Abstract
Background:
The gold standards for assessing ambulation are the Expanded Disability Status Scale (EDSS) and the Timed 25-Foot Walk (T25FW) test. In relation with these measures, we assessed the reliability and validity of four clinical gait measures: the Timed Up and Go (TUG) test, the Dynamic Gait Index (DGI), the 2-Minute Walk Test (2MWT), and the 6-Minute Walk Test (6MWT). Patient self-report of gait was also assessed using the 12-item Multiple Sclerosis Walking Scale (MSWS-12).
Methods:
Individuals 20 years or older with a diagnosis of multiple sclerosis (MS) and an EDSS score of 2.0 to 6.5 completed the MSWS-12, T25FW test, TUG test, DGI, 2MWT, and 6MWT. All the tests were repeated 2 weeks later at the same time of day to establish their reliability and concurrent validity. Predictive validity was established using the EDSS.
Results:
Forty-two patients with MS were included. All measures showed high test-retest reliability. The TUG test, 2MWT, and 6MWT were significantly correlated with the T25FW test (Spearman ρ = −0.902, −0.919, and −0.905, respectively). The EDSS was also significantly correlated with all the walking tests. The MSWS-12 demonstrated the highest correlation to the EDSS (ρ = 0.788).
Conclusions:
The TUG test, the DGI, the 2MWT, and the 6MWT exhibited strong psychometric properties and were found to be significant predictors of the EDSS score. Use of these tests to prospectively monitor the effects of medical and rehabilitation treatment should be considered in the comprehensive care of patients with MS.
Multiple sclerosis (MS) presents in individual patients with a variety of clinical manifestations that can affect functional mobility, especially ambulation. The standardized assessment tools currently used for ambulation are the gait component of the Expanded Disability Status Scale (EDSS)1 and the Timed 25-Foot Walk (T25FW) test, a component of the Multiple Sclerosis Functional Composite.2–4 Limitations have been reported with both the EDSS gait component and the T25FW test.5–8 The gait component of the EDSS is an ordinal scale with criteria for ambulation limited to the distance walked and use of a unilateral or bilateral assistive device. It does not measure fall risk or gait speed. The T25FW test is a ratio scale in which gait speed can be calculated; however, variations may exist in the command for walking (eg, walk as fast as you can or walk at your comfortable speed), which affects the reliability of the test.3 Because the person with MS is asked only to walk in a straight line for a short distance of 25 feet, the examiner may not be able to observe gait deviations and cannot ascertain the ability of the patient to modify gait during turns, the impact of endurance on gait, or fall risk.8 The limitations identified restrict the clinician's ability to comprehensively evaluate the patient's gait. As such, there is a need to establish whether a correlation exists with clinical measures of gait that would include not only speed but also distance, endurance, balance, and fall risk.
A variety of gait measures used in other neurologic conditions can assist in identifying different impairments in gait in MS. The Timed Up and Go (TUG) test is a valid indicator of function9,10; the Dynamic Gait Index (DGI) is a valid indicator of balance during ambulation11; and endurance is often measured using the 2-Minute Walk Test (2MWT) or 6-Minute Walk Test (6MWT). The objective of this study was to assess the test-retest reliability and concurrent validity of these four gait measures: the TUG test,12 the DGI,13 the 2MWT, and the 6MWT.14–16 In addition, predictive validity in relation to the gold standard EDSS was evaluated. The patient self-report of gait as measured by the 12-item Multiple Sclerosis Walking Scale (MSWS-12)17 was also assessed in relation to the EDSS and all the clinical gait measures. This study was conducted based on the recommendations published from the Consensus Conference on Outcome Measures in Gait and Fatigue sponsored by the Consortium of Multiple Sclerosis Centers (CMSC) in November 2007.18
Methods
Study Participants
Participants were referred to this study by their neurologist at the Jacobs Neurological Institute (Buffalo, NY) or by a physical therapist in the MS wellness program at DeGraff Memorial Hospital (North Tonawanda, NY). Individuals were included if they were 20 years or older, had a diagnosis of MS based on the McDonald criteria,19,20 were ambulatory with or without an assistive device, had an EDSS score of 2.0 to 6.5, and did not have a documented relapse in the past 30 days. An EDSS score of 2.0 indicates the beginning of mild disability, and a score of 6.5 indicates the need for bilateral support to ambulate for a minimum of 65 feet. Individuals were excluded if they had limited active range of motion in the lower extremities (which prevented the ability to stand), muscle strength less than fair (3/5 manual muscle test in all muscles of the hip, knee, and ankle), and inability to perform the following full set of activities: rise from a chair, walk 10 feet, turn around, and return to the chair (TUG test). After obtaining approval from the University at Buffalo internal review board, written consent was obtained from all the participants.
Assessments
Overview
Two assessments at the same time of day were conducted 2 weeks apart (days 1 and 2) to evaluate test-retest reliability. At the first appointment, participants who met all the inclusion criteria completed the following: (1) a health history questionnaire describing symptoms associated with MS and current medications; (2) the MSWS-12; (3) physical therapy evaluation, which included a manual muscle test of the lower extremities, Modified Ashworth Scale, proprioception, static standing eyes open and closed, rotational vestibulo-ocular reflex testing, and the Dynamic Visual Acuity Test.21 If the patient was unable to hold gaze during the repetitive rotational vestibulo-ocular reflex testing or had a greater than two-line difference during the Dynamic Visual Acuity Test, a limitation in vestibular processing was noted. These tests, which were not included in the statistical analysis, were performed to establish a comprehensive baseline for each patient. For example, limited proprioception in the lower extremities or vestibulo-ocular deficits may affect the patient's ability to perform a sit-to-stand transfer or to turn 180° safely, which were components of this study. If a deficit was detected, patients were monitored and guarded more closely during completion of the walking tests. (4) Five walking tests: the T25FW test, the TUG test, the DGI, the 2MWT, and the 6MWT.
Walking Tests
The walking tests were performed in a tiled hallway 60 feet in length and free of obstructions. One examiner (S.E.B.) performed all the standardized gait measures during both visits. Mandatory rest breaks were established between specific tests. The specific time for each rest break was established before data collection and was based on the difficulty and energy level required for each preceding test. For example, if the item required one task (such as the T25FW test) or three tasks (such as the TUG test), rest breaks were shorter than for an eight-item task (such as the DGI) or an endurance task. The tests were performed as described below and in this order:
T25FW Test. Two trials were performed. Patients started at a line on the floor and were instructed to “walk as quickly as possible but safely” beyond the second line 25 feet away. Time was recorded in seconds beginning with the first heel strike beyond the start line and ending with the first heel strike after the second line, with the faster time of the two trials used for the analysis.
(There was a 1-minute rest period before proceeding.)
TUG Test. Participants were seated in a chair with two arm rests, were instructed that at the word go they were to rise from the chair, walk as quickly as possible but safely to a mark 10 feet away, turn around, walk back, and sit down. Two trials were performed. The stopwatch was started at the verbal cue go and was stopped when the patient was safely seated in the chair. Time was recorded in seconds, with the faster of the two trials used for the analysis.
(There was a 3-minute rest period before proceeding.)
The DGI. Patients performed the eight walking tasks of the DGI in a hallway with tape markers on the floor every 5 feet for 20 feet total. The examiner used standardized cueing that has been validated in a previous study: normal walking cadence; change in gait speed (normal walk, fast as you can, slow as you can, which was cued by the examiner every 5 feet); walking normal pace for 5 feet, then head turn left for 5 feet, then head turn right 5 feet, then head in midline for 5 feet; walking at a normal pace for 5 feet, then with vertical (up) head turn 5 feet then vertical (down) head turn for 5 feet then head in midline for 5 feet; walking and turning 180° and stopping; walking and stepping over an object; walk around two objects in a figure-eight pattern; and ascend and descend four steps.13 The ordinal score is based on the physical therapist's observation of any gait deviations or imbalance. A normal walk without challenge to balance is scored a 3, and inability to perform the task is 0 (maximum score = 24).
(There was a 5-minute rest period before proceeding.)
The 2MWT. Patients were instructed to “walk at your comfortable pace” back and forth along a 60-foot walkway for 2 minutes.15,16,22
(There was a 5-minute rest period before proceeding.)
The 6MWT. The same protocol as described for the 2MWT test was performed for 6 minutes.
Data Analysis
Based on previous literature and known variances of all the tests, a power analysis identified that 30 participants were needed for 90% statistical power with an expected large effect size (r = 0.50). For the T25FW and TUG tests, two trials of each test were performed on both of the two days separated by 2 weeks (days 1 and 2) to determine whether there were learning or fatigue effects. Two scores of the T25FW and TUG tests were compared using the paired t test. For all the variables, test-retest reliability was examined using intraclass correlation coefficient with a two-way mixed model and consistency type. The distribution of all the variables was tested with a one-sample Kolmogorov-Smirnov test. All the variables were not normally distributed, except for day 1 of the MSWS-12 transformed scores. Therefore, Spearman rho correlation coefficients were used with a one-tailed test to assess concurrent and predictive validity. A statistical software program (IBM SPSS Statistics for Windows, version 22.0; IBM Corp, Armonk, NY) was used, and the significance level was set at P < .05.
Results
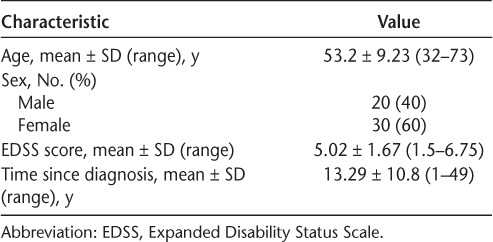
Fifty-four patients were referred from their neurologist or a local MS wellness program to participate in the study. Of the 54 patients, four were excused due to the presence of one or more exclusion criteria. A total of 50 patients completed the study; however, eight patients had incomplete data, and, therefore, 42 patients were used in the analysis. Three patients were lost to follow-up and did not complete day 2. Five patients were unable to complete the higher-level gait measures (DGI, 2MWT, and 6MWT) and, therefore, were excluded from the analysis. Baseline characteristics of all the participants are shown in Table 1.
Table 1.
Characteristics of the 50 study participants
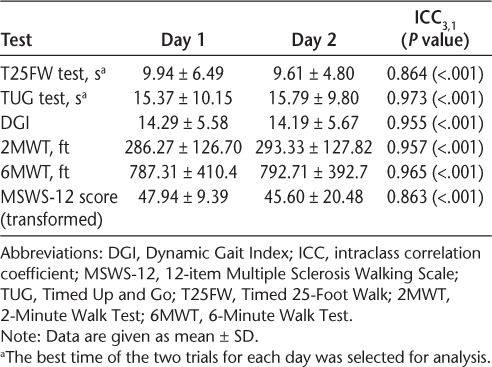
There were no significant differences between trials 1 and 2 for the T25FW test or the TUG test or between days 1 and 2 for all the variables. Test-retest reliability, using intraclass correlation coefficient model 3 for single measures, was very high for all the variables (Table 2).
Table 2.
Test-retest reliability
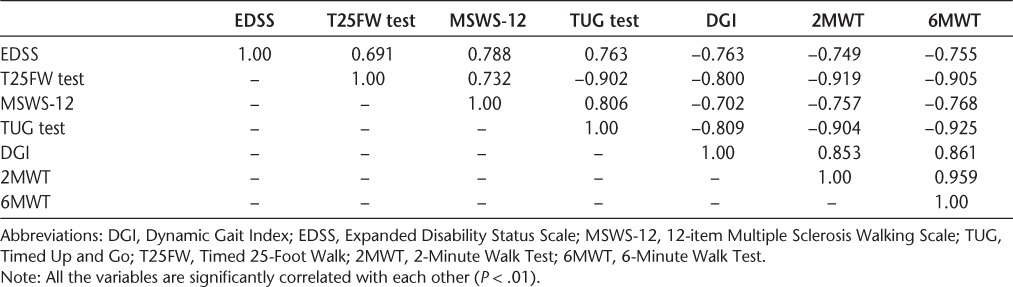
All the gait measures were strongly correlated (P < .01) with each other, establishing concurrent validity. Predictive validity was established given the high correlations between all the gait measures and the EDSS (Table 3). The highest correlation with the EDSS was the self-reported MSWS-12 (ρ = 0.788).
Table 3.
Spearman rho correlations between clinical tests
Discussion
This study provides evidence on the validity and reliability of four clinical gait measures and one self-report measure with the gold standards of the EDSS and the T25FW test. The sample size in this study was larger than required in the power analysis, resulting in many significant findings. Two types of criterion-oriented validity were established as a result of the present protocol. Concurrent validity in which measures are assessed simultaneously and a correlation is computed23 was verified between all functional tests. The direction of the correlations was as expected, taking into account the fact that higher levels of gait limitations are reflected by higher or lower values depending on the measure. Predictive validity in which measures are assessed at separate time points23 was established between all functional tests and the EDSS (the EDSS score was established by the neurologist and not obtained until after all testing procedures were completed). Reliability of the four gait measures was another factor investigated in this study. Participants were retested 2 weeks after the first test at the same time of day to control for variability related to activity through the course of a typical day. Test-retest reliability was good for all measures based on the observed correlation coefficients.
With the increasing options of effective disease-modifying therapies, there is a shift in treatment goals from reducing relapses to a new gold standard of no evidence of disease activity (NEDA).24 The new NEDA guidelines include functional outcomes to assess whether the disease has progressed, pointing out that especially at the lower EDSS score ranges a slight clinical progression would not be identified.25 This underscores the increasing need to comprehensively evaluate the patient with multiple measures, not only to assess various aspects of motor control, but also to detect smaller changes in function. The T25FW test is an appropriate measure of gait velocity but does not include the person's ability to vary his or her gait to perform different tasks required during walking. Daily challenges to walking, such as head scanning, stepping over an obstacle, or turning around, are components of the TUG test and the DGI and can be better indicators of overall function. In fact, in this study all the gait measures as well as the MSWS-12 were stronger predictors of EDSS score than was the T25FW test.
Of equal importance is the assessment of fall risk to establish safety in the home and community. Previous research with the TUG test and DGI has included fall risk for community-dwelling elders and neurologic conditions such as Parkinson's disease.26–28 The TUG test has been established as an indicator of fall risk27 and basic functional mobility because it requires the patient to rise from a chair, walk 10 feet, turn around, and walk back to the chair. The shorter time required in the TUG test is an indicator that the individual is not at risk for falls (<10 seconds).28 The DGI is also able to discriminate between fallers (mean score, 13.3) and nonfallers (mean score, 16.9) in MS. The DGI discriminated better than the Berg Balance Scale but not as well as the Activities-Specific Balance Confidence Scale or the Dizziness Handicap Index.11
In addition to gait measures for fall risk, examining the person's ability to walk safely for a specific time could be an indication of his or her return to community participation. The 2MWT and the 6MWT have been incorporated into research examining patients with cardiac and pulmonary dysfunction, lower-limb amputees, and people with MS.14–16 The verbal instruction used for the 2MWT and the 6MWT followed the protocol of earlier research in respiratory disease.14–16 We chose not to use the verbal instructions cited by Goldman et al.,14 which instruct the patient to “walk as fast and as far as possible” for safety reasons. Goldman et al.14 identified three falls during the 6MWT using that script. During the present study, no falls occurred, and the correlations with the other gait measures were high despite using the previously established command “walk at your comfortable pace.”
Another noteworthy finding was that the MSWS-12 score was a strong predictor of the EDSS score. This finding enforces the value of using a self-report instrument as part of a comprehensive gait analysis. Limited ambulation is a primary consequence of disease progression in MS, and lower-limb function required for ambulation was rated as the most important function for persons with MS.29 Gait limitation is a complex multifactorial impairment and as such should be assessed with multiple valid and reliable outcome measures. This study supports use of the MSWS-12 by all health-care professionals to initiate discussion about the patient's walking ability. Functional measures should then be assessed in a clinical setting to provide a detailed baseline of the patient's gait impairment with the goal of detecting minor functional change earlier in the disease course that would lead to an expedited therapeutic intervention.
As with most study designs, limitations exist, including the fact that these patients were followed up at an MS clinic or in an MS rehabilitation program; therefore, the sample may not represent the general population of patients with MS. Also, owing to the longer disease duration and relatively high level of disability in the sample, the results may not represent patients at an earlier stage of the disease. Finally, the study did not assess sensitivity to change, which is an important quality to ascertain before choosing a measure for clinical monitoring.
Conclusion
With the advances in medical management and rehabilitation in MS, it is imperative that we establish outcome measures that quantify an individual's level of function and gait. This study demonstrated that the TUG test, the DGI, the 2MWT, and the 6MWT exhibit strong psychometric properties. Use of these gait measures, which are easy to perform in any clinical setting, enables the health-care practitioner to monitor the effect and benefit of medical and rehabilitation treatment.
PracticePoints
The Timed Up and Go (TUG) test, the Dynamic Gait Index (DGI), the 2-Minute Walk Test (2MWT), and the 6-Minute Walk Test (6MWT) are valid and reliable clinical gait performance measures in MS.
These four measures also provide clinically important information, such as balance and walking endurance.
Considering their psychometric properties and ease of administration, the TUG test, the DGI, the 2MWT, and the 6MWT should be used more frequently by clinicians to monitor the functional status of patients with MS.
Financial Disclosures
Dr. Bennett has received honoraria for speaking from Acorda Therapeutics, Biogen Idec, and Medtronic Inc; is on the advisory board and has received research funding from Acorda Therapeutics; and is on the Clinical Events Committee of Innovative Neurotronics. Dr. Bromley has received honoraria for speaking and research funding from Acorda Therapeutics. The other authors have no conflicts of interest to disclose.
Funding/Support
This study was supported by a grant from the Foundation of the Consortium of Multiple Sclerosis Centers.
References
- 1. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology. 1983; 33: 1444– 1452. [DOI] [PubMed] [Google Scholar]
- 2. Bin Sawad A, Seoane-Vazquez E, Rodriguez-Monguio R, Turkistani F.. Evaluation of the Expanded Disability Status Scale and the Multiple Sclerosis Functional Composite as clinical endpoints in multiple sclerosis clinical trials: quantitative meta-analyses. Curr Med Res Opin. 2016; 32: 1969– 1974. [DOI] [PubMed] [Google Scholar]
- 3. Rudick RA, Cutter G, Reinold S.. The Multiple Sclerosis Functional Composite: a new clinical outcome measure for multiple sclerosis. Mult Scler. 2002; 8: 359– 365. [DOI] [PubMed] [Google Scholar]
- 4. Kalkers NF, de Groot V, Lazeron RHC, . et al. MS Functional Composite: relation to disease phenotype and disability strata. Neurology. 2000; 54: 1233– 1239. [DOI] [PubMed] [Google Scholar]
- 5. Albrecht H, Wotzel C, Erasmus LP, Kleinpeter M, Konig N, Pollmann W.. Day-to-day variability of maximum walking distance in MS patients can mislead to relevant changes in the Expanded Disability Status Scale (EDSS): average walking speed is a more constant parameter. Mult Scler. 2001; 7: 105– 109. [DOI] [PubMed] [Google Scholar]
- 6. Sharrack B, Hughes RAC, Soudain S, Dunn G.. The psychometric properties of clinical rating scales used in multiple sclerosis. Brain. 1999; 122: 141– 159. [DOI] [PubMed] [Google Scholar]
- 7. Hobart J, Freeman J, Thompson A.. Kurtzke scales revisited: the application of psychometric methods to clinical intuition. Brain. 2000; 123: 1027– 1040. [DOI] [PubMed] [Google Scholar]
- 8. Martin CL, Phillips BA, Kilpatrick TJ, . et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler. 2006; 12: 620– 628. [DOI] [PubMed] [Google Scholar]
- 9. Sebastiao E, Sandroff BM, Learmonth YC, Motl RW.. Validity of the Timed Up and Go test as a measure of functional mobility in persons with multiple sclerosis. Arch Phys Med Rehabil. 2016; 97: 1072– 1077. [DOI] [PubMed] [Google Scholar]
- 10. Cavanaugh JT, Gappmaier VO, Dibble LE, Gappmaier E.. Ambulatory activity in individuals with multiple sclerosis. J Neurol Phys Ther. 2011; 35: 26– 33. [DOI] [PubMed] [Google Scholar]
- 11. Cattaneo D, Regola A, Meotti M.. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil. 2006; 28: 789– 795. [DOI] [PubMed] [Google Scholar]
- 12. Nilsagard YL, Gunnarsson LG, Denison E.. Clinical relevance using timed walk tests and ‘timed up and go’ testing in persons with multiple sclerosis. Physiother Res Int. 2007; 12: 105– 114. [DOI] [PubMed] [Google Scholar]
- 13. McConvey J, Bennett SE.. Reliability of the dynamic gait index in individuals with multiple sclerosis. Arch Phys Med Rehabil. 2005; 86: 130– 133. [DOI] [PubMed] [Google Scholar]
- 14. Goldman MD, Marrie RA, Cohen JA.. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008; 14: 383– 390. [DOI] [PubMed] [Google Scholar]
- 15. Butland RJA, Pang J, Gross ER, Woodcock AA, Geddes DM.. 2-Minute, 6-Minute, and 12-Minute Walking tests in respiratory-disease. BMJ. 1982; 284: 1607– 1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brooks D, Parsons J, Hunter JP, Devlin M, Walker J.. The 2-minute walk test as a measure of functional improvement in persons with lower limb amputation. Arch Phys Med Rehabil. 2001; 82: 1478– 1483. [DOI] [PubMed] [Google Scholar]
- 17. Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology. 2003; 60: 31– 36. [DOI] [PubMed] [Google Scholar]
- 18. Hutchinson B, Forwell SJ, Bennett S, Brown T, Karpatkin H, Miller D.. Toward a consensus on rehabilitation outcomes in MS: gait and fatigue. Report of a CMSC Consensus Conference, November 28–29, 2007. Int J MS Care. 2009; 11: 67– 78. [Google Scholar]
- 19. Polman CH, Reingold SC, Banwell B, . et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69: 292– 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montes J, McDermott MP, Martens WB, . et al. Six-Minute Walk Test demonstrates motor fatigue in spinal muscular atrophy. Neurology. 2010; 74: 833– 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kheradmand A, Zee DS.. The bedside examination of the vestibuloocular reflex (VOR): an update. Rev Neurol. 2012; 168: 710– 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kosak M, Smith T.. Comparison of the 2-, 6-, and 12-minute walk tests in patients with stroke. J Rehabil Res Dev. 2005; 42: 103– 107. [DOI] [PubMed] [Google Scholar]
- 23. Cronbach LJ, Meehl PE.. Construct validity in psychological tests. Psychol Bull. 1955; 52: 281– 302. [DOI] [PubMed] [Google Scholar]
- 24. Bevan CJ, Cree BA.. Disease activity free status: a new end point for a new era in multiple sclerosis clinical research? JAMA Neurol. 2014; 71: 269– 270. [DOI] [PubMed] [Google Scholar]
- 25. Freedman MS, Selchen D, Arnold DL, . et al. Treatment optimization in MS: Canadian MS Working Group updated recommendations. Can J Neurol Sci. 2013; 40: 307– 323. [DOI] [PubMed] [Google Scholar]
- 26. Hebert JR, Corboy JR, Manago MM, Schenkman M.. Effects of vestibular rehabilitation on multiple sclerosis–related fatigue and upright postural control: a randomized controlled trial. Phys Ther. 2011; 91: 1166– 1183. [DOI] [PubMed] [Google Scholar]
- 27. Schoene D, Wu SM, Mikolaizak AS, . et al. Discriminative ability and predictive validity of the timed up and go test in identifying older people who fall: systematic review and meta-analysis. J Am Geriatr Soc. 2013; 61: 202– 208. [DOI] [PubMed] [Google Scholar]
- 28. Hafsteinsdottir TB, Rensink M, Schuurmans M.. Clinimetric properties of the Timed Up and Go Test for patients with stroke: a systematic review. Top Stroke Rehabil. 2014; 21: 197– 210. [DOI] [PubMed] [Google Scholar]
- 29. Heesen C, Bohm J, Reich C, Kasper J, Goebel M, Gold SM.. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008; 14: 988– 991. [DOI] [PubMed] [Google Scholar]


