40/245) | ||
| Lobular | 16% (9/56) | 7% (4/55) | ||
| Differentiation* | 0.010 | 0.002 | ||
| Well/Moderate | 33% (34/103) | 8% (10/130) | ||
| Poor | 50% (61/122) | 21% (32/156) | ||
| Nuclear grade* | 0.058 | 0.0001 | ||
| 1/2 | 37% (42/113) | 5% (7/136) | ||
| 3 | 51% (36/70) | 26% (24/92) | ||
| PR status** | 0.030 | 0.024 | ||
| PR+ | 36% (61/169) | 11% (25/219) | ||
| PR− | 52% (34/66) | 22% (17/78) | ||
unknowns excluded
excludes 4ER−PR+ patients
BCS, breast-conserving surgery; pCR, pathologic complete response; PR, progesterone receptor
Table 3.
Multivariate analysis of rate of breast and axillary downstaging for various pathologic features
| Breast *n=225; 95 events |
Axilla *n=286; 42 events |
|||
|---|---|---|---|---|
| Tumor Pathologic Characteristic | Downstaging to BCS OR |
p-value | Nodal pCR OR |
p-value |
| Histology (lobular vs ductal) | 0.223 | 0.0007 | 0.493 | 0.276 |
| Differentiation (poor vs well/moderate) | 1.30 | 0.397 | 2.667 | 0.014 |
| PR status (− vs +) | 1.59 | 0.139 | 1.89 | 0.077 |
unknowns excluded
OR, odds ratio; BCS, breast-conserving surgery; pCR, pathologic complete response; PR, progesterone receptor
We also performed an analysis examining factors which were significant on univariate analysis, including grade, differentiation, and PR expression to increase the utility of the model in patients with infiltrating ductal carcinoma. The combination of PR status and grade or differentiation identified patients with significantly different likelihoods of downstaging to BCS (p=0.005) or having nodal pCR (p<0.0001) (Table 4). The greatest likelihood of downstaging in the breast was seen in the PR−/high grade or poorly differentiated tumors (62%) compared to 29% in the PR+/non-high grade, non-poorly differentiated tumors. The PR−/high grade or poorly differentiated group also had the highest rate of nodal pCR (35%). Each of the other groups had a less than 15% rate of nodal pCR.
Table 4.
Rates of breast and axillary downstaging by grouped pathologic features
| Breast | Axilla | |||
|---|---|---|---|---|
| Receptor/Grade/Differentiation | BCS Candidate After NAC (n=239)* |
p-value | Nodal pCR (n=301)** |
p-value |
| PR+/High grade or poorly differentiated | 44% (37/85) | 0.005 | 14% (16/117) | <0.0001 |
| PR+/Non-high grade and not poorly differentiated | 29% (24/82) | 8% (8/98) | ||
| PR−/High grade or poorly differentiated | 62% (26/42) | 35% (17/48) | ||
| PR−/Non-high grade and not poorly differentiated | 35% (8/23) | 0% (0/29) | ||
Analysis excludes 3 patients with unknown grade and differentiation and 4 ER−PR+ patients
Analysis excludes 5 patients with unknown grade and differentiation and 4 ER−PR+ patients
BCS, breast-conserving surgery; NAC, neoadjuvant chemotherapy; PR, progesterone receptor
DISCUSSION
The low rate of pCR in patients with ER+/HER2− breast cancers has raised questions regarding the utility of NAC in this subtype. In patients with ER+/HER2− cancers, pCR is not associated with a survival benefit as is seen in other subtypes20, but achieving a pCR is not the only goal of treatment with NAC. Other benefits include increasing eligibility for BCS and decreasing the rate of axillary nodal metastases. In this study, we found that 41% of patients who were not candidates for BCS at presentation were eligible for BCS after NAC. The low pCR rate in patients with ER+/HER2− cancers has not consistently translated to lower rates of BCS after NAC compared to patients with other subtypes. Boughey et al conducted a retrospective analysis of patients enrolled in the ACOSOG Z1071 trial assessing rates of pCR and BCS based on ER, PR, and HER2 status, and found a significantly lower rate of BCS in HR+/HER2− patients compared to other subtypes (p=0.019).4 In contrast, Straver et al conducted a similar study restricted to the cohort of patients initially requiring mastectomy and found no significant difference in rates of BCS when comparing subtypes (p=0.11).9
The majority of studies assessing BCS rates after NAC have compared the rate of BCS as the final surgical procedure, regardless of patient eligibility for BCS at presentation.4,10–12 We were able to identify whether patients required a mastectomy or were candidates for BCS pre-treatment by surgeon documentation both pre- and post-NAC, and excluded those who had a priori contraindications to BCS, allowing us to more accurately analyze the rate of downstaging and potential benefit of NAC.
While we found a low rate of pCR in the breast of 7%, the use of histologic tumor type, PR expression, and differentiation or nuclear grade identified subsets of patients significantly more likely to respond to NAC, with PR-, poorly differentiated or high-grade cancers having a 62% rate of downstaging to BCS compared to 29% in patients lacking these features. The pooled studies by Cortazar et al20 and von Minckwitz et al21 incorporated tumor grade as a surrogate marker to distinguish luminal A and B subtypes when assessing pCR in HR+ cancers. Both studies found that although HR+/HER2− patients were the least likely to have pCR, the luminal A-like tumors (HR+/HER2−, grade 1 or 2) were approximately 50% less likely to have a pCR than the luminal B - like tumors (HR+/HER2−, grade 3) (Cortazar et al 8% vs 16%, von Minckwitz et al 9% vs 15%, respectively). Lips et al retrospectively analyzed the impact of PR expression on the response to NAC in ER+/HER2− patients22 and found that PR− tumors had a 35% rate of breast-only pCR and near pCR, significantly higher than the 12% seen in PR+ tumors (p<0.001). The group’s neoadjuvant response index (NRI), calculated from breast and axillary response scores, was found to be significantly higher for PR− and luminal B tumors. In the combined PR+/luminal A group, 50% of patients had an NRI below the median compared to only 25% of the PR−/luminal B group. However, they concluded that because excellent responses occurred in both groups, they could not identify a chemo-resistant subgroup.
Our findings support these results; we saw higher chemosensitivity in PR negative and high-grade or poorly differentiated tumors. Our results also confirm that the subset of patients who are both PR negative and high grade or poorly differentiated are the most likely to have primary tumor downstaging as well as axillary pCR. Although Lips et al showed similar results22, the clinical applicability of their NRI score is unknown. We were able to corroborate their results using clinically relevant endpoints. Other investigators have found Ki-67 measurement either initially, or after a short course of endocrine therapy, to be a predictor of response to NAC in ER+/HER2− patients.23,24 Measurement of Ki-67 is not a part of routine pathologic evaluation at Memorial Hospital, so we were unable to evaluate whether Ki-67 results would further refine the identification of a subset of patients most likely to benefit from NAC.
While there are many factors in addition to response to NAC that determine patient suitability for BCS after NAC25, only patients who obtain a nodal pCR are able to avoid axillary dissection. The rate of nodal pCR in our study was 15% among women who initially presented with cN+ disease. When PR expression and grade or differentiation were included in the analysis, we found a 35% rate of nodal pCR in patients with tumors that both lack PR expression and are high grade or poorly differentiated, with a nodal pCR of <15% in each of the other subsets of patients. We observed that nodal pCR was more frequent than breast pCR, even when breast pCR was defined as the absence of invasive carcinoma alone. This is similar to a previous observation from our institution in a smaller cohort of HR+/HER2− patients18 where Mamtani et al observed a nodal pCR rate of 21% for ER+/HER2− patients compared to a breast pCR rate of 5% (p=0.03). This finding suggests a clear role for NAC in ER+ HER2− patients presenting with nodal metastases.
We also attempted to determine if patients with lobular histology obtain any significant benefit from NAC and found that 16% of lobular carcinomas downstaged to become eligible for BCS after NAC, a significantly lower rate than the 48% of ductal carcinomas that downstaged. We saw similar trends when comparing overall pCR and nodal pCR rates for ILC versus IDC. In our study, patients with ILC had a 7% rate of nodal pCR, which was less than half that seen in IDC, and only a 1% overall pCR rate versus 6% in IDC. Delpech et al retrospectively evaluated the pCR rates and BCS rates after NAC in ER+ ductal versus lobular carcinomas.13 They found that BCS was significantly less frequent in ILC than IDC patients (19% vs 34%, p<0.001) and histology remained a predictor of mastectomy after adjusting for age, tumor grade, size, multifocality, stage, and nodal status. Lips et al also evaluated the response to NAC in ILC versus IDC and stratified by subtype.14 When adjusted for HR and HER2 status, lobular histology was not associated with a lower pCR rate than ductal, with HR+/HER2− tumors having a 7% vs 5% pCR rate, respectively (p=0.76). They also found that BCS rates did not significantly differ when evaluated within T stage.
One of the limitations of our study was that several patients were missing data, mainly for nuclear grade or differentiation. Because multivariate analyses were restricted to patients with complete data, they had limited power, limiting the conclusions that can be drawn from their results. However, even with smaller numbers, histology and differentiation were significant predictors of tumor downstaging and nodal PCR, respectively. An important caveat is that patients in our cohort were selected for NAC based on clinicopathologic features or locally advanced disease, and the results observed may not be applicable to all HR+/HER2− breast cancer patients.
Conclusions
Clinically meaningful variation in response to NAC is seen based on features routinely available in core biopsy pathology reports. Knowledge of these response rates in subsets of ER+ patients may assist in determining whether patients should have NAC or surgery as an initial treatment approach.
Synopsis.
Herein we determine whether pathologic features can identify subsets of patients with ER+, HER2− breast cancers likely to benefit from NAC. We conclude that PR and differentiation can be used to identify those ER+ patients most likely to benefit from NAC.
Acknowledgments
The preparation of this manuscript was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Footnotes
The authors have no conflict of interest disclosures to report.
This study was supported by Judy Guitelman, Dan Epstein, and the Dan J. Epstein Family Foundation, and presented in part in podium format at the Society of Surgical Oncology 2017 Annual Cancer Symposium, March 15–18, 2017, Seattle WA.
References
- 1.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 2.van Nes JG, Putter H, Julien JP, Tubiana-Hulin M, van de Vijver M, Bogaerts J, de Vos M, et al. Preoperative chemotherapy is safe in early breast cancer, even after 10 years of follow-up; clinical and translational results from the EORTC trial 10902. Breast Cancer Res Treat. 2009;115(1):101–13. doi: 10.1007/s10549-008-0050-1. [DOI] [PubMed] [Google Scholar]
- 3.Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94(10):1189–200. doi: 10.1002/bjs.5894. [DOI] [PubMed] [Google Scholar]
- 4.Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;260(4):608–14. doi: 10.1097/SLA.0000000000000924. discussion 14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caudle AS, Yu TK, Tucker SL, Bedrosian I, Litton JK, Gonzalez-Angulo AM, Hoffman K, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012;14(3):R83. doi: 10.1186/bcr3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, Hudis C, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242–9. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarneri V, Broglio K, Kau SW, Cristofanilli M, Buzdar AU, Valero V, Buchholz T, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24(7):1037–44. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 8.Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48(18):3342–54. doi: 10.1016/j.ejca.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Straver ME, Rutgers EJ, Rodenhuis S, Linn SC, Loo CE, Wesseling J, Russell NS, et al. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol. 2010;17(9):2411–8. doi: 10.1245/s10434-010-1008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loibl S, Volz C, Mau C, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Response and prognosis after neoadjuvant chemotherapy in 1,051 patients with infiltrating lobular breast carcinoma. Breast Cancer Res Treat. 2014;144(1):153–62. doi: 10.1007/s10549-014-2861-6. [DOI] [PubMed] [Google Scholar]
- 11.Petrelli F, Barni S. Response to neoadjuvant chemotherapy in ductal compared to lobular carcinoma of the breast: a meta-analysis of published trials including 1,764 lobular breast cancer. Breast Cancer Res Treat. 2013;142(2):227–35. doi: 10.1007/s10549-013-2751-3. [DOI] [PubMed] [Google Scholar]
- 12.Truin W, Vugts G, Roumen RM, Maaskant-Braat AJ, Nieuwenhuijzen GA, van der Heiden-van der Loo M, Tjan-Heijnen VC, et al. Differences in Response and Surgical Management with Neoadjuvant Chemotherapy in Invasive Lobular Versus Ductal Breast Cancer. Ann Surg Oncol. 2016;23(1):51–7. doi: 10.1245/s10434-015-4603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delpech Y, Coutant C, Hsu L, Barranger E, Iwamoto T, Barcenas CH, Hortobagyi GN, et al. Clinical benefit from neoadjuvant chemotherapy in oestrogen receptor-positive invasive ductal and lobular carcinomas. Br J Cancer. 2013;108(2):285–91. doi: 10.1038/bjc.2012.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lips EH, Mukhtar RA, Yau C, de Ronde JJ, Livasy C, Carey LA, Loo CE, et al. Lobular histology and response to neoadjuvant chemotherapy in invasive breast cancer. Breast Cancer Res Treat. 2012;136(1):35–43. doi: 10.1007/s10549-012-2233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, Meterissian S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 16.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–61. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, Lebeau A, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 18.Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M, El-Tamer M, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg Oncol. 2016;23(11):3467–74. doi: 10.1245/s10434-016-5246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 20.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 21.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 22.Lips EH, Mulder L, de Ronde JJ, Mandjes IA, Vincent A, Vrancken Peeters MT, Nederlof PM, et al. Neoadjuvant chemotherapy in ER+ HER2− breast cancer: response prediction based on immunohistochemical and molecular characteristics. Breast Cancer Res Treat. 2012;131(3):827–36. doi: 10.1007/s10549-011-1488-0. [DOI] [PubMed] [Google Scholar]
- 23.Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ, DeSchryver K, et al. Ki67 Proliferation Index as a Tool for Chemotherapy Decisions During and After Neoadjuvant Aromatase Inhibitor Treatment of Breast Cancer: Results From the American College of Surgeons Oncology Group Z1031 Trial (Alliance) J Clin Oncol. 2017;35(10):1061–9. doi: 10.1200/JCO.2016.69.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XB, Krishnamurti U, Bhattarai S, Klimov S, Reid MD, O'Regan R, Aneja R. Biomarkers Predicting Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer. Am J Clin Pathol. 2016;145(6):871–8. doi: 10.1093/ajcp/aqw045. [DOI] [PubMed] [Google Scholar]
- 25.Criscitiello C, Curigliano G, Burstein HJ, Wong S, Esposito A, Viale G, Giuliano M, et al. Breast conservation following neoadjuvant therapy for breast cancer in the modern era: Are we losing the opportunity? Eur J Surg Oncol. 2016;42(12):1780–6. doi: 10.1016/j.ejso.2016.10.011. [DOI] [PubMed] [Google Scholar]