Abstract
In October 2012, a cluster of illnesses and deaths was reported in Uganda and was confirmed to be an outbreak of Marburg virus disease (MVD). Patients meeting the case criteria were interviewed using a standard investigation form, and blood specimens were tested for evidence of acute or recent Marburg virus infection by reverse transcription–polymerase chain reaction (RT-PCR) and antibody enzyme-linked immunosorbent assay. The total count of confirmed and probable MVD cases was 26, of which 15 (58%) were fatal. Four of 15 laboratory-confirmed cases (27%) were fatal. Case patients were located in 4 different districts in Uganda, although all chains of transmission originated in Ibanda District, and the earliest case detected had an onset in July 2012. No zoonotic exposures were identified. Symptoms significantly associated with being a MVD case included hiccups, anorexia, fatigue, vomiting, sore throat, and difficulty swallowing. Contact with a case patient and attending a funeral were also significantly associated with being a case. Average RT-PCR cycle threshold values for fatal cases during the acute phase of illness were significantly lower than those for nonfatal cases. Following the institution of contact tracing, active case surveillance, care of patients with isolation precautions, community mobilization, and rapid diagnostic testing, the outbreak was successfully contained 14 days after its initial detection.
Keywords: Marburg virus, filoviruses, hemorrhagic fever, zoonotic disease, outbreak response
Viruses within the family Filoviridae, genera Ebolavirus and Marburgvirus, can cause viral hemorrhagic fever in humans with a high case-fatality rate (CFR; 23%–90%) [1, 2]. The Marburgvirus genus contains 2 closely related viruses, which, together, will be simply referred to here as Marburg virus. Outbreaks of Ebola virus disease (EVD) and Marburg virus disease (MVD) begin when an initial zoonotic transmission from an infected animal to a human occurs, and the transmission is then amplified through close human-to-human contact. Direct and unprotected contact with blood and other body fluids from viremic patients or corpses is the chief means of transmission among humans, and transmission via droplets and fomites is also possible [3, 4].
Repeated detection of Marburg virus–specific RNA and isolation of virus from bats captured in the wild has demonstrated that the reservoir of currently known Marburg viruses is the Egyptian fruit bat (Rousettus aegyptiacus) [5]. Previous cases and several outbreaks of MVD have been linked to exposure in caves and/or mines inhabited by Egyptian fruit bats [6–9]. Previous outbreaks of MVD in Uganda occurred in 2007 and 2008, with 4 cases among miners entering the Kitaka mine in Ibanda District [7] and 2 isolated cases among tourists entering Python Cave in Queen Elizabeth National Park [8, 9]. Primates are also susceptible to viruses in the Ebolavirus and Marburgvirus genera, which cause clinical signs of EVD and MVD, respectively, and transmission to humans has occurred following exposure to sick or dead primates [10–13].
Previous EVD and MVD outbreaks with high case counts (315 cases of EVD were reported in the Democratic Republic of the Congo during 1995, and 425 cases of EVD were reported in Uganda during 2000) [4, 14] and high CFRs (88% among individuals with MVD in Angola during 2005) [15] have led the international community to develop well-described procedures for rapidly investigating and extinguishing confirmed outbreaks by halting the chain of human-to-human transmission [16–19]. Outbreak measures coordinated by national and subnational multisectoral, multidisciplinary outbreak coordination committees seek to halt the chain of virus transmission by identifying all current cases, instituting barrier nursing practices in hospital facilities designed to contain patients in isolation, and closely following contacts of cases, with prompt isolation and testing of patients if clinical signs develop. Engagement of the community through education and social mobilization are essential for the success of responses. Community members must be aware of disease transmission risks and comfortable with sending patients to treatment centers and providing information to the investigation teams to ensure complete ascertainment of cases and identification of all contacts [4, 17].
The presence of real-time diagnostic capacity in the field is vital to providing rapid information regarding patient infection status, prognosis, and risk of virus transmission. The symptoms of EVD and MVD are frequently nonspecific, with fever, fatigue, and gastrointestinal signs predominating, thus making mandatory laboratory confirmation of infection necessary for discerning these diseases from other, more common causes of febrile illness. Detection of virus RNA (via reverse transcription– polymerase chain reaction [RT-PCR]) or antigen (via enzyme-linked immunosorbent assay [ELISA]) in serum or blood indicates that the patient is currently viremic and can be infectious to others [20], which is important for determining whether a patient should be placed in or remain under isolation conditions. Quantitative RT-PCR (qRT-PCR) also provides a means to determine the relative virus load, which can inform the survival prognosis [21].Immunoglobulin M(IgM) antibody (detected via ELISA) is a marker of recent infection, owing to the disappearance of IgM 2–3 months after infection onset, whereas immunoglobulin G (IgG) can persist for ≥10 years [22].
On 16 October 2012, a cluster of illnesses and deaths was reported in Kabale District, western Uganda. Blood specimens were collected from 3 affected individuals, and on 18 October, testing at the Uganda Virus Research Institute (UVRI)/Centers for Disease Control and Prevention (CDC) hemorrhagic fever laboratory confirmed Marburg virus infection in these patients. The outbreak response was led by the Uganda Ministry of Health, with assistance from the World Health Organization, the CDC, Médecins sans Frontières, the Uganda Peoples Defense Forces, the Uganda Red Cross, and the African Field Epidemiology Network. In this article, we describe findings of the outbreak investigation and diagnostic testing and review the clinical symptoms of cases.
MATERIALS AND METHODS
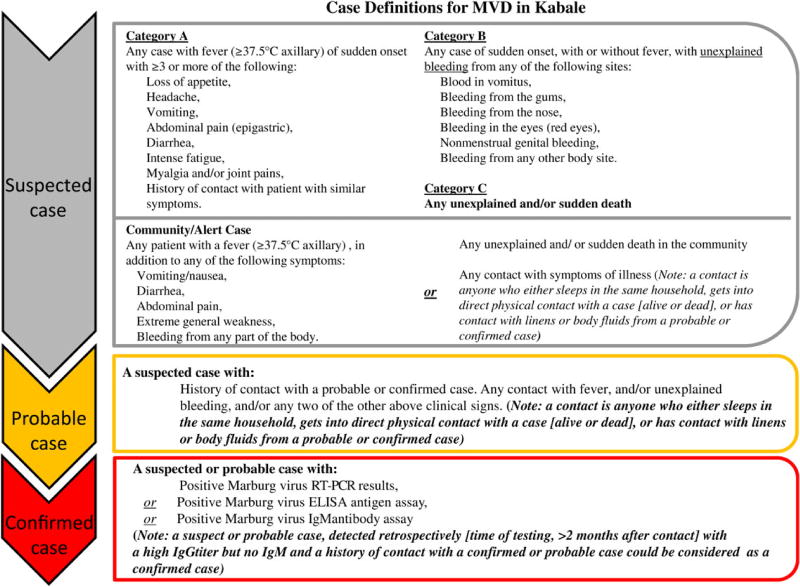
The case definitions for MVD in this outbreak were as follows (Figure 1). A suspected case patient had either (1) fever (axillary temperature, ≥37.5°C) and ≥3 symptoms indicative of MVD, (2) any unexplained bleeding, or (3) any unexplained or sudden death. A probable case patient had illness meeting the suspected case definition and a history of contact with a patient who had probable or confirmed MVD. A confirmed case patient was any suspected or probable case patient with laboratory-confirmed evidence of acute or previous Marburg virus infection, as detected by RT-PCR, antigen-capture ELISA, or IgM/IgG ELISA. Suspected and probable case patients were reclassified as “not a case” if diagnostic test results for a specimen collected during the appropriate period were negative.
Figure 1.
Official case definitions that were used during the 2012 Marburg virus disease outbreak, as approved by Uganda Ministry of Health. Abbreviations: ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; IgM, immunoglobulin M; MVD, Marburg virus disease; RT-PCR, reverse transcription–polymerase chain reaction.
A standard case investigation form was used to collect epidemiologic and clinical information about each potential case patient. In the initial phases of the investigation, efforts were made to identify and test all persons who were currently ill and fit the suspected case patient definition in designated hospital facilities with isolation precautions. Persons who had direct or indirect contact with confirmed or probable case patients while they were symptomatic were systematically identified and visited daily for 21 days after their last contact. If they developed symptoms during the surveillance period, the contacts were hospitalized under isolation precautions and tested for acute Marburg virus infection. Once the current case patients and their contacts had been identified, a retrospective investigation sought to identify cases that had occurred prior to the outbreak’s detection, and surviving persons were tested for previous Marburg virus infection, through IgM and IgG serological analysis. Symptoms were recorded at the time of admission to the isolation ward for current cases or, for retrospective cases, were summarized throughout the illness.
Blood and serum specimens from acutely ill patients were tested in the field by RT-PCR as follows. Total RNA was extracted with the BeadRetriever system (Invitrogen), using the MagMax Viral RNA isolation kit (Applied Biosystem). Marburg virus–specific qRT-PCR assays targeting the VP40 gene and polymerase gene were performed on each specimen. Blood and serum specimens from patients with acute illness and patients who had recovered were also tested for anti–Marburg virus IgM and IgG by ELISA, using previously described methods [23].
All confirmed case patients who were acutely ill (ie, those with positive qRT-PCR results) were hospitalized in treatment centers, using barrier nursing techniques. Confirmed case patients remained under isolation until Marburg virus RNA was not detected in their blood, as measured by qRT-PCR. Suspected case patients who had clinically improved were allowed to be discharged from the treatment center if they had negative blood qRT-PCR results on day 3 or later after the onset of symptoms, and they were classified as “not a case.” Case patients who died were buried by a trained team, using appropriate infection control measures.
Case investigation forms were tabulated in a computerized spreadsheet (Excel), and summary statistics were calculated using statistical analysis software (Epi Info 7; SAS). A bivariate χ2 comparison of symptoms was performed by classifying confirmed and probable case patients as MVD case patients and by classifying patients with negative test results and complete information as controls. P values were corrected for 32 simultaneous comparisons, using the technique of Benjamini and Hochberg [24], to balance power and type 1 error. Statistical significance was accepted at a P value of ≤.01. Similarly, recorded symptoms of confirmed and probable cases were compared by the Fisher exact test and evaluated for association with fatal outcome. Maps of affected regions were created using geospatial information system software (ESRI ArcMap 10.0).
RESULTS
In total, 15 laboratory-confirmed cases of MVD were identified, with 9 detected during acute illness (RT-PCR results were positive) and 6 detected during convalescence (RT-PCR results were negative, and IgM/IgG ELISA results were positive). Four confirmed cases were fatal. Eleven probable cases were identified, all of which were fatal cases. The total count of confirmed and probable MVD cases was 26, of which 15 (58%) were fatal. Ages ranged from newborn to 64 years; 16 case patients (62%) were female.
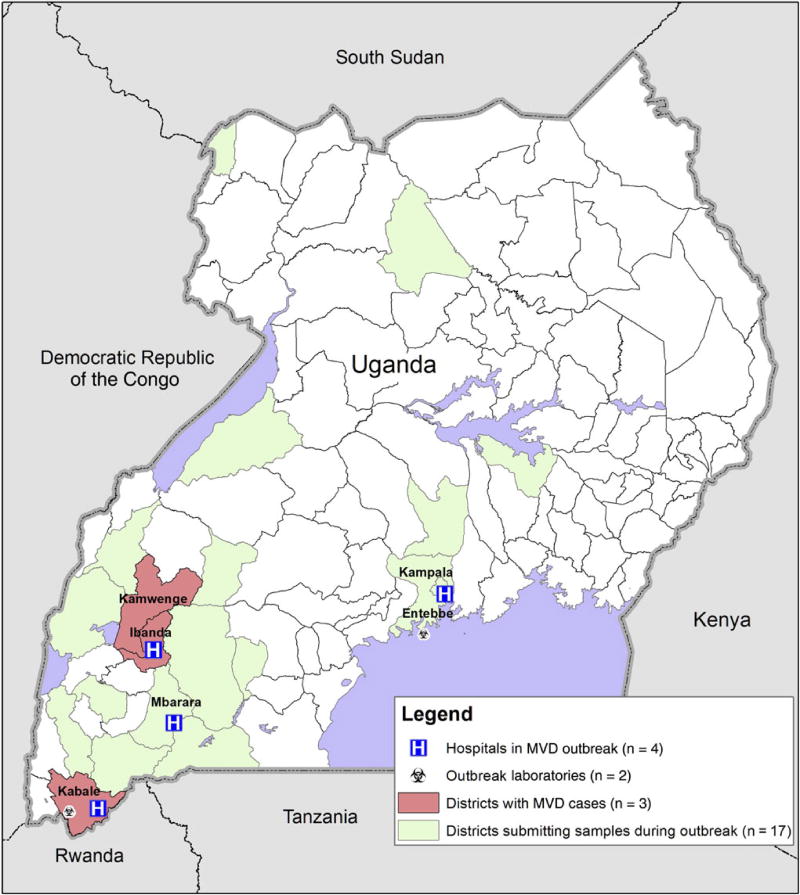
Confirmed case patients resided in 3 districts of Uganda (Kabale, Ibanda, and Kamwenge; Figure 2) and were hospitalized in treatment centers in 3 districts (Kabale, Ibanda, and Mbarara) and Kampala. To facilitate rapid specimen testing, a field laboratory was set up in Kabale to perform same-day qRT-PCR testing. Additional laboratory support was provided by the viral hemorrhagic fever laboratory in UVRI (Entebbe), which performed ELISAs to determine Marburg virus–specific IgM and IgG titers and qRT-PCR testing on specimens from outside Kabale and Ibanda districts. During the outbreak investigation, 198 specimens from 173 individuals were tested. In addition to the 3 affected districts, diagnostic specimens from suspected case patients were submitted from 17 districts across Uganda.
Figure 2.
Map portraying the districts with MVD case-patients, districts who submitted specimens for testing from suspect cases during the outbreak, hospitals who had isolation facilities, and laboratories that performed diagnostic testing in support of the outbreak response. Abbreviation: MVD, Marburg virus disease.
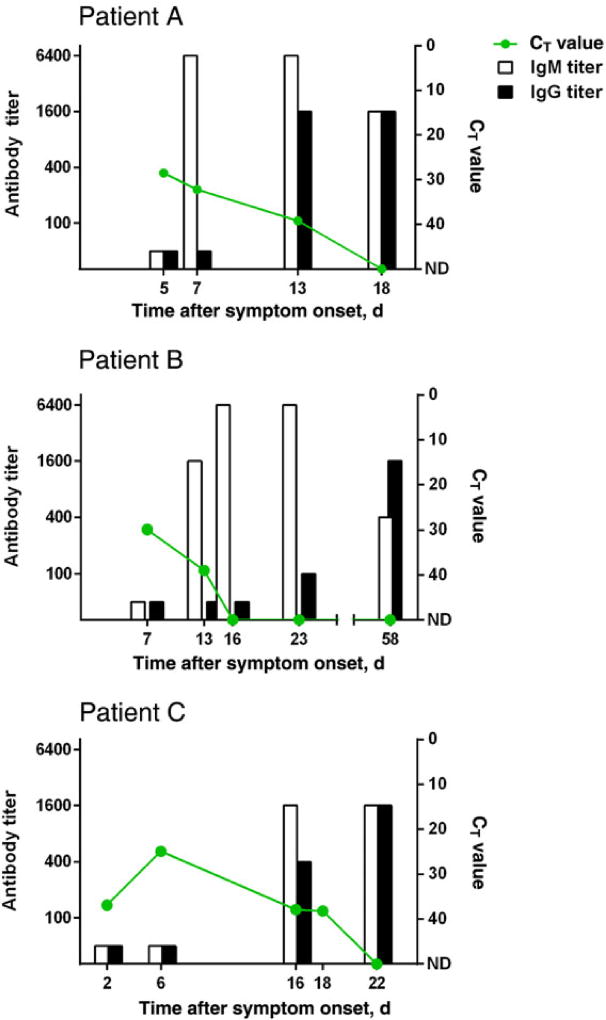
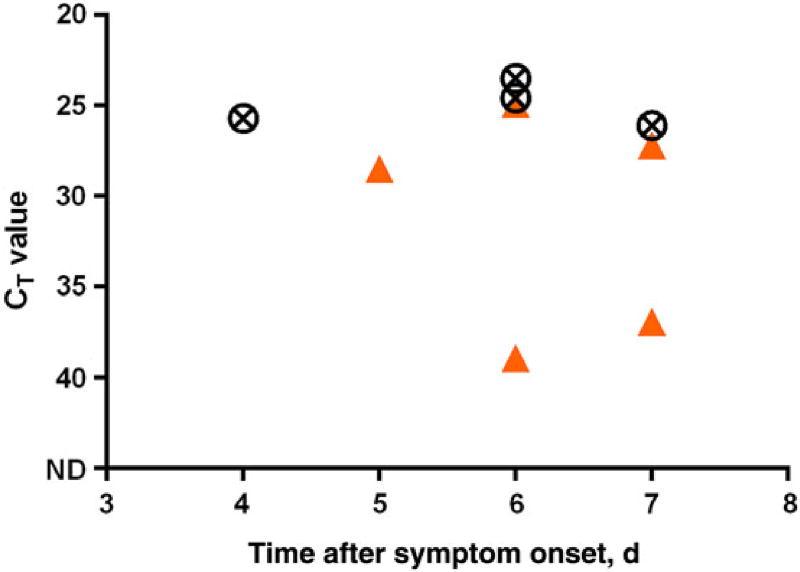
Serial blood specimens were collected from 3 MVD case patients who survived infection (Figure 3). Among the specimens available for testing, qRT-PCR threshold cycle (CT) values were lowest (signifying the highest quantity of virus load) at the earliest point of sampling (6–7 days after symptom onset) and declined to undetectable levels 16–22 days after symptom onset. IgM antibody was first detected 7–16 days after onset of symptoms, while IgG was first detected 13–23 days after onset. CT values from blood specimens collected <8 days after symptom onset from 4 surviving case patients were compared with values for 5 case patients who died (Figure 4). Average CT values from case patients who died were significantly lower than those for surviving case patients (P = .02 by the t test, assuming equal variances).
Figure 3.
Reverse transcription-polymerase chain reaction cycle threshold (Ct) value (green line), Immunoglobulin (Ig)G (black bar), and IgM (white bar) values from serial blood specimens in three laboratory-confirmed case-patients.
Figure 4.
Reverse transcription–polymerase chain reaction cycle threshold (Ct) values from the first blood specimen collected (range: 4–7 days post onset) in laboratory-confirmed case-patients. Circles: patients with fatal outcome (n = 4), Triangles: patients who recovered (n = 5). Abbreviation: ND, not detected (ie, negative RT-PCR result).
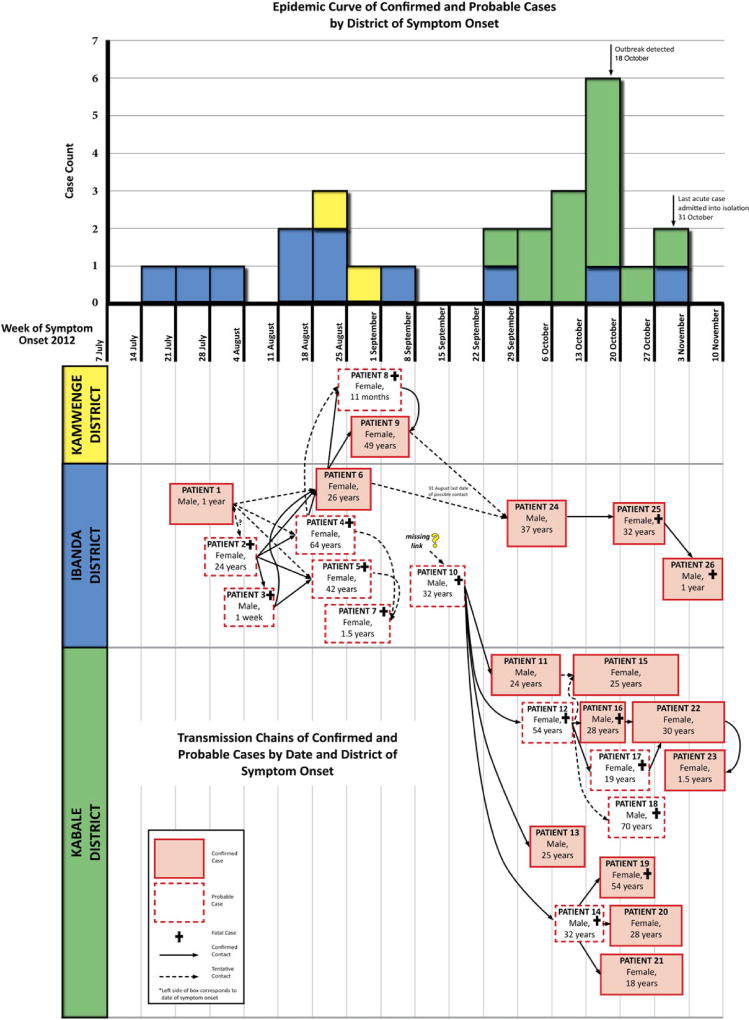
The outbreak had 2 separate chains of transmission at the time of the investigation, in October 2012 (Figure 5), which could not be directly linked to each other epidemiologically. However, both chains occurred in Ibanda District contemporaneously, and molecular analysis of isolates from each chain yielded nearly identical virus sequences [25], indicating that the 2 chains were likely connected and originated from the same unidentified spillover event. The earliest confirmed case patient (detected retrospectively via IgG ELISA) had an estimated onset of illness in mid-July 2012, nearly 3 months before the outbreak was detected, in mid-October 2012. Subsequent infections in family members occurred in Ibanda and Kamwenge districts during August–October 2012, affecting 6 confirmed and 6 probable case patients. The last case patient in this first detected chain of transmission was admitted to a treatment center on 31 October 2012. The first case patient in the second chain of transmission was a probable case patient who worked in Ibanda District and traveled to Kabale District to be cared for by family when he became ill in mid-September 2012; the infection spread to family members, close associates (a religious leader and her child), and 1 healthcare worker located in Kabale District, affecting 9 confirmed and 5 probable case patients. The last case patient in this second chain of transmission was admitted to the Kabale treatment center on 29 October 2012. No case patients in either transmission chain reported activities that placed them in contact with bats or other potential wildlife sources.
Figure 5.
A, Epidemic curve of confirmed and probable cases by district of symptom onset. Blue bars: cases with onset in Ibanda district, Yellow bars: cases with onset. Kamwenge district, Green bars: cases with onset in Kabale district. B, Transmission chains of confirmed and probable cases by date and District of symptom onset. Pink boxes: confirmed cases, White boxes: probable cases. The left boundary of the box is set at the case’s date of onset, and the right boundary of the box is set at the approximate date of death or recovery. Solid arrows indicate contact between cases that suggest Marburg virus disease transmission, while dashed arrows are a tentative suggestion of transmission. Black crosses indicate the case was fatal.
Symptoms recorded for all 26 confirmed and probable case patients and 125 controls are reported in Table 1. Reported symptoms for confirmed and probable case patients were also analyzed separately, and no significant differences were found in the proportions of symptoms reported between these 2 groups. Nearly all confirmed and probable cases (96%) had fever; anorexia, fatigue, headache, and vomiting were present in >75%. Half of confirmed and probable case patients (13 [50%]) had hemorrhagic symptoms, which included vaginal bleeding (4 female case patients [29%]), epistaxis (5 case patients [21%]), bleeding gums (4 case patients [17%]), hematemesis (4 case patients [17%]), petechiae (2 case patients [11%]), hemoptysis (2 case patients [8%]), and hemorrhage indicative of disseminated intravascular coagulation (1 case patient [6%]). Conjunctival hemorrhage or “red eyes” were noted in 3 case patients (13%). Hiccups and anorexia had the strongest associations with being a confirmed or probable case patient (OR, 22.9 and 19.3, respectively; P < .0001 for both), and fatigue, vomiting, sore throat, and difficulty swallowing were also significantly associated with being a confirmed or probable case patient. Attending a funeral prior to illness onset (OR, 5.3; P = .0006) and having direct contact with another confirmed, probable, or suspected case patient (OR, 33.4; P < .0001) were risk factors significantly associated with being a confirmed or probable case patient (Table 2). Fatal outcome was compared between confirmed case patients (n = 9) and controls, and this was significantly associated with being a case patient (OR, 6.9; P = .003). No symptoms or risk factors were significantly associated with a fatal outcome among confirmed and probable case patients (data not shown).
Table 1.
Comparison of Symptoms in Case Patients With Confirmed or Probable Marburg Virus Disease (MVD) and Test-Negative Controls Initially Suspected of Having MVD
| Symptom/ Outcome |
Cases, %a |
Controls, %a |
P Value |
Odds Ratio (95% CI) |
|---|---|---|---|---|
| Fever | 24 (96) | 75 (77) | .03 | 7.4 (.9, 57.4) |
| Anorexia | 22 (92) | 44 (36) | <.0001 | 19.3 (4.3, 85.8) |
| Fatigue | 19 (86) | 59 (50) | .002 | 6.3 (1.8, 22.6) |
| Headache | 18 (86) | 79 (65) | .06 | 3.3 (.9, 11.7) |
| Vomiting | 19 (76) | 52 (41) | .002 | 4.4 (1.6, 11.7) |
| Abdominal pain | 14 (67) | 63 (52) | .2 | 1.8 (.7, 4.9) |
| Muscle/joint pain | 11 (55) | 50 (42) | .27 | 1.7 (.7, 4.4) |
| Any hemorrhagic condition | 13 (50) | 49 (40) | .36 | 1.5 (.64, 3.5) |
| Deathc | 4 (44) | 13 (10) | .003 | 6.9 (1.9, 24.8) |
| Diarrhea | 11 (44) | 52 (42) | .87 | 1.1 (.5, 2.5) |
| Hiccups | 8 (36) | 3 (2) | <.0001 | 22.9 (5.4, 96.3) |
| Sore throat | 4 (29) | 6 (5) | .01 | 7.3 (1.8, 30.1) |
| Vaginal bleeding | 4 (29) | 6 (10) | .09 | 3.6 (.85, 15.1) |
| Difficulty breathing | 6 (26) | 13 (11) | .08 | 3.0 (1.0, 8.9) |
| Difficulty swallowing | 4 (21) | 3 (2) | .01 | 10.6 (2.2, 51.9) |
| Nose bleeding | 5 (21) | 18 (15) | .54 | 1.5 (.5, 4.6) |
| Gum bleeding | 4 (17) | 5 (4) | .04 | 4.9 (1.2, 19.8) |
| Blood in vomit | 4 (17) | 25 (20) | 1.0 | 0.8 (.25, 2.6) |
| Conjunctivitis | 3 (14) | 15 (13) | .74 | 1.1 (.3, 4.4) |
| Conjunctival bleeding | 3 (13) | 2 (2) | .03 | 8.9 (1.4, 56.3) |
| Petechiae | 2 (11) | 1 (1) | .05 | 13.6 (1.2, 159) |
| Rash | 1 (4) | 4 (3) | .6 | 1.4 (.1, 12.7) |
| Conjuctival injection site bleeding | 0 | 3 (2.5) | 1.0 | NA |
| Melena | 0 | 23 (19) | .03 | NA |
| Blood in urine | 0 | 7 (6) | .6 | NA |
Abbreviations: CI, confidence interval; NA, not applicable.
There were 26 cases and 125 controls. Owing to varying response rates, statistical calculations were based on the number of responses for each symptom; the number responding “yes” are reported.
By χ2 analysis or the Fisher exact test. A P value of ≤ .01 is considered significantly significant.
Calculated only for case patients who were acutely ill when disease was confirmed by laboratory analysis (n = 9).
Table 2.
Comparison of Risk Factors in Case Patients With Confirmed or Probable Marburg Virus Disease (MVD) and Test-Negative Controls Initially Suspected of Having MVD
| Risk Factor | Cases, % |
Controls, % |
P Valuea |
Odds Ratio (95% CI) |
|---|---|---|---|---|
| Contact with a case | 24 (96) | 41 (42) | <.0001 | 33.4 (4.3, 256.7) |
| Attending a funeral | 13 (62) | 20 (23) | .0006 | 5.3 (1.9, 14.8) |
| Male sex | 10 (38) | 65 (55) | .13 | 0.5 (.3, 1.2) |
| Travel before illness | 8 (36) | 22 (25) | .30 | 1.7 (.6, 4.6) |
Abbreviation: CI, confidence interval.
By χ2 analysis or the Fisher exact test. A P value of ≤ .01 is considered significantly significant.
Nine confirmed case patients were admitted to treatment centers, of whom 3 (33%) died, compared with deaths among 12 of 15 confirmed and probable case patients (71%) who were not cared for in an isolation facility (P = .1). Among confirmed case patients who died, death occurred a mean of 9 days after onset of symptoms, while death among probable case patients occurred a mean of 6.5 days after onset. For confirmed and probable case patients, hospitalization occurred a mean of 4 days after symptom onset. The 6 surviving confirmed case patients were discharged from isolation a mean of 22 days (range, 16–30 days) after onset of symptoms and spent a mean of 14.3 days (range, 4–22 days) in isolation. Many of the controls suspected of having MVD were hospitalized while laboratory testing was performed, and of 11 with information on isolation duration available, discharge from isolation occurred a mean of 4 days after admission (range, 0–12 days). For confirmed case patients, the distribution of days after symptom onset that a patient was reported or detected was bimodal: acutely detected cases were identified 0–6 days after onset, while convalescent cases detected during the retrospective case investigation were identified 29–105 days after onset.
Once the outbreak was detected, 327 persons were listed as having had contact with confirmed or probable case patients and were followed for 21 days after their last date of contact. Only 1 direct contact that was under follow-up and the child of this case patient developed MVD. All other case patients were discovered prior to the commencement of contact tracing.
DISCUSSION
The outbreak described in this article represents the first epidemic of MVD with multiple generations of human-to-human transmission to occur in Uganda, affecting 26 patients in 3 districts. This most likely resulted from low-level and undetected community transmission from the outbreak’s beginning (late July or earlier) to early October 2012. Similar to the MVD outbreak in Uige, Angola [26], full-length genome sequences of virus from 4 case patients (2 from each transmission chain) in the 2012 Uganda MVD outbreak were nearly identical (>99%), strongly suggesting a single spillover event from wildlife, with subsequent human-to-human transmission [25]. This is in contrast with the occurrence of MVD in Watsa, Democratic Republic of the Congo, where 9 different virus lineages were found in case patients, demonstrating a rather different picture of multiple spillover events beginning with gold miners who were exposed to bats in the Goroumbwa mine [6].
The zoonotic spillover event initiating the 2012 Uganda MVD outbreak was not identified, although the earliest confirmed case originated in Ibanda District, the same district housing the Kitaka mine, where the 2007 MVD epidemic occurred and where a large R. aegyptiacus population infected with Marburg virus was found [7]. The mine was closed following the 2007 outbreak but was reopened during 2009, and full mining activity had resumed in 2010. No links were found between any case in the 2012 outbreak and mining activity or cave exposures, but the close proximity of the mine and its recent reopening is a striking coincidence.
Among 3 confirmed case patients who survived, we found that the highest viremia level, based on the CT value determined by qRT-PCR, occurred approximately 5–7 days after onset of symptoms and persisted to approximately 16–22 days after onset in survivors. IgM titers were detectable 7–16 days after symptom onset, while IgG became detectable 13–16 days after onset. While the number of case patients with serial blood specimens was quite limited and specimens were collected at variable times, the timing of viral RNA detection in blood and anti–Marburg IgM and IgG detection roughly corresponded to findings in previous case reports of Ebola virus and Sudan virus infection [21, 22].Viral load in acute blood specimens (ie, specimens obtained <8 days after symptom onset), as measured by the qRT-PCR CT value, was also significantly associated with outcome, with case patients who died having significantly lower CT values than surviving case patients, in agreement with findings for patients infected with Sudan virus [21]. In this analysis, there were no associations between the presence of particular clinical symptoms and fatal outcome, which may be related to the small number of cases available for comparison. In the Watsa outbreak, hiccups, conjunctival injection, and hemorrhagic symptoms were all associated with a fatal outcome [6].
The CFR for the outbreak described in this report was calculated in 2 different ways. If all 26 confirmed and probable cases were included, the CFR was 58%. However, inclusion of probable and retrospectively confirmed case patients in this calculation had considerable potential for bias, owing to the incomplete nature of retrospective investigations—fatal cases occurring prior to the outbreak’s detection were not able to be confirmed, and not all cases were able to be found. Therefore, we also calculated the CFR among confirmed case patients in whom MVD was confirmed only after the detection of the outbreak (ie, only case patients [4 of 9] with acute clinic disease), and we arrived at a CFR of 44%. However, this second estimate of CFR also has potential for bias because nearly all of these patients (8 of 9) received prompt care in isolation facilities, which may improve a patient’s chances for survival. However, the ascertainment of case patients identified during acute infection was complete, in that we were able to follow the clinical course of these patients from the time of diagnosis to discharge or death.
Regardless of which CFR calculation method is used, the case patients in the 2012 Uganda MVD outbreak had a much lower CFR, and there were fewer cases than in the 2 previous large-scale MVD outbreaks occurring in Africa—154 persons (CFR, 83%) during the 1998–2000 outbreak in Durba-Watsa, Democratic Republic of Congo [6], and 252 persons (CFR, 90%) during the 2005 outbreak in Uige, Angola (both calculated by including confirmed and probable case patients). The reasons for the large differences in CFR are not clear. Among extrinsic factors that could explain patient outcomes, both the Uige and Watsa outbreaks occurred in areas with histories of civil unrest, which led to a reduction in the overall health of the population and fewer resources for patient care at the affected hospitals. Access to adequate health care could potentially contribute to worse survival rates. However, it is also possible that recalculating the CFRs for these 2 outbreaks and including only confirmed cases with acute infection may also demonstrate a reduced CFR.
The relative virulence of the virus strains is another potential explanation for differences in CFR. The genome sequences that were described in the 2012 MVD outbreak (Ibanda-423 2012 and Kabale-422 2012) were most closely related to those of strains that were found in bats in Uganda and 2 previous single cases in humans (Leiden 2008, 98% homology; and Musoke 1980, 94% homology) [25], so it is difficult to draw conclusions about the relative virulence of these strains in humans, compared with the virulence of the less closely related strains that caused the Uige outbreak (Ang 1382 2005, 93% homology) and the Durba outbreak (07DRC99 1999, 92% homology).
Although the difference was not statistically significant, it is noteworthy that the CFR of case patients admitted to an isolation ward (33%) was less than half the CFR of patients who were sick prior to the institution of isolation wards (71%). This could be due to the imperfect nature of retrospective case finding, in which recall bias may occur: interviewees may be more likely to remember the persons who became sick and died than to remember the persons who became sick and recovered [27]. Furthermore, we have no way to confirm that the probable cases were indeed a part of the outbreak, beyond the presence of epidemiologic links to confirmed cases; some of these cases may have died from unrelated causes, although the timing and exposures fit the criteria for MVD transmission. Regardless, the relatively low CFR of case patients treated in facilities with isolation precautions can hopefully be used to encourage the community to embrace these facilities as places for quality care of patients, rather than places for the community to fear [18].
In addition to the differing CFRs when comparing the 2012 Uganda case patients to the Uige and Watsa case patients, we also found differences in symptoms reported. While anorexia (92%) was nearly universally reported by 2012 Uganda confirmed and probable case patients, 66% and 77% of Uige and Watsa case patients, respectively, reported anorexia [6, 15]. The 2012 Uganda case patients were similar to Watsa case patients with respect to the proportion reporting fever (96% and 93%, respectively), vomiting (76% and 76%), and hiccups (36% and 40%), whereas 85% of Uige case patients reported fever, 34% reported vomiting, and 5% had hiccups. The presence of hiccups was associated with being a case patient in the 2012 Uganda outbreak and was associated with fatal outcome in the Watsa outbreak. The 2012 Uganda case patients, however, had greater similarity to Uige case patients with respect to the proportion reporting diarrhea (44% and 46%, respectively) and any kind of hemorrhage (50% and 51%), whereas 57% of Watsa case patients reported diarrhea and 69% reported any hemorrhage. Melena was also more frequent in Watsa case patients (55%) than in case patients from the 2012 Uganda (0%) and Uige (17%) outbreaks. Limitations exist in attempting to compare frequencies of symptoms across outbreaks, because the data collection instruments and times at which symptoms were assessed may differ. However, the clinical picture portrayed by these 3 outbreaks shows a variety of nonspecific symptoms that can be present in case patients, further underlining the need for a broad case definition and laboratory confirmation of MVD cases during ongoing outbreaks.
Risk factors associated with being a confirmed or probable case patient were activities that put a person in close contact with other sick persons, such as participating in a funeral or having direct contact with another confirmed, probable, or suspected case patient prior to illness onset. This association was also found with Uige case patients [15] and is supported by molecular evidence that, in this outbreak, a single strain of Marburg virus was transmitted through several generations of case patients. The importance of halting chains of transmission through institution of isolation precautions for all currently ill case patients and through active surveillance of all potentially exposed case contacts cannot be overstated. Following the institution of these public health measures, the outbreak was successfully and rapidly contained only 14 days after its initial detection.
In addition to MVD, Uganda has experience with several previous EVD outbreaks and cases [2, 14, 28] and has a well-developed infrastructure for responding to these outbreaks quickly. Scientific, logistic, and psychosocial support systems rapidly fell into place, leading to a successful effort despite the challenging multidistrict nature of this outbreak response. It was somewhat surprising in the retrospective investigation to find that case patients were identified with dates of onset tracing back to July 2012, meaning that the outbreak had gone undetected for >2 months, at a time when there was an EVD outbreak response occurring <200 km to the north, in Kibaale District [29] (Shoemaker, unpublished data). Furthermore, following the 2007 MVD outbreak in Ibanda, a district hospital had received specific training on viral hemorrhagic fever detection, control, and reporting in an attempt to improve disease detection. Clearly, identification of suspected MVD patients can be difficult, especially when the initial clinical manifestation mimics other common febrile illnesses and when hemorrhage and death is not universally observed.
Since 2011, a permanent laboratory capable of safely identifying patients infected with the region’s endemic hemorrhagic fever viruses (Ebola, Bundibugyo, Sudan, Marburg, Crimean- Congo hemorrhagic fever, and Rift Valley fever viruses) has been functional in Uganda. This is a joint effort through the UVRI and the CDC’s Viral Special Pathogens Branch, and it has contributed greatly to the ability to rapidly detect and confirm viral hemorrhagic fever outbreaks. During the MVD outbreak response described in this report, specimens from districts all over the country were sent to the CDC/UVRI laboratory for detection of agents responsible for viral hemorrhagic fever, including 2 specimens from individuals in Luwero District who were confirmed as having acute infection with Sudan ebolavirus [30], thus identifying the country’s third filovirus outbreak within 5 months. The 2012 Luwero outbreak was very small, with only 6 cases, and may have not been detected if there were a lower level of awareness of viral hemorrhagic fevers in general or if it had been more difficult to pursue diagnostic testing. Alternatively, the Luwero outbreak might have continued, with additional persons becoming ill and dying. Clearly, having laboratory capacity present in Uganda contributed to the early detection and response and to the rapid containment of these epidemics.
In conclusion, the 2012 Marburg virus outbreak in Uganda occurred over multiple districts but was traced back to Ibanda District. The origin of the outbreak could not be determined, although contact with R. aegyptiacus fruit bats or other infected wildlife at least 3 months prior to the outbreak’s detection was suspected. The CFR (58%) was lower than observed in previous MVD outbreaks in Africa. Rapid response, including initiation of contact-tracing activities, as soon as the outbreak was detected quickly brought the outbreak to a close.
Acknowledgments
We thank Patrick Tusiime (district health officer, Kabale District, Uganda) and Craig Manning (Viral Special Pathogens Branch, Centers for Disease Control and Prevention [CDC]).
The outbreak response was funded through emergency budgets of the CDC, the Uganda Ministry of Health, Médecins sans Frontières, the World Health Organization, and other relevant responding agencies.
Footnotes
Presented in part: 2013 Epidemic Intelligence Service Conference; 2013 American Society for Tropical Medicine and Hygiene, Abstract #910; 2014 Filoviruses Symposium.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204:S810–6. doi: 10.1093/infdis/jir299. [DOI] [PubMed] [Google Scholar]
- 2.MacNeil A, MacNeil E, Farnon J, et al. Proportion of deaths and clinical features in bundibugyo Ebola virus infection, Uganda. Emerg Infect Dis. 2010;16:1969–72. doi: 10.3201/eid1612.100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters CJ. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, democratic Republic of the Congo, 1995. J Infect Dis. 1999;179:S87–91. doi: 10.1086/514284. [DOI] [PubMed] [Google Scholar]
- 4.Khan AS, Tshioko FK, Heymann DL, et al. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis. 1999;179(suppl 1):S76–86. doi: 10.1086/514306. [DOI] [PubMed] [Google Scholar]
- 5.Towner J, Amman B, Sealy T, et al. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009;5:e1000536. doi: 10.1371/journal.ppat.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bausch D, Nichol ST, Muyembe-Tamfum JJ, et al. Marburg hemorrhagic fever associated with multiple genetic lineages of virus. N Engl J Med. 2006;355:909–19. doi: 10.1056/NEJMoa051465. [DOI] [PubMed] [Google Scholar]
- 7.Adjemian J, Farnon EC, Tschioko F, et al. Outbreak of marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007. J Infect Dis. 2011;204:S796–9. doi: 10.1093/infdis/jir312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timen A. Response to imported case of Marburg Hemorrhagic fever, the Netherlands. Emerg Infect Dis. 2009;15:1171–5. doi: 10.3201/eid1508.090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC. Imported case of Marburg hemorrhagic fever - Colorado, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1377–81. [PubMed] [Google Scholar]
- 10.Georges A-J, Leroy EM, Renaut AA, et al. Ebola hemorrhagic fever outbreaks in Gabon, 1994–1997: epidemiologic and health control issues. J Infect Dis. 1999;179:S65–75. doi: 10.1086/514290. [DOI] [PubMed] [Google Scholar]
- 11.Formenty P, Hatz C, Le Guenno B, et al. Human infection due to Ebola virus, subtype Côte d’Ivoire: clinical and biologic presentation. J Infect Dis. 1999;179(suppl 1):S48–53. doi: 10.1086/514285. [DOI] [PubMed] [Google Scholar]
- 12.Leroy EM, Rouquet P, Formenty P, et al. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science. 2004;303:387–90. doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- 13.Brauburger K, Hume AJ, Mühlberger E, Olejnik J. Forty-five years of marburg virus research. Viruses. 2012;4:1878–927. doi: 10.3390/v4101878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC. Outbreak of Ebola hemorrhagic fever Uganda, August 2000–January 2001. MMWR Morb Mortal Wkly Rep. 2001;50:73–7. [PubMed] [Google Scholar]
- 15.Roddy P, Thomas SL, Jeffs B, et al. Factors associated with Marburg Hemorrhagic fever: analysis of patient data from Uige, Angola. J Infect Dis. 2010;201:1909–18. doi: 10.1086/652748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterk E. Filovirus Haemorrhagic Fever Guidelines. Barcelona: Medecins Sans Frontieres. 2008 [Google Scholar]
- 17.Roddy P, Weatherill D, Jeffs B, et al. The Medecins Sans Frontieres intervention in the Marburg hemorrhagic fever epidemic, Uige, Angola, 2005. II. lessons learned in the community. J Infect Dis. 2007;196(suppl 2):S162–7. doi: 10.1086/520544. [DOI] [PubMed] [Google Scholar]
- 18.Jeffs B, Roddy P, Weatherill D, et al. The Medecins Sans Frontieres intervention in the Marburg hemorrhagic fever epidemic, Uige, Angola, 2005. I. Lessons learned in the hospital. J Infect Dis. 2007;196(suppl 2):S154–61. doi: 10.1086/520548. [DOI] [PubMed] [Google Scholar]
- 19.Raabe V, Borchert M. Infection control during filoviral hemorrhagic Fever outbreaks. J Glob Infect Dis. 2012;4:69–74. doi: 10.4103/0974-777X.93765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grolla A, Jones SM, Fernando L, et al. The use of a mobile laboratory unit in support of patient management and epidemiological surveillance during the 2005 Marburg Outbreak in Angola. PLoS Negl Trop Dis. 2011;5:e1183. doi: 10.1371/journal.pntd.0001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Towner JS, Rollin PE, Bausch DG, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol. 2004;78:4330–41. doi: 10.1128/JVI.78.8.4330-4341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ksiazek TG, Rollin PE, Williams AJ, et al. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(suppl 1):S177–87. doi: 10.1086/514321. [DOI] [PubMed] [Google Scholar]
- 23.Ksiazek TG, West CP, Rollin PE, Jahrling PB, Peters CJ. ELISA for the detection of antibodies to Ebola viruses. J Infect Dis. 1999;179:S192–8. doi: 10.1086/514313. [DOI] [PubMed] [Google Scholar]
- 24.Benjamini Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 25.Albarino CG, Shoemaker T, Khristova ML, et al. Genomic analysis of filoviruses associated with four viral hemorrhagic fever outbreaks in Uganda and the Democratic Republic of the Congo in 2012. Virology. 2013;442:97–100. doi: 10.1016/j.virol.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Towner JS, Khristova ML, Sealy TK, et al. Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola. J Virol. 2006;80:6497–516. doi: 10.1128/JVI.00069-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francesconi P, Yoti Z, Declich S, et al. Ebola hemorrhagic fever transmission and risk factors of contacts, Uganda. Emerg Infect Dis. 2003;9:1430. doi: 10.3201/eid0911.030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoemaker T, Shoemaker A, MacNeil S, et al. Reemerging Sudan Ebola Virus Disease in Uganda, 2011. Emerg Infect Dis. 2012;18:1480–3. doi: 10.3201/eid1809.111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO Outbreak news. Ebola haemorrhagic fever, Uganda. Wkly Epidemiol Rec. 2012;87:339. [PubMed] [Google Scholar]
- 30.WHO Outbreak news. Ebola haemorrhagic fever, Uganda, update. Wkly Epidemiol Rec. 2012;87:493. [PubMed] [Google Scholar]