Abstract
Background:
Understanding the relationship between perceived fatigue and performance fatigability could lead to more effective interventions to manage multiple sclerosis (MS)–related fatigue. However, the relationship between self-perceived fatigue measured using the Neurological Fatigue Index (NFI-MS) and performance fatigability in people with MS is unknown. We sought to explore the relationship between the NFI-MS and performance fatigability in people with MS.
Methods:
Fifty-two participants (mean ± SD age, 46.8 ± 10.1 years) completed the study. Three measures of performance fatigability were used: percent change in meters walked from first to last minute of the 6-Minute Walk Test, percent change in force exerted from first to last trial on a repetitive maximal hand grip test, and response speed variability on the Continuous Performance Test. Perceived physical and cognitive fatigue were measured using the NFI-MS. The state level of fatigue was examined immediately before and after performing the fatigability measures using a one-item visual analogue fatigue scale.
Results:
Of the three performance fatigability measures, only the attentional task (response speed variability) was significantly associated with NFI-MS physical (r = 0.326, P = .020) and cognitive (r = 0.276, P = .050) domain scores. Participants demonstrated significantly higher state levels of fatigue after performing all performance fatigability measures (P ≤ .001).
Conclusions:
The NFI-MS and the performance fatigability measures used in this study are easy to administer. We encourage wider use of these measures in clinical and research settings for comprehensive assessment of MS-related fatigue.
Fatigue is the most debilitating symptom of multiple sclerosis (MS).1 It interferes with daily function, affects workload, and hampers interpersonal relationships, often leading to reduced quality of life.2 Fatigue related to MS is multidimensional, consisting of different components, such as perceived physical and cognitive fatigue and performance fatigability.3–5 It is thought that MS-related fatigue results from disruptions in corticosubcortical brain regions6 as well as being due to other comorbidities, such as depression7 and cognitive impairments.8
A recent study by Kluger et al.9 introduced a unified taxonomy to guide the assessment and management of fatigue in neurologic populations. The taxonomy distinguished between fatigue that is perceived by the individual, referred to as perceived fatigue, and fatigue that can be objectively quantified by the researcher or clinician, referred to as fatigability. Perceived fatigue in people with MS is defined as a lack of motivation or a sense of tiredness that makes it difficult to efficiently perform daily physical and cognitive tasks.10 Perceived fatigue can be measured using a variety of self-report scales that assess perceived fatigue under different constructs, such as physical or cognitive or state versus trait.9 Researchers frequently use the Modified Fatigue Impact Scale (MFIS)11 to assess MS-related fatigue.12 However, a recommendation from the Multiple Sclerosis Council for Clinical Practice Guidelines suggests that the MFIS needs further psychometric evaluation.13 The MFIS does not fit into the Rasch model analysis,14 which uses a psychometric approach to develop and refine patient-reported outcomes and renders the MFIS score invalid. This might partially explain the conflicting results in previous studies that attempted to explore the relationship between fatigue assessed using MFIS and fatigability in people with MS.15
Fatigability, also called performance fatigability to distinguish it from perceived fatigability,4,5 is defined as a measure of change in the performance of a physical or a cognitive task over time9,10 and can be objectively quantified by the clinician or researcher. There is no established measure of performance fatigability, and research is ongoing in terms of the measurement and classifications of fatigability.16 Previous studies have attempted to measure performance fatigability in people with MS in two ways: physically, through changes in walking speed or repetitive maximal upper and lower limb contractions over time, and cognitively, through changes in cognition over time.15,17,18 Perceived fatigue can be related to fatigability if items in the self-report measure objectify the individual's fatigue levels as a deterioration in performing physical or cognitive activities.9 Nevertheless, fatigability is generally distinguished from perceived fatigue by the concept of change (a measurable difference in the performance of a task over time)19 and how it is measured (quantified by the clinician/researcher vs. reported by the patient).
Mills et al.20 developed the Neurological Fatigue Index (NFI-MS) to assess fatigue in people with MS. The NFI-MS fits the Rasch model analysis, was developed following guidelines from the US Food and Drug Administration,21 and has good external validity compared with commonly used scales in MS (the MFIS and the Fatigue Severity Scale). This makes the NFI-MS more psychometrically sound than commonly used fatigue scales in measuring perceived fatigue in people with MS.
The relationship between perceived fatigue assessed using the NFI-MS and performance fatigability is unknown in people with MS. Therefore, the main aim of this study was to explore the relationship between perceived fatigue, as measured using the NFI-MS, and performance fatigability in people with MS. Both perceived fatigue and fatigability interfere with the performance of household activities, can lead to deterioration in the performance of physical and cognitive tasks, and can worsen other symptoms, such as depression, sleepiness, and attention-related problems.9,22,23 Understanding the relationship between performance fatigability and perceived fatigue may help answer the following question: “Are people with MS with high levels of perceived fatigue more fatigable?” This could lead to more effective interventions to address these constructs and encourage wider use of these measures in clinical and research settings.
Methods
This study was performed in accordance with the University of Kansas Medical Center's institutional review board. The inclusion criteria include 1) 18 to 60 years of age, 2) relapsing-remitting or secondary progressive MS,24 3) able to ambulate with or without an assistive device, and 4) score greater than 24 on the Mini-Mental State Examination.25 The exclusion criteria include 1) a history of alcohol/drug abuse or a nervous system disorder other than MS; 2) severe physical, neurologic, or sensory impairments that would interfere substantially with testing; 3) a developmental history of learning disability or attention-deficit/hyperactivity disorder; 4) relapse or corticosteroid use within 4 weeks of assessment; 5) a known untreated sleep disorder (such as sleep apnea); 6) uncorrected vision loss that would interfere considerably with testing; 7) an acute ischemic cardiovascular event or coronary artery bypass surgery fewer than 3 months ago; and 8) uncontrolled blood pressure with medication (>190/110 mm Hg). People with MS were recruited to participate in this study at the MS clinic located at the University of Kansas Medical Center and through personal referrals from participants and physicians. Informed consent was obtained from all the participants.
Fifty-two individuals completed the study procedures. Medical history, medication use, and demographic characteristics were obtained from these participants. Before testing, participants were asked to refrain from taking medications other than what they typically take and from consuming caffeine beyond their typical daily consumption. Participants were instructed to refrain from exercise for 24 hours before testing. On the day of the assessment, participants first completed a battery of self-reported questionnaires and then the fatigability measures, which were randomized in order.
Perceived fatigue was measured using the NFI-MS,20 a validated scale for use with people with MS that assesses fatigue during the past 2 weeks. It consists of 23 questions, each on a Likert scale from 0 to 3, with higher score indicating more fatigue. The NFI-MS measures fatigue in three domains: physical, cognitive, and sleep quality. It also consists of a summary scale that includes the physical and cognitive domains. For the purposes of this study, only the physical domain, the cognitive domain, and the summary scale were used in data analysis. A validated ordinal to interval transformation of the NFI-MS raw scores developed by Mills et al.20 was used.
Performance fatigability was assessed using the 6-Minute Walk Test (6MWT), a grip strength test, and response speed on a cognitive test. The 6MWT is a frequently used measure of physical performance and endurance.26 It has been previously modified in administration and scoring to assess physical fatigability in people with MS.15,27,28 The version used in this study was used by Goldman et al.28 Specifically, instructions regarding permitted rest and encouragement phrases were eliminated and instructions regarding speed were emphasized. The administration was further modified for the present study by eliminating reminders every minute of how much time was remaining, and the participants were not informed that they would be walking for 6 minutes. Participants were instructed to walk as fast and as safely as they could back and forth along a 15-m path marked with tape in a hallway. A cone marked the turnaround at each end. The tape was marked with a red marker every meter to ease calculating the distance walked. Participants were allowed to use an assistive device if they used one for community ambulation. During the test, the administrator marked on the tape using a sticky tab where the participant was located at the end of every minute. Performance fatigability was calculated as a percent change in the distance walked between the first and sixth minutes.
The second measure to assess performance fatigability was a grip strength test. Grip strength is a frequently used method to measure hand grip strength29 and has been previously used to assess physical fatigability in people with MS by measuring change in grip force in kilograms through repetitive maximal hand grip over time.18 A hydraulic handheld dynamometer (JAMAR Technologies, Inc, Hatfield, PA)30 was used in this study; the handle was first adjusted according to the participant's grip size.30 The participant was then instructed to sit upright with shoulder adducted to neutral, elbow flexed at 90°, and forearm and wrist in the neutral position.31 Each participant was instructed to squeeze the handheld dynamometer with maximum strength when the examiner said “squeeze now” and to continue to squeeze the handle maximally until the examiner said “stop.” Participants performed 15 trials of maximal hand grip contractions, holding each contraction for 5 seconds, with a 5-second rest between each repetition. The participants were not informed of the number of trials or the length of each trial. A metronome heard only by the examiner using a headset was used to maintain the 5-second intervals. The maximal force exerted for each trial was recorded. Performance fatigability was calculated by measuring the percent change between the first and last trials. The test was first administered using the dominant hand and then repeated using the nondominant hand. Because of recent evidence demonstrating no significant difference in grip fatigability between dominant and nondominant hands in people with MS,18 data are reported for the dominant hand only in the present study.
The third measure of performance fatigability was a cognitive test called the Continuous Performance Test (CPT) (Conners CPT 3),32 which is a well-known computerized measure of sustained attention. Participants were seated in front of a computer screen and instructed to press the space bar when any letter of the alphabet except the letter X appeared on the monitor. To assess fatigability, the test was modified by eliminating instructions that emphasize that the participants are to respond as fast as they can, and participants were not informed how long the test lasted. The test takes 14 minutes to complete, with no rest provided. Performance fatigability was measured using the response speed variability score, which was previously found to be effective in detecting fatigability in people with MS.17 The response speed variability score measures the consistency of how fast the participant responds throughout the test. The mean response speed variability T-scores of the participants were used as the main outcome variable.
The state level of fatigue was assessed immediately preceding and following each fatigability measure using the one-item visual analogue fatigue scale.33 The participants were instructed to indicate on a 100-mm line their current level of fatigue from “not at all fatigued” to “extremely fatigued.” The outcome measure was the value of the length in millimeters along the line where the participants placed the mark.
Owing to their associations with MS-related fatigue, depression, quality of life, functional status, and disease severity were also assessed using the Beck Depression Inventory (BDI [fast screen]),34 the Multiple Sclerosis Quality of Life–54 (MSQOL-54),35 the Functional Status Questionnaire (FSQ),36 and the Patient-Determined Disease Steps (PDDS) scale.37
Data were analyzed using IBM SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). Descriptive statistics were calculated for the demographic characteristics. Assumptions of normality were tested using the Shapiro-Wilk test and normal Q-Q plots. When assumptions of normality were met, Pearson product correlations were used to examine the associations between perceived fatigue, fatigability measures, and demographics. If the assumptions of normality were not met, Spearman product correlations were used. Wilcoxon signed rank tests were used to examine the differences between the first minute and the last minute on the 6MWT, between the first trial and the last trial on the grip strength test, and in current fatigue measured using the one-item visual analogue fatigue scale from before to after each of the fatigability measures. Stepwise multivariate linear regression was used to examine which factors significantly predict perceived fatigue in people with MS using the summary scale score of the NFI-MS as the dependent variable. The alpha level was set at .05.
Results
Fifty-two participants with a mean ± SD age of 46.8 ± 10.1 years were included in the analysis. There were 44 female participants and eight male participants, 47 with relapsing-remitting MS and five with secondary progressive MS. Mean ± SD disease duration was 12.5 ± 7.6 years. Participants presented mostly with mild disease (mean ± SD PDDS scale score, 1.8 ± 1.6), minimal to mild depression (mean ± SD BDI score, 3.7 ± 3.1), and no severe global cognitive impairments (mean ± SD Mini-Mental State Examination score, 28.7 ± 1.6). Mean ± SD FSQ score was 79.7 ± 13.9. Mean ± SD physical and mental domains of the MSQOL-54 were 60.3 ± 18.6 and 67.6 ± 20.3, respectively.
Change in Performance on Fatigability Measures and State Levels of Fatigue
Total meters walked on the 6MWT in the last minute compared with the first minute decreased by 12.7%, and the total force in kilograms exerted in the last trial in the grip strength test compared with the first trial decreased by 35.9%. Figure 1 illustrates performance at every minute on the 6MWT and during each trial on the grip test of the dominant hand. Meters walked in the sixth minute were significantly lower than those walked in the first minute in the 6MWT (z score = −6.130, P ≤ .001). The force exerted at trial 15 was significantly lower than the force exerted in the first trial in the grip strength test of the dominant hand (z score = −6.303, P ≤ .001). The visual analogue fatigue scale score was significantly higher after performance of each fatigability measure compared with current perceived fatigue measured before performing the measures (grip strength test: z score = −5.691, P ≤ .001; 6MWT: z score = −5.906, P ≤ .001; CPT: z score = −6.150, P ≤ .001) (Figure 2).
Figure 1.
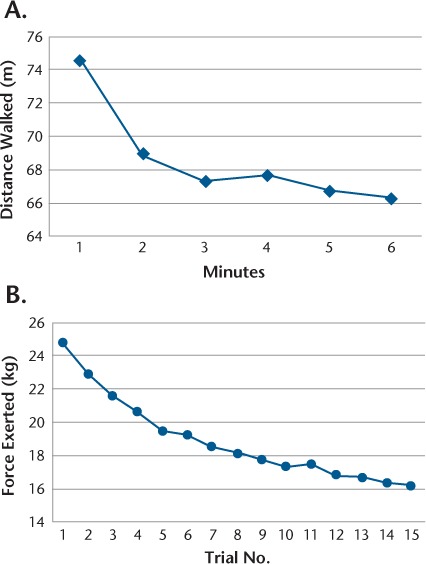
Physical fatigability for 6-Minute Walk Test (6MWT) and grip strength test
A, Meters walked every minute for 6 minutes on 6MWT. B, Force exerted every trial for 15 trials on grip strength test.
Figure 2.
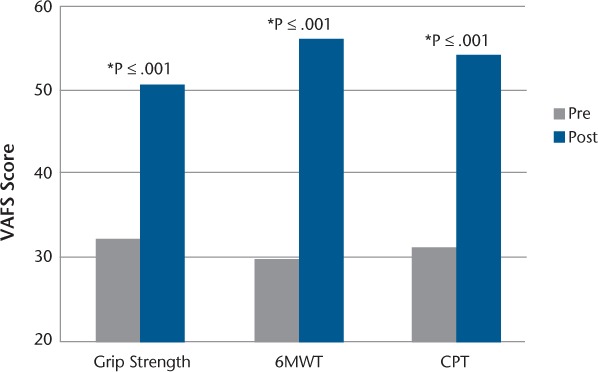
Difference in current perceived fatigue (visual analogue fatigue scale [VAFS]) before and after performing fatigability measures
*Difference is significant at α ≤ .001
6MWT, 6-Minute Walk Test; CPT, Continuous Performance Test.
NFI-MS and Fatigability
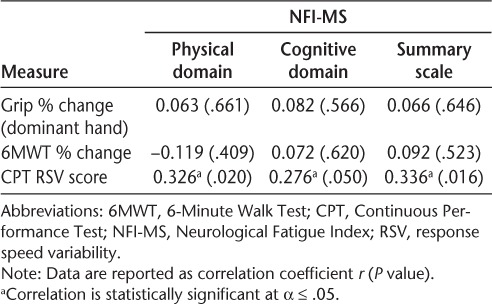
The percent change score of the 6MWT was not significantly associated with the NFI-MS physical domain (r = −0.119, P = .409), cognitive domain (r = 0.072, P = .620), or the summary scale (r = −0.092, P = .523) scores. The grip strength test change scores for the dominant hand were not significantly associated with the NFI-MS physical domain (r = 0.063, P = .661), cognitive domain (r = 0.082, P = .566), or summary scale (r = 0.066, P = .646) scores. In contrast, cognitive fatigability was significantly associated with the NFI-MS physical domain (r = 0.326, P = .020), cognitive domain (r = 0.276, P = .050), and summary scale (r = 0.336, P = .016) scores (Supplementary Figure 1, which is published in the online version of this article at ijmsc.org). The bivariate correlation analyses between the NFI-MS and the fatigability measures are shown in Table 1.
Table 1.
Bivariate correlations between NFI-MS and fatigability measures
NFI-MS and Clinical Characteristics
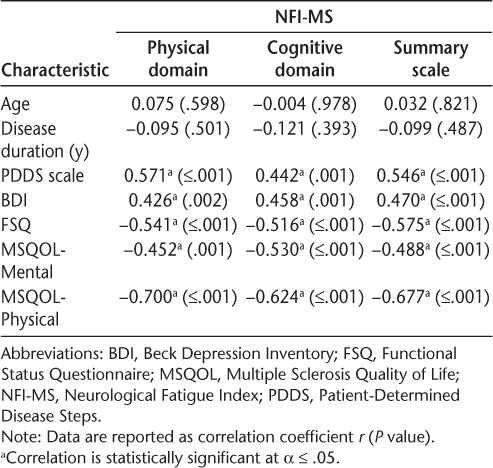
Depression was significantly associated with the NFIMS domain scores (physical: r = 0.426, P = .002; cognitive: r = 0.458, P = .001) and the summary scale score (r = 0.470, P ≤ .001). Disease severity as assessed using the PDDS scale was also significantly associated with the NFI-MS domain scores (physical: r = 0.571, P ≤ .001; cognitive: r = 0.442, P = .001) and the summary scale score (r = 0.546, P ≤ .001). Further correlation analysis indicated that the NFI-MS domain scores were significantly and strongly associated with subjective functional status as measured using the FSQ (physical domain: r = −0.541, P ≤ .001; cognitive domain: r = −0.516, P ≤ .001; summary scale: r = −0.575, P ≤ .001). Mental quality of life as measured using the MSQOL-54 was significantly and negatively associated with the NFIMS domain scores (physical: r = −0.452, P = .001; cognitive: r = −0.530, P ≤ .001; summary scale: r = −0.488, P ≤ .001). Physical quality of life as measured using the MSQOL-54 was also significantly and negatively associated with the NFI-MS domain scores (physical: r = −0.700, P ≤ .001; cognitive: r = −0.624, P ≤ .001; summary scale: r = −0.677, P ≤ .001). Age and disease duration were not statistically significantly associated with either the physical or cognitive domain scores or the summary scale score of the NFI-MS. Table 2 displays the bivariate correlation analyses between the NFI-MS scores and the clinical characteristics.
Table 2.
Bivariate correlations between NFI-MS and clinical characteristics
NFI-MS Regression Analysis
Variables that were significantly associated with the NFI-MS summary scale score (PDDS scale, FSQ, BDI, physical and mental domains of the MSQOL-54, and response speed variability scores) were included in the regression model. The analysis retained only physical quality of life as a significant predictor of perceived fatigue, explaining 45.8% of the variance in the NFIMS summary scale score (R2= 0.458, P ≤ .001). Owing to the confounding effect of depression on fatigue, in which fatigue can be a symptom of depression or vice versa,7 the regression analysis was repeated including the BDI score as a covariate. After controlling for depression, physical quality of life remained a significant predictor, explaining 34.3% of the variance in the NFI-MS summary scale score (β = −.586, R2= 0.343, P ≤ .001).
Discussion
To our knowledge, this is the first study to explore the relationship between perceived fatigue assessed using the NFI-MS and performance fatigability in people with MS. The findings of this study indicate that higher performance fatigability on an attentional task is associated with higher physical and cognitive perceived fatigue and overall perceived fatigue. Interestingly, performance fatigability assessed using a physical task was not associated with perceived physical fatigue, perceived cognitive fatigue, or overall perceived fatigue. Another important finding is the strong significant association between physical quality of life and overall perceived fatigue in the study sample even after controlling for depression.
Performance fatigability measured using an attentional task was significantly associated with perceived physical fatigue, perceived cognitive fatigue, and overall perceived fatigue. Only one previous study17 also used the response speed variability score as a measure of performance fatigability, and it too found an association between fatigability and perceived cognitive fatigue measured using the FIS. The response speed variability score was associated with perceived physical fatigue in the present study. Perhaps the nature of the CPT (in which participants sat continuously for 14 minutes without rest and used their finger to tap on the space bar continuously) contributed to the association with perceived physical fatigue. Furthermore, functional neuroimaging studies found that response variability was associated with central factors such as disruptions in the thalamocortical circuits and decreased white matter volume,38,39 which might explain the involvement of physical perceptions of fatigue.
Performance fatigability assessed using a physical task was not associated with perceived physical fatigue, perceived cognitive fatigue, or overall perceived fatigue. This lack of association is supported by previous studies that also failed to find an association between these constructs,15,18 but other studies have found an association between perceived physical fatigue and performance fatigability.28,40 The conflicting results may be due to different scoring and administration methods to calculate performance fatigability.15,28 Similar to the results of the present study, Leone et al.15 found no association between performance fatigability (measured using 6MWT percent change scores) and fatigue (measured using the MFIS). However, Goldman et al.28 found that higher perceived physical fatigue (measured using the MFIS) was associated with fewer meters walked on the 6MWT. Although the latter study recorded meters walked every minute, the main outcome measure used in the analysis was total meters walked, not percent change as used in the present study and in the study by Leone et al.15 Severijns et al.18 found that fatigue (measured using the MFIS) was not associated with grip fatigability in people with MS, which is consistent with the present study findings. However, a recent study by Wolkorte et al.40 found that physical fatigue measured by the MFIS was weakly associated with index finger muscle fatigability measured using a force transducer. Due to the variability in methods to assess performance fatigability and perceived fatigue in people with MS, future studies should establish a valid measure of performance fatigability in people with MS and expand use of the NFI-MS as a measure of fatigue in research and clinical settings.
As an umbrella term, MS-related fatigue encompasses both perceived fatigue and fatigability. Therefore, based on the findings of the present study, it seems that the NFI-MS captures the cognitive aspect of MS-related fatigue (ie, it captures both perceived cognitive fatigue and performance fatigability assessed using an attentional task) but not the physical aspect (ie, it captures perceived physical fatigue only, not performance fatigability assessed using a physical task). Larger-scale studies are needed to verify these conclusions. One possible explanation is that perhaps the items on the NFI-MS physical domain are not worded in a manner that objectifies the individual's performance physical fatigability; hence the lack of association. However, items on the NFI-MS cognitive domain such as “my coordination gets worse as the day goes on” and “mental effort really takes it out of me” are worded in a manner that captures both perceived fatigue and performance fatigability assessed using an attentional task. In addition, the confounding effect of peripheral fatigue might be another reason for the lack of association with perceived physical fatigue,10 which is the decline or complete failure to excite muscles often due to changes in muscle tissue or deficits in the function of the neuromuscular junction.10 Although several studies have proposed that perceived fatigue and fatigability in people with MS are due to disease-caused physiologic alterations in the central nervous system,8,9 peripheral components may also contribute.10 Therefore, to capture the physical aspect of MS-related fatigue, perhaps both perceived physical fatigue measures and performance fatigability measures are needed collectively to capture the peripheral and central components of physical MS-related fatigue. Future studies with adequate sample size are needed to confirm these conclusions.
Most of the variability of perceived fatigue was explained by lower physical quality of life in this study sample even after controlling for the confounding effect of depression. This is an important finding that affirms the serious effects that perceived fatigue has on physical quality of life in people with MS. Only one previous study explored the relationship between fatigue assessed using the NFI-MS and MS-related clinical characteristics.41 The lack of associations between fatigue and age or disease duration is similar to the findings of Mills and Young,41 who observed no associations between fatigue and age or disease duration but found strong associations with disease severity. Mills and Young found that higher fatigue was associated with a higher physical and psychological impact of MS measured using the 29-item Multiple Sclerosis Impact Scale,41 which is somewhat similar to our finding in which reduced physical quality of life is associated with higher fatigue in people with MS. The present study findings differ from those of Mills and Young in that we found that depression was strongly associated with fatigue, in contrast to the weak association found in their study. However, previous studies found significant associations between depression and fatigue in people with MS.7,8 This might be due to the clinical overlap between depression and fatigue7 because fatigue can be a symptom of depression and vice versa.
The finding that state levels of fatigue increased significantly after performing the fatigability tests in a sample of individuals with mild disease severity is relevant for daily life. The fatigability measures used in this study resemble activities of daily living, and the finding that those tasks were fatiguing the participants reflects how an individual with MS might be struggling functionally on a daily basis. The 6MWT is a walking task that resembles daily activities such as community ambulation. A strong, sustained grip is often needed to carry groceries or shopping bags. Sustained attention (CPT) is necessary for individuals to effectively perform continuous and repetitive activities, such as following clinician or therapist instructions. Being fatigued may affect the performance of these tasks and limit the individual's functional abilities. Therapists and clinicians may need to consider structuring their interventions to limit increasing MS-related fatigue. For example, Karpatkin et al.27 suggested that people with MS might exhibit less fatigue if they walk intermittently instead of continuously. This study showed that people with MS who walked intermittently for 6 minutes (ie, walked every 2 minutes and rested another 2 minutes) had less fatigue and walked longer distances compared with those who continuously walked for 6 minutes.
There are some limitations to the present study. First, its cross-sectional design makes it difficult to interpret the associations as a cause-effect relationship. In addition, the study findings are not generalizable to individuals with MS with moderate-to-severe disease severity because the study sample, on average, had mild disease severity. Participants were permitted to take their usual medications the day of testing, which might have affected their performance on the tests. Furthermore, results should be interpreted with caution because, due to the exploratory nature of the study, correction for multiple comparisons has not been made.
In summary, perceived fatigue is associated with performance fatigability assessed using an attentional task but not with performance fatigability assessed using a physical task in people with MS, and decreased physical quality of life is a large contributor to perceived fatigue in people with MS with mild disease severity. For a comprehensive and multidimensional assessment of MS-related fatigue, the measures used in this study can be easily administered in clinical and research settings. Due to the exploratory nature of the present study, larger-scale future studies are needed to verify these findings and to explore the association between perceived fatigue and fatigability in those with more severe disability due to MS.
PracticePoints
Study participants reported increased levels of state fatigue after completing three performance fatigability tasks (walking, hand grip, and attention).
Of the three performance fatigability tasks, only attention was significantly correlated with perceived fatigue, illustrating that increased perceived fatigue is not always associated with a decrease in performance.
The Neurological Fatigue Index and the walking, hand grip, and attention performance fatigability measures used in the present study can be easily administered and scored in clinical settings for a comprehensive assessment of MS-related fatigue.
Supplementary Material
Acknowledgments
We thank Drs. Patricia Kluding, Jeff Radel, Jessie Huisinga, and Sharon Lynch for their contributions to this study and the creators of the NFI-MS for granting us permission to use the questionnaire for this study.
Financial Disclosures
Dr. Bruce provides unbranded talks for the Novartis speakers' bureau and has served on the Novartis MS and Cognition Medical Advisory Board. The other authors have no conflicts of interest to disclose.
References
- 1. Berger JR, Pocoski J, Preblick R, Boklage S.. Fatigue heralding multiple sclerosis. Mult Scler. 2013; 19: 1526– 1532. [DOI] [PubMed] [Google Scholar]
- 2. Glanz BI, Degano IR, Rintell DJ, Chitnis T, Weiner HL, Healy BC.. Work productivity in relapsing multiple sclerosis: associations with disability, depression, fatigue, anxiety, cognition, and health-related quality of life. Value Health. 2012; 15: 1029– 1035. [DOI] [PubMed] [Google Scholar]
- 3. Krupp LB, Serafin DJ, Christodoulou C.. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010; 10: 1437– 1447. [DOI] [PubMed] [Google Scholar]
- 4. Enoka RM, Duchateau J.. Translating fatigue to human performance. Med Sci Sports Exerc. 2016; 48: 2228– 2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zijdewind I, Prak RF, Wolkorte R.. Fatigue and fatigability in persons with multiple sclerosis. Exerc Sport Sci Rev. 2016; 44: 123– 128. [DOI] [PubMed] [Google Scholar]
- 6. Bisecco A, Caiazzo G, d'Ambrosio A, . et al. Fatigue in multiple sclerosis: the contribution of occult white matter damage. Mult Scler. 2016; 22: 1676– 1684. [DOI] [PubMed] [Google Scholar]
- 7. Kinsinger SW, Lattie E, Mohr DC.. Relationship between depression, fatigue, subjective cognitive impairment, and objective neuropsychological functioning in patients with multiple sclerosis. Neuropsychology. 2010; 24: 573– 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bol Y, Duits AA, Hupperts RM, Vlaeyen JW, Verhey FR.. The psychology of fatigue in patients with multiple sclerosis: a review. J Psychosom Res. 2009; 66: 3– 11. [DOI] [PubMed] [Google Scholar]
- 9. Kluger BM, Krupp LB, Enoka RM.. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013; 80: 409– 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finsterer J, Mahjoub SZ.. Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care. 2014; 31: 562– 575. [DOI] [PubMed] [Google Scholar]
- 11. Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D.. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Mult Scler. 1999; 5: 251– 259. [DOI] [PubMed] [Google Scholar]
- 12. Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF.. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994; 18 suppl 1: S79– S83. [DOI] [PubMed] [Google Scholar]
- 13. Larson RD. Psychometric properties of the modified fatigue impact scale. Int J MS Care. 2013; 15: 15– 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mills RJ, Young CA, Pallant JF, Tennant A.. Rasch analysis of the Modified Fatigue Impact Scale (MFIS) in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010; 81: 1049– 1051. [DOI] [PubMed] [Google Scholar]
- 15. Leone C, Severijns D, Dolezalova V, . et al. Prevalence of walking-related motor fatigue in persons with multiple sclerosis: decline in walking distance induced by the 6-Minute Walk Test. Neurorehabil Neural Repair. 2016; 30: 373– 383. [DOI] [PubMed] [Google Scholar]
- 16. Eldadah BA. Fatigue and fatigability in older adults. Phys Med Rehabil. 2010; 2: 406– 413. [DOI] [PubMed] [Google Scholar]
- 17. Bruce JM, Bruce AS, Arnett PA.. Response variability is associated with self-reported cognitive fatigue in multiple sclerosis. Neuropsychology. 2010; 24: 77– 83. [DOI] [PubMed] [Google Scholar]
- 18. Severijns D, Lamers I, Kerkhofs L, Feys P.. Hand grip fatigability in persons with multiple sclerosis according to hand dominance and disease progression. J Rehabil Med. 2015; 47: 154– 160. [DOI] [PubMed] [Google Scholar]
- 19. Schnelle JF, Buchowski MS, Ikizler TA, Durkin DW, Beuscher L, Simmons SF.. Evaluation of two fatigability severity measures in elderly adults. J Am Geriatr Soc. 2012; 60: 1527– 1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mills RJ, Young CA, Pallant JF, Tennant A.. Development of a patient reported outcome scale for fatigue in multiple sclerosis: the Neurological Fatigue Index (NFI-MS). Health Qual Life Outcomes. 2010; 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. US Food and Drug Administration. . Draft Guidance for Industry on Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims (Docket 2006D-0044). Rockville, MD: US Food and Drug Administration; 2006. [Google Scholar]
- 22. Goksel Karatepe A, Kaya T, Gunaydn R, Demirhan A, Ce P, Gedizlioglu M.. Quality of life in patients with multiple sclerosis: the impact of depression, fatigue, and disability. Int J Rehabil Res. 2011; 34: 290– 298. [DOI] [PubMed] [Google Scholar]
- 23. Murphy S, Niemiec SS.. Aging, fatigue, and fatigability: implications for occupational and physical therapists. Curr Geriatr Rep. 2014; 3: 135– 141. [Google Scholar]
- 24. McDonald WI, Compston A, Edan G, . et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001; 50: 121– 127. [DOI] [PubMed] [Google Scholar]
- 25. Pangman VC, Sloan J, Guse L.. An examination of psychometric properties of the mini-mental state examination and the standardized mini-mental state examination: implications for clinical practice. Appl Nurs Res. 2000; 13: 209– 213. [DOI] [PubMed] [Google Scholar]
- 26. Solway S, Brooks D, Lacasse Y, Thomas S.. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001; 119: 256– 270. [DOI] [PubMed] [Google Scholar]
- 27. Karpatkin H, Cohen ET, Rzetelny A, . et al. Effects of intermittent versus continuous walking on distance walked and fatigue in persons with multiple sclerosis: a randomized crossover trial. J Neurol Phys Ther. 2015; 39: 172– 178. [DOI] [PubMed] [Google Scholar]
- 28. Goldman MD, Marrie RA, Cohen JA.. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008; 14: 383– 390. [DOI] [PubMed] [Google Scholar]
- 29. Bohannon RW. Dynamometer measurements of hand-grip strength predict multiple outcomes. Percept Mot Skills. 2001; 93: 323– 328. [DOI] [PubMed] [Google Scholar]
- 30. Fess EE. A method for checking Jamar dynamometer calibration. J Hand Ther. 1987; 1: 28– 32. [Google Scholar]
- 31. Mathiowetz V, Rennells C, Donahoe L.. Effect of elbow position on grip and key pinch strength. J Hand Surg. 1985; 10: 694– 697. [DOI] [PubMed] [Google Scholar]
- 32. Conners CK. Conners 3rd Edition (Conners 3). North Tonawanda, NJ: Multi-Health System; 2008. [Google Scholar]
- 33. Lee KA, Hicks G, Nino-Murcia G.. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991; 36: 291– 298. [DOI] [PubMed] [Google Scholar]
- 34. Steer RA, Cavalieri TA, Leonard DM, Beck AT.. Use of the Beck Depression Inventory for Primary Care to screen for major depression disorders. Gen Hosp Psychiatry. 1999; 21: 106– 111. [DOI] [PubMed] [Google Scholar]
- 35. Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW.. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995; 4: 187– 206. [DOI] [PubMed] [Google Scholar]
- 36. Jette AM, Cleary PD.. Functional disability assessment. Phys Ther. 1987; 67: 1854– 1859. [DOI] [PubMed] [Google Scholar]
- 37. Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D.. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol. 2013; 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bellgrove MA, Hester R, Garavan H.. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004; 42: 1910– 1916. [DOI] [PubMed] [Google Scholar]
- 39. Walhovd KB, Fjell AM.. White matter volume predicts reaction time instability. Neuropsychologia. 2007; 45: 2277– 2284. [DOI] [PubMed] [Google Scholar]
- 40. Wolkorte R, Heersema DJ, Zijdewind I.. Muscle fatigability during a sustained index finger abduction and depression scores are associated with perceived fatigue in patients with relapsing-remitting multiple sclerosis. Neurorehabil Neural Repair. 2015; 29: 796– 802. [DOI] [PubMed] [Google Scholar]
- 41. Mills RJ, Young CA.. The relationship between fatigue and other clinical features of multiple sclerosis. Mult Scler. 2011; 17: 604– 612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


