Abstract
Purpose
To analyze pooled clinical data using different radiobiological models and to understand the relationship between biologically effective dose (BED) and tumor control probability (TCP) for stereotactic body radiotherapy (SBRT) of early-stage non-small cell lung cancer (NSCLC).
Method and Materials
The clinical data of 1-, 2-, 3-, and 5-year actuarial or Kaplan-Meier TCP from 46 selected studies were collected for SBRT of NSCLC in the literature. The TCP data were separated for Stage T1 and T2 tumors if possible, otherwise collected for combined stages. BED was calculated at isocenters using six radiobiological models. For each model, the independent model parameters were determined from a fit to the TCP data using the least chi-square (χ2) method with either one set of parameters regardless of tumor stages or two sets for T1 and T2 tumors separately.
Results
The fits to the clinic data yield consistent results of large α/β ratios of about 20 Gy for all models investigated. The regrowth model that accounts for the tumor repopulation and heterogeneity leads to a better fit to the data, compared to other 5 models where the fits were indistinguishable between the models. The models based on the fitting parameters predict that the T2 tumors require about additional 1 Gy physical dose at isocenters per fraction (≤5 fractions) to achieve the optimal TCP when compared to the T1 tumors.
Conclusion
This systematic analysis of a large set of published clinical data using different radiobiological models shows that local TCP for SBRT of early-stage NSCLC has strong dependence on BED with large α/β ratios of about 20 Gy. The six models predict that a BED (calculated with α/β of 20) of 90 Gy is sufficient to achieve TCP ≥ 95%. Among the models considered, the regrowth model leads to a better fit to the clinical data.
INTRODUCTION
Surgery has been the preferred modality for operable early stage non-small cell lung cancer (NSCLC) patients with a 5-year overall survival rates of 50–80% (1, 2). For medically inoperable early stage NSCLC, conventional fractionated radiotherapy resulted in disappointing long-term local control and overall survival rates of 30–70% and 15–30%, respectively (3). Stereotactic body radiotherapy (SBRT) appears to provide outcomes that are comparable with surgery (4). With improvement in technology including image guidance and respiratory motion management, SBRT is increasingly becoming a standard of care for inoperable early stage lung cancer, and possibly an option for medically resectable patients as well. Hypo-fractionated RT of 3–5 Gy per fraction may also be a reasonable option for some patients inappropriate for SBRT (5).
A wide variety of doses and dose fractionation schemes have been used for SBRT. A method to compare different schemes is to use radiobiologic/physical modeling, as the fraction size and overall treatment time have an impact on treatment outcomes and a simple comparison based on physical dose is not adequate. A variety of models have been suggested to express the biological effects of different radiation fractionation schemes. These models typically consider the dose per fraction, number of fractions, total dose, and overall elapsed treatment time. A commonly used overall figure of merit to express cell killing is the biologically effective dose (BED). The linear quadratic (LQ) model has been the most widely applied within the context of conventional doses per fraction. However, the continuously bending survival curve predicted by this model raises concerns of potential overestimation of tumor cell killing at the larger doses per fraction often employed with SBRT. Guerrero and Li (6) proposed a modification of the LQ model that could better handle higher dose ranges with a survival curve that features linear quadratic and linear (LQL) relation between the logarithm of the cell survival and dose in low and high fraction dose ranges, respectively. Park et al. (7) proposed the universal survival curve (USC) model by combining the LQ model in the low dose range and the single hit multi-target model (SHMT) of linear behavior in the high dose range with a smooth transition from the LQ to SHMT model for SBRT. Tai et al. (8) proposed a LQ-inspired regrowth model (hereafter regrowth model) by introducing inter-tumor heterogeneity and tumor regrowth after radiation treatment to describe the survival rate dependence of follow up time. McKenna and Ahmad proposed a modification of the LQ model (mLQ) for SBRT (9). Guckenberger et al. applied a modified linear quadratic and linear (mLQL) model (10,11) to a multi-institution dataset. However, the utility and suitability of these bio-physical models for SBRT are still under debate (12, 13). In addition, dose and fractionation schemes for SBRT remain to be optimized.
This work is to model radiation dose responses in SBRT for early stage NSCLC by analyzing the available published clinical local-control data using various bio-physical models proposed for SBRT or hypofractionation schemes. The model parameters are determined by fitting the clinical data. The results from this modeling study may be informative in guiding clinicians to optimize dose and/or dose fractionation for SBRT of early stage lung cancers.
METHODS
A. Clinical data
A total of 160 reported studies, identified by a thorough literature search of medical journals through May 2014 on SBRT and hypofractionation RT for NSCLC, were reviewed. Among these reports, 46 were identified (14–59) to contain the necessary information based on the selection criteria including tumor control probability (TCP) of primary tumors, detailed radiation dose specification, and the number of patients greater than 10. From each of the reports identified, the 1-, 2-, 3-, and 5-year actuarial or Kaplan-Meier TCP data, obtained directly from the paper or indirectly by extracting from the graphs in the paper, were collected for stages T1 and T2 NSCLC. The data from patients with metastatic disease to the lung were excluded. Among all the patients analyzed, 90% were staged as T1/2N0M0 and 10% were unspecified. Tumor controls were evaluated based on follow-up diagnostic quality CT with very limited aid of positron emission tomography (PET). The TCP data consists of 77% of patient population following the guidelines by Response Evaluation Criteria in Solid Tumors (RECIST) (60), 11% similar to, but slight different from RECIST or the World Health Organization (WHO) (61), 10% unspecified, and 2% neither RECIST nor WHO. It is well known that the pencil beam dose calculation algorithm used in some identified reports is inaccurate at the lung-tissue interface, resulting in erroneous periphery doses (62). In this analysis, all prescribed radiation doses were converted to the isocenter doses based on the specified isodose lines (either from the papers or via private communications with authors) or 80% if unspecified in the paper (6% of the studies analyzed did not specify the prescribed isodose lines). The isocenter doses were preferred in other studies (10, 63). The hypo-fractionated data of at least 3 Gy per fraction were also included to increase the data points at low doses. The dose rates of 1/5 to 1/2 Gy per minute with the same or similar delivery techniques from available data are used to calculate the beam-on time if unavailable for the LQL model (see details below). For studies not reporting TCP data specifically for tumor stage, dose or fractionation, the patient population averaged or median values of prescribed doses, fractionations, or treatment times were used. For studies not reporting the percentage of patient/tumor in a particular stage (T1 or T2), the T1 portion of 50% was assumed if unavailable, since the average of T1 portion was 52% in all selected studies that reported tumor stage details. The selected 46 studies contain 3479 early stage NSCLC patients with prescription doses ranging from 15 to 70 Gy in 1 to 20 fractions, out of which, 211 patients received hypo-fractionated RT with doses from 50 to 60 Gy at 3–4 Gy per fraction, and 3268 patients received RT of at least 6 Gy per fraction.
B. Radiobiological models
The following six models expressed in BED formula were used in this analysis.
-
LQ model:
(1) where α and β characterize intrinsic radiosensitivity of cells, D and d are the total and fractional doses, respectively.
-
The LQL model (6): relating the BED and constant dose-rate exposure with the dose protraction factor G for dose-rate effects as
(2) where G(x) = 2(x + e−x −1)/x2, λ=ln2/Tr is the repair rate (Tr repair half-time) and T the treatment delivery time, Δ is calculated by adjusting the low-dose and the high-dose regions of the model to reproduce lethal–potentially lethal behavior for infinite dose rates.
-
The USC model (7):
(3) where −1/D0 and Dq are the slope and x-intercept of the logarithm survival curve, n the number of fraction. The smooth transition at dose dT from the LQ to SHMT yields and .
-
The mLQ model (9):
(4) where γ is a model parameter to take high fractionation dose effects into account. The model reduces to the LQ model at low fractional doses.
-
where dT is the transition dose between the LQ model at low doses and linear behavior at high doses.
For the above five models, the TCP has the form(6) where K0 is the number of tumor cells at the beginning of radiotherapy.
- The regrowth model (8):
(7)
For this model, TCP has the form:
| (8) |
with
| (9) |
Γ is elapsed treatment time; Tp potential effective tumor doubling time; τ the follow-up time after treatment; δ a parameter characterizing the speed of tumor cell regrowth after radiation treatment, Tp is the conventional doubling time when δ→1; Kcr the critical tumor number that defines the control of an individual tumor; σk the Gaussian width for the distribution of tumor cell numbers. The six independent model parameters are: α, α/β, , Tp, and δ.
The key formula and model parameters of these 6 models are summarized in a table in the supplementary material.
C. Model fitting to the clinical data
To model the regrowth of the residual tumor cells after treatment, the term in Eq. (9) is used. The regrowth model takes the follow-up time into account, and it was used to fit the TCP data as a function of serial follow-up times. Other models were used to fit the data only at a given follow up time. For each of the models considered, the independent model parameters were determined from a simultaneous fit to the TCP data using the least chi-square (χ2) fitting method. The parameter sets were obtained for tumor stage T1 and T2 separately or combined for the regrowth model. For all other models, one set of the model parameters was obtained with stages T1 and T2 tumor combined due to limited statistics on the TCP at the individual follow-up time. The TCP predicted by the models (Eqs. 6 or 8) is TCPmodel = fT1 * TCPT1model + fT2 * TCPT2model, where fT1 and fT2are the T1 and T2 portions and fT1 + fT2 =1. The χ2 has the form:
with the TCP uncertainty . Ni is the number of patients for the ith data point. The uncertainty for TCP→1 is calculated using the maximum TCP value other than 1 in the clinic data. The fit is weighted by of each data point, so that high-quality data points were considered preferably. Given large statistics of the TCP data, χ2 is expected to be Gaussian-distributed. The goodness of the fitting was justified by χ2/ndf, here ndf is the number of degree of freedom (ndf) defined as the total number of data points minus the number of free parameters in the fit. During the fitting process, BED, model predicted TCP, and χ2 are recalculated if model parameters change until the least χ2 is found. The fitting procedures have been described in details elsewhere using the regrowth model (64) and had been validated in Ref. (8). The regrowth model takes into account the correlations of TCP at different follow-up time automatically as shown in Eq. 8 and it can be used to fit the 1, 2, 3, and 5 year TCP data simultaneously, while all other models are used to fit the TCP data at different follow-up time separately since those correlations are not taken into account.
RESULTS
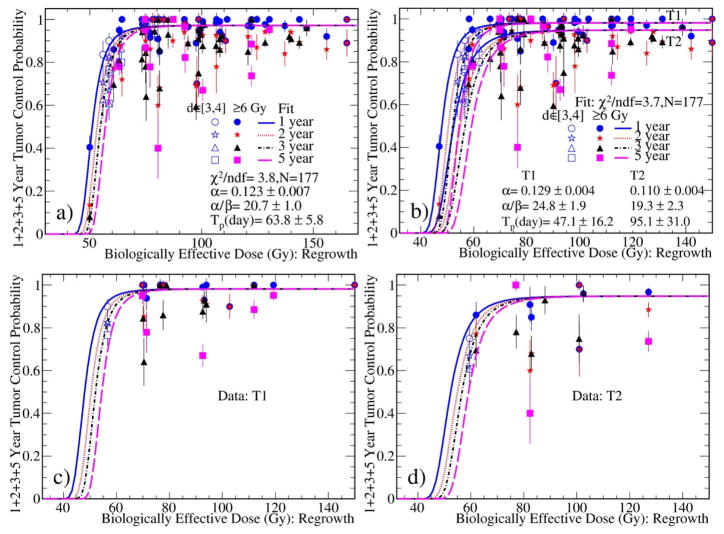
Figure 1a presents the fit to all TCP data with one set of parameters regardless of the tumor stages using the regrowth model. The data points are presented with error bars of one standard deviation. Unless otherwise specified, BED is calculated with the corresponding α/β ratio. Fig. 1b shows the fit to all TCP data with two different sets of parameters for the T1 and T2 tumors separately. It is seen that TCP reached an asymptotic plateau at a BED of about 90 Gy and 110 Gy for T1 and T2 tumors, respectively. Figures 1c and 1d depict the fit curves superimposed over the T1 and T2 sub-datasets. The model parameters determined from the fits with the regrowth model are summarized in Table 1 along with other data.
Fig. 1.
Simultaneous fits to the 1, 2, 3, and 5 year TCP data with a) one set of parameters regardless of the tumor stages, and b) two sets for the stage T1 and T2 tumors, respectively. The data points are shown with error bars of one standard deviation. The BED was calculated using the α/β obtained during the fitting. In Fig. 1b, the BED is calculated using the parameters for the T1 tumors. The fit curves from Fig. 1b are overlaid with the c) T1 and d) T2 sub-datasets.
Table 1.
Model parameters determined from simultaneous fits to 1-, 2-, 3-, and 5-year TCP data with one set of parameters regardless of tumor stages and two sets for Stage T1 and T2 tumors separately using the regrowth model and from the fits to the 1-, 2-, and 3-year TCP using the remaining five models.
| Fit | regrowth | Fit | LQ | USC | mLQ | LQL | mLQL | ||
|---|---|---|---|---|---|---|---|---|---|
| Fits to the data with the same model parameters for the T1 and T2 tumors | χ2/ndf | 3.8 | 1 year | 1.5 | 1.6 | 1.6 | 1.6 | 1.6 | |
| α (Gy−1) | 0.123±0.007 | 0.215±0.022 | 0.215±0.006 | 0.215±0.017 | 0.215±0.017 | 0.215±0.002 | |||
| α/β (Gy) | 20.7±1.0 | 17.9±0.9 | D0=1.1±0.1 Gy | β =0.0120±0.003 Gy−2 | 17.9±1.1 | 17.9±0.6 | |||
| Tp(days)/Dx/γ | Tp=63.8±5.8 days, δ=0.253±0.025, Kcr/K0=0.008±0.003, σK/K0=0.004±0.002 | - | Dq=11.3±0.5 Gy | γ=873±2932 Gy−1 | Δ≈0, Tr=40.5±16.9 days | dT=90.2±9.1 Gy | |||
| Fits to the data with T1 two sets parameters for the T1 and T2 tumors separately | χ2/ndf | 3.7 | 2 year | 4.8 | 4.9 | 4.9 | 5.0 | 4.9 | |
| T1 | α (Gy−1) | 0.129±0.004 | 0.185±0.013 | 0.185±0.022 | 0.185±0.010 | 0.184±0.012 | 0.185±0.001 | ||
| α/β (Gy) | 24.8±1.9 | 26.0±2.5 | D0=1.5±0.1 Gy | β=0.007±0.0007 Gy−2 | 24.4±1.6 | 26.0±0.7 | |||
| Tp(days)/Dx/γ | Tp=47.1±16.2 days, δ=0.267±0.041, Kcr/K0=0.010±0.002, σK/K0=0.005±0.001 | - | Dq=12.2±2.5 Gy | γ=1623±3938 Gy−1 | Δ≈0, Tr=0.3±0.3 | dT=84.0±8.1 Gy | |||
| χ2/ndf | - | 3 year | 7.1 | 7.2 | 7.2 | 7.4 | 7.2 | ||
| T2 | α (Gy−1) | 0.110±0.004 | 0.163±0.010 | 0.163±0.005 | 0.163±0.007 | 0.160±0.010 | 0.163±0.010 | ||
| α/β (Gy) | 19.3±2.3 | 32.5±3.5 | D0=1.7±0.1 Gy | β=0.0050±0.0005 Gy−2 | 29.1±4.1 | 32.5±3.7 | |||
| Tp(days)/Dx/γ | Tp=95.1±31.0 days, δ=0.278±0.035, Kcr/K0=0.012±0.001, σK/K0=0.007±0.001 | - | Dq=16.1±1.2 Gy | γ=884±2781 Gy−1 | Δ≈0, Tr=0.1±0.2 days | dT =57.3±16.8 Gy | |||
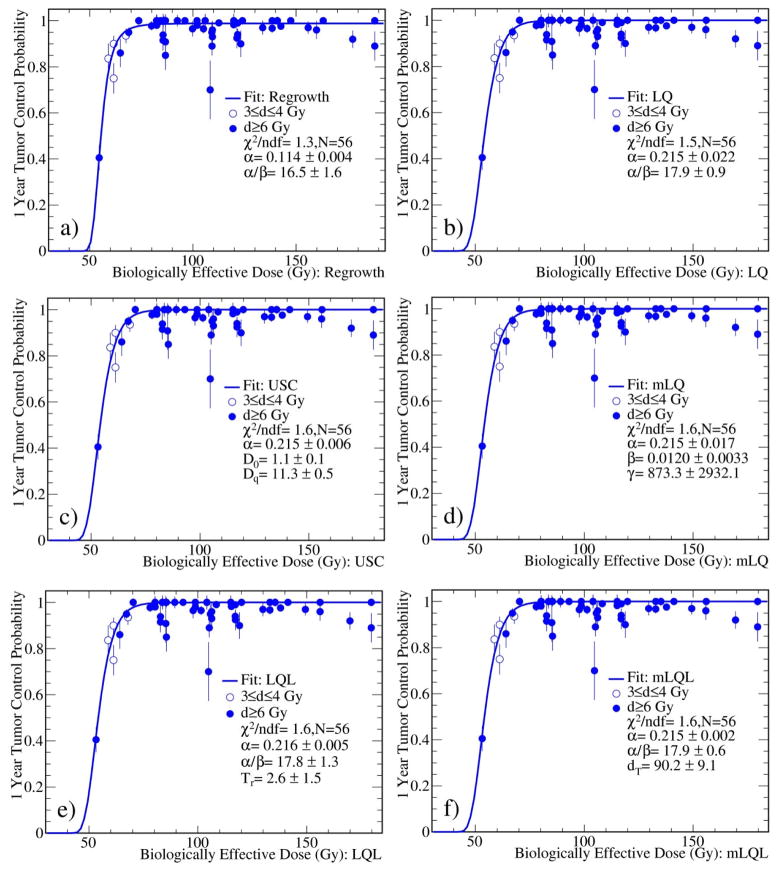
Figure 2 shows the fits to the 1 year TCP data with all six models. The fit with the regrowth model is presented for comparison. All model fits consistently yield large α/β ratios of about 20 Gy. The results are summarized in Table 1. The numbers of cells K0 at the beginning of radiotherapy were found to vary from 104 to 106 for the models considered. It is clear that the fit with the regrowth model yields the lowest χ2/ndf value, indicating the best fit among the six models considered. All other models give indistinguishable fits to the clinical data and consistent radiobiological parameters. For LQL model, the fit results were insensitive to the delivery time since it is a higher order correction.
Fig. 2.
Fits to the 1 year TCP data with different models. The fit with the regrowth model is also shown for comparison.
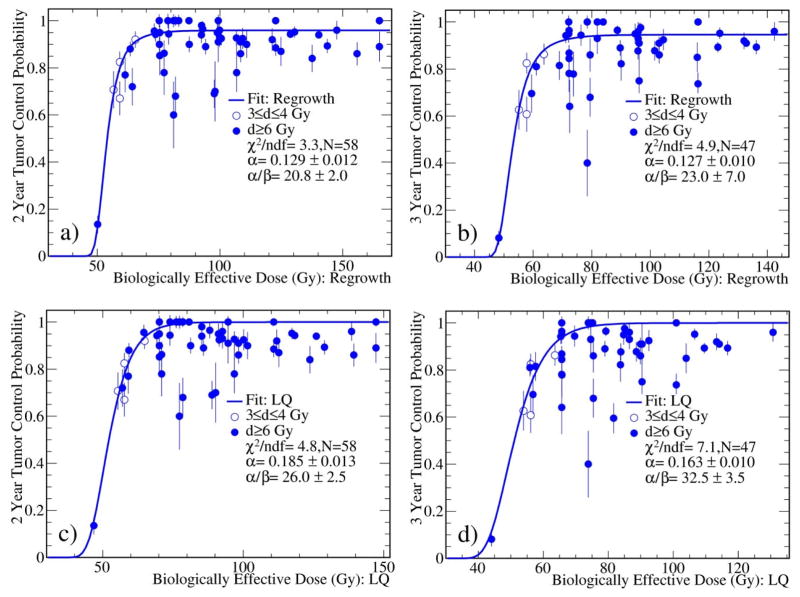
The 2 and 3 year TCP data were also fitted using the six models. The regrowth model again yields the best fits to the data as indicated by the smaller χ2/ndf values. As an example, the fits to the 2 and 3 year TCP data using the regrowth and LQ models were shown in Fig. 3. The fit results are summarized in Table 1. From the table, the α values tend to decrease and α/β ratios tend to increase as the follow-up time increases, reflecting the fact that the tumor control rate decreases as a function of the follow up time.
Fig. 3.
Fits to the 2 (left) and 3 (right) year TCP data with the regrowth and LQ models. The fits to other four models (not shown) are similar to the fits with the LQ model. The fits with the regrowth model are also shown for comparison. The BED was calculated using the α/β obtained during the fitting.
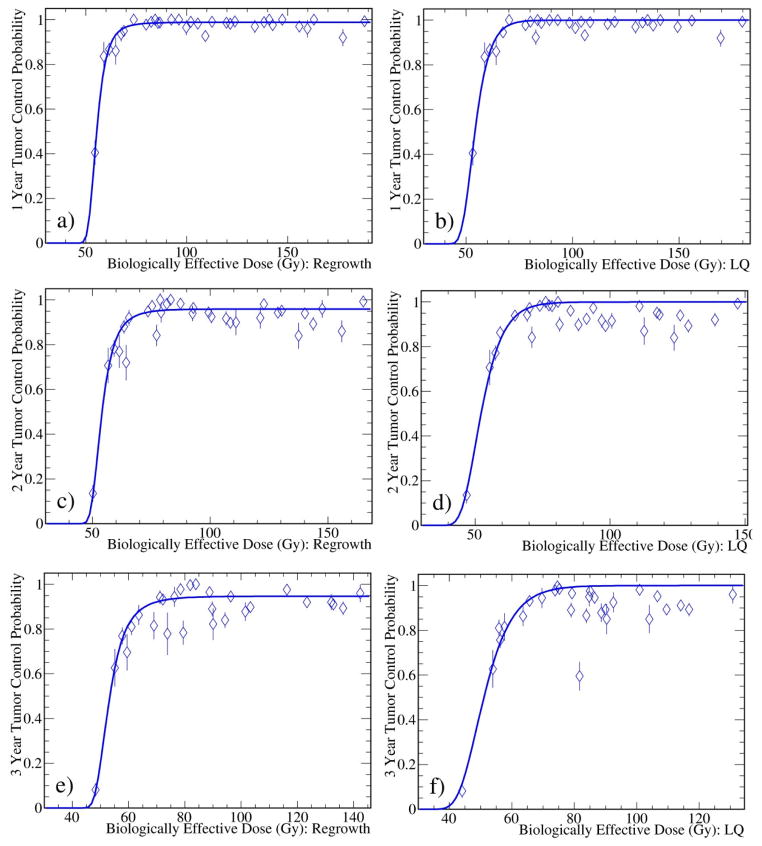
Due to limited statistics of some data points, there exist large fluctuations of the TCP data for a given BED. In Fig. 4, the weighted averages of 1, 2, and 3 year TCP data in BED bins are overlaid with the fit curves of the regrowth and LQ models shown in Figs. 2 and 3. The LQ model is indistinguishable with the remaining four models (not shown). It clearly shows that the regrowth model describes the data better.
Fig. 4.
Weighted averages of 1, 2, and 3 year TCP data in BED bins overlaid with the fit curves in Figs. 2 and 3 for the regrowth (left) and LQ (right) models. The remaining four models show similar patterns (not shown) as the LQ model.
DISCUSSION
We have fitted the selected clinic data using six models by assigning high weights to high-quality data points (studies with large patient numbers) and adjusting a number of fitting parameters including α/β ratio. The fit lead to steep TCP-BED relationships for SBRT of NSCLC, showing that TCP increases drastically with BEDs of 50–60 Gy and reaches an asymptotic plateau around a BED of 90 Gy (see Fig. 1a). This implies that a BED (calculated with the fitted value of α/β = 20) of larger than 90 Gy may lead to a TCP ≥ 95% for T1 tumors based on these 6 models investigated. The regrowth model yields a better fit compared to other models. This is not attributed to a few more model parameters, the reason is that it allows TCP<1 for high BED, permitting the model crosses the data points by their weights, but all other models always have asymptotic plateau of TCP=1 for large BED, while most (~80%) clinical TCP data are <1.
To achieve the optimal TCP, the T2 tumors require approximately additional 1 Gy dose per fraction (up to 5 fractions) compared to the T1 tumors based on the regrowth model. This finding is similar to that for oropharyngeal tumors where additional 3 Gy is required for each increase in T stage (65) for a given TCP.
The maximum likelihood method (ML) is another well-known fitting method. However, when ML was used to fit the TCP data, it run into a divergence problem when a model predicts a TCP=1.0 at high BED. In addition, the ML fitting method does not weight each clinical TCP data based on its statistical error. It was found that the ML fitting yielded a result similar to the χ2 fitting with equal weighting to all TCP data for the growth model. To fit TCP data collected from studies of various number of patients, the least chi-square (χ2) fitting method was therefore chosen to determine the best model parameters.
Various attempts were made to ensure that the steep dose response obtained is not due to the modeling process used. For example, we tested the fitting with empirical formulism, TCP=1/[1+(BED50/BED)κ] and 1/[1+exp[−(BED−BED50)/κ], where BED50 is the BED required to achieve 50% TCP and κ a fitting parameter. Those tests yielded the consistent α/β ratio and the steep TCP-BED relationship as those obtained with the six models. To test whether the steep dose response is attributed mainly by several low BED data points (Fig. 1a), we re-fitted the data with those low BED data points removed and still found the consistent steep dose response (e.g., α=0.110±0.004 Gy−1 and α/β=20.6±3.1 Gy). These fits are different from an analysis (66) where a monotonic relationship between TCP and BED with nearly no asymptotic plateau was reported with empirical α=0.33 Gy−1 and α/β=8.6 Gy for early stage NSCLC. It might be attributed to the different radiobiological parameters used. We also varied the prescribed isodose from 75% to 85% if unspecified in the paper, we found consistent results since only 6% papers did not specify the prescribed isodoses.
The fits to the TCP data with the regrowth model give Kcr/K0≈0 and σK/K0≈0 due to large K0 of 104 to 106, while Kcr/σK is of order of 2 as expected. The fits to the TCP data using the LQL model (6) yield Δ≈0 (Eq. 2), suggesting that the high fractional dose term Δ may be omitted in the LQL model with the large α/β ratio for SBRT of NSCLC. The fits to the data using the USC and mLQL models give high thresholds (dT >30 Gy), implying that the LQ-based BED (see Eqs. 3 and 5) with large α/β ratios can lead to a reasonably fit to the data. The fits using the mLQ model result in large γ values (Eq. 4) indicate that the higher order correction term β/γ is negligible. Amongst all models considered, the regrowth model fits the data with the lowest χ2/ndf and can provide extra information on the potential tumor doubling time (47±16 and 95±31 days for the T1 and T2 tumors, respectively, and 64±6 days regardless of tumor stages), consistent with those in Ref. (67). The T1 tumors grow much faster than the T2 tumors. The regrowth model, accounting for the tumor repopulation, can also predict TCP dependence on the follow-up time as a function of BED and as well as the TCP difference on tumor stage as seen in the clinic data even at higher BED in the plateau region (the term in Eq. 9), while all other models have an asymptotic value of 1 in the plateau region. All fits to the clinical data with different models yield consistently a large α/β ratio of about 20 Gy. The values of α and α/β for the T1 tumors are systematically larger than those for the T2 tumors, meaning that the T1 tumors are more sensitive to radiation. A slightly higher dose is needed to achieve the optimal TCP for T2 tumors when compared to T1 tumors as shown in Table 2. As shown in Table 2, the fits of different models to the TCP data at individual follow up time also yield consistent large α/β ratios.
Table 2.
Fractionation regimens of least doses at isocenters to reach asymptotic plateau for the regrowth model. The 1, 3, 4, and 5 fractions are assumed to be delivered in 1 day, 7 days, 10 days, and 14 days, respectively.
| Regrowth | asymptotic plateau (optimal) | |||
|---|---|---|---|---|
| Isocenter Dose (Gy) | 1 fx | 3 fx | 4 fx | 5 fx |
| T1 | 36.4±1.0 | 53.0±1.0 | 57.6±0.9 | 61.3±0.7 |
| T2 | 37.4±1.1 | 56.1±1.0 | 61.6±0.9 | 65.9±0.9 |
It has been debated whether the widely used LQ model in the conventional dose fractionation schemes be applicable to the SBRT regimens. It is well-known that the LQ model of continuous bending behavior overestimates the cell-killing in the high dose region. However, Fowler (68) pointed out that an α/β ratio greater than 10 Gy could make the LQ model work better in the high dose region. Maciejewski et al (65) reported a good biological evidence of α/β ratios greater than 20 Gy in rapidly proliferating and hypoxic oropharyngeal tumors from the best fits to their clinical data. It was found (69) that NSCLC repopulates approximately as fast as oropharyngeal tumors and therefore was likely to have α/β ratios higher than 10 Gy. The α/β values from our study are of about 20 Gy, agreed in general with the qualitative findings in Refs. 69 and 70 which were not able to provide quantitative α/β ratios for SBRT of NSCLC. The current results demonstrate that the LQ formulism with an α/β value of 20 Gy including tumor heterogeneity and tumor regrowth can describe the tumor dose response after SBRT. Our study shows that the alternative USC, mLQ, LQL, and mLQL models become indistinguishable with the LQ model for the SBRT regimens for NSCLC. Mehta et al (66) also found that the clinical data could not distinguish the LQ and USC models. Brown et al (72) showed in their Figure 1 that, in an idealized case, the LQ model with large α/β ratio can be comparable to the LQL and USC models.
Out of six models considered, the regrowth model specifically takes the population heterogeneity into account. Carlone et al. (73) showed that the population-based TCP model in case of dominant heterogeneity in radio-sensitivity has the form: , where erfc is the complementary error function , σh the combination of the standard deviations of radiobiological parameters, ξ the Euler’s gamma constant with ξ ≈ 0.577. The fits to the clinical data with the population-based TCP using the models other than the regrowth model yield smaller α when compared to those without heterogeneity. The standard deviations σh of different models vary from 0.4 to 1.0. The α/β ratios remain stable and consistent with those from the fits to the data without population heterogeneity as discussed in Ref. 73.
The potential role of hypoxia and reoxygenation were not explicitly incorporated in the models used in this study. It is possible that the extracted value of α/β may be different when hypoxia and reoxygenation are included (74). Therefore, the presently determined α/β value should be considered as an effective radiobiological parameter that is associated with the formulism of a model.
The major sources of inaccuracy in this analysis include the lack of sufficient information and the wide variations in patient selection, dose prescription and fractionations, treatment techniques, radiation dose actually delivered, and outcome assessment in the selected studies. The models used may not be capable of fully considering these data heterogeneities. Therefore the relation between TCP and BED from this meta-data-based analysis may not be the same as the relation between individual patient TCP and BED. Standardization in data report for clinical SBRT study is critically needed. In addition, normal tissue toxicity is not taken into account in this study, although low or mild normal tissue toxicity was reported. Due to the limited information on tumor size or volume from the selected studies, the tumor size-corrected model (75) was not considered in this study.
CONCLUSION
We performed a systematic study to analyze the pooled TCP data for SBRT of early stage NSCLC using six different radiobiological models. The fit to the pooled TCP data with these models yield steep relationships between TCP with BED with a large α/β value of about 20 Gy. The models predict that a BED of 90 Gy, as calculated with α/β of 20 Gy, is sufficient to achieve a TCP of greater than 95% for T1 tumors. The regrowth model leads a better fit than other five models, while the LQ model was the simplest. However, caution should be excised when using the data generated by the models, as insufficient information and/or incomplete models might be used.
Supplementary Material
Acknowledgments
We thank members of the American Association of Physicists in Medicine (AAPM) Working Group on Biological Effects of Hypofractionated Radiotherapy/SBRT (WGSBRT) for helpful discussion
Footnotes
Conflict of Interest
There is no conflict of interest for all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mountain CF. Surgical treatment of lung cancer. Crit Rev Oncol hematol. 1991;11:179–207. doi: 10.1016/1040-8428(91)90020-d. [DOI] [PubMed] [Google Scholar]
- 2.Dominioni L, Imperatori A, Rovera F, et al. Stage I nonsmall cell lung carcinoma: Analysis of survival and implications for screening. Cancer. 2000;89:S2334–S2344. doi: 10.1002/1097-0142(20001201)89:11+<2334::aid-cncr4>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Qiao X, Tullgren O, Lax I, et al. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung cancer. 2003;41:1–11. doi: 10.1016/s0169-5002(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 4.Robinson CG, DeWees TA, El Naqa IM, et al. Patterns of failure after stereotactic body radiation therapy or lobar resection for clinical stage I non-small-cell lung cancer. J Thorac Oncol. 2013;8:192–201. doi: 10.1097/JTO.0b013e31827ce361. [DOI] [PubMed] [Google Scholar]
- 5.Lucas JT, Kuremsky JG, Soike M, et al. Comparison of accelerated hypofractionation and stereotactic body radiotherapy for Stage 1 and node negative Stage 2 non-small cell celllung cancer (NSCLC) Lung Cancer. 2014;85:59–65. doi: 10.1016/j.lungcan.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerrero M, Li XA. Extending the linear-quadratic model for large fraction doses pertinent to stereotactic radiotherapy. Phys Med Biol. 2004;49:4825–4835. doi: 10.1088/0031-9155/49/20/012. [DOI] [PubMed] [Google Scholar]
- 7.Park C, Papiez L, Zhang SC, Story M, Timmerman RD. Universal Survival Curve and Single Fraction Equivalent Dose: Useful Tools in Understanding Potency of Ablative Radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 8.Tai A, Liu F, Gore E, Li XA. An analysis of tumor control probability of stereotactic body radiation therapy for lung cancer with a regrowth model. Phys Med Biol. 2016;61:3903–3913. doi: 10.1088/0031-9155/61/10/3903. [DOI] [PubMed] [Google Scholar]
- 9.McKenna F, Ahmad S. Toward a Unified Survival Curve: in Regard to Kavanagh and Newman (Int J Radiat Oncol Biol Phys 2008; 71: 958–959) and Park et al (Int J Radiat Oncol Biol Phys 2008; 70: 847–852) Int J Radiat Oncol Biol Phys. 2009;73:640. doi: 10.1016/j.ijrobp.2008.08.063. [DOI] [PubMed] [Google Scholar]
- 10.Guckenberger M, Klement RJ, Allgäuer M, et al. Applicability of the linear-quadratic formalism for modeling local tumor control probability in high dose per fraction stereotactic body radiotherapy for early stage non-small cell lung cancer. Radiother Oncol. 2013;109:13–20. doi: 10.1016/j.radonc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Astrahan M. Some implications of linear-quadratic-linear radiation doseresponse with regard to hypofractionation. Med Phys. 2008;35:4161–4672. doi: 10.1118/1.2969065. [DOI] [PubMed] [Google Scholar]
- 12.Brenner DJ. The Linear-Quadratic Model Is an Appropriate Methodology for Determining Isoeffective Doses at Large Doses per Fraction. Semin Radiat Oncol. 2008;18:234–239. doi: 10.1016/j.semradonc.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkpatrick JP, Meyer JJ, Marks LB. The Linear-Quadratic Model Is Inappropriate to Model High Dose per Fraction Effects in Radiosurgery. Semin Radiat Oncol. 2008;18:240–243. doi: 10.1016/j.semradonc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Cheung PCF, Yeung LTF, Basrur V, Ung YC, Balogh J, Danjoux C. Accelerated hypofractionation for early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54:1014–1023. doi: 10.1016/s0360-3016(02)03045-6. [DOI] [PubMed] [Google Scholar]
- 15.Onimaru R, Shirato H, Shimizu S, et al. Tolerance of organs at risks in small-volume, hypofractionated image-guided radiotherapy for primary and metastatic lung cancer. Int J Radiat Oncol Biol Phys. 2003;56:126–135. doi: 10.1016/s0360-3016(03)00095-6. [DOI] [PubMed] [Google Scholar]
- 16.Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63:1427–1431. doi: 10.1016/j.ijrobp.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann FB, Geinitz H, Schill S, et al. Stereotactic hypofractionated radiation therapy for stage I non- small cell lung cancer. Lung Cancer. 2005;48:107–114. doi: 10.1016/j.lungcan.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Xia T, Li H, Sun Q, et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66:117–125. doi: 10.1016/j.ijrobp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 20.Baumann P, Nyman J, Lax I, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer a retrospective analysis of patients treated in the Nordic countries. Acta Oncol. 2006;45:787–795. doi: 10.1080/02841860600904862. [DOI] [PubMed] [Google Scholar]
- 21.Hoyer M, Roed H, Hansen AT, et al. Prospective study of stereotactic radiotherapy of limited-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66:S128–S135. [Google Scholar]
- 22.Koto M, Takai Y, Ogawa Y, et al. A phase II study on stereotactic body radiotherapy for stage I non-small cell lung cancer. Radiother Oncol. 2007;85:429–434. doi: 10.1016/j.radonc.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Hof H, Muenter M, Oetzel D, et al. Stereotactic Single-Dose Radiotherapy (Radiosurgery) Stereotactic Single-Dose Radiotherapy (Radiosurgery) Cancer. 2007;119:148–155. doi: 10.1002/cncr.22763. [DOI] [PubMed] [Google Scholar]
- 24.Salazar OM, Sandhu TS, Lattin PB, et al. Once-weekly high-dose stereotactic body radiotherapy for lung cancer: 6-year analysis of 60 early-stage, 42 locally advanced, and 7 metastatic lung cancers. Int J Radiat Oncol Biol Phys. 2008;72:707–715. doi: 10.1016/j.ijrobp.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 25.Fritz P, Kraus HJ, Blaschke T, et al. Stereotactic, high single-dose irradiation of stage I non-small cell lung cancer (NSCLC) using four-dimensional CT scans for treatment planning. Lung Cancer. 2008;60:193–199. doi: 10.1016/j.lungcan.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Onimaru R, Fujino M, Yamazaki K, et al. Steep dose-response relationship for Stage I non-small-cell lung cancer using hypofractionated high-dose irradiation by real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:374–378. doi: 10.1016/j.ijrobp.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 27.Baumann P, Nyman J, Hoyer M, et al. Outcome in prospective phase II trail of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–3296. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 28.van Zyp Voort, Prevost JB, Hoogeman MS, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: Clinical outcome. Radiothera Oncol. 2009;91:296–300. doi: 10.1016/j.radonc.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 29.McCammon R, Schefter TE, Gaspar LE, Zaemisch R, Gravdahl D, Kavanagh B. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2009;73:112–118. doi: 10.1016/j.ijrobp.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 30.Kopek N, Paludan M, Petersen J, et al. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiother Oncol. 2009;93:402–407. doi: 10.1016/j.radonc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Takeda A, Sanuki N, Kunieda E, et al. Stereotactic body radiotherapy for primary lung cancer at a dose of 50 Gy total in 5 fractions to the periphery of the planning target volume calculated using a superposition algorithm. Int J Radiat Oncol Biol Phys. 2009;73:442–448. doi: 10.1016/j.ijrobp.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 32.Stephans KL, Djemil T, Reddy CA, et al. A Comparison of Two Stereotactic Body Radiation Fractionation Schedules for Medically Inoperable Stage I Non-small Cell Lung Cancer. J Thorac Oncol. 2009;4:976–982. doi: 10.1097/JTO.0b013e3181adf509. [DOI] [PubMed] [Google Scholar]
- 33.Timmerman R, Paulus R, Galvin J, et al. Stereotactic Body Radiation Therapy for inoperable Early Stage Lung Cancer. J Am Med Assoc. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunlap NE, Larner JM, Read PW. Size matters: a comparison of T1 and T2 peripheral non-small-cell lung cancers treated with stereotactic body radiation therapy (SBRT) J Thorac Cardiovasc Surg. 2010;140:583–589. doi: 10.1016/j.jtcvs.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 35.Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung cancer. 2010;68:72–77. doi: 10.1016/j.lungcan.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Bradley JD, El Naqa I, Drzymala RE. Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: the pattern of failure is distant. Int J Radiat Oncol Biol Phys. 2010;77:1146–50. doi: 10.1016/j.ijrobp.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Videtic GM, Stephans K, Reddy C, et al. Intensity-modulated radiotherapy-based stereotactic body radiotherapy for medically inoperable early-stage lung cancer: excellent local control. Int J Radiat Oncol Biol Phys. 2010;77:344–349. doi: 10.1016/j.ijrobp.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Haasbeek CJ, Lagerwaard FJ, Antonisse ME, Slotman BJ, Senan S. Stage I Nonsmall Cell Lung Cancer in Patients Aged 75 Years. Cancer. 2010;116:406–414. doi: 10.1002/cncr.24759. [DOI] [PubMed] [Google Scholar]
- 39.Bral S, Gevaert T, Linthout N, et al. Prospective, Risk-adapted Strategy of Stereotactic Body Radiotherapy for early-stage non-small-cell lung cancer: results of a phase II trial. Int J Radiat Oncol Biol Phys. 2011;80:1343–1349. doi: 10.1016/j.ijrobp.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 40.Nath SK, Sandhu AP, Kim D, et al. Locoregional and distant failure following image-guided stereotactic body radiation for early-stage primary lung cancer. Radiother Oncol. 2011;99:12–17. doi: 10.1016/j.radonc.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Mohammed N, Grills IS, Wong CY, et al. Radiographic and metabolic response rates following image-guided stereotactic radiotherapy for lung tumors. Radiother Oncol. 2011;99:18–22. doi: 10.1016/j.radonc.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Matsuo Y, Shibuya K, Yasushi N, et al. Prognostic factors in stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:1104–1111. doi: 10.1016/j.ijrobp.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 43.Soliman H, Cheung P, Yeung L, et al. Accelerated Hypofractionated Radiotherapy for Early-stage Non-small-cell Lung Cancer: Long-term Results. Int J Radiat Oncol Biol Phys. 2011;79:459–465. doi: 10.1016/j.ijrobp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Olsen JR, Robinson CG, El Naqa I, et al. Dose-response for stereotactic body radiotherapy in early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:e299–e303. doi: 10.1016/j.ijrobp.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 45.Westover KD, Seco J, Adams JA, Lanuti M, Choi NC, Engelsman M, Willers H. Proton SBRT for medically inoperable stage I NSCLC. J Thorac Oncol. 2012;7:1021–1025. doi: 10.1097/JTO.0b013e31824de0bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grills IS, Hope AJ, Guckenberger M, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. J Thorac Oncol. 2012;7:1382–1393. doi: 10.1097/JTO.0b013e318260e00d. [DOI] [PubMed] [Google Scholar]
- 47.Rowe BP, Boffa DJ, Wilson LD, Kim AW, Detterbeck FC, Decker RH. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol. 2012;7:1394–1399. doi: 10.1097/JTO.0b013e3182614bf3. [DOI] [PubMed] [Google Scholar]
- 48.Bibault JE, Prevost B, Dansin E, Mirabel X, Lacornerie T, Lartigau E. Image-guided robotic stereotactic radiation therapy with fiducial-free tumor tracking for lung cancer. Radiat Oncol. 2012;7:102. doi: 10.1186/1748-717X-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clarke K, Taremi M, Dahele M, et al. Stereotactic body radiotherapy (SBRT) for non-small cell lung cancer (NSCLC): is FDG-PET a predictor of outcome? Radiother Oncol. 2012;104:62–66. doi: 10.1016/j.radonc.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 50.Trakul N, Chang CN, Harris J, et al. Tumor Volume-Adapted Dosing in Stereotactic Ablative Radiotherapy of Lung Tumors. Int J Radiat Oncol Biol Phys. 2012;84:231–237. doi: 10.1016/j.ijrobp.2011.10.071. [DOI] [PubMed] [Google Scholar]
- 51.Yung T, Giuliani ME, Le LW, et al. Outcomes of accelerated hypofractionated radiotherapy in stage I non-small-cell lung cancer. Curr Oncol. 2012;19:e264–e269. doi: 10.3747/co.19.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shirata Y, Jingu K, Koto M, et al. Prognostic factors for local control of Stage I non-small cell lung cancer in stereotactic radiotherapy: a retrospective analysis. Radiation Oncology. 2012;7:182. doi: 10.1186/1748-717X-7-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoue T, Katoh N, Onimaru R, et al. Stereotactic body radiotherapy using gated radiotherapy with real-time tumor-tracking for stage I non-small cell lung cancer. Radiat Oncol. 2013;8:69. doi: 10.1186/1748-717X-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duncker-Rohr V, Nestle U, Momm F, et al. Stereotactic ablative radiotherapy for small lung tumors with a moderate dose. Favorable results and low toxicity. Strahlenther Onkol. 2013;189:33–40. doi: 10.1007/s00066-012-0224-y. [DOI] [PubMed] [Google Scholar]
- 55.Robinson CG, DeWees TA, El Naqa IM, et al. Patterns of failure after stereotactic body radiation therapy or lobar resection for clinical stage I non-small-cell lung cancer. J Thorac Oncol. 2013;8:192–201. doi: 10.1097/JTO.0b013e31827ce361. [DOI] [PubMed] [Google Scholar]
- 56.Baschnagel AM, Mangona VS, Robertson JM, Welsh RJ, Kestin LL, Grills IS. Lung Metastases Treated with Image-guided Stereotactic Body Radiation Therapy. Clin Oncol (R Coll Radiol) 2013;25:236–241. doi: 10.1016/j.clon.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Bush DA, Cheek G, Zaheer S, et al. High-Dose Hypofractionated Proton Beam Radiation Therapy Is Safe and Effective for Central and Peripheral Early-Stage Non-Small Cell Lung Cancer: Results of a 12-Year Experience at Loma Linda University Medical Center. Int J Radiat Oncol Biol Phys. 2013;86:964–968. doi: 10.1016/j.ijrobp.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 58.Lucas JT, Kuremsky JG, Soike M, et al. Comparison of accelerated hypofractionation and stereotactic body radiotherapy for Stage 1 and node negative Stage 2 non-small cell celllung cancer (NSCLC) Lung Cancer. 2014;85:59–65. doi: 10.1016/j.lungcan.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang JY, Li QQ, Xu QY, et al. Stereotactic Ablative Radiation Therapy for Centrally Located Early Stage or Isolated Parenchymal Recurrences of Non-Small Cell Lung Cancer: How to Fly in a “No Fly Zone”. Int J Radiat Oncol Biol Phys. 2014;88:1120–1128. doi: 10.1016/j.ijrobp.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 60.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 61.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 62.Kavanagh B, Ding MS, Schefter T, et al. The dosimetric effect of inhomogeneity correction in dynamic conformal arc stereotactic body radiation therapy for lung tumors. J Appl Clin Med Phys. 2006;7:58–63. doi: 10.1120/jacmp.v7i2.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shuryak I, Carlson DJ, Brown JM, Brenner DJ. High-dose and fractionation effects in stereotactic radiation therapy: Analysis of tumor control data from 2965 patients. Radiother Oncol. 2015;115:327–334. doi: 10.1016/j.radonc.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Tai A, Erickson B, Khater KA, Li XA. Estimate of Radiobiologic Parameters From Clincal Data for Biologically Based Treatment Planning for Liver Irradaition. Int J Radiat Oncol Biol Phys. 2008;70:900–907. doi: 10.1016/j.ijrobp.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 65.Maciejewski B, Withers HR, Taylor JMG, Hliniak A. Dose fractionation and regeneration in radiotherapy for cancer of the oral cavity and oropharynx: tumor dose-response and repopulation. Int J Radiat Oncol Biol Phys. 1989;16:831–843. doi: 10.1016/0360-3016(89)90503-8. [DOI] [PubMed] [Google Scholar]
- 66.Mehta N, King CR, Agazaryan N, et al. Stereotactic body radiation therapy and 3- dimensional conformal radiotherapy for stage I nonsmall cell lung cancer: A pooled analysis of biological equivalent dose and local control. Practical Radiat Oncol. 2012;2:288–295. doi: 10.1016/j.prro.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 67.Murai T, Shibamoto Y, Baba F, et al. Progression of Non–Small-Cell Lung Cancer During the Interval Before Stereotactic Body Radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:463–467. doi: 10.1016/j.ijrobp.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Fowler JF. Linear Quadratics is Alive and Well: In Regard to Park et al. (Int. J. Radiat Oncol. Biol. Phys. 2008; 70: 847–852) Int J Radiat Oncol Biol Phys. 2008;72:957–959. doi: 10.1016/j.ijrobp.2008.06.1929. [DOI] [PubMed] [Google Scholar]
- 69.Fowler JF, Chappell RJ. Non–small-cell lung tumors repopulate rapidly during radiation therapy. Int J Radiat Oncol Biol Phys. 2000;46:516–517. doi: 10.1016/s0360-3016(99)00364-8. [DOI] [PubMed] [Google Scholar]
- 70.Chi A, Wen S, Liao ZX, et al. What Would Be the Most Appropriate Ratio in the Setting of Stereotactic Body Radiation Therapy for Early Stage Non-Small Cell Lung Cancer. BioMed Research International. 2013 doi: 10.1155/2013/391021. Article ID 391021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Yang FJ, Li BS, et al. Which Is the Optimal Biologically Effective Dose of Stereotactic Body Radiotherapy for Stage I Non–Small-Cell Lung Cancer? A Meta-Analysis. Int J Radiat Oncol Biol Phys. 2011;81:e305–e316. doi: 10.1016/j.ijrobp.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 72.Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88:254–262. doi: 10.1016/j.ijrobp.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carlone MC, Warkentin B, Stavrev P, Fallone BG. Fundamental form of a population TCP model in the limit of large heterogeneity. Med Phys. 2006;33:1634–1642. doi: 10.1118/1.2193690. [DOI] [PubMed] [Google Scholar]
- 74.Carlson DJ, Keall PJ, Loo BW, JR, et al. Hypofractionation Results in Reduced Tumor Cell Kill Compared to Conventional Fractionation for Tumors With Regions of Hypoxia. Int J Radiat Oncol Biol Phys. 2012;79:1188–1195. doi: 10.1016/j.ijrobp.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohri N, Werner-Wasik M, Grills IS, et al. Modeling Local Control After Hypofractionated Stereotactic Body Radiation Therapy for Stage I Non-Small Cell Lung Cancer: A Report from the Elekta Collaborative Lung Research Group. Int J Radiat Oncol Biol Phys. 2012;84:e379–e384. doi: 10.1016/j.ijrobp.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.