Summary
Background and Objective
Upon stimulation, endothelial cells release von Willebrand factor (VWF) enriched in ultra-large (UL) forms that are rapidly cleaved by ADAMTS-13. The zinc metalloprotease fits in the consensus for members of the ADAMTS family, but also contains two unique C-terminal CUB domains. There are 5 and 2 cysteine residues in the CUB-1 and CUB-2 domains, respectively, instead of four as deducted from the consensus. In this study, we investigated the role of cysteine residues in the CUB-1 domain in ADAMTS-13 synthesis and activity.
Methods and Results
CUB-1 and cysteine mutations were expressed in mammalian cell lines and examined for synthesis, secretion, stability, and VWF-cleaving activity. When expressed as isolated domain, CUB-1, but not CUB-2, covalently aggregated. Converting any of the four cysteines that fit in the CUB consensus (C1192, C1213, C1236 and C1254) reduced the secretion of the mutants to the conditioned medium, but not to extracellular matrix. The mutations also resulted in a moderate increase in proteolytic degradation and decrease in cleaving plasma VWF under static, but not flowing conditions. In contrast, replacing C1275, which was found to be in the thiol form, with a serine residue prevented covalent aggregation of CUB-1, but had no effect on secretion and VWF-cleaving activity. C1275S is also markedly resistant to proteolytic degradation.
Conclusion
The data illustrate the importance of consensus cysteines in the secretion and proteolytic activity of ADAMTS-13. They also identify an ADAMTS-13 mutant that is resistant to proteolytic degradation, while maintaining a normal VWF-cleaving activity.
Keywords: TTP, ADAMTS-13, CUB domains, cysteine, biosynthesis, stability
Introduction
Upon secretion from activated endothelial cells, the ultra-large (UL) and hyperactive forms of von Willebrand factor (VWF) multimers undergo rapid, but partial proteolysis by ADAMTS-13 to reduce their hyper-adhesiveness, while maintaining their hemostatic potency. ADAMTS-13 deficiency, caused by either mutations in ADAMTS13 gene or autoantibodies to the metalloprotease, is associated with thrombotic thrombocytopenia purpura (TTP) characterized by systemic and platelet-VWF-rich microvascular thrombosis (1-3).
ADAMTS-13 is a member of the A Disintegrin and Metalloprotease with Thrombospondin type 1 repeat (ADAMTS) metalloprotease family. It has a domain structure similar to other family members, except that it also contains two C-terminal CUB domains (Complement components C1r/C1s, Uegf, and Bone morphogenic protein-1) (2). The structural consensus of a CUB domain is composed of ten β-strands arranged into two β-sheets and the overall structure is stabilized by two disulfide bonds (C1-C2 and C3-C4) among four conserved cysteine residues (4). The entire CUB domain is hydrophobic and believed to be independently folded as an immunoglobulin-like domain (5;6). However, the CUB domains in ADAMTS-13 do not precisely fit in this consensus because CUB-1 and CUB-2 contain five and two cysteine residues, respectively (2). The unusual disulfide bond pattern and a potential unpaired cysteine in the CUB-1 domain may allow a more flexible secondary structure. It has been previously demonstrated that the lack of disulfide bonds allows the CUB domain of the third component of complement (C3) to be extended, a conformational change pivotal for activating C3 (7).
Although the ADAMTS-13 CUB domains are structurally unknown, their function has been studied in vitro with inconsistent results. On one hand, the recombinant (r) CUB-1 domain and peptides derived from it bind VWF and partially inhibit the cleavage of ULVWF-platelet strings under flow conditions (8), suggesting that it may dock ADAMTS-13 to the substrate. Removal of the CUB domains significantly reduces the binding affinity of the metalloprotease for the VWF substrate under static conditions (9) and upon exposure to mechanical forces (10). Consistent with these in vitro data, mutations in CUB domains have been reported in patients with familial TTP (3;11-14), but it remains unclear as to whether these mutations result in defective transcription/translation or expression of a dysfunctional metalloprotease. On the other hand, a truncated ADAMTS-13 lacking the CUB domains (and 7 TSP-1 motifs) cleaves (UL)VWF in vitro under static and flow conditions (15;16). Binding of truncation mutants lacking CUB domains to VWF is reduced, but not entirely abolished under static and flow conditions (9;10).
For its physiological function, a recent report demonstrates that the CUB domains interact with membrane lipid microdomains to direct ADAMTS-13 secretion to the apical surface of endothelial cells and Madin-Darby canine kidney fibroblasts (17). A mutation in the CUB-1 domain (4143-4144insA) that removes the C-terminal part of the CUB-2 domain reverses the secretion polarity in the ADAMTS-13 transfected Madin-Darby canine kidney cells.
To further study the physiological role of the ADAMTS-13 CUB domains, we have constructed 1) mutations that converted each of the five cysteine residues in the CUB-1 domain to serine, 2) isolated CUB domains, and 3) truncation mutants of ADAMTS-13. These mutants and isolated domain constructs were used to study how cysteine residues in the CUB-1 domain regulate the biosynthesis, secretion, stability, and VWF cleaving activity of the metalloprotease.
Material and Methods
Plasmids and mutagenesis
An ADAMTS-13 cDNA (containing the native signal peptide) was subcloned into the mammalian expression vector pSecTag-hygromycin (Invitrogen, Carlsbad, CA) that contains C-terminal His- and Myc-tags (8). Several ADAMTS-13 constructs were then generated by either site-directed mutagenesis using a commercial kit (Stratagene, La Jolla, CA) or polymerase chain reaction (PCR). The mutants included: 1) six isolated domains as CUB-1 (C1192 – E1298, apparent MW of 15 kDa), CUB-2 (C1299 – T1427, apparent MW of 16 kDa), CUB-1+2 (C1192 – T1427, apparent MW of 32 kDa), CUB-1C1275S, CUB-1C1275S+2, and CUB-1+ the eighth thrombospondin motif (CUB-1+TSP-18, W1076 – E1298, apparent MW of 37 kDa); 2) two truncated ADAMTS-13 that missed either TSP-18 and two CUB domains (MDTST7) or the catalytic domain (DTSTC); 3) five full-length ADAMTS-13 that had each of the five cysteine residues in the CUB-1 domain converted to serine (C1192S, C1213S, C1236S, C1254S, and C1275S). Since the first four cysteine residues are expected to form two disulfide bonds based on the consensus, we named them as consensus cysteine mutants. The accuracy of all constructs was confirmed by direct DNA sequencing.
Transfection and stable cell lines
cDNAs were introduced to Hela or CHO cells by liposome-mediated DNA transfer (Lipofectamine ™ 2000; Invitrogen), as previously described (18). The transfected cells were maintained in Dulbecco-modified eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/ml streptomycin at 37°C with 95% air and 5% CO2. Cells expressing wild-type (WT) ADAMTS-13 or mutants were selected in medium containing hygromycin-B (500 μg/ml, final concentration). For production, confluent cells were incubated with the serum-free Opti-Pro SFM with 4mM L-glutamine (Invitrogen) for 24 hrs and the conditioned media were collected. The recombinant proteins were purified through a Ni2+ column and resolved by 10% SDS polyacrylamide gel electrophoresis (SDS-PAGE) under reducing or non-reducing conditions. ADAMTS-13, its mutants, and domain constructs were detected by immunoblotting using either a monoclonal Myc-antibody (Sigma, St. Louis, MO) or a goat antibody made against a synthetic peptide derived from CUB-1 (Bethyl Laboratories, Montgomery, TX).
Analysis of ADAMTS-13 secretion
The conditioned medium of transiently transfected CHO cells was collected 72 hours after transfection. WT rADAMTS-13 and mutants released into the medium were detected by the monoclonal Myc-antibody and quantified by densitometry. The adherent cells were then washed, lysed and analyzed for intracellular pools of rADAMTS-13 (protein loading was adjusted by total protein measured at OD 280nm and actin was used as a loading control). In a separate set of experiments, rADAMTS-13 secreted into the extracellular matrix (ECM) was measured to determine the secretion polarity as previously described (17). For this, cells in confluent culture were first detached by EDTA (0.5 mM) and ECM was then dissolved with SDS sample buffer. The concentration of total proteins in cell lysates and ECM was first measured and used to adjust for equal loading for SDS-PAGE and probed with the Myc-antibody.
Stability of rADAMTS-13 during storage
WT rADAMTS-13 and its mutants were aliquoted and stored for up to four weeks. Fresh and stored samples were resolved by 10% of SDS-PAGE followed by immunoblotting with the Myc antibody. A degradation rate was calculated based on a ratio of degraded to total ADAMTS-13 detected by immunoblots at different time points. Two sets of protease inhibitors were used to determine the nature of ADAMTS-13 degradation: EDTA at 10 mM or a protease inhibitor cocktail containing 0.5 mM of AEBSF (serine protease inhibitor), 25 μM of Bestatin (aminopeptidase inhibitor), 7.5 μM of E-64 (cysteine protease inhibitor), 10 μM of Pepstatin (aspartic protease inhibitor), and 1 μM of Phosphoramidon (metalloendopeptidase inhibitor; Calbiochem, Gibbstown, NJ).
To compare the rate and degree of ADAMTS-13 cleavage by thrombin, the recombinant ADAMTS13 and C1275S (35 nM each) were incubated with 1U human thrombin at 37°C in 1× Tris-buffered saline (TBS) with 5 mM CaCl2 and 150 mM NaCl (25). Aliquots of samples was taken after 1, 2 and 6 hrs incubation and subjected to SDS-PAGE. The thrombin cleavage products were detected by immunobloting with anti-myc monoclonal antibody.
ADAMTS-13 activity under static and flow conditions
ADAMTS-13 activity was first measured under static conditions using a method modified from that of Tsai et al, with VWF multimers purified from human plasma cryoprecipitate as the substrate (18;19). Briefly, rADAMTS-13 was diluted (1:5) with low-ionic-strength Tris-buffered saline (5 mM of NaCl) and then activated with 1 mM of BaCl2 for 5 min. It was mixed with VWF, dialyzed against 1.5 M of urea for up to 24 hrs at 37°C and then subjected to 6% SDS-PAGE under reducing conditions. The separated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane and immunoblotted for cleaved VWF fragments by a polyclonal VWF antibody (DakoCytomation, Carpinteria, CA).
For the flow assay, human umbilical vein endothelial cells (HUVECs) grown in a 35 mm culture dish were first stimulated with 25 mM histamine for 3 minutes and then attached to a parallel-plate flow chamber system. Using HUVECs and human platelets were approved by the IRB of Baylor College of Medicine on research involving human subjects. HUVECs were perfused with a Tyrode's buffer containing washed human platelets in the presence of rADAMTS-13 at a flow rate of 0.2 ml/min, which generated a calculated wall shear stress of 2.5 dyn/cm2 (with an apparent viscosity of 1 cp). Perfusion of washed platelets resulted in the formation of long and platelet-decorated strings that were cleaved by rADAMTS-13 (20). VWF cleaving activity was defined as a percent reduction of ULVWF strings in 20 continuous view fields (x 200) in the presence of rADAMTS-13 as compared to its absence.
Thiol-reactive chromatography
To determine which cysteine residue(s) in the CUB domains is in a thiol form, activated thiol Sepharose 6B beads were used to capture WT rADAMTS-13 (21). Briefly, beads (2 mg) were washed and incubated with rADAMTS-13 (5 μg) in 1 ml of binding buffer (0.2M NaCl, 0.1M Tris-HCl, pH7.5) for 15 min at room temperature with constant rotation. The beads with bound ADAMTS-13 were washed 5 times with binding buffer and then incubated with 10 μg of trypsin (Roche Applied Science, Mannhein, Germany) for 10 hrs at 37°C in a 0.1M Tris-HCl buffer. The beads were again washed with binding buffer to remove unbound tryptic peptide and incubated with 20 mM of DTT to release rADAMTS-13 tryptic peptides that remained bead bound (contain thiols).
Mass spectrometry
The free thiol containing ADAMTS-13 tryptic peptides released from the beads were identified by mass spectrometry (21). Briefly, tryptic peptides were acidified with trifluoroacetic acid to ∼ pH 3, desalted on a C18 ZipTip column (Millipore, Billerica, MA) and eluted from the ZipTip with 3-5 μl of 50% acetonitrile and 2% formic acid. The solution was spotted on a MALDI target plate, with matrix (CHCA, alpha-cyano-4-hydroxycinnamic acid), dried and analyzed in reflector mode on a 4700 Proteomics Analyzer MALDI TOF/TOF mass spectrometer (Applied Biosystems, Foster City, CA). The spectra were examined for protonated monoisotopic peptide masses of Cys containing peptides predicted from a tryptic digest of ADAMTS-13. Selected peptide precursor ions were subjected to high-energy collision-induced dissociation to generate MS/MS data, which were analyzed to define the sequence.
ADAMTS-13 binding to VWF
VWF interaction with rADAMTS-13 was measured by an ELISA (18). Briefly, purified VWF multimers (5 μg) were immobilized onto wells of microtiter plates by overnight incubation at 4°C. The coated plates were then incubated with 3% bovine serum albumin at 37°C for 60 minutes to block nonspecific sites. After washing with phosphate-buffered saline (PBS), 100 μl of BSA (as a control) or rADAMTS-13 (0.5, 1.0, 2.0 μg/ml) were incubated with immobilized VWF for 30 minutes at room temperature. After washing the wells with PBS, VWF-bound ADAMTS-13 was detected with a polyclonal ADAMTS-13 antibody (156, Bethyl Lab, Montgomery, TX) and chemiluminescence.
Statistical Analysis
Experimental data were presented as either representative image from multiple experiments or as mean ± SEM of multiple data sets. The Student's t test was used for quantitative data analysis and a p value of less than 0.05 was considered to be statistically significant.
Results
Recombinant CUB-1 formed covalent aggregation
When resolved by 10% SDS-PAGE, rCUB-1 domain was detected as a smeared band of greater than 190 kDa and a single band of monomeric CUB-1 band under non-reducing and reducing conditions, respectively (Figure 1A & B, Lane 2). In comparison, CUB-2 was predominantly detected as a single band corresponding to a monomeric polypeptide under non-reducing and reducing conditions (Figure 1A & B, Lane 1). A polypeptide containing CUB-1 and CUB-2 domains (CUB-1+2) was also predominantly detected as a single band on non-reducing and reducing conditions (Figure 1A & B, Lane 3). However, a recombinant polypeptide containing CUB-1 and the eighth thrombospondin motif (CUB-1+TSP-18) again formed aggregates under non-reducing, but not reducing conditions (Figure 1A & B, Lane 4).
Figure 1. Interaction between the CUB-1 and CUB-2 domains.
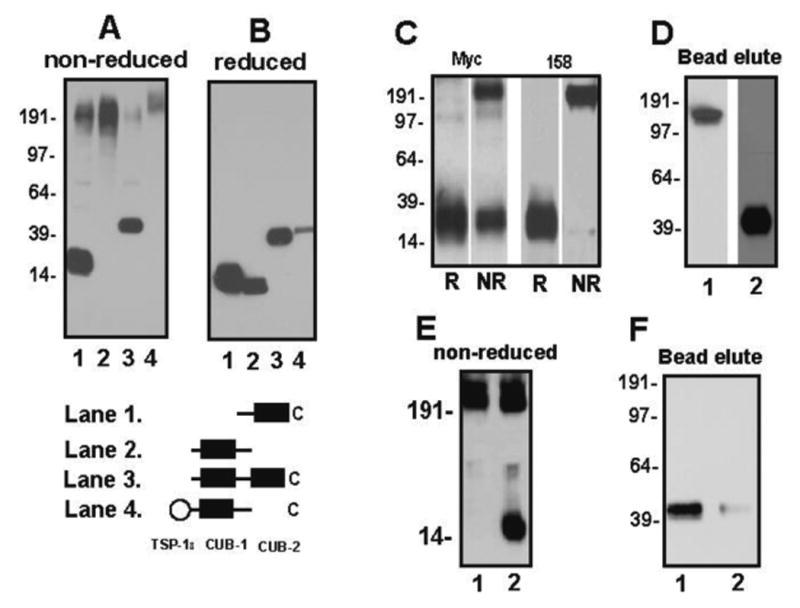
The ADAMTS-13 CUB domains were expressed in CHO cells and recombinant proteins were immunoblotted by a monoclonal antibody against Myc-tag (both CUB domains) or a goat antibody made against a synthetic peptide derived from CUB-1 under non-reducing (A) and reducing conditions (B). CUB-1 and CUB-2 were co-expressed in CHO cells and immunoblotted under reducing and non-reducing conditions by antibodies against Myc-tag or clone 158, which only binds CUB-1 (C, R: reduced; NR: non-reduced). The activated thiol beads captured rADAMTS-13 (D, lane 1) and rCUB-1+2 (D, lane 2) that were eluted by DTT and detected by immunoblotting. Recombinant CUB-1 (E, lane 1) and CUB-1C1275S (E, lane 2) were separated on SDS-PAGE under non-reducing conditions and were immunoblotted with Myc antibody. Conversion of C1275 (F, lane 1) to serine (F, lane 2) reduced CUB-1+2 captured by thiol bead by more than 90%. The figure was a representative of 3 ∼11 separate experiments.
When cDNAs for CUB-1 and CUB-2 were cotransfected, the CUB-1 antibody detected a monomeric band of 15 kDa under reducing conditions, but a high molecule weight band greater than 190 kDa under non-reducing conditions. In contrast, the monoclonal Myc antibody, which binds both domains (that were expressed as Myc-tagged polypeptides), detected CUB-2 as a monomer and CUB-1 as a high molecular weight oligomer (Figure 1C), indicating that coexpressing CUB-2 did not prevent CUB-1 aggregation. Together, these data demonstrate that CUB-1 requires CUB-2 at its C-terminal, but not TSP-18 at its N-terminal flank to maintain a monomeric structure, potentially by regulating CUB-1 folding.
The finding also suggests that the CUB-1 domain may contain surface exposed and active thiol(s); a notion that is consistent with that CUB-1 contains five cysteine residues instead of four in the consensus sequence. We therefore used active thiol beads to capture rADAMTS-13 and rCUB-1+2 polypeptide, a technique that we have previously used to identify surface exposed thiols on VWF multimers (21). As shown in Figure 1D, the thiol beads captured both WT ADAMTS-13 and CUB-1+2 construct, suggesting that CUB domains contain surface exposed thiol(s). To further identify the thiol form of cysteine residues in the CUB domains, we digested the thiol-bead captured CUB-1+2 with trypsin and then analyzed the thiol-containing tryptic peptides by mass spectrometry. As expected, thiol beads captured only one tryptic peptide from the CUB domains (C1275GRPGGGVLR), indicating that the fifth cysteine (C1275) is in the thiol form. Consistent with the mass spectrometry data, converting the fifth cysteine (C1275) to serine allows a significant portion of CUB-1C1275S to become a monomeric form under non-reducing conditions (Figure 1E) and reduced the thiol bead-capturing of CUB 1C1275S+2 by more than 90% (Figure 1F), suggesting C1275 is involved in forming covalent aggregation of the isolated CUB-1 domain. The finding that a large portion of CUB-1C1275S remained as aggregates further suggests that the structure of CUB-1 has been altered when it was expressed as isolated domain.
CUB-1 cysteine mutations affected ADAMTS-13 secretion
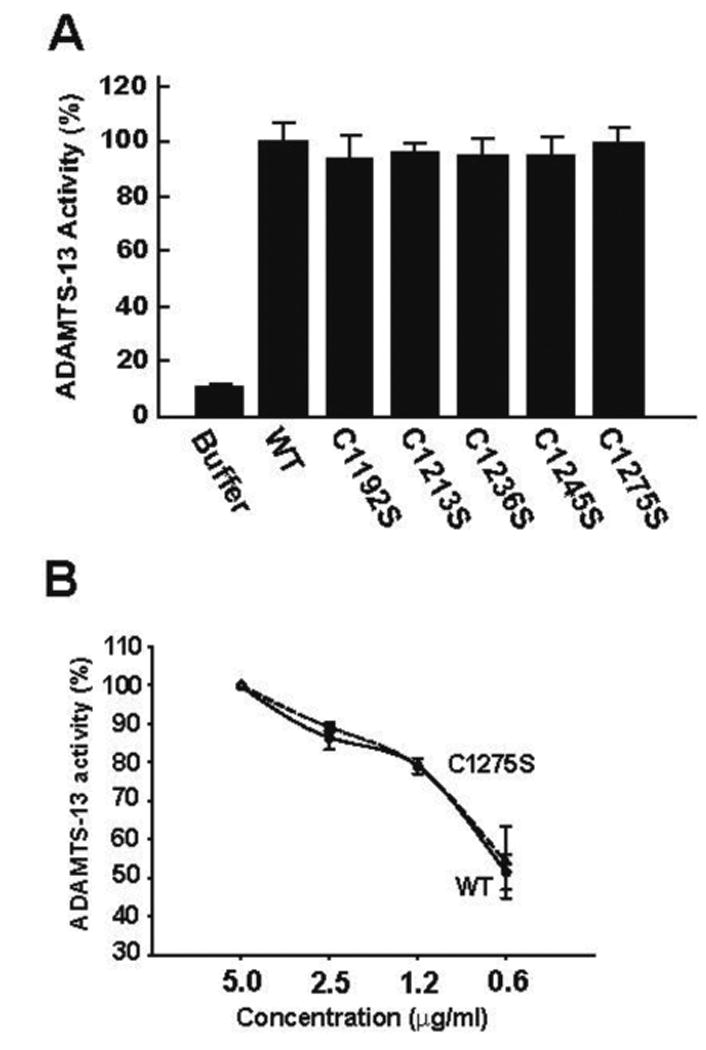
Consistent with previous reports (22), the data presented in Figure 1 suggest that cysteine residues in the CUB-1 domain are critical for its stability, which may in turn affect ADAMTS-13 biosynthesis and stability. To examine this possibility, we transiently expressed in CHO cells ADAMTS-13 mutants that converted each of the five cysteine residues (C1192, C1213, C1236, C1254 and C1275) to serine. We used transient, instead of stable transfection to eliminate possible interference caused by the incorporation of different copies of ADAMTS-13 cDNA into the host genome (23). Expression and secretion of rADAMTS-13 and its mutants were measured in the conditioned media, cell lysates and extracellular matrix (ECM) of transfected cells. As shown in Figure 2, ADAMTS-13 mutants of the four consensus cysteine residues (C1192S, C1213S, C1236S and C1254S), which are predicted to form two disulfide bonds (C1192-C1213 and C1236-C1254), were detected in significantly reduced amounts in the conditioned media, counting for 21% - 28% of the WT as determined by densitometry. In contrast, C1275S was secreted in the amount comparable to WT rADAMTS-13.
Figure 2. The CUB-1 consensus cysteine mutants impaired ADAMTS-13 secretion.
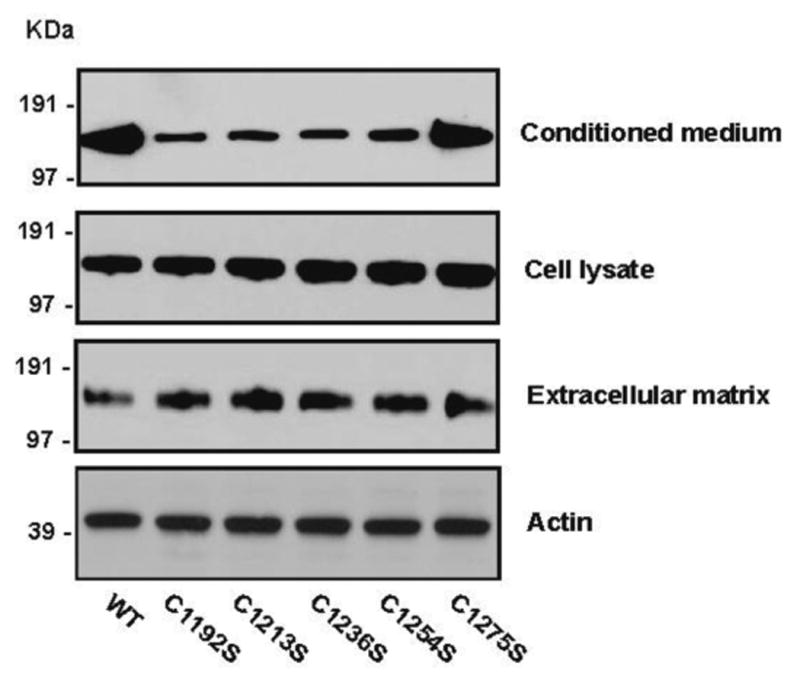
ADAMTS-13 cDNAs were transiently transfected into CHO cells and their expression evaluated in the conditioned media, cell lysates, and ECM 72 hrs after transfection by immunoblotting. Secretion of four consensus cysteine mutants was significantly reduced in the conditioned media (top panel), but not to ECM (bottom panel). In contrast, C1275S was secreted in the amount comparable to WT. Intracellular pool of the ADAMTS-13 mutants was comparable to that of WT (middle panel). Actin was used as sample loading controls. The figure was a representative of five separate experiments.
There may be at least four possible reasons for the decreased secretion of ADAMTS-13 mutants: 1) impaired DNA transcription, 2) intracellular retention of aberrant metalloprotease, 3) disruption of a secretion polarity, and 4) proteolytic degradation. To distinguish among these possibilities, we first quantified the intracellular pool of ADAMTS-13 mutants by immunoblotting and densitometry. We found that the amounts of mutants in cell lysates were comparable to WT ADAMTS-13 (Figure 2), indicating that the mutants were synthesized at a normal rate.
Shang et al (17) have previously demonstrated that CUB domains interact with cell membrane microdomains (lipid rafts) to direct ADAMTS-13 secretion primarily to the luminal surface of endothelial cells and transfected Madin-Darby canine kidney fibroblasts. In our experiments, CHO cells which are also fibroblast origin are used to study the secretion polarity of ADAMTS-13. Deletion of the CUB domains reverses the secretion polarity. It is, therefore, possible that some or all the ADAMTS-13 mutants in CUB-1 disrupt or reverse this secretion polarity to reduce secretion to the conditioned medium, while increase it to ECM. We therefore quantified the amount of rADAMTS-13 secreted into ECM and calculated the ratio of rADAMTS-13 detected in the medium to that in ECM. Four consensus cysteine mutants were detected in greater amounts in ECM than the WT (Figure 2), resulting in lower ratios of ADAMTS-13 in the medium to that in ECM (Table 1). However, C1275S was secreted at a level similar to WT in the conditioned medium and ECM. The data suggest that the consensus cysteine mutants reverse the polarity of ADAMTS-13 secretion from luminal to ECM of ADAMTS-13 expressing cells.
Table 1. Ratios of ADAMTS-13 secretion and mutants to medium/extracelluar matrix.
| Mutation | Densitometry | Medium/ECM ratio | |
|---|---|---|---|
| medium | ECM | ||
| WT | 1.00 | 1.00 | 1.00 |
| C1192S | 0.21 ± 0.01 | 1.52 ± 0.10 | 0.14 |
| C1213S | 0.25 ± 0.04 | 1.60 ± 0.09 | 0.16 |
| C1236S | 0.23 ± 0.03 | 1.53 ± 0.09 | 0.15 |
| C1254S | 0.28 ± 0.02 | 1.50 ± 0.08 | 0.19 |
| C1275S | 0.89 ± 0.01 | 1.24 ± 0.06 | 0.72 |
CUB-1 mutations affected ADAMTS-13 stability
Although under-reported, previous studies (16;24), including ours (8), have shown that rADAMTS-13 undergoes significant degradation, even when the metalloprotease was stored at -80°C. We therefore measured the degradation rate of rADAMTS-13 and mutants that were purified through a Ni2+ column to 80-90% purity (Figure 3A) and calculated it to be approximately 10-30% per week, regardless if the metalloprotease was in the conditioned medium or a purified form (Figure 3B). It was detected in rADAMTS-13 secreted from transfected CHO cells and Hela cells, but with different degradation products (39 kDa and 53 kDa for CHO and Hela cells, respectively, Figure 3C). The degradation products contained the C-terminal part of ADAMTS-13 as they were recognized by a monoclonal antibody against the C-terminal Myc tag as well as a CUB-1 specific antibody (data not shown).
Figure 3. rADAMTS-13 was degraded during storage.
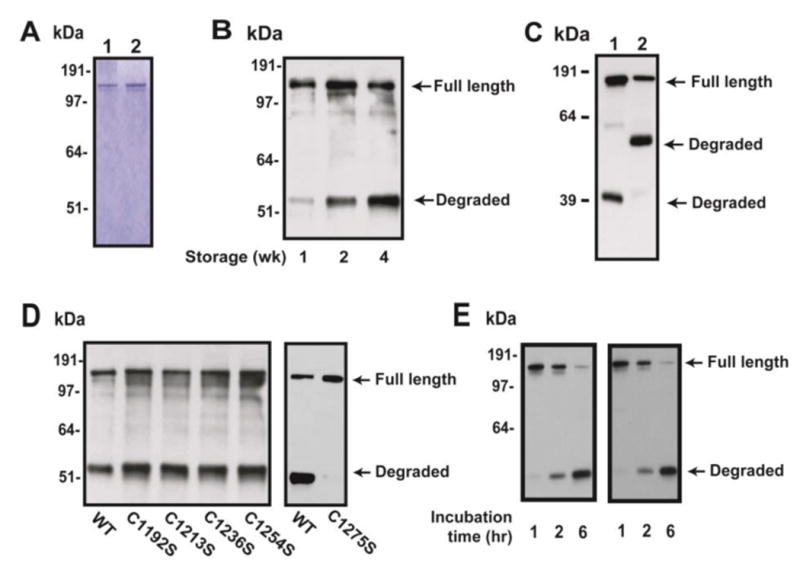
(A) Coomassie blue staining of WT ADAMTS-13 and C1275S mutant after Ni-column purification of the condition medium. (B) Purified rADAMTS-13 was stored at -80°C for up to 4 weeks before subjected to SDS-PAGE and immunoblots with antibody against the C-terminal Myc tag. WT rADAMTS-13 underwent time-dependent degradation. (C) ADAMTS-13 expressed in CHO (lane 1) or Hela cells (lane 2) was significantly degraded when they were stored for two weeks, yielded different products of 39 kDa and 53 kDa, respectively. (D) Four mutants of consensus cysteine residues resulted in increased degradation, whereas no degradation of C1275S was detected. (E) rADAMTS-13 and C1275S mutant were incubated with thrombin and aliquots of samples taken at 1, 2, and 6 hrs. The denatured samples were separated on SDS-PAGE and immunoblotted with a monoclonal anti-Myc antibody. The figure is a representative of 1 (A), 3 (B), 5 (C), 3 (D), and 2 (E) independent experiments.
As shown in Figure 3D, converting any of the four consensus cysteine residues resulted in a faster rate of degradation as compared to the WT metalloprotease, but C1275S remained stable during the four week of storage. However, the serine protease thrombin, which has been shown to cleave ADAMTS-13 (25), cleaved C1275S mutant and WT ADAMTS-13 at a comparable rate (Figure 3E). These data indicate that ADAMTS-13 is sensitive to proteolysis and the CUB-1 domain plays a role in the process. Consistent with the hypothesis, an ADAMTS-13 truncation that removed the CUB domains (MDTST7) was also resistant to proteolysis, whereas the one that removed catalytic domain (DTSTC) had similar proteolytic rate as WT ADAMTS-13 (Figure 4A). Finally, degradation was inhibited by a cocktail of protease inhibitors, but not by EDTA (Figure 4B), indicating that the degradation is not dependent on divalent cations.
Figure 4. Resistant to degradation by C-terminal truncation without CUB-domains.
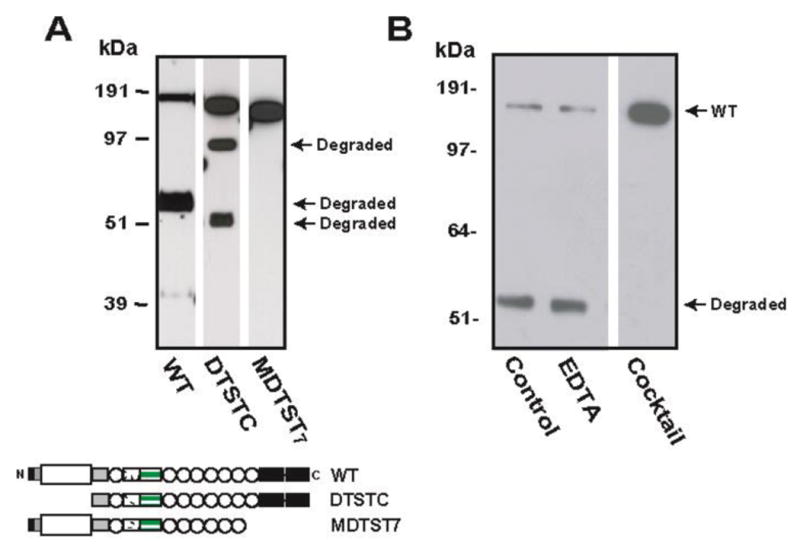
(A) Two truncation mutants lacking the catalytic (DTSTC) or CUB domains (MDTST7) were expressed in CHO cells and recombinant proteins purified from the conditioned medium and stored for two weeks. DTSTC underwent proteolysis, whereas MDTST7 was resistant. (B) Degradation of WT rADAMTS-13 was inhibited by a protease inhibitor cocktail, but not by EDTA. The figure is a representative of three separate experiments.
The mutants cleaved (UL)VWF under static and flow conditions
When tested under static conditions, all ADAMTS-13 mutants cleaved VWF purified from human cryoprecipitate. However, the cleavage was 100% for the wild-type and C1275S after 24 hrs incubation, but was 70-80% for the four consensus cysteine mutants (Figure 5A and 5B), indicating that the consensus cysteine mutants have a moderately reduced VWF-cleaving activity (20-30%). The VWF-cleaving activity of C1275 remained unchanged after four weeks in storage (Figure 5C).
Figure 5. The CUB-1 cysteine mutants maintained VWF-cleaving activity.
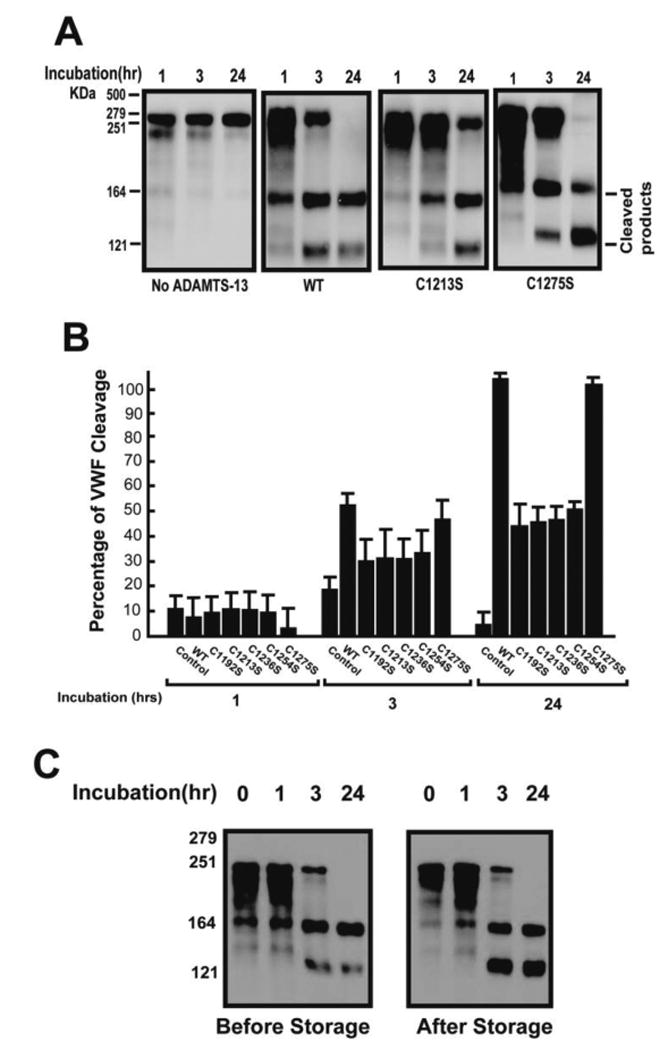
(A) Recombinant WT ADAMTS-13 and its mutants (5 μg/ml each) were first activated by barium and then incubated with plasma VWF (5 μg) for 1 – 24 hrs and tested for their (UL)VWF cleaving activity in the presence of urea. The reaction mix was then separated on 7% SDS-PAGE and immunoblotted with a polyclonal VWF antibody. Because the consensus mutants had similar activity, only C1213S was showed in Panel A. (B) Summary of the VWF-cleaving activity of WT ADAMTS-13 and cysteine mutants. ADAMTS-13 was absent in the control experiments (n = 5). (C) C1275S was tested for cleaving pVWF multimers under static conditions before and after four weeks of storage (need add the molecular weight marker and time points.).
We then measured the ability of rADAMTS-13 to cleave ULVWF strings formed on activated endothelial cells using a previously described method (20). The wild-type and mutated rADAMTS-13 were equally active in cleaving ULVWF strings (90-100%, Figure 6A). Furthermore, C1275S was as efficient as the WT metalloprotease in cleaving ULVWF strings at multiple doses (Figure 6B).
Figure 6. Cleavage of ULVWF strings by CUB-1 cysteine mutants under flow conditions.
Recombinant WT ADAMTS-13 and its cysteine mutants (5 μg/ml each) were perfused over histamine-activated HUVEC at a 2.5 dyn/cm2 of wall shear stress for 3 min. The number of platelet-decorated ULVWF strings was counted in 20 continuous review-fields (200×). ADAMTS-13 activity was defined as a percent of reduction in the number of ULVWF strings after ADAMTS-13 perfusion as compared to controls (perfused with buffer only). (A) The VWF-cleaving activity was tested at 5 μg/ml of ADAMTS-13 or cysteine mutants (ANOVA, n = 3, p = 0.559). (B) WT rADAMTS-13 and C1275S mutant were tested for VWF-cleaving activity at 0.6 – 5 μg/ml (the Student's t test, n = 3, p = 0.674 between WT and C1275S).
Consistent with data on VWF-cleaving activity, rADAMTS-13 mutants bound immobilized VWF dose-dependently and at levels comparable to WT rADAMTS-13 (Figure 7). The data indicate that the cysteine mutants did not affect the interaction between ADAMTS-13 and VWF substrate.
Figure 7. Binding of rADAMTS-13 to immobilized VWF.
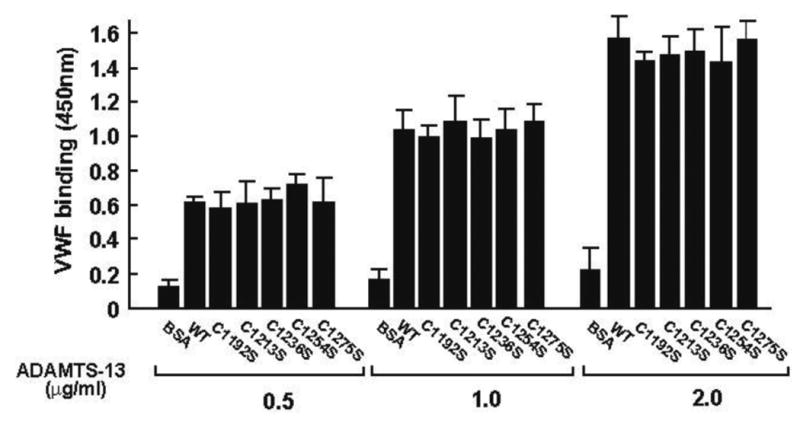
VWF multimers purified from human cryoprecipitate were immobilized onto the wells of microtiter plate overnight at 4°C. rADAMTS-13 was then incubated with immobilized VWF for 30 min and bound ADAMTS-13 detected by a polyclonal antibody against the fourth TSP-1 motif (clone 156) (ANOVA test, n = 3, p > 0.05 among WT and mutants).
Discussion
We have shown that, when individually expressed, the CUB-1, but not CUB-2 domain of ADAMTS-13, covalently aggregated (Figure 1). The aggregation was prevented or reduced when CUB-1 and CUB-2 were expressed as a single polypeptide, but not when two domains were expressed as separate polypeptides in the same cells. Furthermore, aggregation remained when a construct containing CUB-1 with the 8th TSP-1 motif, which constitutes the N-terminal flanking sequence of CUB-1. The data suggest that CUB-1 is not stable as an isolated domain, but can be stabilized by CUB-2 through an intrinsic interaction that may have helped CUB-1 folding. The finding differs from early studies that CUB domains in other proteins can be independently folded (4;26) and individually expressed (27). Such a structural stability is mostly attributed to two disulfide bonds formed among four consensus cysteine residues (4). However, the CUB-1 domain in ADAMTS-13 contains five cysteine residues. Based on the consensus sequence and available structural data for the CUB domain of other proteins, one would expect the first four cysteine residues in the CUB-1 domain to form two disulfide bonds (C1192-C1213 and C1236-C1254) (4), whereas C1275, remains as a surface exposed thiol that is sandwiched between two disulfide bonds. C1275 is likely to be active in forming interchain disulfide bonds and could also disrupt other disulfide bonds when CUB-1 is expressed as an isolated domain. However, this thiol becomes less active when CUB-1 is expressed as a part of either CUB-1+2 construct, suggesting an interaction between the two CUB domains is required to stabilize the overall structure of CUB-1 domain. Consistent with the notion, the C1275S mutant in CUB-1 domain, which replaced the active C1275 to a serine residue, also reduced a covalent aggregation of the recombinant.
The first four cysteine residues could be structurally critical for the stability of the CUB-1 domain. To test this possibility, we have constructed and expressed five rADAMTS-13 mutants with each of the five cysteine residues in the CUB-1 domain individually converted to serine. We found that the secretion of four consensus cysteine mutants into the conditioned medium was significantly reduced, whereas C1275S was secreted normally (Figure 2). The reduced secretion is not caused by defects in synthesis because the intracellular pool of the mutants was similar to that of WT ADAMTS-13. Instead, it is partially due to a reversed secretion polarity because the consensus cysteine mutants were secreted relatively more to ECM than to the conditioned medium (Table 1). This targeted secretion and its impairments caused by the cysteine mutants may be important for the VWF-cleaving activity associated with endothelial cells (17;28). We would like to point out that the data are generated using CHO cells. However, ADAMTS-13 is reported to be produced in humans hepatic stellate cells, endothelial cells and megakaryocytic/platelets. This expression diversity suggests that a polarized expression is intrinsic for ADAMTS-13.
In addition to secretion defects, the consensus cysteine mutants were also less active in cleaving plasma VWF under static conditions (Figure 5), but maintained normal activities in cleaving ULVWF strings under flow conditions (Figure 6). In contrast, C1275S that removes the free thiol in CUB-1 retained a full VWF-cleaving activity under static and flow conditions.
Although ADAMTS-13 is a relatively stable metalloprotease with a half-life of 2-3 days in vivo (29), we find that WT rADAMTS-13 was proteolytically cleaved at a rate of 10-30% per week (Figure 3 and Figure 4B). The degradation is unlikely to be caused by autocatalysis because it 1) was not inhibited by EDTA, 2) was not prevented by deleting the catalytic domain, and 3) occurred at different cleavage sites on rADAMTS-13 produced from two fibroblast cell lines (CHO and Hela, Figure 3B). The degradation of rADAMTS-13 has also been noticed in previous studies, but the cause is unknown (8;16;24). We find that all consensus cysteine mutants were more sensitive to proteolysis as compared WT metalloprotease, whereas the C1275S mutant was remarkably resistant. Consistent with the results of cysteine mutants, a truncated ADAMTS-13 lacking the CUB domains was also resistant to proteolysis. The exact reason for the resistance to degradation remains unknown, but the observation indicates a critical role for the unpaired C1275 in the process.
In summary, we have demonstrated that the five cysteine residues in the CUB-1 domain differentially regulate ADAMTS-13 functions. Because of their structural importance, the first four consensus cysteine residues are critical for synthesis, the polarized secretion and the static VWF-cleaving activity of the metalloprotease. However, conversion of C1275, which is less crucial for the biosynthesis, to serine makes the metalloprotease highly resistant to proteolytic degradation through an unknown mechanism.
Footnotes
Disclosure of Conflict of Interests: The authors have no conflict of interest to disclose.
This work was supported by NIH grant HL71895 and HL82808.
References
- 1.Moake JL, Rudy CK, Troll JH, Weinstein MJ, Colannino NM, Azocar J, Seder RH, Hong SL, Deykin D. Unusually large plasma factor VIII: von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;307:1432–1435. doi: 10.1056/NEJM198212023072306. [DOI] [PubMed] [Google Scholar]
- 2.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 3.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Jr, Ginsburg D, Tsai HM. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 4.Romero A, Romao MJ, Varela PF, Kolln I, Dias JM, Carvalho AL, Sanz L, Topfer-Petersen E, Calvete JJ. The crystal structures of two spermadhesins reveal the CUB domain fold. Nat Struct Biol. 1997;4:783–788. doi: 10.1038/nsb1097-783. [DOI] [PubMed] [Google Scholar]
- 5.Bork P, Beckmann G. The CUB domain. A widespread module in developmentally regulated proteins. J Mol Biol. 1993;231:539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg H, Uitdehaag JC, Davies JM, Wallis R, Drickamer K, Weis WI. Crystal structure of the CUB1-EGF-CUB2 region of mannose-binding protein associated serine protease-2. EMBO J. 2003;22:2348–2359. doi: 10.1093/emboj/cdg236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdul AA, Gunasekaran K, Volanakis JE, Narayana SV, Kotwal GJ, Murthy HM. The structure of complement C3b provides insights into complement activation and regulation. Nature. 2006;444:221–225. doi: 10.1038/nature05258. [DOI] [PubMed] [Google Scholar]
- 8.Tao Z, Peng Y, Nolasco L, Cal S, Lopez-Otin C, Li R, Moake JL, Lopez JA, Dong JF. Recombinant CUB-1 domain polypeptide inhibits the cleavage of ULVWF strings by ADAMTS13 under flow conditions. Blood. 2005;106:4139–4145. doi: 10.1182/blood-2005-05-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majerus EM, Anderson PJ, Sadler JE. Binding of ADAMTS13 to von Willebrand factor. J Biol Chem. 2005;280:21773–21778. doi: 10.1074/jbc.M502529200. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Pan W, Rux AH, Sachais BS, Zheng XL. The cooperative activity between the carboxyl-terminal TSP1 repeats and the CUB domains of ADAMTS13 is crucial for recognition of von Willebrand factor under flow. Blood. 2007;110:1887–1894. doi: 10.1182/blood-2007-04-083329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Licht C, Stapenhorst L, Simon T, Budde U, Schneppenheim R, Hoppe B. Two novel ADAMTS13 gene mutations in thrombotic thrombocytopenic purpura/hemolytic-uremic syndrome (TTP/HUS) Kidney Int. 2004;66:955–958. doi: 10.1111/j.1523-1755.2004.00841.x. [DOI] [PubMed] [Google Scholar]
- 12.Pimanda JE, Maekawa A, Wind T, Paxton J, Chesterman CN, Hogg PJ. Congenital thrombotic thrombocytopenic purpura in association with a mutation in the second CUB domain of ADAMTS13. Blood. 2004;103:627–629. doi: 10.1182/blood-2003-04-1346. [DOI] [PubMed] [Google Scholar]
- 13.Assink K, Schiphorst R, Allford S, Karpman D, Etzioni A, Brichard B, van de KN, Monnens L, van den Heuvel L. Mutation analysis and clinical implications of von Willebrand factor-cleaving protease deficiency. Kidney Int. 2003;63:1995–1999. doi: 10.1046/j.1523-1755.63.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 14.Riksen NP, Luken BM, Klasen IS, Voorberg J, Crama N, van Deuren M. Antibodies against the CUB1-2 domains of ADAMTS13 in a patient with benign monoclonal gammopathy: no causal relationship. Haematologica. 2007;92:e74–e76. doi: 10.3324/haematol.11475. [DOI] [PubMed] [Google Scholar]
- 15.Tao Z, Wang Y, Choi H, Bernardo A, Nishio K, Sadler JE, Lopez JA, Dong JF. Cleavage of ultra-large multimers of Von Willebrand factor by C-terminal truncated mutants of ADAMTS-13 under flow. Blood. 2005;106:141–143. doi: 10.1182/blood-2004-11-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng X, Nishio K, Majerus EM, Sadler JE. Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. J Biol Chem. 2003;278:30136–30141. doi: 10.1074/jbc.M305331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang D, Zheng XW, Niiya M, Zheng XL. Apical sorting of ADAMTS13 in vascular endothelial cells and Madin-Darby canine kidney cells depends on the CUB domains and their association with lipid rafts. Blood. 2006;108:2207–2215. doi: 10.1182/blood-2006-02-002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao Z, Anthony K, Peng Y, Choi H, Nolasco L, Rice L, Moake JL, Dong JF. Novel ADAMTS-13 mutations in an adult with delayed onset thrombotic thrombocytopenic purpura. J Thromb Haemost. 2006;4:1931–1935. doi: 10.1111/j.1538-7836.2006.02098.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87:4235–4244. [PubMed] [Google Scholar]
- 20.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 21.Choi H, Aboulfatova K, Pownall HJ, Cook R, Dong JF. Shear-induced disulfide bond formation regulates adhesion activity of von willebrand factor. J Biol Chem. 2007;282:35604–35611. doi: 10.1074/jbc.M704047200. [DOI] [PubMed] [Google Scholar]
- 22.Yammani RR, Seetharam S, Seetharam B. Identification and characterization of two distinct ligand binding regions of cubilin. J Biol Chem. 2001;276:44777–44784. doi: 10.1074/jbc.M106419200. [DOI] [PubMed] [Google Scholar]
- 23.Dong JF, Li CQ, Sae-Tung G, Hyun W, Afshar-Kharghan V, Lopez JA. The cytoplasmic domain of glycoprotein (GP) Ibalpha constrains the lateral diffusion of the GP Ib-IX complex and modulates von Willebrand factor binding. Biochemistry. 1997;36:12421–12427. doi: 10.1021/bi970636b. [DOI] [PubMed] [Google Scholar]
- 24.Majerus EM, Zheng X, Tuley EA, Sadler JE. Cleavage of the ADAMTS13 propeptide is not required for protease activity. J Biol Chem. 2003;278:46643–46648. doi: 10.1074/jbc.M309872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crawley JT, Lam JK, Rance JB, Mollica LR, O'Donnell JS, Lane DA. Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. Blood. 2005;105:1085–1093. doi: 10.1182/blood-2004-03-1101. [DOI] [PubMed] [Google Scholar]
- 26.Sieron AL, Tretiakova A, Jameson BA, Segall ML, Lund-Katz S, Khan MT, Li S, Stocker W. Structure and function of procollagen C-proteinase (mTolloid) domains determined by protease digestion, circular dichroism, binding to procollagen type I, and computer modeling. Biochemistry. 2000;39:3231–3239. doi: 10.1021/bi992312o. [DOI] [PubMed] [Google Scholar]
- 27.Hintze V, Howel M, Wermter C, Grosse BE, Becker-Pauly C, Beermann B, Yiallouros I, Stocker W. The interaction of recombinant subdomains of the procollagen C-proteinase with procollagen I provides a quantitative explanation for functional differences between the two splice variants, mammalian tolloid and bone morphogenetic protein 1. Biochemistry. 2006;45:6741–6748. doi: 10.1021/bi060228k. [DOI] [PubMed] [Google Scholar]
- 28.Turner N, Nolasco L, Tao Z, Dong JF, Moake J. Human endothelial cells synthesize and release ADAMTS-13. J Thromb Haemost. 2006;4:1396–1404. doi: 10.1111/j.1538-7836.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- 29.Furlan M, Robles R, Morselli B, Sandoz P, Lammle B. Recovery and half-life of von Willebrand factor-cleaving protease after plasma therapy in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 1999;81:8–13. [PubMed] [Google Scholar]


